Electricity Production from Yeast Wastewater in Membrane-Less Microbial Fuel Cell with Cu-Ag Cathode
Abstract
:1. Introduction
2. Materials and Methods
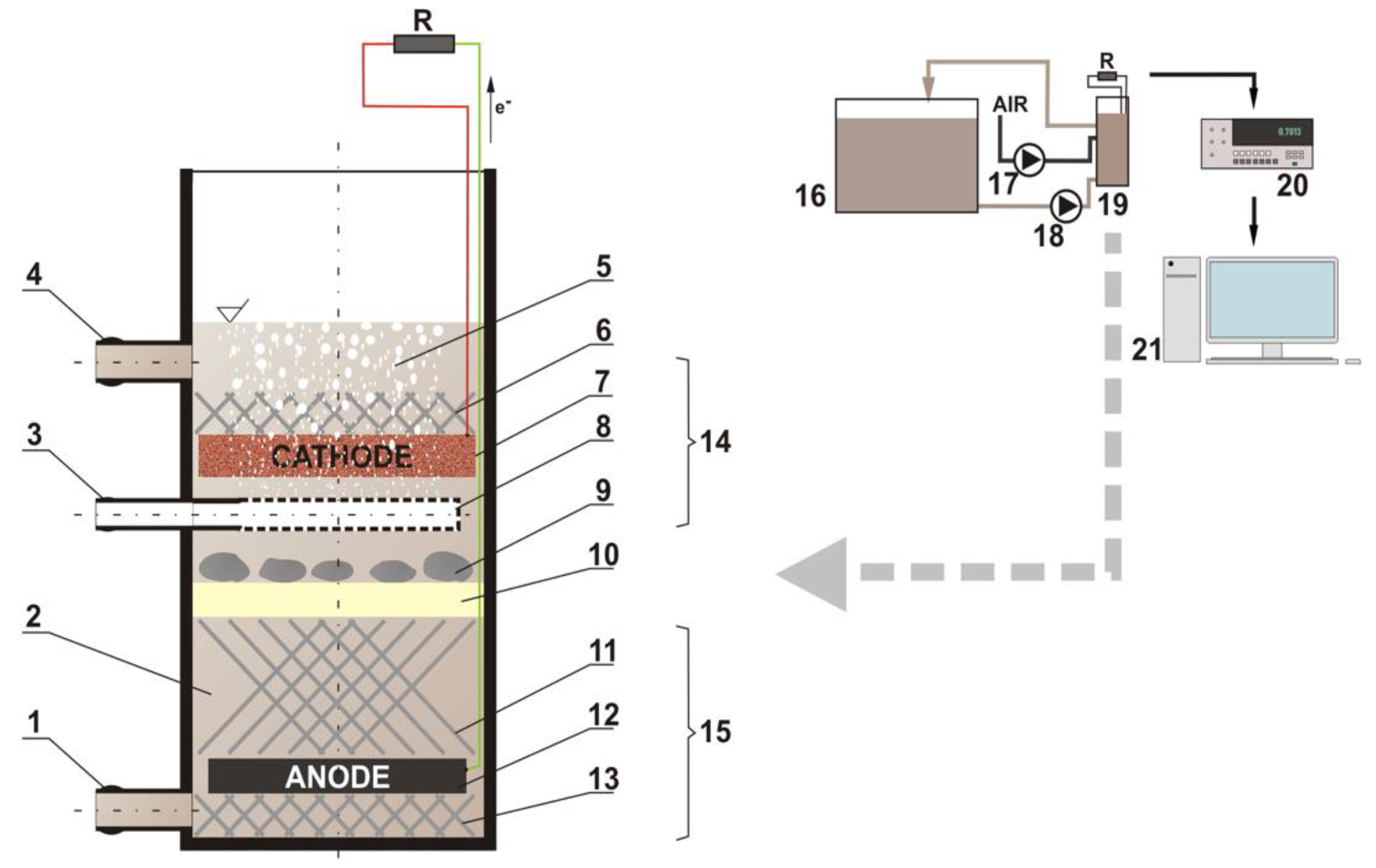
2.1. Preparation of a Cathode for ML-MFC
2.2. Selection of the Electrodes with Cu-Ag Catalyst for Use in the ML-MFC
2.3. Measurements of Voltage and Power during the Operation of ML-MFC
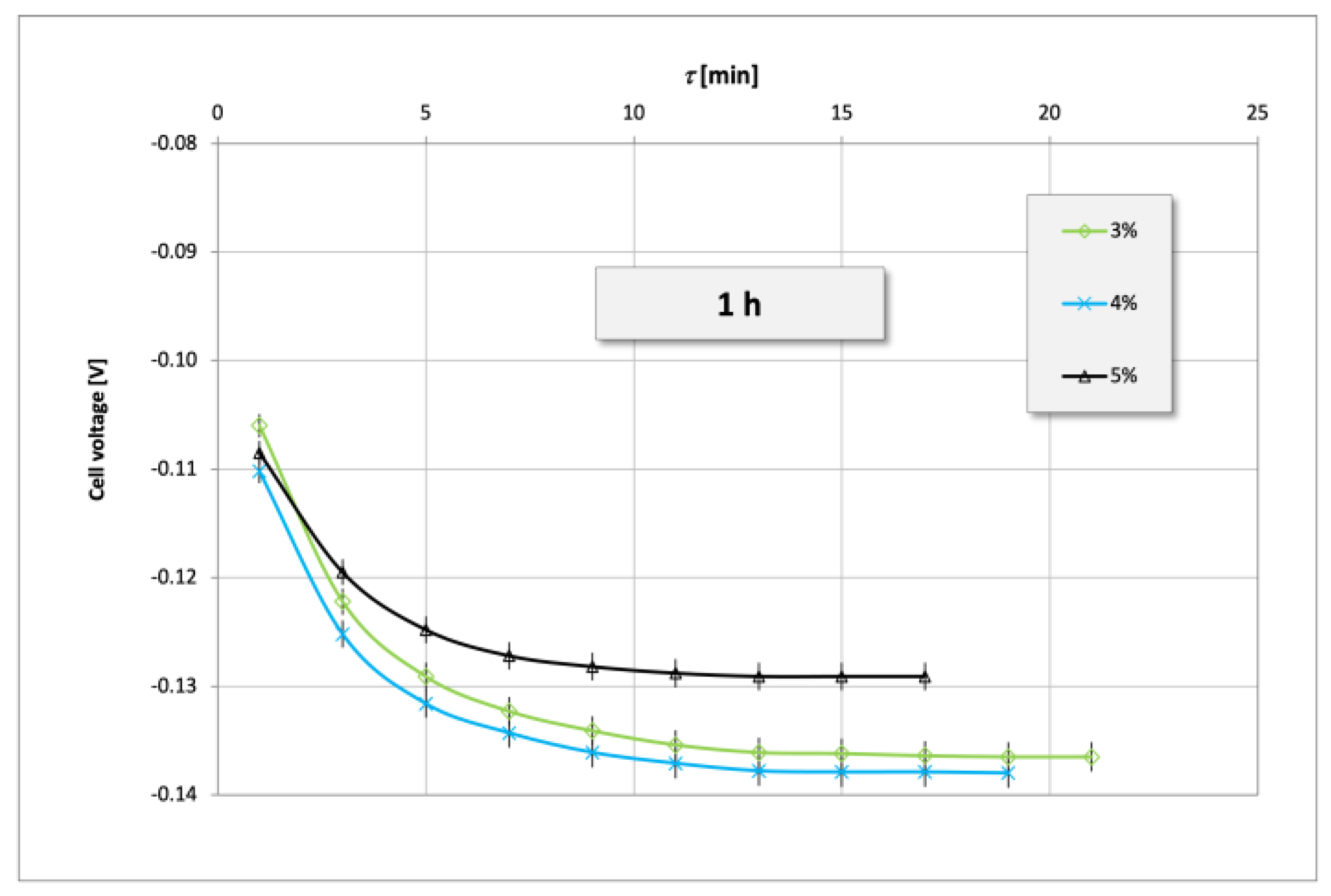
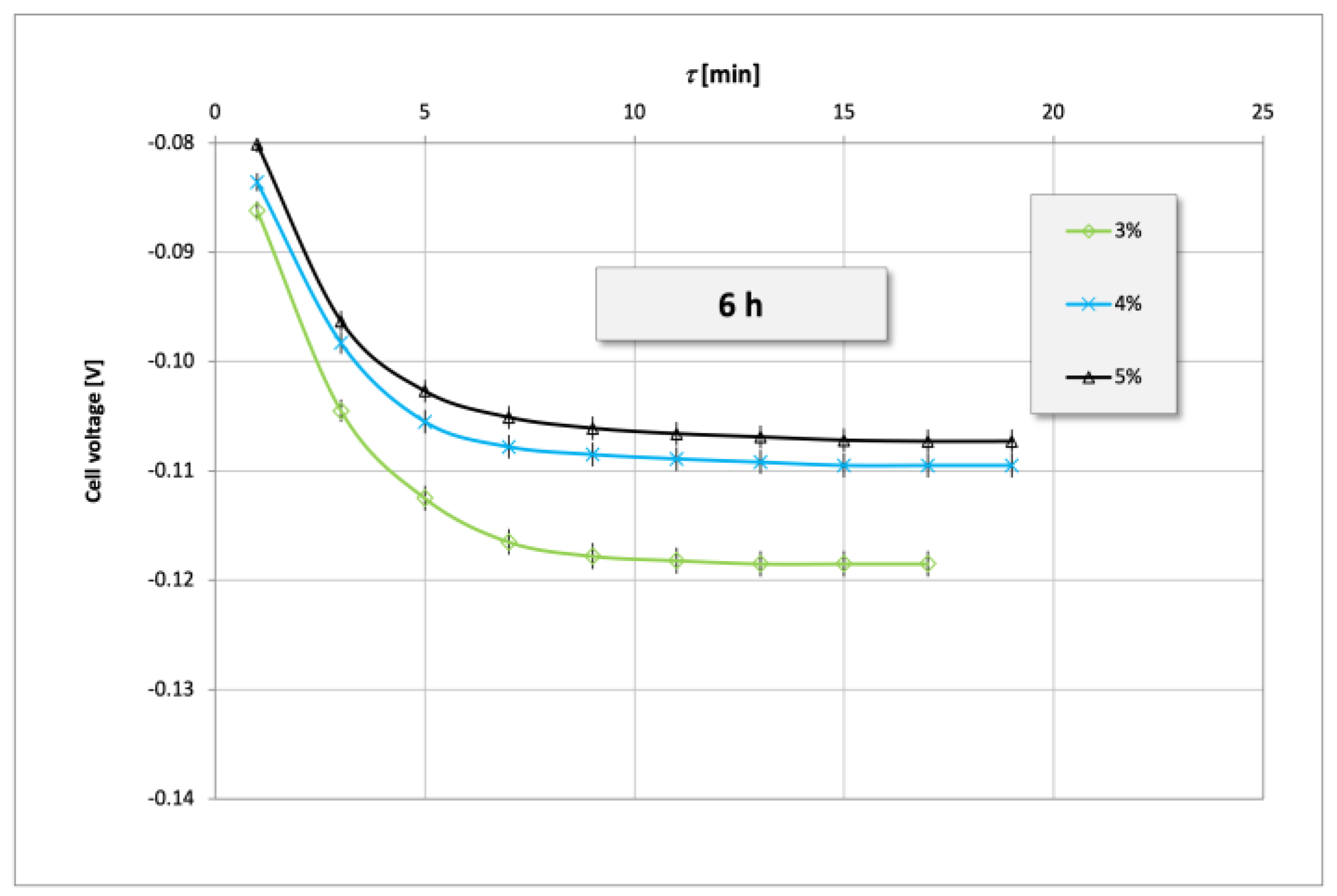
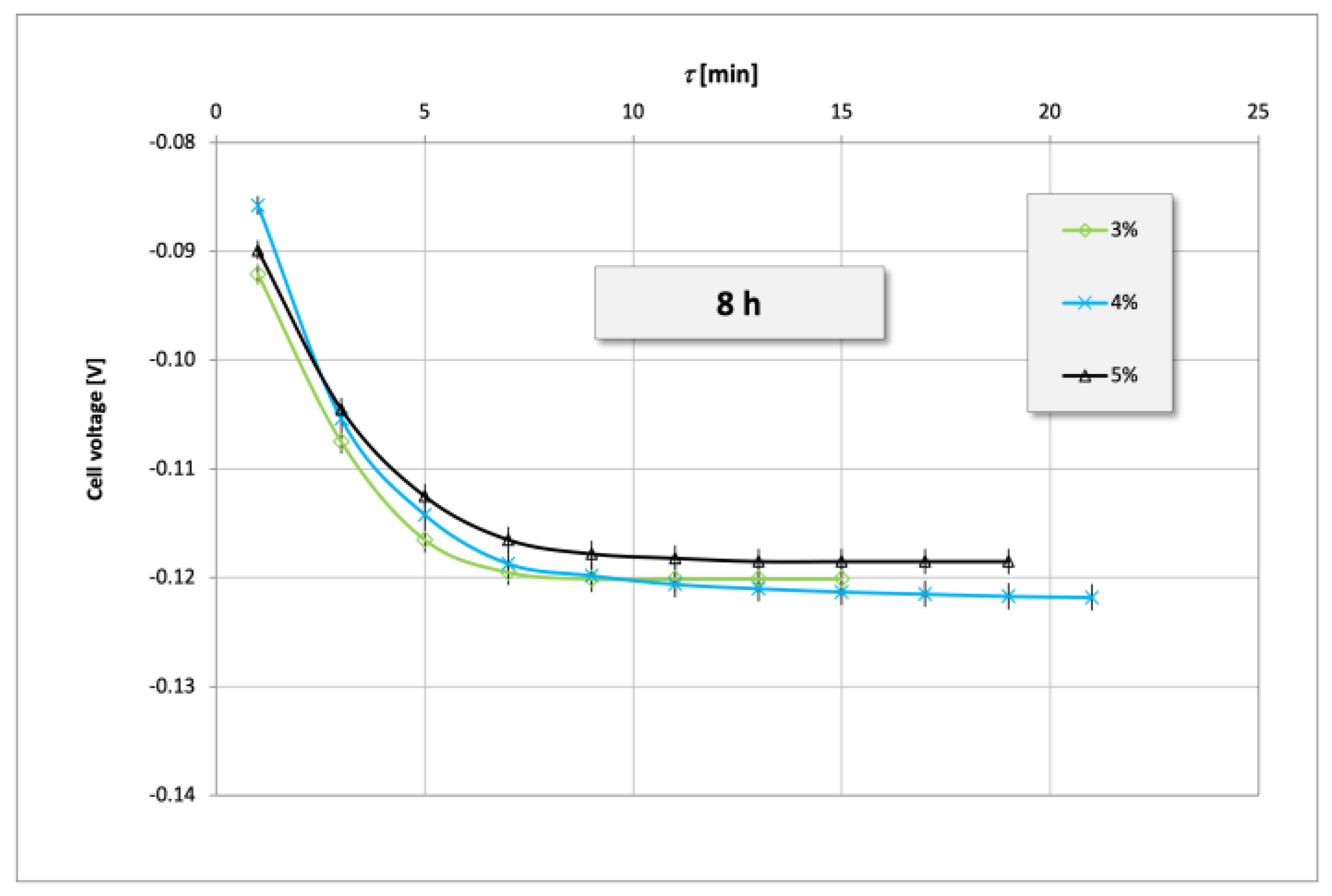
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sadecka, Z. Podstawy Biologicznego Oczyszczania Ścieków; Wydawnictwo Seidel-Przywecki: Józefosław, Poland, 2010. [Google Scholar]
- Sikora, J.; Miksch, K. Biotechnologia Ścieków; Wydawnictwa Naukowe PWN: Warszawa, Poland, 2010. [Google Scholar]
- Bartkiewicz, B.; Umiejewska, K. Oczyszczanie Ścieków Przemysłowych; Wydawnictwa Naukowe PWN: Warszawa, Poland, 2020. [Google Scholar]
- Logan, B. Microbial Fuel Cells; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef] [Green Version]
- Selihin, N.M.; Tay, M.G. A review on future wastewater treatment technologies: Micro-nanobubbles, hybrid electro-Fenton processes, photocatalytic fuel cells, and microbial fuel cells. Water Sci. Technol. 2022, 85, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C. Electrical effects accompanying the decomposition organic compounds. Proc. R. Soc. Lond. Ser. B 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Bond, D.R.; Lovley, D.R. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 2005, 71, 2186–2189. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Kim, B.H.; Kim, H.S.; Kim, H.J.; Kim, G.T.; Kim, M.; Chang, I.S.; Park, Y.K.; Chang, H.I. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 2001, 7, 297–306. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A Mediator-Less Microbial Fuel Cell Using a Metal Reducing Bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to incre- ased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Pham, C.A.; Jung, S.J.; Phung, N.T.; Lee, J.; Chang, I.S.; Kim, B.H.; Yi, H.; Chun, J. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 2003, 223, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Saha, T.C.; Protity, A.T.; Zohora, F.T.; Shaha, M.; Ahmed, I.; Barua, E.; Sarker, P.K.; Mukherjee, S.K.; Barua, A.; Salimullah, M.; et al. Microbial Fuel Cell (MFC) Application for Generation of Electricity from Dumping Rubbish and Identification of Potential Electrogenic Bacteria. Adv. Ind. Biotechnol. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Halim, M.A.; Rahman, M.O.; Eti, I.A.; Shefa, N.R.; Ibrahim, M.; Alam, M.J. Electricity generation in different cell connections with optimized anodic materials in microbial fuel cells. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Sharan, S.; Khare, P.; Shankar, R.; Patel Ratnesh, K.; Mondal, P. Simultaneous removal of organics and bioenergy production by microbial fuel cell: Modeling approach. Int. J. Chem. React. Eng. 2021, 19, 1351–1362. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Preparation and Analysis of Ni–Co Catalyst Use for Electricity Production and COD Reduction in Microbial Fuel Cells. Catalysts 2019, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Gupta, P. Economical and sustainable microbial peroxide-producing cell utilizing domestic sewage water and its contemporaneous treatment. Fuel Cells 2022, 22, 186–196. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Benites, S.M.; Delfín-Narciso, D.; Luis, A.-S.; Díaz, F.; Luis, C.-C.; Moises, G.C. Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells. Molecules 2022, 27, 5198. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Microbial Fuel Cell with Ni–Co Cathode Powered with Yeast Wastewater. Energies 2018, 11, 3194. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, B.; Włodarczyk, P.P. The Membrane-Less Microbial Fuel Cell (ML-MFC) with Ni-Co and Cu-B Cathode Powered by the Process Wastewater from Yeast Production. Energies 2020, 13, 3976. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Biryol, I.; Ayol, A. Yeast industry wastewater treatment with microbial fuel cells: Effect of electrode materials and reactor configurations. Int. J. Hydrog. Energy 2022, in press. [CrossRef]
- Wilberforce, T.; Sayed, E.T.; Abdelkareem, M.A.; Elsaid, K.; Olabi, A.G. Value added products from wastewater using bioelectrochemical systems: Current trends and perspectives. J. Water Process Eng. 2021, 39, 101737. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Machuca-Martínez, F.; Barajas-Solano, A.F. Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules 2021, 26, 3222. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, T.; Uthirakrishnan, U.; Sivakumar, S.V.; Authilingam, S.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. Processing of electroplating industry wastewater through dual chambered microbial fuel cells (MFC) for simultaneous treatment of wastewater and green fuel production. Int. J. Hydrog. Energy 2022, 47, 37569–37576. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz Noriega, M.; Benites, S.M.; Gonzales, G.A.; Salinas, A.S.; Palacios, F.S. Generation of bioelectricity from fruit waste. Energy Rep. 2020, 6, 37–42. [Google Scholar] [CrossRef]
- Segundo, R.-F.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Angelats-Silva, L.; Díaz, F. Golden Berry Waste for Electricity Generation. Fermentation 2022, 8, 256. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Benites, S.M.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Valdiviezo-Dominguez, F.; Quezada Álvarez, M.A.; Vega-Ybañez, V.; Angelats-Silva, L. Bioelectricity Production from Blueberry Waste. Processes 2021, 9, 1301. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Angelats-Silva, L.; Murga-Torres, E. Use of Banana Waste as a Source for Bioelectricity Generation. Processes 2022, 10, 942. [Google Scholar] [CrossRef]
- Segundo, R.-F.; De La Cruz-Noriega, M.; Milly Otiniano, N.; Benites, S.M.; Esparza, M.; Nazario-Naveda, R. Use of Onion Waste as Fuel for the Generation of Bioelectricity. Molecules 2022, 27, 625. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wu, Y.; Cao, D.; Zhao, L.; Sun, Q. Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour. Technol. 2009, 100, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, S.; Zhuang, L. Polypyrrole/carbon black composite as a novel oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2010, 195, 3490–3493. [Google Scholar] [CrossRef]
- Zuo, K.; Liang, S.; Liang, P.; Zhou, X.; Sun, D.; Zhang, X.; Huang, X. Carbon filtration cathode in microbial fuel cell to enhance wastewater treatment. Bioresour. Technol. 2015, 185, 426–430. [Google Scholar] [CrossRef]
- Liu, Y.; Harnisch, F.; Fricke, K.; Schroeder, U.; Climent, V.; Feliu, J.M. The study of electrochemically active microbial biofilms on different carbon-based anode materials in microbial fuel cells. Biosens. Bioelectron. 2010, 25, 2167–2171. [Google Scholar] [CrossRef]
- Sanchez, D.V.P.; Huynh, P.; Kozlov, M.E.; Baughman, R.H.; Vidic, R.D.; Yun, M. Carbon Nanotube/Platinum (Pt) Sheet as an Improved Cathode for Microbial Fuel Cells. Energy Fuels 2010, 24, 5897–5902. [Google Scholar] [CrossRef]
- Kosimaningrum, W.E.; Ouis, M.; Holade, Y.; Buchari, B.; Noviandri, I.; Kameche, M.; Cretin, M.; Innocent, C. Platinum Nanoarrays Directly Grown onto a 3D-Carbon Felt Electrode as a Bifunctional Material for Garden Compost Microbial Fuel Cell. J. Electrochem. Soc. 2021, 168, 025501. [Google Scholar] [CrossRef]
- Santoro, C.; Kodali, M.; Herrera, S.; Serov, A.; Ieropoulos, I.; Atanassov, P. Power generation in microbial fuel cells using platinum group metal-free cathode catalyst: Effect of the catalyst loading on performance and costs. J. Power Sources 2018, 378, 169–175. [Google Scholar] [CrossRef]
- Park, H.I.; Mushtaq, U.; Perello, D.; Lee, I.; Cho, S.K.; Star, A.; Yun, M. Effective and Low-Cost Platinum Electrodes for Microbial Fuel Cells Deposited by Electron Beam Evaporation. Energy Fuels 2007, 21, 2984–2990. [Google Scholar] [CrossRef]
- Liew, K.B.; Daud, W.R.W.; Ghasemia, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S.; Wang, J.; Zhu, C.; Urynowiczcz, M.A. Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem. Commun. 2007, 9, 1730–1734. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Zhuang, L.; Li, W.; Zhou, S.; Zhang, J. Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells. Biosens. Bioelectron. 2009, 24, 2825–2829. [Google Scholar] [CrossRef]
- Santoro, C.; Lei, Y.; Li, B.; Cristianid, P. Power generation from wastewater using single chamber microbial fuel cells (MFCs) with platinum-free cathodes and pre-colonized anodes. Biochem. Eng. J. 2012, 62, 8–16. [Google Scholar] [CrossRef]
- Arkatkar, A.; Mungray, A.M.; Sharma, P. Biological modification in air-cathode microbial fuel cell: Effect on oxygen diffusion, current generation and wastewater degradation. Chemosphere 2021, 284, 131243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, S.; Pant, D.; Van Bogaert, G.; Logan, B.E. Power generation using an activated carbon and metal mesh cathode in a microbial fuel cell. Electrochem. Commun. 2009, 11, 2177–2179. [Google Scholar] [CrossRef]
- Taşkan, B. Increased power generation from a new sandwich-type microbial fuel cell (ST-MFC) with a membrane-aerated cathode. Biomass Bioenergy 2020, 142, 105781. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Power Densities Using Different Cathode Catalysts (Pt and CoTMPP) and Polymer Binders (Nafion and PTFE) in Single Chamber Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 364–369. [Google Scholar] [CrossRef]
- Lilloja, J.; Mooste, M.; Kibena-Põldsepp, E.; Sarapuu, A.; Kikas, A.; Kisand, V.; Käärik, M.; Kozlova, J.; Treshchalov, A.; Paiste, P.; et al. Cobalt-, iron- and nitrogen-containing ordered mesoporous carbon-based catalysts for anion-exchange membrane fuel cell cathode. Electrochim. Acta 2023, 439, 141676. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Xu, M. Graphene oxide supported magnesium oxide as an efficient cathode catalyst for power generation and wastewater treatment in single chamber microbial fuel cells. Chem. Eng. J. 2017, 328, 106–116. [Google Scholar] [CrossRef]
- Kodali, M.; Gokhale, R.; Santoro, C.; Serov, A.; Artyushkova, K.; Atanassov, P. High Performance Platinum Group Metal-Free Cathode Catalysts for Microbial Fuel Cell (MFC). J. Electrochem. Soc. 2017, 164, H3041–H3046. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Xu, Q.; Sun, J.; Hou, B.; Zhang, Y. Iron- and nitrogen-functionalized graphene as a non-precious metal catalyst for enhanced oxygen reduction in an air-cathode microbial fuel cell. J. Power Sources 2012, 213, 265–269. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Q.; Ding, J.; Wang, K.; Jiang, J. Development of an MFC-powered BEF system with novel Fe-Mn-Mg/CF composite cathode to degrade refractory pollutants. J. Clean. Prod. 2021, 326, 129348. [Google Scholar] [CrossRef]
- Martin, E.; Tartakovsky, B.; Savadogo, O. Cathode materials evaluation in microbial fuel cells: A comparison of carbon, Mn2O3, Fe2O3 and platinum materials. Electrochim. Acta 2011, 58, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Parveen, N.; Hiep Han, T.; Ali Ansari, S.; Lee, M. Sustainable Bio-Energy Production in Microbial Fuel Cell Using MnO2 Nanoparticle-Decorated Hollow Carbon Nanofibers as Active Cathode Materials. J. Nanoelectron. Optoelectron. 2021, 16, 127–135. [Google Scholar] [CrossRef]
- Włodarczyk, B.; Włodarczyk, P.P. Comparsion of Cu-B alloy and stainless steel as electrode material for microbial fuel cell. In Renewable Energy Sources: Engineering, Technology, Innovation ICORES 2018; Wróbel, M., Jewiarz, M., Szlęk, A., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2020; pp. 1057–1063. [Google Scholar] [CrossRef]
- Włodarczyk, B.; Włodarczyk, P.P. Methanol electrooxidation with Cu-B catalyst. Infrastruct. Ecol. Rural Areas (Polish Acad. Sci. Crac.) 2016, 4, 1483–1492. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Wastewater Treatment and Electricity Production in a Microbial Fuel Cell with Cu-B Alloy as the Cathode Catalyst. Catalysts 2019, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, P.P.; Włodarczyk, B. Feasibility of Waste Engine Oil Electrooxidation with Ni-Co and Cu-B Catalysts. Energies 2022, 15, 7686. [Google Scholar] [CrossRef]
- Antolini, E.; Cardellini, F. Formation of carbon supported Pt-Ru alloys: An XRD analysis. J. Alloys Compd. 2001, 315, 118–122. [Google Scholar] [CrossRef]
- Motl, N.E.; Ewusi-Annan, E.; Sines, I.T.; Jensen, L.; Schaak, R.E. Au-Cu Alloy Nanoparticles with Tunable Compositions and Plasmonic Properties: Experimental Determination of Composition and Correlation with Theory. J. Phys. Chem. C 2010, 114, 19263–19269. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Cardellini, F.; Passalacqua, E. Physical and morphological characteristics and electrochemical behaviour in PEM fuel cells of PtRu/C catalysts. J. Solid State Electrochem. 2001, 5, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Antolini, E.; Cardellini, F.; Giorgi, L. Effect of Me (Pt + Ru) content in Me/C catalysts on PtRu alloy formation: An XRD analysis. J. Mater. Sci. Lett. 2000, 19, 2099–2103. [Google Scholar] [CrossRef]
- Tench, D.M.; White, J.T. A New Periodic Displacement Method Applied to Electrodeposition of Cu-Ag Alloys. J. Electrochem. Soc. 1992, 139, 443. [Google Scholar] [CrossRef]
- Bernasconi, R.; Hart, J.L.; Lang, A.C.; Magagnin, L.; Nobili, L.; Taheri, M.L. Structural properties of electrodeposited Cu-Ag alloys. Electrochim. Acta 2017, 251, 475–481. [Google Scholar] [CrossRef]
- Shao, W.; Sun, Y.; Zangari, G. Electrodeposition of Cu-Ag Alloy Films at n-Si(001) and Polycrystalline Ru Substrates. Coatings 2021, 11, 1563. [Google Scholar] [CrossRef]
- Holtzer, M.; Staronka, A. Chemia fizyczna: Wprowadzenie; Wydawnictwa AGH: Cracow, Poland, 2000. [Google Scholar]
- Permana, D. Performance of Single Chamber Microbial Fuel Cell (SCMFC) for biological treatment of tofu wastewater. Proc. IOP Conf. Ser. Earth Environ. Sci. 2019, 277, 012008. [Google Scholar] [CrossRef]
- Pham, T.H.; Rabaey, K.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Boon, N.; Verstraete, W. Microbial Fuel Cells in Relation to Conventional Anaerobic Digestion Technology. Eng. Life Sci. 2006, 6, 285–292. [Google Scholar] [CrossRef]
- Włodarczyk, B.; Włodarczyk, P.P. Analysis of the Potential of an Increase in Yeast Output Resulting from the Application of Additional Process Wastewater in the Evaporator Station. Appl. Sci. 2019, 9, 2282. [Google Scholar] [CrossRef] [Green Version]
- Huggins, T.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Energy and performance comparison of microbial fuel cell and conventional aeration treating of wastewater. J. Microb. Biochem. Technol. 2013, S6, 1–5. [Google Scholar] [CrossRef] [Green Version]



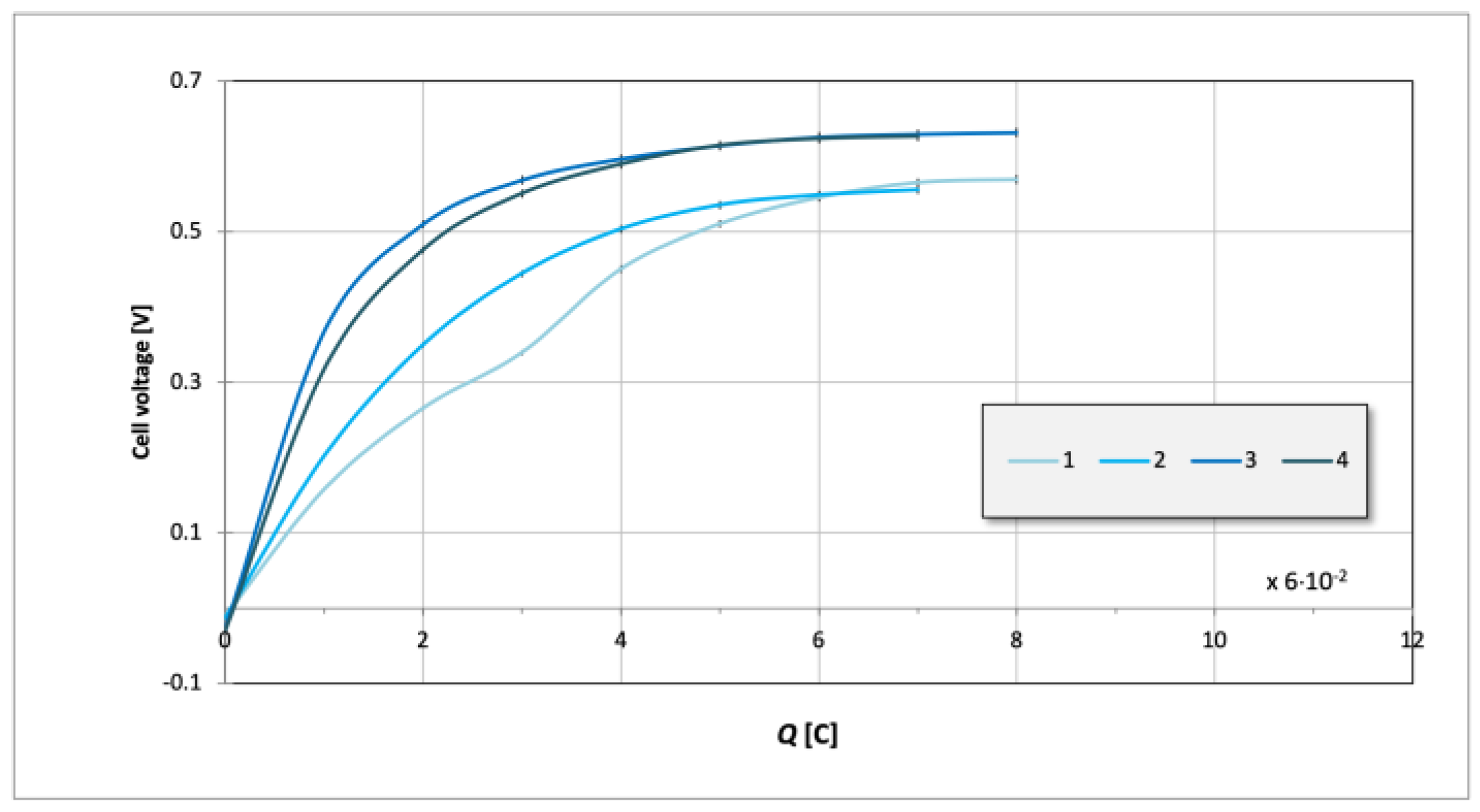

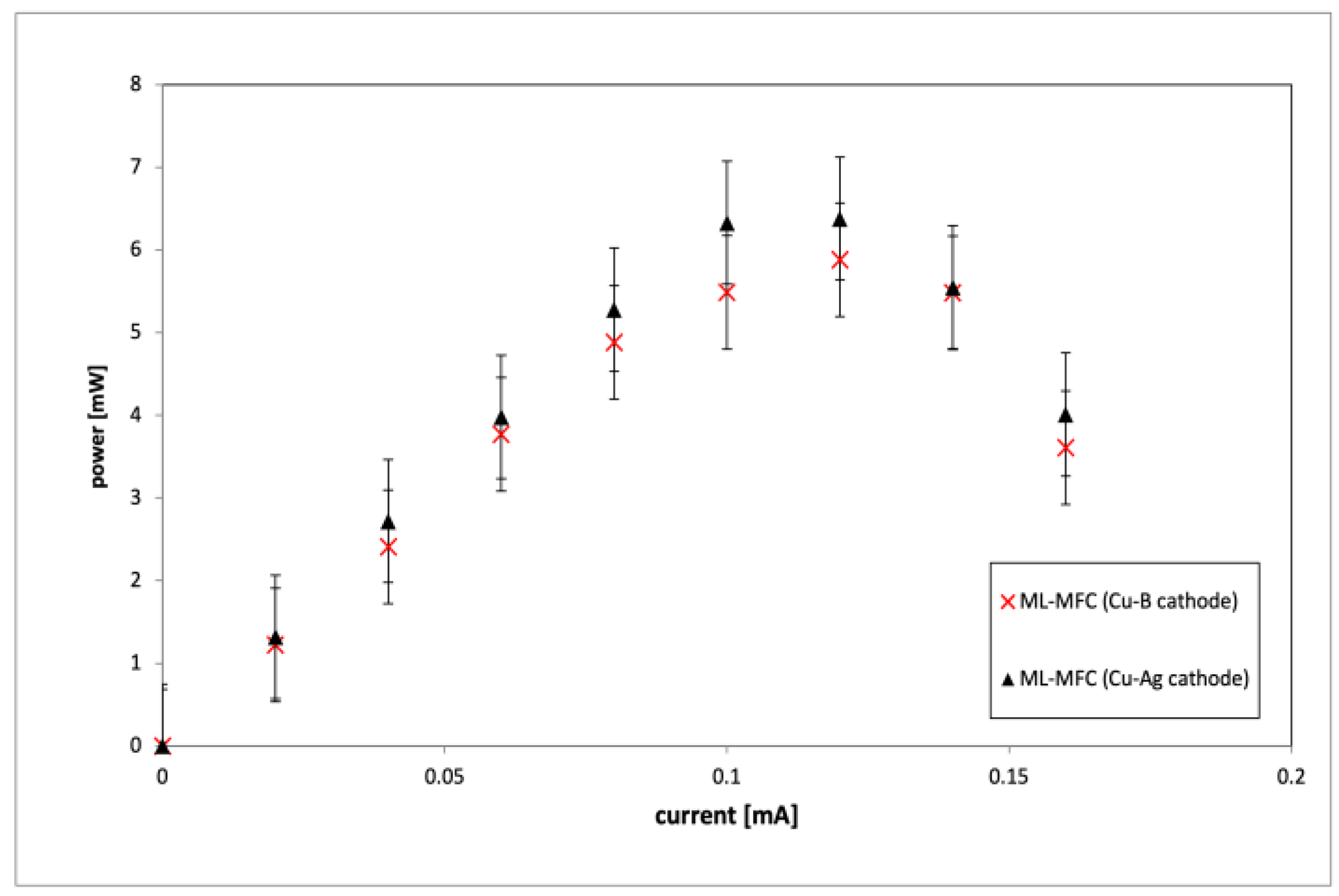
| Component | Volume |
|---|---|
| AgNO3 | 0.02 mol·L−1 |
| CuSO4·7H2O | 0.05 mol·L−1 |
| Trilon B | 0.12 mol·L−1 |
| NaOH | 1.00 mol·L−1 |
| Oxidizing Time of the Cu-Ag [h] | Max Power Obtained in ML-MFC [mW] | Average Voltage of the ML-MFC [V] | ||||
|---|---|---|---|---|---|---|
| Content of Ag in the Alloy (%) | Content of Ag in the Alloy (%) | |||||
| 3 | 4 | 5 | 3 | 4 | 5 | |
| 1 | 4.01 | 3.98 | 4.03 | 0.82 | 0.81 | 0.72 |
| 3 | 4.05 | 4.16 | 4.31 | 0.84 | 0.83 | 0.78 |
| 6 | 4.11 | 4.46 | 5.11 | 0.86 | 0.85 | 0.89 |
| 8 | 4.26 | 5.03 | 5.01 | 0.87 | 0.88 | 0.81 |
| Content of Ag [%] | Max Power Obtained in ML-MFC [mW] | Average Voltage of the ML-MFC [V] | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Anodic Charge | Number of Anodic Charge | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 3 | 4.99 | 5.15 | 5.49 | 5.12 | 0.84 | 0.85 | 0.87 | 0.91 |
| 4 | 5.65 | 5.81 | 5.99 | 5.46 | 0.91 | 0.92 | 0.99 | 0.94 |
| 5 | 5.79 | 5.95 | 6.38 | 5.92 | 0.91 | 0.91 | 1.09 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, B.; Włodarczyk, P.P. Electricity Production from Yeast Wastewater in Membrane-Less Microbial Fuel Cell with Cu-Ag Cathode. Energies 2023, 16, 2734. https://doi.org/10.3390/en16062734
Włodarczyk B, Włodarczyk PP. Electricity Production from Yeast Wastewater in Membrane-Less Microbial Fuel Cell with Cu-Ag Cathode. Energies. 2023; 16(6):2734. https://doi.org/10.3390/en16062734
Chicago/Turabian StyleWłodarczyk, Barbara, and Paweł P. Włodarczyk. 2023. "Electricity Production from Yeast Wastewater in Membrane-Less Microbial Fuel Cell with Cu-Ag Cathode" Energies 16, no. 6: 2734. https://doi.org/10.3390/en16062734





