Heterogeneous Catalytic Conversion of Terpenes into Biofuels: An Open Pathway to Sustainable Fuels
Abstract
1. Introduction
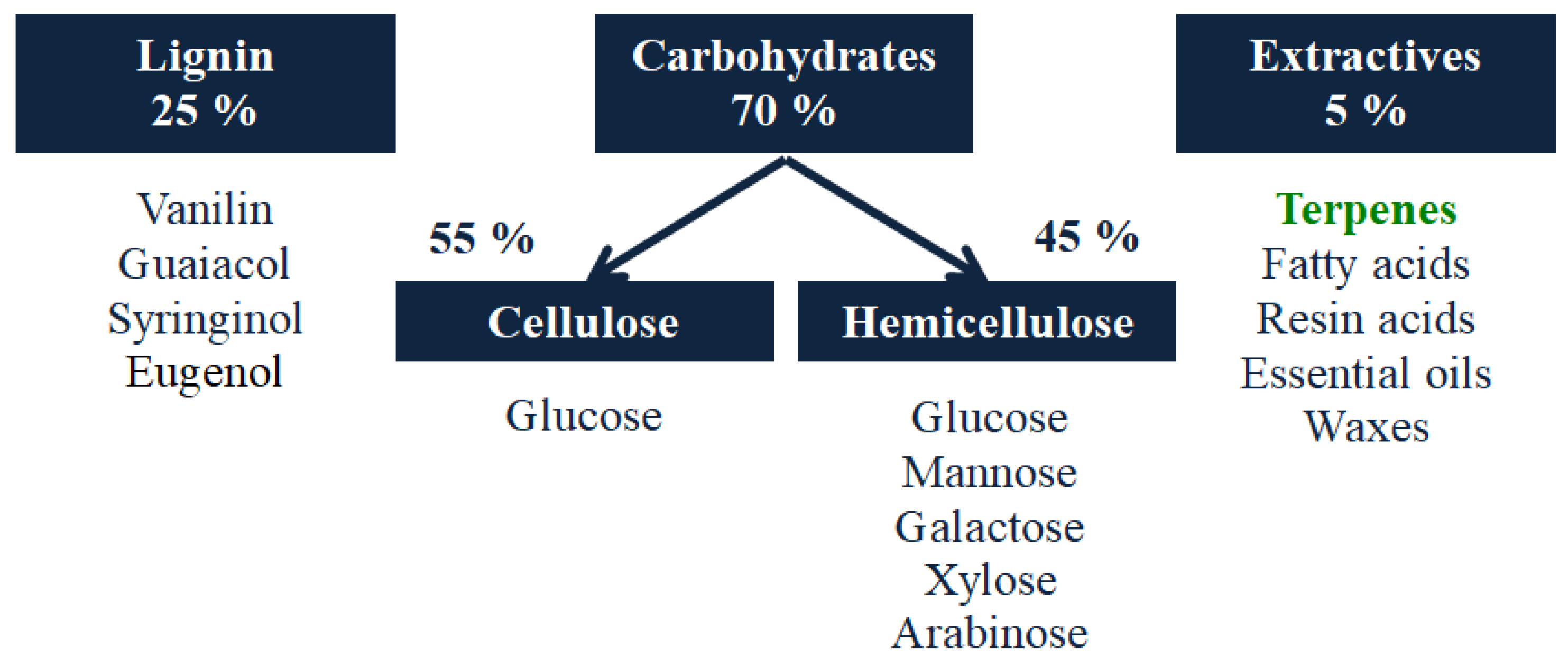
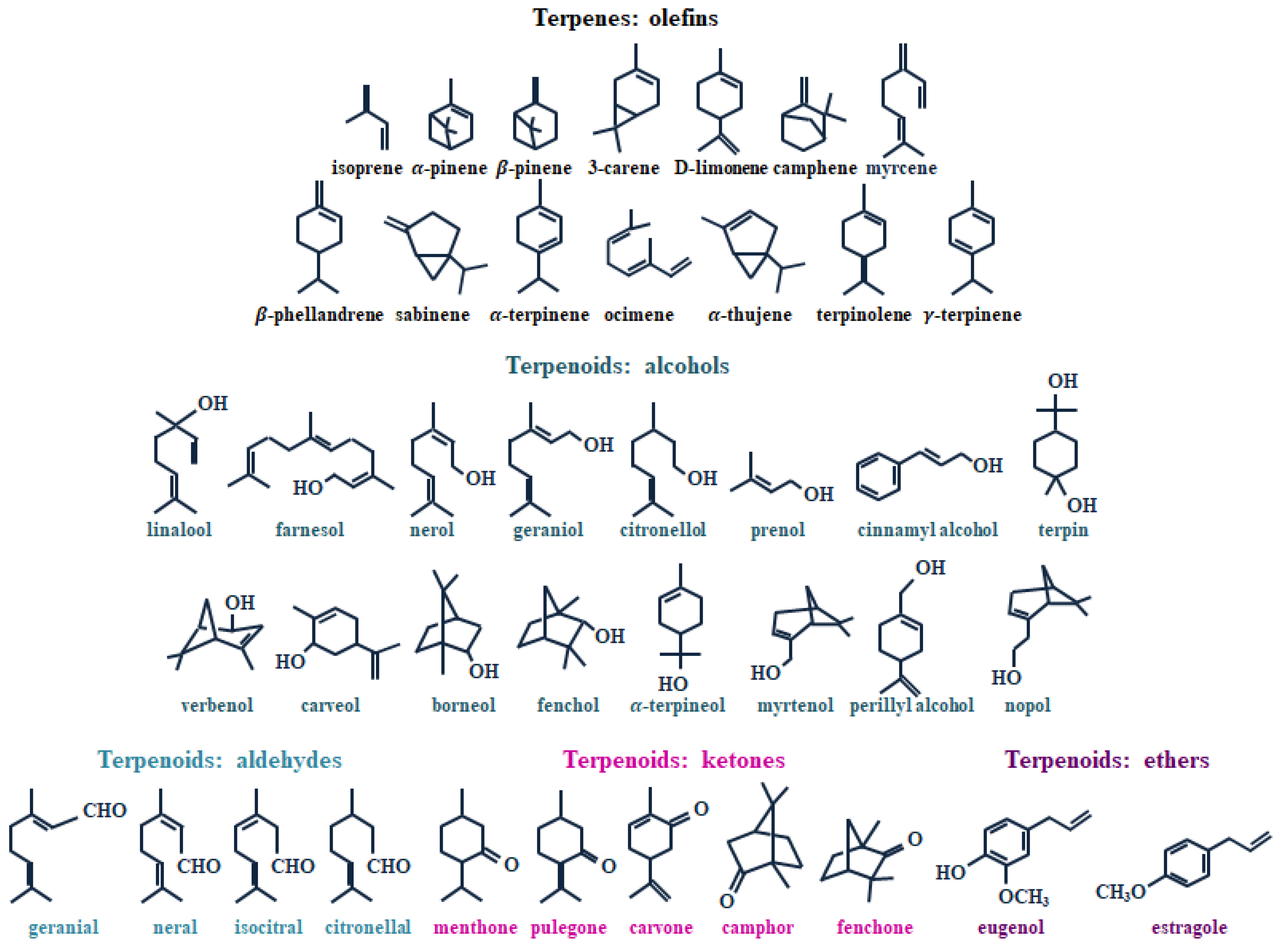
2. Terpenes and Turpentine
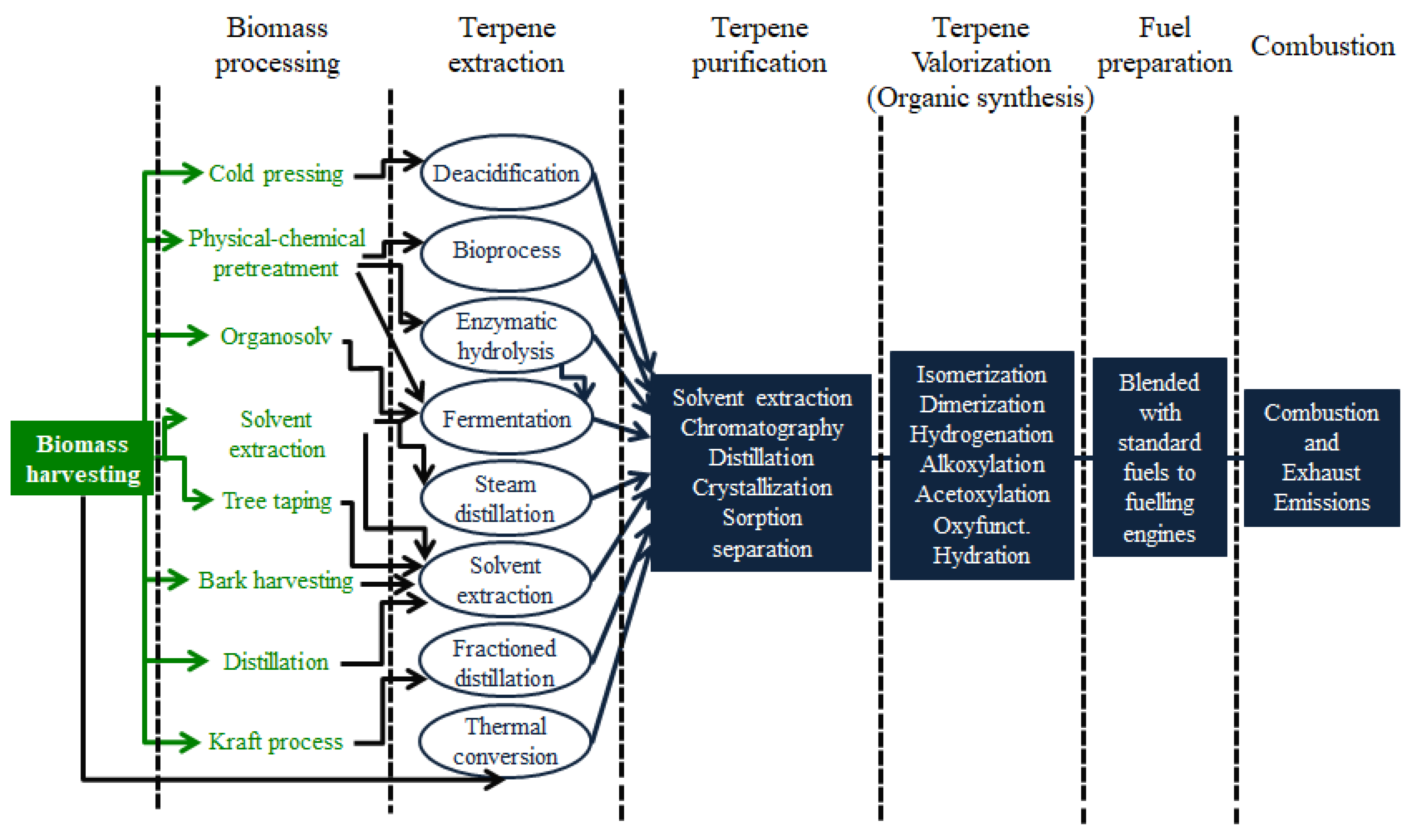
3. Processes for Valorization of Terpenic Feedstock as Fuels
4. Heterogeneous Catalysis for Terpene Conversion
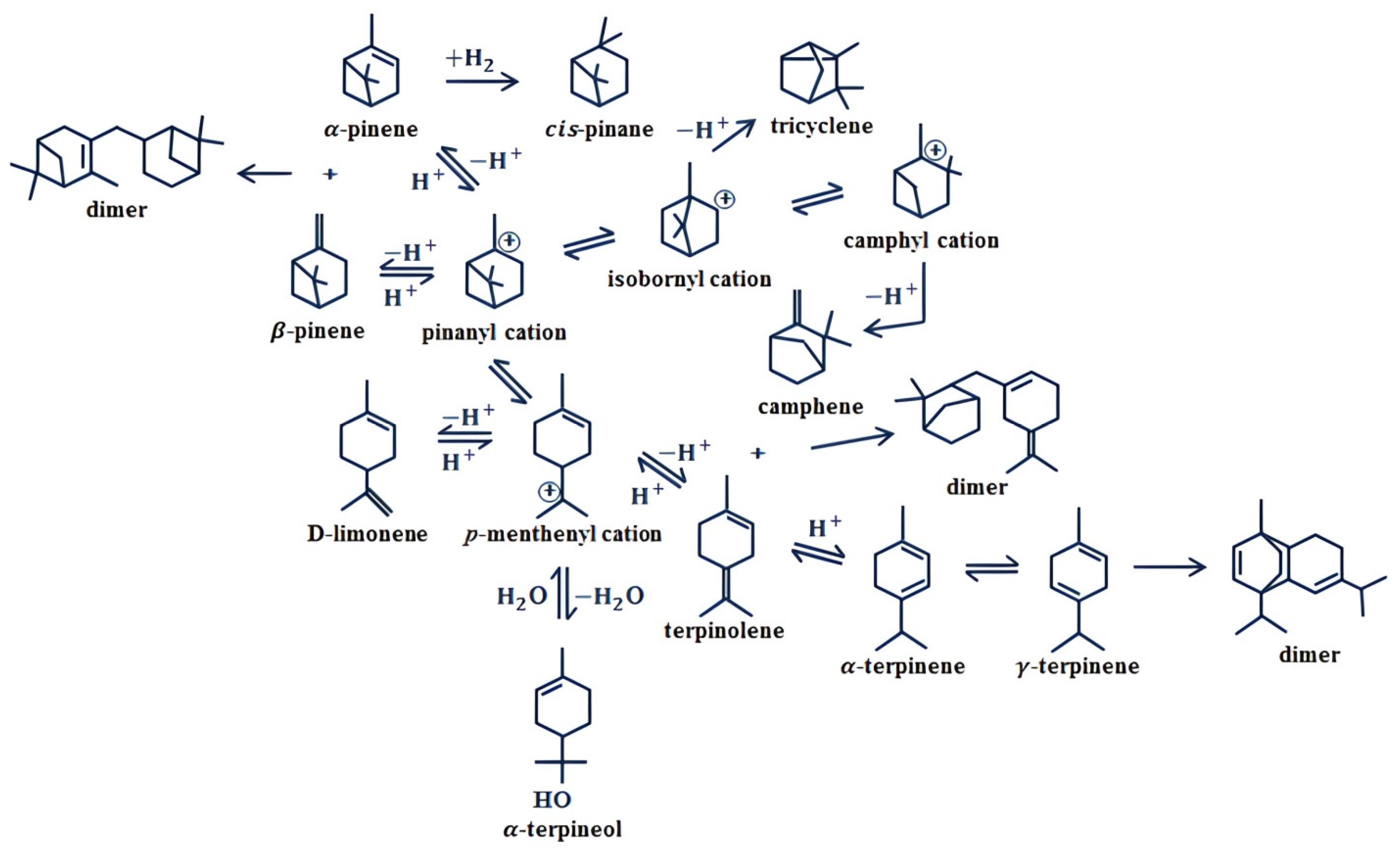
4.1. Isomerization and Dimerization
4.2. Hydrogenation
4.3. Alkoxylation
4.4. Acetoxylation
4.5. Oxyfunctionalization
4.6. Hydration
| Process | Reactant | Catalyst | Load (wt.%) | Temp. (°C) | Time (h) | X (%) | Y (%) | S (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Hydration | α-pinene | Sulphonated carbon | 21.9–46.5 | 50 | nr | 95 | 55.1 7 | 58 | [96] |
| TCA/ZrO2·nH2O | 15 | 80 | 2 | 57 | 32.49 7* | 57 | [56] | ||
| Kraft lignin | 15 | 80 | 24 | 97.8 | 52.2 7* | 53.4 | [94] | ||
| Amberlyst 15 | 20 | 70 | nr | 93.12 | 35.2 7* | 39.21 | [59] | ||
| Rice straw ct 240 °C | 11.6 | 80 | 24 | 57.23 | 20.18 7* | 35.27 | [95] | ||
| Rice straw ct 300 °C | 11.6 | 80 | 24 | 67.60 | 38.58 7* | 57.07 | [95] | ||
| Rice straw ct 350 °C | 11.6 | 80 | 24 | 84.16 | 11.10 7* | 13.20 | [95] | ||
| Rice straw ct 300 °C | 11.6 | 50 | 24 | 55.33 | 20.39 7* | 36.85 | [95] | ||
| Rice straw ct 300 °C | 11.6 | 120 | 24 | 74.05 | 25.19 7* | 34.02 | [95] | ||
| Oxyfunctionalization | Cr-APO5 | nr | 80 | nr | 85 | 65.6 2 | 77 | [24] | |
| Pt-Nb2O5 | nr | 170 | 360 | 99 | nr | 95 | [86] | ||
| Alkoxylation | PW1-SBA15 | 1.8 | 80 | 85 | 62 | 32.9 6 | 53 | [85] | |
| PW2-SBA15 | 6.5 | 80 | 85 | 73 | 38 6 | 52 | [85] | ||
| PW3-SBA15 | 7.7 | 80 | 85 | 84 | 45.4 6 | 54 | [85] | ||
| PW4-SBA15 | 10.2 | 80 | 85 | 93 | 51.2 6 | 55 | [85] | ||
| PW5-SBA15 | 19.3 | 80 | 85 | 79 | 41.9 6 | 53 | [85] | ||
| -pinene | PW4-SBA15 | 10.2 | 80 | 85 | 99 | 48.5 6 | 49 | [85] | |
| limonene | PW4-SBA15 | 10.2 | 80 | 85 | 55 | 35.8 6 | 65 | [85] | |
| Hydrogenation | pinane-2-hydro peroxide | Pd/C | 4 | 20–80 (1–11 bar) | nr | 100 | 90.5 3 | 90.5 | [98] |
| α-pinene | Pd-Charcoal | 10 | 25 | 240 | 100 | 68 9 | nr | [88] | |
| -pinene | Pd-Charcoal | 10 | 25 | 240 | 100 | 72 9 | nr | [88] | |
| α-pinene | Pt-Alumina | 5 | 25 | 2880 | 95 | 93 | nr | [88] | |
| -pinene | Pt-Alumina | 5 | 25 | 2880 | 96 | 88 | nr | [88] | |
| α-pinene | Pd/C | 4 | 20 (11 bar) | nr | nr | 83 4 | nr | [98] | |
| Hydrodeoxygenation | Menthol | Nb2O5 | 0.5 c/m | 190 (20 bar) | 4 | 100 | 3 5 | 3 | [86] |
| Ru/Nb2O5 | 0.5 c/m | 190 (20 bar) | 4 | 71 | 38 5 | 54 | [86] | ||
| Ru/SiO2 | 0.5 c/m | 190 (20 bar) | 4 | 12 | 2 5 | 17 | [86] | ||
| Pd/Nb2O5 | 0.5 c/m | 190 (20 bar) | 4 | 100 | 88 5 | 88 | [86] | ||
| Pd/SiO2 | 0.5 c/m | 190 (20 bar) | 4 | 18 | 11 5 | 59 | [86] | ||
| Pt/Nb2O5 | 0.5 c/m | 190 (20 bar) | 4 | 100 | 86 5 | 86 | [86] | ||
| Low dispersion Pt/Nb2O5 | 0.5 c/m | 190 (20 bar) | 4 | 99 | 94 5 | 95 | [86] | ||
| Low dispersion Pt/Nb2O5 | 0.5 c/m | 170 (20 bar) | 4 | 99 | 94 5 | 95 | [86] | ||
| Low dispersion Pt/Nb2O5 | 0.5 c/m | 150 (20 bar) | 4 | 18 | 10 5 | 57 | [86] | ||
| conversion to methyl cyclopentadiene | linalool | Ru-based organic | 0.1–5 mol.% | 25–60 | 15 min-16 h | nr | 0–100 8 | nr | [80] |
5. Production and Commercialization of Terpenes: Challenges for Their Use as Fuels
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhongnan, R.; Hadlich, R.R.; Yang, R.; Dayton, D.C.; Mante, O.D.; Assanis, D. Experimental investigation of naphthenic biofuel surrogate combustion in a compression ignition engine. Fuel 2022, 312, 122868. [Google Scholar] [CrossRef]
- García, D.; Ramos, A.; Rodríguez-Fernández, J.; Bustamante, F.; Alarcón, E.; Lapuerta, M. Impact of oxyfunctionalized turpentine on emissions from a Euro 6 diesel engine. Energy 2020, 201, 117645. [Google Scholar] [CrossRef]
- Hocko, M.; Al-Rabeei, S.; Košcáková, M. Effects of FAME Biofuel and Jet A-1 Aviation Kerosene Blends on the Operating Characteristics of Aircraft Jet Engines. Appl. Sci. 2023, 13, 971. [Google Scholar] [CrossRef]
- Donoso, D.; Ballesteros, R.; Bolonio, D.; García-Martínez, M.J.; Lapuerta, M.; Canoira, L. Hydrogenated Turpentine: A Biobased Component for Jet Fuel. Energy Fuels 2021, 35, 1465–1475. [Google Scholar] [CrossRef]
- Wilson, G.R.; Edwards, T.; Corporan, E.; Freerks, R.L. Certification of alternative aviation fuels and blend components. Energy Fuels 2013, 27, 962–966. [Google Scholar] [CrossRef]
- Duong, L.H.; Reksowardojo, I.K.; Soerawidjaja, T.H.; Fujita, O.; Neonufa, G.F.; Nguyen, T.T.; Prakoso, T. Soap-derived biokerosene as an aviation alternative fuel: Production, composition, and properties of various blends with jet fuel. Chem. Eng. Process 2020, 153, 107980. [Google Scholar] [CrossRef]
- Harvey, B.G.; Wright, M.E.; Quintana, R.L. High-density renewable fuels based on the selective dimerization of pinenes. Energy Fuels 2010, 24, 267–273. [Google Scholar] [CrossRef]
- Woodroffe, J.D.; Lupton, D.V.; Garrison, M.D.; Nagel, E.M.; Siirila, M.J.; Harvey, B.G. Synthesis and fuel properties of high-energy density cyclopropanated monoterpenes. Fuel Process Technol. 2021, 222, 106952. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, N.; Lapkin, A.A. Towards circular economy: Integration of bio-waste into chemical supply chain. Curr. Opin. Chem. Eng. 2019, 26, 148–156. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-Based Natural Deep Eutectic Systems as Efficient Solvents To Recover Astaxanthin from Brown Crab Shell Residues. ACS Sustain. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Külheim, C.; Peter, G.F.; Tuskan, G.A. Plant-derived terpenes: A feedstock for specialty biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef]
- Knuuttila, P. Wood sulphate turpentine as a gasoline bio-component. Fuel 2013, 104, 101–108. [Google Scholar] [CrossRef]
- Krishna, G.; Prasad, A.R.S.; Krishna, M.V.; Kotebavi, V. The effects of turpentine on engine and emission characteristics of SI and CI engines. AIP Conf. Proc. 2021, 2358, 110003. [Google Scholar] [CrossRef]
- Yumrutaş, R.; Alma, M.H.H.; Özcan, H.; Kaşka, Ö. Investigation of purified sulfate turpentine on engine performance and exhaust emission. Fuel 2008, 87, 252–259. [Google Scholar] [CrossRef]
- Yang, X.; Li, T.; Tang, K.; Zhou, X.; Lu, M.; Ounkham, W.L.; Spain, S.M.; Frost, B.J.; Lin, H. Highly efficient conversion of terpenoid biomass to jet-fuel range cycloalkanes in a biphasic tandem catalytic process. Green Chem 2017, 19, 3566–3573. [Google Scholar] [CrossRef]
- Cho, S.M.; Choi, J.H.; Kim, J.H.; Koo, B.; Choi, I.G. Catalytic Conversion of α-Pinene to High-Density Fuel Candidates Over Stannic Chloride Molten Salt Hydrates. Appl. Sci. 2020, 10, 7517. [Google Scholar] [CrossRef]
- Raman, V.; Sivasankaralingam, V.; Dibble, R.; Sarathy, S.M. α-Pinene-a High Energy Density Biofuel for SI Engine Applications; SAE Technical Paper 2016-01-2171; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, Y.; Choi, J.W.; Jae, J.; Ha, J.M.; Suh, D.J.; Choi, J.; Lee, K.Y. Production of high-energy-density fuels by catalytic β-pinene dimerization: Effects of the catalyst surface acidity and pore width on selective dimer production. Energy Convers. Manag. 2016, 116, 72–79. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, P.; Liu, X.; Hou, Y.; Yang, X.; Shan, S.; Ma, Y.; Pan, D.; Dong, B.; Guo, Z. Preparation of High-Density Fuel Through Dimerization of 𝛽-Pinene. Chem. Eng. Technol. 2020, 43, 2259–2265. [Google Scholar] [CrossRef]
- Tippmann, S.; Chen, Y.; Siewers, V.; Nielsen, J. From flavors and pharmaceuticals to advanced biofuels: Production of isoprenoids in Saccharomyces cerevisiae. Biotechnol. J. 2013, 8, 1435–1444. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Lai, Y.; Mo, Q.; Yuan, J. High-yielding terpene-based biofuel production in Rhodobacter capsulatus. ACS Synth Biol. 2021, 10, 1545–1552. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Recent progress in synthesis of fine and specialty chemicals from wood and other biomass by heterogeneous catalytic processes. Catal. Rev. 2007, 49, 197–340. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Díaz, Y.; Rodríguez, L.; Pohl, S.; Lapuerta, M.; Piloto-Rodríguez, R. Biomass-based heterogeneous catalysts for biodiesel production: A comprehensive review. Int. J. Energy Res. 2021, 46, 3782–3809. [Google Scholar] [CrossRef]
- Kumar, V.; Agarwal, A.K. A review on catalytic terpene transformation over heterogeneous catalyst. IJCRCPS 2014, 1, 78–88. [Google Scholar]
- Gusevskaya, E.V. Reactions of terpenes catalyzed by heteropoly compounds: Valorization of biorenewables. ChemCatChem 2014, 6, 1506–1515. [Google Scholar] [CrossRef]
- Pahima, E.; Hoz, S.; Ben-Tzion, M.; Major, D.T. Computational design of biofuels from terpenes and terpenoids. Sustain. Energy Fuels 2019, 3, 457–466. [Google Scholar] [CrossRef]
- Simakova, I.L.; Simakov, A.V.; Murzin, D.Y. Valorization of Biomass Derived Terpene Compounds by Catalytic Amination. Catalysts 2018, 8, 365. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Quintana, R.L.; Harvey, B.G. Efficient conversion of pure and mixed terpene feedstocks to high density fuels. Fuel 2012, 97, 560–568. [Google Scholar] [CrossRef]
- Yanowitz, J.; Ratcliff, M.A.; McCormick, R.L.; Taylor, J.D.; Murphy, M.J. Compendium of Experimental Cetane Numbers NREL/TP-5400-67585; National Renewable Energy Lab.(NREL): Golden, CO, USA, 2017. [Google Scholar]
- Comelli, F.; Ottani, S.; Francesconi, R.; Castellari, C. Densities, viscosities, and refractive indices of binary mixtures containing n-hexane+ components of pine resins and essential oils at 298.15 K. J. Chem. Eng. Data 2002, 47, 93–97. [Google Scholar] [CrossRef]
- Hellier, P.; Al-Haj, L.; Talibi, M.; Purton, S.; Ladommatos, N. Combustion and emissions characterization of terpenes with a view to their biological production in cyanobacteria. Fuel 2013, 111, 670–688. [Google Scholar] [CrossRef]
- Gonçalves, D.; Paludetti, M.F.; Florido, P.M.; Tonetti, C.; Gonçalves, C.B.; Rodrigues, C.E.C. Physical behavior of the phases from the liquid-liquid equilibrium of citrus essential oils systems at 298.2 K. J. Chem. Eng. Data 2018, 63, 2718–2737. [Google Scholar] [CrossRef]
- Monica, F.D.; Kleij, A.W. From terpenes to sustainable and functional polymers. Polym. Chem. 2020, 1, 5109–5127. [Google Scholar] [CrossRef]
- García, D.; Bustamante, F.; Villa, A.L.; Lapuerta, M.; Alarcón, E. Oxyfunctionalization of turpentine for fuel applications. Energy Fuel 2020, 34, 579–586. [Google Scholar] [CrossRef]
- García, D.; Bustamante, F.; Alarcón, E.; Donate, J.M.; Canoira, L.; Lapuerta, M. Improvements of thermal and thermochemical properties of rosin by chemical transformation for its use as biofuel. Waste Biomass Valorization 2020, 11, 6383–6394. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a natural base chemical in sustainable chemistry: A critical review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Tsolakis, N.; Bam, W.; Srai, J.S.; Kumar, M. Renewable chemical feedstock supply network design: The case of terpenes. J. Clean Prod. 2019, 222, 802–822. [Google Scholar] [CrossRef]
- Donoso, D.; García, D.; Ballesteros, R.; Lapuerta, M.; Canoira, L. Hydrogenated or oxyfunctionalized turpentine: Options for automotive fuel components. RSC Adv. 2021, 11, 18342–18350. [Google Scholar] [CrossRef]
- Alsharif, A.; Smith, N.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydroisomerisation of α-Pinene and Limonene to ρ-Cymene over Silica-Supported ZnO in the Gas Phase. Catalysts 2021, 11, 1245. [Google Scholar] [CrossRef]
- Erman, M.B.; Kane, B.J. Chemistry around pinene and pinane: A facile synthesis of cyclobutanes and oxatricyclo-derivative of pinane from cis- and trans-pinanols. Chem. Biodivers. 2008, 5, 910–919. [Google Scholar] [CrossRef]
- Swift, K.A. Catalytic transformations of the major terpene feedstocks. Top. Catal. 2004, 27, 143–155. [Google Scholar] [CrossRef]
- Woodroffe, J.; Harvey, B.G. High-performance, biobased, jet fuel blends containing hydrogenated monoterpenes and synthetic paraffinic kerosenes. Energy Fuels 2020, 34, 5929–5937. [Google Scholar] [CrossRef]
- Yu, F.; Xie, L.; Wu, F.; Yuan, B.; Xie, C.; Yu, S.; Liu, X.; Wang, L.; Wang, D. Mild Hydrogenation of α-Pinene Catalyzed by Ru Nanoparticles Loaded on Boron-doped Amphiphilic Core-Shell Mesoporous Molecular Sieves. ChemCatChem 2019, 11, 1518–1525. [Google Scholar] [CrossRef]
- Casella, M.L.; Santori, G.F.; Moglioni, A.; Vetere, V.; Ruggera, J.F.; Iglesias, G.M.; Ferretti, O.A. Stereoselective hydrogenation of terpenes using platinum-based catalysts. Appl. Catal. A-Gen. 2007, 318, 1–8. [Google Scholar] [CrossRef]
- Bernas, H.; Bernas, A.; Mäki-Arvela, P.; Leino, R.; Murzin, D.Y. Hydrogenation of geraniol using ruthenium-BINAP catalysts. Catal. Sci. Technol. 2012, 2, 1901–1907. [Google Scholar] [CrossRef]
- Huang, Y.; Qiu, S.; Xu, J.; Lian, H. Hydrogenation of Citral to Citronellal Catalyzed by Waste Fluid Catalytic Cracking Catalyst Supported Nickel. ACS Omega 2020, 6, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Arvela, P.; Kumar, N.; Paseka, I.; Salmi, T.; Murzin, D.Y. Support effects in nerol hydrogenation over Pt/SiO2, Pt/H-Y and Pt/H-MCM-41 catalysts. Catal. Lett. 2004, 98, 173–179. [Google Scholar] [CrossRef]
- Keller, C.L.; Doppalapudi, K.R.; Woodroffe, J.D.; Harvey, B.G. Solvent-free dehydration, cyclization, and hydrogenation of linalool with a dual heterogeneous catalyst system to generate a high-performance sustainable aviation fuel. Commun. Chem. 2022, 5, 113. [Google Scholar] [CrossRef]
- Alsalme, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. α-Pinene isomerisation over heteropoly acid catalysts in the gas-phase. Appl. Catal. A-Gen. 2010, 390, 219–224. [Google Scholar] [CrossRef]
- Comelli, N.A.; Grzona, L.; Masini, O.; Ponzi, E.N.; Ponzi, M.I. Obtention of camphene with H3PW12O40 catalysts supported on TiO2, SiO2 and ZrO2nH2O. J. Chil. Chem. Soc. 2004, 49, 245–250. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wróblewska, A. The isomerization of limonene over the Ti-SBA-15 catalyst-the influence of reaction time, temperature, and catalyst content. Catalysts 2017, 7, 273. [Google Scholar] [CrossRef]
- Zou, J.J.; Chang, N.; Zhang, X.; Wang, L. Isomerization and dimerization of pinene using Al-incorporated MCM-41 mesoporous materials. ChemCatChem 2012, 4, 1289–1297. [Google Scholar] [CrossRef]
- Polo, H.P.; Lopes, N.P.G.; da Silva, M.J. Exploring the Keggin-Type Heteropolyacid-Catalyzed Reaction Pathways of the 𝛽-Pinene with Alkyl Alcohols. Catal. Lett. 2019, 149, 2844–2853. [Google Scholar] [CrossRef]
- Ávila, M.C.; Comelli, N.A.; Rodríguez-Castellón, E.; Jiménez-López, A.; Flores, R.C.; Ponzi, E.N.; Ponzi, M.I. Study of solid acid catalysis for the hydration of α-pinene. J. Mol. Catal. A Chem. 2010, 322, 106–112. [Google Scholar] [CrossRef]
- Mochida, T.; Ohnishi, R.; Horita, N.; Kamiya, Y.; Okuhara, T. Hydration of α-pinene over hydrophobic zeolites in 1,4-dioxane-water and in water. Microporous Mesoporous Mater. 2007, 101, 176–183. [Google Scholar] [CrossRef]
- Wijayati, N.; Pranowo, H.D.; Jumina, J.; Triyono, T. Syntheis of terpineol from α-pinene catalyzed by TCA/Y-Zeolite. Indones. J. Chem. 2011, 11, 234–237. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Zhou, Z.; Zhang, Z. Kinetic study of the direct hydration of turpentine. Chem. Eng. J. 2011, 168, 351–358. [Google Scholar] [CrossRef]
- da Silva, K.A.; Rodrigues, N.V.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly acid catalysts in the valorization of the essential oils: Acetoxylation of β-caryophyllene. Appl. Catal A-Gen. 2010, 374, 87–94. [Google Scholar] [CrossRef]
- Zargar, A.; Bailey, C.B.; Haushalter, R.W.; Eiben, C.B.; Katz, L.; Keasling, J.D. Leveraging microbial biosynthetic pathways for the generation of ‘drop-in’ biofuels. Curr. Opin. Biotechnol. 2017, 45, 156–163. [Google Scholar] [CrossRef]
- Liu, C.L.; Tian, T.; Alonso-Gutierrez, J.; Garabedian, B.; Wang, S.; Baidoo, E.E.K.; Benites, V.; Chen, Y.; Petzold, C.J.; Adams, P.D.; et al. Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 2018, 11, 285. [Google Scholar] [CrossRef]
- Beşün, N.; Özkan, F.; Gündüz, G. Alpha-pinene isomerization on acid-treated clays. Appl. Catal. A-Gen. 2002, 224, 285–297. [Google Scholar] [CrossRef]
- Breen, C.; Moronta, A. Characterization and catalytic activity of aluminium-and aluminum/tetramethylammonium-exchanged bentonites. J. Phys. Chem. B 2000, 104, 2702–2708. [Google Scholar] [CrossRef]
- Handojo, L.; Putra, I.A.; Azis, M.M.; Prakoso, T.; Soerawidjaja, T.H.; Indarto, A. Isomerization of turpentine using various heterogeneous and homogeneous acid catalysts. AIP Conf. Proc. 2019, 2085, 020056. [Google Scholar] [CrossRef]
- Agabekov, V.E.; Sen’kov, G.M.; Sidorenko, A.Y.; Tuyen, N.D.; Tuan, V.A. New α-pinene isomerization catalysts. Catal. Ind. 2011, 3, 319–330. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tanaka, T.; Matsuyama, T.; Funabiki, T.; Yoshida, S. Alumina-supported rare-earth oxides charecterized by acid-catalyzed reactions and spectroscopic methods. J. Phys. Chem. B 2001, 105, 1908–1916. [Google Scholar] [CrossRef]
- Comelli, N.; Grzona, L.; Masini, O.; Ponzi, E.N.; Ponzi, M.I. Catalyst deactivation during α-pinene isomerization. Fe-Mn-promoted sulfated zirconium oxide. React. Kinet. Catal. Lett. 2000, 71, 27–32. [Google Scholar] [CrossRef]
- Luo, J.Y.; Zhang, X.P.; An, X.N. Study on α-pinene isomerization catalyzed by MoO3/ZrO2 solid superacid. Linchan Huaxue Yu Gongye 2004, 24, 26–30. [Google Scholar]
- Luo, J.Y.; Yang, Y.; Fan, Y.M.; An, X.N. Study on α-pinene isomerization catalyzed by SO42-/ZrO2-TiO2 solid superacid. Linchan Huaxue Yu Gongye 2004, 24, 15–19. [Google Scholar]
- Wang, Y.M.; Xie, H.D.; Huang, W.L. Study on α-pinene isomerization catalyzed by SO42-/ZrO2 solid superacid. Linchan Huaxue Yu Gongye 2002, 22, 10–14. [Google Scholar]
- Yadav, M.K.; Chadasama, C.D.; Jasra, R.V. Isomerization of α-pinene using modified montmorillonite clays. J. Mol. Catal. A Chem. 2004, 216, 51–59. [Google Scholar] [CrossRef]
- Özkan, F.; Gündüz, G.; Akpolat, O.; Besün, N.; Murzin, D.Y. Isomerization of α-pinene over ion-exchanged natural zeolites. Chem. Eng. J. 2003, 91, 257–269. [Google Scholar] [CrossRef]
- Xu, X.T.; Li, B.; Li, J.L.; Wei, C.Q. Catalytic isomerization of α-pinene on Ag/NiY zeolite. Guangxi Huagong 2000, 29, 7–8. [Google Scholar]
- Ünveren, E.; Gündüz, G.; Cakicioğlu-Özkan, F. Isomerization of alpha-pinene over acid treated natural zeolite. Chem. Eng. Commun. 2005, 192, 386–404. [Google Scholar] [CrossRef]
- Chimal-Valencia, O.; Robau-Sanchez, A.; Collins-Martinez, V.; Aguilar-Elguezabal, A. Ion exchange resins as catalyst for the isomerization of α-pinene to camphene. Bioresour. Technol. 2004, 93, 119–123. [Google Scholar] [CrossRef]
- Ecormier, M.A.; Wilson, K.; Lee, A.F. Structure-reactivity correlations in sulphated zirconia catalysts for the isomerization of α-pinene. J. Catal. 2003, 215, 57–65. [Google Scholar] [CrossRef]
- Flores-Moreno, J.L.; Baraket, L.; Figueras, F. Isomerisation of α-pinene oxide on sulfated oxides. Catal. Lett. 2001, 77, 113–117. [Google Scholar] [CrossRef]
- Ravindra, D.B.; Nie, Y.T.; Jaenicke, S.; Chuah, G.K. Isomerization of α-pinene oxide over B2O3/SiO2 and Al-MSU catalysts. Catal. Today 2004, 96, 147–153. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Quintana, R.L.; Goldsmith, B.R.; Harvey, B.G. Solvent-free conversion of linalool to methylcyclopentadiene dimers: A route to renewable high-density fuels. ChemSusChem 2011, 4, 465–469. [Google Scholar] [CrossRef]
- Harun, F.W.; Almadani, E.A.; Radzi, S.M. Metal cation exchanged montmorillonite K10 (MMT K10): Surface properties and catalytic activity. J. Sci. Res. Dev. 2016, 3, 90–96. [Google Scholar]
- Kaźmierczak, J.; Hreczycho, G. Nafion as effective and selective heterogeneous catalytic system in O-metalation of silanols and POSS silanols. J. Catal. 2018, 367, 95–103. [Google Scholar] [CrossRef]
- Wright, M.E.; Harvey, B.G. Process and Apparatus for the Selective Dimerization of Terpenes and Alphaolefin Oligomers with a Single-Stage Reactor and a Single-Stage Fractionation System. U.S. Patent US9266792B2, 23 February 2016. [Google Scholar]
- Jaramillo, H.; Palacio, L.A.; Sierra, L. Characterization of a heteropolyacid supported on mesoporous silica and its application in the aromatization of α-pinene. Stud. Surf. Sci. Catal. 2002, 142, 1291–1298. [Google Scholar] [CrossRef]
- Castanheiro, J.E.; Fonseca, I.M.; Ramos, A.M.; Vital, J. Tungstophosphoric acid immobilised in SBA-15 as an efficient heterogeneous acid catalyst for the conversion of terpenes and free fatty acids. Microporous Mesoporous Mater. 2017, 249, 16–24. [Google Scholar] [CrossRef]
- Gabriel, C.B.; Canhaci, S.J.; Borges, L.E.; Fraga, M.A. Aviation biofuel range cycloalkane from renewables: Liquid-phase catalytic conversion of menthol on niobia-supported catalysts. Fuel 2020, 277, 118288. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, B.; Yu, F.; Liu, Y.; Xie, C.; Yu, S. Hydrogenation of α-Pinene over Platinum Nanoparticles Reduced and Stabilized by Sodium Lignosulfonate. ACS Omega 2020, 5, 8902–8911. [Google Scholar] [CrossRef]
- Moutombi, F.J.N.; Selka, A.; Fabiano-Tixier, A.S.; Foucher, D.; Clarisse, O.; Chemat, F.; Touaibia, M. Highly selective solvent-free hydrogenation of pinenes to added-value cis-pinane. Comptes Rendus Chim. 2018, 21, 1035–1042. [Google Scholar] [CrossRef]
- de Meireles, A.L.; dos Santos, M.; da Silva, K.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly acid catalysts for the synthesis of fragrance compounds from biorenewables: The alkoxylation of monoterpenes. ChemCatChem 2014, 6, 2706–2711. [Google Scholar] [CrossRef]
- Sutijan, D.; Budiman, A. Kinetics of acetoxylation of Indonesian crude turpentine over Amberlyst 36 wet: Pseudo-homogeneous approach. AIP Conf. Proc. 2016, 1755, 050003. [Google Scholar] [CrossRef]
- Yadav, M.K.; Patil, M.V.; Jasra, R.V. Acetoxylation and hydration of limonene and α-pinene using cation-exchanged zeolite beta. J. Mol. Catal. A Chem. 2000, 297, 101–109. [Google Scholar] [CrossRef]
- Robles-Dutenhefner, P.A.; da Silva, K.A.; Siddiqui, M.R.H.; Kozhevnikov, I.V.; Gusevskaya, E.V. Hydration and acetoxylation of monoterpenes catalyzed by heteropoly acid. J. Mol. Catal. A Chem. 2001, 175, 33–42. [Google Scholar] [CrossRef]
- Aguas, I.E.; Alarcon, E.; Villa, A.L. Turpentine valorization by its oxyfunctionalization to nopol through heterogeneous catalysis. Heliyon 2020, 6, e03887. [Google Scholar] [CrossRef]
- Xie, J.; Han, Q.; Wang, J.; Bai, L.; Lu, J.; Liu, Z. Enhanced α-terpineol yield from α-pinene hydration via synergistic catalysis using carbonaceous solid acid catalysts. Ind. Eng. Chem. Res. 2019, 58, 22202–22211. [Google Scholar] [CrossRef]
- Wei, Z.; Xiong, D.; Duan, P.; Ding, S.; Li, Y.; Li, L.; Niu, P.; Chen, X. Preparation of carbon-based solid acid catalysts using rice straw biomass and their application in hydration of α-pinene. Catalysts 2020, 10, 213. [Google Scholar] [CrossRef]
- Vital, J.; Ramos, A.M.; Silva, I.F.; Valente, H.; Castanheiro, J.E. Hydration of α-pinene over zeolites and activated carbons dispersed in polymeric membranes. Catal. Today 2000, 56, 167–172. [Google Scholar] [CrossRef]
- Vital, J.; Ramos, A.M.; Silva, I.F.; Castanheiro, J.E. The effect of α-terpineol on the hydration of α-pinene over zeolites dispersed in polymeric membranes. Catal. Today 2001, 67, 217–223. [Google Scholar] [CrossRef]
- Semikolenov, V.A.; Ilyna, I.I.; Simakova, I.L. Linalool synthesis from α-pinene: Kinetic peculiarities of catalys steps. Appl. Catal. A-Gen. 2001, 211, 91–107. [Google Scholar] [CrossRef]
- Yang, A.C.; Liu, X.; Yin, D.; Zhang, Z.; Lei, D.; Yang, J. A recyclable Pd colloidal catalyst for liquid phase hydrogenation of α-pinene. J. Ind. Eng. Chem. 2014, 26, 333–334. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Pájaro, E.; Villa, A.L.; Martínez-O, F. Selective synthesis of camphene from isomerization of α- and β-pinene over heterogeneous catalysts. Microporous Mesoporous Mater. 2021, 324, 111273. [Google Scholar] [CrossRef]
- Akgül, M.; Özyağcı, B.; Karabakan, A. Evaluation of Fe- and Cr-containing clinoptilolite catalysts for the production of camphene from α-pinene. J. Ind. Eng. Chem. 2013, 19, 240–249. [Google Scholar] [CrossRef]
- Kumar, S.; Sinhmar, P.S.; Gogate, P.R. Ultrasound assisted improved synthesis of TiO2 catalyst and subsequent evaluation for isomerization of alpha pinene. Chem. Eng. Process. -Process Intensif. 2021, 169, 108591. [Google Scholar] [CrossRef]
- Golets, M.; Ajaikumar, S.; Mohln, M.; Wärnå, J.; Rakesh, S.; Mikkola, J.-P. Continuous production of the renewable ρ-cymene from α-pinene. J. Catal. 2013, 307, 305–315. [Google Scholar] [CrossRef]
- Szücs-Balázs, J.-Z.; Coroş, M.; Woiczechowski-Pop, A.; Blăniţă, G.; Vlassa, M. Supported H4SiW12O40 catalysts for α-pinene isomerization. Cent. Eur. J. Chem. 2012, 10, 1208–1217. [Google Scholar] [CrossRef]
- Sinhmar, P.S.; Gogate, P.R. Improved Activation of Titanium Dioxide Catalyst for Isomerization of Alpha Pinene and Understanding into Effect of Isomerization Parameters. Arab. J. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- da Silva, K.A.; Robles-Dutenhefner, P.A.; Kozhevnikov, I.V.; Gusevskaya, E.V. Phosphotungstic heteropoly acid as efficient heterogeneous catalyst for solvent-free isomerization of α-pinene and longifolene. Appl. Catal. A Gen. 2009, 352, 188–192. [Google Scholar] [CrossRef]
- Lossano, L.; Toma, S.H.; Pereira, H.; Avanzi, L.H.; dos Santos, R.; Peffi, L.F.; Araki, K.; Cella, R.; Makoto, M. Phosphotungstic acid impregnated niobium coated superparamagnetic iron oxide nanoparticles as recyclable catalyst for selective isomerization of terpenes. RSC Adv. 2021, 11, 14203. [Google Scholar] [CrossRef]
- Selka, A.; Levesque, N.A.; Foucher, D.; Clarisse, O.; Chemat, F.; Touaibia, M. A Comparative Study of Solvent-Free and Highly Efficient Pinene Hydrogenation over Pd on Carbon, Alumina, and Silica Supports. Org. Process Res. Dev. 2016. [Google Scholar] [CrossRef]
- Schmçger, C.; Stolle, A.; Bonrath, W.; Ondruschka, B.; Keller, T.; Jandt, K.D. A Practical Approach for Ambient-Pressure Hydrogenations Using Pd on Porous Glass. ChemSusChem 2009, 2, 77–82. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, C.; Zhai, G.; Zhao, M.; Xian, M.; Jia, Y.; Yu, Z.; Liu, F.; Jian, F.; Sun, W. Bifunctional catalyst Pd–Al-MCM-41 for efficient dimerization-hydrogenation of β-pinene in one pot. RSC Adv. 2017, 7, 47539–47546. [Google Scholar] [CrossRef]
- Mehta, J.P.; Parmar, D.K.; Nakum, H.D.; Godhani, D.R.; Desai, N.C. Enhanced catalytic oxidation of monoterpenes by zeolite-Y entrapped iron complex: Spectral studies and mechanistic vision. J. Porous Mater. 2018. [Google Scholar] [CrossRef]
- Parmar, D.K.; Butani, P.M.; Thumar, N.J.; Jasani, P.M.; Padaliya, R.V.; Sandhiya, P.R.; Nakum, H.D.; Khan, N.; Makwana, D. Oxy-functionalization of olefins with neat and heterogenized binuclear V(IV)O and Fe(II) complexes: Effect of steric hindrance on product selectivity and output in homogeneous and heterogeneous phase. Mol. Catal. 2019, 474, 110424. [Google Scholar] [CrossRef]
- Golets, M.; Ajaikumar, S.; Larsson, W.; Blomberg, D.; Grundberg, H.; Wärnå, J.; Salmi, T.; Mikkola, J.-P. A Kinetic Study of the Liquid Phase Acetoxylation of α-Pinene. Top. Catal. 2012, 55, 649–656. [Google Scholar] [CrossRef]
- Costa, V.V.; da Silva, K.A.; Mesquita, R.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly Acid Catalysts for the Synthesis of Fragrance Compounds from Biorenewables: Cycloaddition of Crotonaldehyde to Limonene, α-Pinene, and β-Pinene. ChemCatChem 2013, 5, 3022–3026. [Google Scholar] [CrossRef]
- Caiado, M.; Machado, A.; Santos, R.N.; Matos, I.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Valente, A.A.; Castanheiro, J.E. Alkoxylation of camphene over silica-occluded tungstophosphoric acid. Appl. Catal. A Gen. 2013, 451, 36–42. [Google Scholar] [CrossRef]
- da Silva, K.A.; Kozhevnikov, I.V.; Gusevskaya, E.V. Isomerisation of α-pinene oxide over silica supported heteropoly acid H3PW12O40. Appl. Catal. A Gen. 2005, 294, 106–110. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2013. [Google Scholar] [CrossRef]
- Hua, L.S.; Chen, L.W.; Antov, P.; Kristak, L.; Tahir, P.M. Engineering Wood Products from Eucalyptus spp. Adv. Mater. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for bio-based solvents created as petrochemical and fuel products transition towards renewable resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef]
- Lange, B.M.; Ahkami, A. Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes-current status and future opportunities. Plant Biotechnol. J. 2013, 11, 169–196. [Google Scholar] [CrossRef]
- Wu, H.; Fu, Q.; Giles, R.; Bartle, J. Production of mallee biomass in Western Australia: Energy balance analysis. Energy Fuels 2008, 22, 190–198. [Google Scholar] [CrossRef]
- Joyce, B.L.; Stewart, C.N. Designing the perfect plant feedstock for biofuel production: Using the whole buffalo to diversify fuels and products. Biotechnol. Adv. 2012, 30, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Zalesny, R.S.; Cunningham, M.W.; Hall, R.B.; Mirck, J.; Rockwood, D.L.; Stanturf, J.; Volk, T.A. Woody biomass from short rotation energy crops. In Sustainable production of fuels, chemicals, and fibers from forest biomass. Am. Chem. Soc. 2011, 1067, 27–63. [Google Scholar] [CrossRef]
- Miądlicki, P.; Wróblewska, A.; Kiełbasa, K.; Koren, Z.C.; Michalkiewicz, B. Sulfuric acid modified clinoptilolite as a solid green catalyst for solvent-free α-pinene isomerization process. Microporous Mesoporous Mater. 2021, 324, 111266. [Google Scholar] [CrossRef]

| Compound | Chemical Formula | Density (g/mL) | Kinematic Viscosity (mm2/s at 40 °C) | Cetane Number |
|---|---|---|---|---|
| camphene | C10H16 | 0.842 [30] | 34.96 [30] | 29 [31] |
| -caryophyllene | C15H24 | 0.900 (15 °C) [28] | 29 [31] | |
| 1,8-cineole | C10H18O | 0.920 (25 °C) [32] | ||
| (-)--citronellene | C10H18O | 0.760 (20 °C) [32] | ||
| /-farnesene | C15H24 | 0.841 (20 °C) [32] | 32 [28] | |
| Geranial | C10H16O | 0.887 (20 °C) [33] | ||
| Geraniol | C10H18O | 0.874 (25 °C) [34] | 7.652 (25 °C) [34] | 19.25 [31] |
| D-limonene | C10H16 | 0.841 (25 °C) [34] | 1.089 (25 °C) [34] | 18.9 [34] |
| Linalool | C10H18O | 0.857 (25 °C) [34] | 5.324 (25 °C) [34] | 16.45 [34] |
| -pinene | C10H16 | 0.855 (25 °C) [34] | 1.502 (25 °C) [34] | 23.13 [34] |
| -pinene | C10H16 | 0.867 (25 °C) [34] | 1.766 (25 °C) [34] | 21.25 [31] |
| Sabinene | C10H16 | 0.844 (20 °C) | ||
| Squalene | C30H50 | 0.858 (20 °C) [33] | ||
| -bisabolene | C15H30 | 0.809 (25 °C) [34] | 32.2 [31] | |
| -terpinene | C10H16 | 0.845 (25 °C) [34] | 1.008 (25 °C) [34] | 20.3 [31] |
| Nerol | C10H18O | 0.876 (20 °C) [33] | ||
| 2-carene | C10H16 | 0.862 (25 °C) | ||
| 3-carene | C10H16 | 0.857 [8] | 27 [31] | |
| -terpineol | C10H18O | 0.931 (25 °C) [34] | 40.08 (25 °C) [34] |
| Process | Terpenes | Source | Solid Catalysts | Yield (%) | Reuse Cycles | References |
|---|---|---|---|---|---|---|
| Hydrogenation | Myrcene | Pine resin | Pd-charcoal/Ni-Raney | nr | nr | [4,43] |
| Limonene | Millipore | Ni-Raney/Pd/PtO2 | 89.4 | nr | [4,44] | |
| Terpinolene | nr | Ni-Raney | nr | nr | [43] | |
| -pinene | Millipore | Amberlyst-15/Ni-Raney/molecular sieve | 85.8 | nr | [44,45] | |
| -pinene | Pine resin | Ni-Raney | nr | nr | [4,43] | |
| Camphene | Pine resin | Ni-Raney | nr | nr | [4,43] | |
| Verbenone | nr | NiO-MgO/Sn-Pt/PtSiO2 | nr | nr | [43,46] | |
| Geraniol | Aldrich | Pt/PtO2/Ni/MoS3/Rh-based/Ru-based | 98 | nr | [43,47] | |
| Citral | nr | Pd/MoS3/Ni/Cr-based/Pt-SiO2 | 80–92 | nr | [43,48] | |
| Nerol | Fluka | SiO2/Pt/SiO2/Pt/H-Y | 22–66 | nr | [49] | |
| Linalool | nr | Amberlyst 15/Pd-C | 81 | nr | [50] | |
| Isomerization | -pinene | nr | Ankalite KT-3/TiO2/kaolin/natural zeolite/HCl-activated Montmorillonite/Al2O3/cation-exchanged bentonite/Ag-NiY zeolite/H3PW12O40-SiO2;TiO2;ZrO2·nH2O/Cs2.5H0.5PW12O40-Nb2O5;ZrO2;TiO2 | 35.9–80 | nr | [27,43,51,52] |
| -pinene | nr | HCl-activated Montmorillonite/TA-4/MA-4 | 35.9–83 | nr | [43] | |
| Dipentene | nr | MoS3/-Al2O3 | nr | nr | [43] | |
| Limonene | nr | Ti-SBA-15 | 0–33 | nr | [53] | |
| Dimerization | -pinene | nr | Al-MCM-41 | 43–87 | 4 | [54] |
| Addition of alcohols | -pinene | Sigma | H3PW12O40·12·H2O | nr | 3 | [55] |
| Hydration | -caryophyllene | copaiba oil | PW/PW-SiO2 | 70 | nr | [27] |
| dihydromyrcene | nr | PW/PW-SiO2 | nr | nr | [27] | |
| α-pinene | Sigma Turpentine | HPW12O40/trichloroacetic acid-SiO2;TiO2;SiO2;Al2O3/Amberlyst 15/Y-Zeolite | 10.2–35.5 | 3–8 | [44,56,57,58,59] | |
| Oxyfunctionalization | β-pinene | GMP | Amberlyst 15 | nr | nr | [36] |
| Acetoxylation | -caryophyllene | copaiba oil | PW/SiO2 | 70 | nr | [27,60] |
| α-terpineol | terpenic alcohols | AIPW12O40 | 92–95 | nr | [27] | |
| Alkylation | Limonene | nr | Amberlite IR120 | nr | nr | [43] |
| Camphene | nr | PW/SiW-TiO2/SiW-ZrO2 | nr | nr | [27] |
| Reactant | Catalyst | Load (wt.%) | Temp. (°C) | Time (h) | X (%) | Y (%) | S (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| -pinene | TiO2-SO42 | nr | nr | nr | 91 | 61 1 | 67 | [24] |
| Fe-Mn-SZrO2 | 1 | 130 | 3 | 95 | 57 1 | 60 | [68] | |
| MoO3/ZrO2 | nr | 120 | nr | 93.5 | 56.8 | 60.7 | [69] | |
| SO42/ZrO2-TiO2 | nr | 130 | nr | 96.6 | 55.4 | 57.4 | [70] | |
| Al3+/TMA+/MMT | nr | 120 | 1 | 83 | 55.6 1 | 67 | [64] | |
| NH4+/exchanged HY | 3 | 150 | 1–4 min. | 99 | 50.5 1 | 51 | [24] | |
| SO42−/ZrO2 | nr | 130 | nr | 88.8 | 49.2 | 55.4 | [71] | |
| Kaolin | 5.8 | 150 | 2 | 86 | 45.6 1 | 53 | [72] | |
| Clinoptilolite-BaO | 2.7 | 155 | 3 | 100 | 39.5 1* | 39.5 | [73] | |
| Clinoptilolite | 2.5 | 155 | 2 | 92 | 43.73 1 | 48 | [24] | |
| Raw clay | 1 | 155 | 3 | 87 | 43.5 1 | 50 | [63] | |
| Silica-alumina | 3 | 30 | 0.5 | 99.8 | 42.9 1 | 43 | [24] | |
| Y-zeolite | 5.8 | 200 | 1 | 84 | 41.2 1 | 49 | [24] | |
| Mordenite | 5.8 | 120 | 1 | 92 | 35 1 | 38 | [24] | |
| Ce3+/MMT | 5.8 | 150 | 2 | 99 | 48.5 1 | 49 | [72] | |
| Ag/NiY | nr | 156 | 94.5 | 46.1 | 48.7 | [74] | ||
| natural zeolite | nr | 155 | 3.25 | 100 | 37.8 1 | 37.8 | [75] | |
| Yb/Al2O3 | 3 | 50 | 3 | 63.2 | 32 1 | 49 | [67] | |
| ion exchange resin | 1 | 120 | 2 | 80 | 29.6 1 | 37 | [76] | |
| Sulphated zirconia | 9 | 60 | 4.03 | 66 | 21 1 | 32 | [77] | |
| Yb/SiO2 | 3 | 50 | 3 | 26.5 | 6 1 | 23 | [24] | |
| Y/SiO2 | 3 | 50 | 3 | 28.3 | 5 1 | 17 | [24] | |
| HPWZr | 1 | 130 | 20 min | 8 | 3.6 1* | 45 | [52] | |
| HPWZr | 1 | 130 | 2 | 14 | 7.14 1* | 51 | [52] | |
| HPWTi | 1 | 130 | 20 min | 12 | 6.12 1* | 51 | [52] | |
| HPWTi | 1 | 130 | 2 | 55 | 25.85 1* | 47 | [52] | |
| HPWSi | 1 | 130 | 20 min | 96 | 8.64 1* | 9 | [52] | |
| HPWSi | 1 | 80 | 20 min | 42 | 21.84 1* | 52 | [52] | |
| HPWSi | 1 | 80 | 2 | 93 | 34.41 1* | 37 | [52] | |
| HPWSi | 1 | 45 | 20 min | 8 | 4.16 1* | 52 | [52] | |
| HPWSi | 1 | 45 | 2 | 20 | 10.40 1* | 52 | [52] | |
| Al-Si RB | 3 | 130 | 6 | 85 | 46 1 | 54.12 * | [61] | |
| Al-Si RB (50 mL of 10% HCl) | 0.5 | 130 | 5.5 | 85 | 48 1 | 56.47 * | [66] | |
| Al-Si RB (50 mL of 10% H3PO4) | 1 | 130 | 1.7 | 85 | 49.11 | 57.76 * | [66] | |
| Al-Si RB | 3 | 140 | 5.5 | 85 | 51.5 1 | 60.59 * | [66] | |
| Al/Diatomite | 1 | 140 | 4 | 85 | 43 1 | 50 | [66] | |
| ZSM-5/Bentonite | 1 | 140 | 7 | 85 | 43.5 1 | 51.18 * | [66] | |
| -pinene | -Al2O3-Na | nr | nr | nr | 100 | 99.5 2 | 99.5 | [24] |
| CsxO/-Al2O3 | 3 | 25 | 2 | 98 | 98 2 | 100 | [24] | |
| MgO | 3 | 80 | 3 | 83.5 | 81.8 1 | 98 | [24] | |
| 3-carene | 0.1 wt.% Sn 2.6 wt.% Ni/SiO2 | 2.7 | 120 | 4 | 48 | 37 3 | 77 | [24] |
| 2.7 wt.% Ni/SiO2 | 2.7 | 120 | 1 | 61 | 30.5 3 | 50 | [24] | |
| -pinene oxide | Sulphated Al2O3 | nr | 0 | 1 | 100 | 76 3 | 76 * | [78] |
| B2O3/SiO2 | 15 | 25 | 4 | 84 | 58 3 | 69 | [79] |
| Process | Catalyst | Reuse Cycles | Conversion Loss (%) | Ref. |
|---|---|---|---|---|
| α-pinene isomerization | Pd colloidal | 10 | 5 | [99] |
| Titanates | 4 | 1 | [100] | |
| Clinoptilolite with Fe and Cr | 4 | 3 | [101] | |
| TiO2 | 8 | 5 | [102] | |
| Pd-Zn supported on Al-SBA15 | 3 | 36 | [103] | |
| H4SiW12O40 | 3 | 36 | [104] | |
| TiO2 | 8 | 6 | [105] | |
| H3PW12O40 | 3 | 4 | [106] | |
| β-pinene isomerization | Titanates | 7 | 0 | [100] |
| Terpenes isomerization | SPION-Nb30 | 5 | 18 | [107] |
| Caryophyllene isomerization | Cs2.5H0.5PW12O40 | 2 | 1.5 | [89] |
| α-pinene hydrogenation | Pd/C and Pd/alumina | 13 | 1 | [108] |
| β-pinene hydrogenation | Pd/glass | 3 | 4 | [109] |
| β-pinene hydrogenation | Pd–Al-MCM-41 | 5 | 8 | [110] |
| β-pinene dimerization | SiO2/Al2O3/Al-MCM-41 | 4 | 3 | [54] |
| α-pinene oxyfunctionalization | [Fe(L)2(H2O)2]–Y | 4 | 3 | [111] |
| α-pinene oxyfunctionalization | zeolite-Y immobilized binuclear complexes | 2 | 2 | [112] |
| Limonene oxyfunctionalization | zeolite-Y immobilized binuclear complexes | 2 | 3 | [112] |
| β-caryophyllene acetoxylation | H3PW12O40 | 2 | 0 | [60] |
| α-pinene acetoxylation | Amberlyst 70 | 3 | 60 | [113] |
| Turpentine oxyfunctionalization | Sn-MCM-41 | 4 | 0 | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapuerta, M.; Tobío-Pérez, I.; Ortiz-Alvarez, M.; Donoso, D.; Canoira, L.; Piloto-Rodríguez, R. Heterogeneous Catalytic Conversion of Terpenes into Biofuels: An Open Pathway to Sustainable Fuels. Energies 2023, 16, 2526. https://doi.org/10.3390/en16062526
Lapuerta M, Tobío-Pérez I, Ortiz-Alvarez M, Donoso D, Canoira L, Piloto-Rodríguez R. Heterogeneous Catalytic Conversion of Terpenes into Biofuels: An Open Pathway to Sustainable Fuels. Energies. 2023; 16(6):2526. https://doi.org/10.3390/en16062526
Chicago/Turabian StyleLapuerta, Magín, Indira Tobío-Pérez, Marianela Ortiz-Alvarez, David Donoso, Laureano Canoira, and Ramón Piloto-Rodríguez. 2023. "Heterogeneous Catalytic Conversion of Terpenes into Biofuels: An Open Pathway to Sustainable Fuels" Energies 16, no. 6: 2526. https://doi.org/10.3390/en16062526
APA StyleLapuerta, M., Tobío-Pérez, I., Ortiz-Alvarez, M., Donoso, D., Canoira, L., & Piloto-Rodríguez, R. (2023). Heterogeneous Catalytic Conversion of Terpenes into Biofuels: An Open Pathway to Sustainable Fuels. Energies, 16(6), 2526. https://doi.org/10.3390/en16062526












