Latent Heat Storage Systems for Thermal Management of Electric Vehicle Batteries: Thermal Performance Enhancement and Modulation of the Phase Transition Process Dynamics: A Literature Review
Abstract
:1. Introduction
- 1.
- A two-stage literature survey process was conducted as described below:
- 1.1.
- Recent studies related to thermal behavior, thermal regime requirements and thermal issues for EV battery systems were identified. These issues were shortly covered in the Introduction and they are essential to understanding the challenges for the BTMSs. Search terms and Boolean expressions employed were:
- 1.1.1.
- “Electric vehicle battery system”
- 1.1.2.
- “Battery system” + “capacity degradation”
- 1.1.3.
- “Battery system” + “thermal runaway”
- 1.1.4.
- “Electric vehicle” + “cold startup”
- 1.2.
- Relevant BTMSs studies were identified, both review and research articles. The terms and Boolean expressions used were as follows:
- 1.2.1.
- “Battery thermal management system”
- 1.2.2.
- “Battery thermal control”
- 2.
- Research and review articles related to BTMSs based on latent heat storage. The main focus was identification of thermal performance enhancement techniques and their adequacy for EV battery systems. The search expressions employed were the following:
- 2.1.
- “Electric vehicle battery system” + “phase change material”
- 2.2.
- “Electric vehicle battery system” + “phase change material” + “enhancement”
- 2.3.
- “Electric vehicle battery system” + “phase change material” + “fin”
- 2.4.
- “Electric vehicle battery system” + “phase change material” + “additives”
- 2.5.
- “Electric vehicle battery system” + “phase change material” + “heat pipe”
- 2.6.
- “Electric vehicle battery system” + “phase change material” + “enhancement”
- 2.7.
- “Electric vehicle battery system” + “phase change material” + “metal foam”
- 2.8.
- “Electric vehicle battery system” + “phase change material” + “active enhancement”
- 2.9.
- “Electric vehicle battery system” + “phase change material” + “field”
- 2.10.
- “Electric vehicle battery system” + “phase change material” + “vibration”
- 2.11.
- “Phase change material” + “enhancement” + “modulation”
- 2.12.
- “Phase change material” + “tuning”
- -
- The main topic of the article was a BTMS;
- -
- The BTMSs were based on PCMs;
- -
- A thermal performance enhancement effect was reported (no matter what the reporting form was);
- -
- Preference was given to references from 2019 and later; older references were included, however, provided that they reported results worth further investigation.
2. Battery Systems Thermal Issues
- Chemical reactions between electrolyte and cathode are initiated;
- film forms on the electrode interface;
- decomposition of anode and electrolyte occurs.
2.1. Power/Capacity Degradation
2.2. Thermal Runaway
2.3. Electrical Imbalance
2.4. BS Behavior at Sub-Zero Temperatures
2.5. Battery Thermal Management Systems (BTMS)
2.5.1. General Issues of BTMSs
2.5.2. BTMSs Based on Latent Heat Storage
3. Passive Techniques for Heat Transfer Enhancement in BTMSs Based on PCMs
3.1. Heat Transfer Area Extension
3.1.1. Nanocarbon Materials
- The RT44HC/fumed silica composite, which has a lower thermal conductivity, extended the cooling period to a greater extent than the RT44HC/EG composite;
- The RT44HC/fumed silica composite was deemed unsuitable for the thermal management of a multi-cell battery pack. Due to the low thermal conductivity, an extremely high temperature difference (>12 °C) in the battery pack occurred. The highly uneven temperature distribution led to a high voltage difference between the cells, which triggered an early stop of the 20-cycle test of this pack at −10 °C and led to a loss of capacity;
- Compared with the no-PCM case, the RT44HC/EG composite prevented battery overheating during the single-discharge test and suppressed the battery temperature fluctuation in the 20-cycle test. In both tests, the RT44HC/EG composite improved the temperature uniformity in the battery pack. The maximum temperature difference was reduced by up to 6 °C. The more uniform temperature distribution reduced the voltage differences between the battery cells, especially when the ambient temperature was dropped from 5 to −10 °C.
- The optimum nano-additive mass ratio;
- Preparation methods and prevention of particles clogging and agglomeration;
- Long term stability.
3.1.2. Nanoparticles
3.1.3. Fins and Complex Structures
- The fin material replaces an equivalent volume of PCM; this results in decreasing the thermal storage capacity of the enhanced system;
- An enhancement limit exists given by the fin efficiency; increasing the fin length/height beyond this limit does not result in further heat transfer enhancement;
- Some fin layouts can prevent the development of buoyancy driven flows, thus inhibiting the natural convection of the molten fraction;
- Most studies reviewed discuss the melting process; the effect of fins on the solidification process was not properly addressed;
- For EVs the mass systems could be an important constraint; it is important to assess the tradeoff between thermal performance of the BTMS and the mass penalty.
3.2. Porous Media
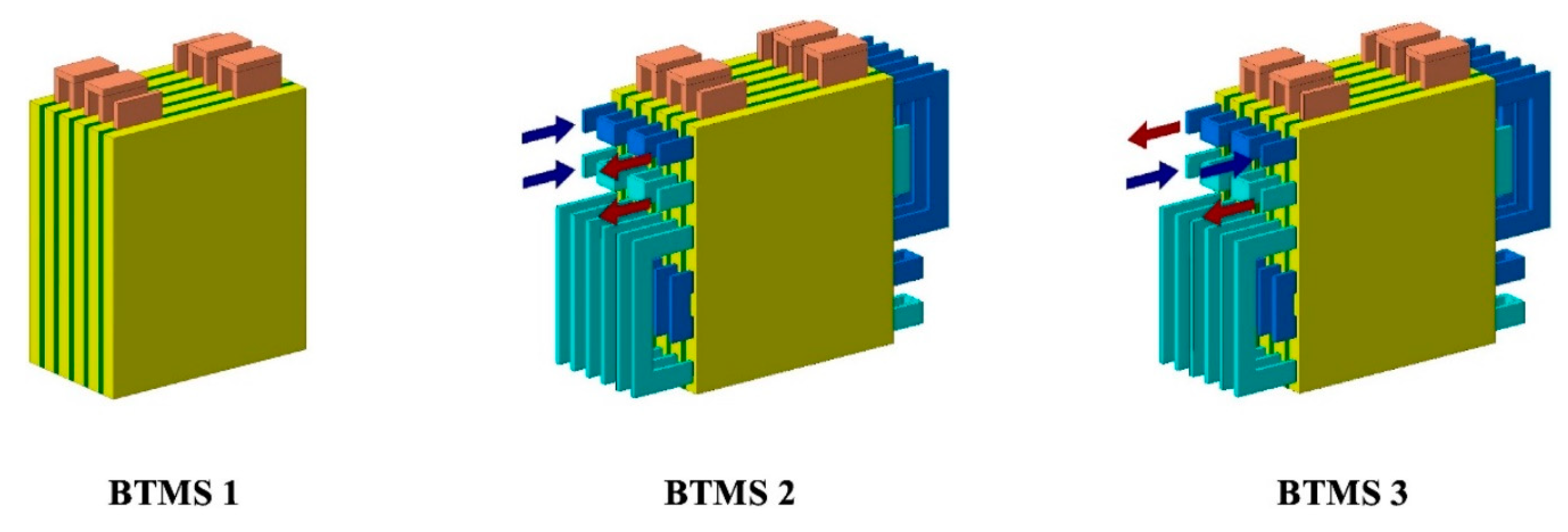
3.3. Heat Pipes
4. Combined Passive Techniques
5. Hybrid and Combined Systems
6. Modulation of PCMs Thermo-Physical Properties and Phase Transition Behavior
- The optimum operational temperature range is 20 to 40 °C. Outside this range, the battery performance is reduced, and RUL degradation occurs;
- The heat dissipation profile is random: no periodicity or amplitude can be predicted;
- The battery can be exposed to extreme temperatures, depending on the environmental temperature. Thus, not only heat dissipation during discharge can be an issue but also heating up of the battery from temperatures below the minimum operational temperature.
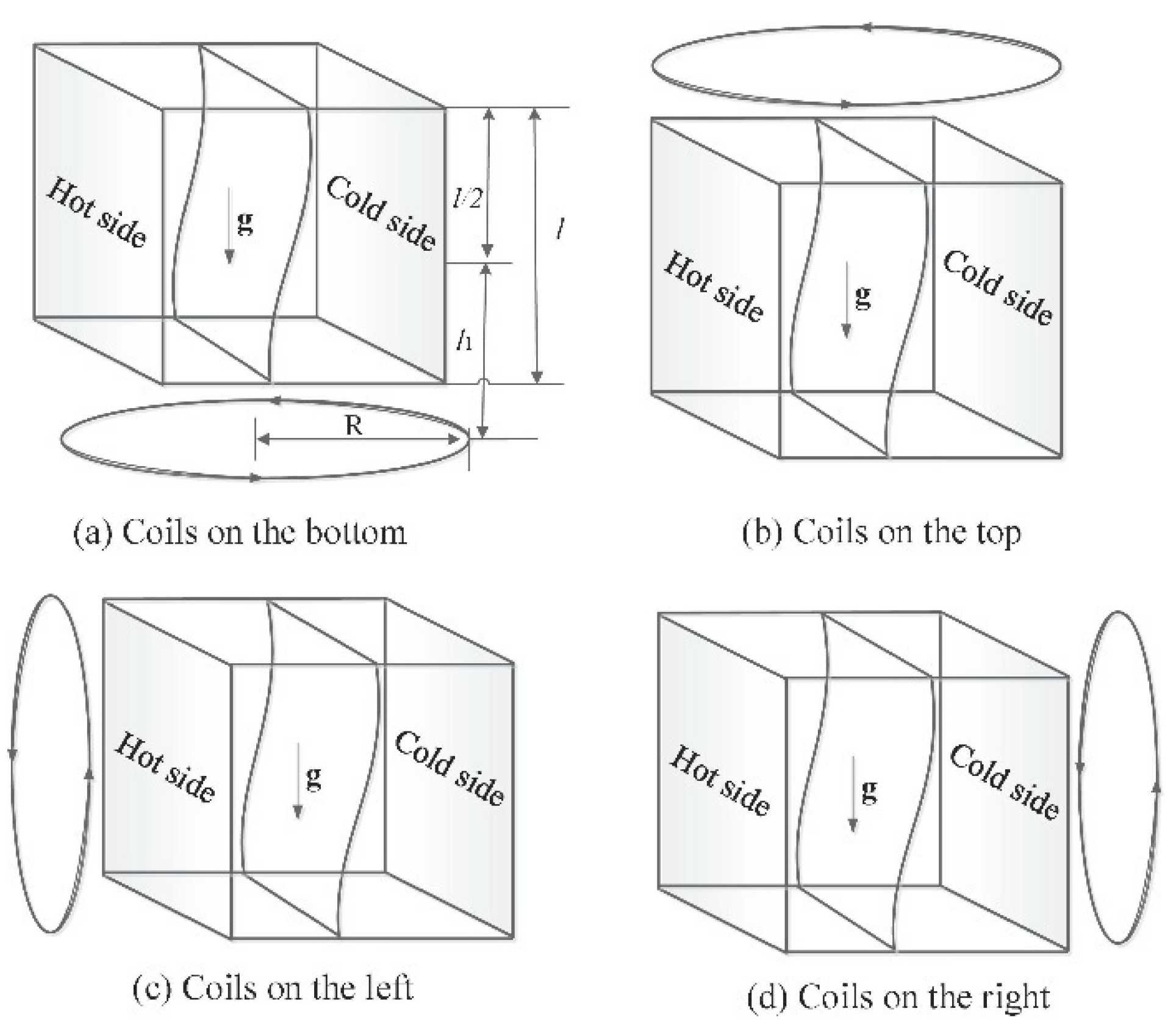
6.1. Magnetic Fields
6.2. Electric Fields
6.3. Mechanical Vibration
7. Conclusions
- The capacity of handling high rates of heat dissipation. This may result in the incapacity of the BTMS to limit the battery temperature, and eventually in thermal runaway;
- The capacity to remove the heat accumulated before the beginning of a new cycle. The PCM is not regenerated before the beginning of the next accumulation cycle, leading eventually to thermal runaway.
- No reports were identified in the analyzed articles discussing the BTMSs tests in real life (with EV under typical usage scenarios);
- If PCM-based passive techniques are used in hybrid BTMSs, it would be interesting to assess the energy savings that an enhancement technique produces.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| BTMS | Battery Thermal Management System |
| CMC | Carboxymethyl Cellulose |
| CPCM | Composite Phase Change Material |
| COP | Coefficient of Performance |
| DA | Decanoic Acid |
| DHPD | Disodium Hydrogen Phosphate Dodecahydrate |
| DSC | Differential Scanning Calorimeter |
| EG | Expanded Graphite |
| EV | Electric Vehicle |
| HTF | Heat Transfer Fluid |
| LF | Liquid Fraction |
| MF | Metal Foam |
| MP | Melting Point |
| OBC | Olefin Block Copolymer |
| PA | Paraffin |
| PW | Paraffin Wax |
| PCM | Phase Change Material |
| PEG | Polyethylene Glycol |
| RUL | Remaining Useful Life |
| SAT | Sodium Acetate Trihydrate |
| TC | Thermal Conductivity (Coefficient) |
| TCE | Thermal Conductivity Enhancer |
| TEC | Thermoelectric Cooler |
References
- Amjad, S.; Neelakrishnan, S.; Rudramoorthy, R. Review of design considerations and technological challenges for successful development and deployment of plug-in hybrid electric vehicles. Renew. Sustain. Energy Rev. 2010, 14, 1104–1110. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy storage technologies and real life applications—A state of the art review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef] [Green Version]
- Goutam, S.; Timmermans, J.-M.; Omar, N.; Van den Bossche, P.; Mierlo, J.M. Comparative Study of Surface Temperature Behavior of Commercial Li-Ion Pouch Cells of Different Chemistries and Capacities by Infrared Thermography. Energies 2015, 8, 8175–8192. [Google Scholar] [CrossRef] [Green Version]
- Andwari, A.M.; Pesiridis, A.; Rajoo, S.; Martinez-Botas, R.; Esfahanian, V. A review of Battery Electric Vehicle technology and readiness levels. Renew. Sustain. Energy Rev. 2017, 78, 414–430. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Li, B.; Yan, Y. A critical review of thermal management models and solutions of lithium-ion batteries for the development of pure electric vehicles. Renew. Sustain. Energy Rev. 2016, 64, 106–128. [Google Scholar] [CrossRef]
- Jaguemont, J.; Van Mierlo, J. A comprehensive review of future thermal management systems for battery-electrified vehicles. J. Energy Storage 2020, 31, 101551. [Google Scholar] [CrossRef]
- Thakur, A.K.; Prabakaran, R.; Elkadeem, M.; Sharshir, S.W.; Arıcı, M.; Wang, C.; Zhao, W.; Hwang, J.-Y.; Saidur, R. A state of art review and future viewpoint on advance cooling techniques for Lithium–ion battery system of electric vehicles. J. Energy Storage 2020, 32, 101771. [Google Scholar] [CrossRef]
- Shukla, K.; Mohammad Siddique, A.R.; Venkateshwar, K.; Mohaghegh, M.R.; Tasnim, S.H.; Mahmud, S. Experimental investigation on thermal field measurement of lithium-ion batteries under vibration. J. Energy Storage 2022, 53, 105110. [Google Scholar] [CrossRef]
- Lai, C.; Shan, S.; Feng, S.; Chen, Y.; Zeng, J.; Song, J.; Fu, L. Numerical investigations on heat transfer enhancement and energy flow distribution for interlayer battery thermal management system using Tesla-valve mini-channel cooling. Energy Convers. Manag. 2023, 280, 116812. [Google Scholar] [CrossRef]
- Shen, X.; Cai, T.; He, C.; Yang, Y.; Chen, M. Thermal analysis of modified Z-shaped air-cooled battery thermal management system for electric vehicles. J. Energy Storage 2023, 58, 106356. [Google Scholar] [CrossRef]
- Jilte, R.; Afzal, A.; Islam, T.; Manokar, A.M. Hybrid cooling of cylindrical battery with liquid channels in phase change material. Int. J. Energy Res. 2021, 45, 11065–11083. [Google Scholar] [CrossRef]
- Jilte, R.; Kumar, R.; Ahmadi, M.H.; Chen, L. Battery thermal management system employing phase change material with cell-to-cell air cooling. Appl. Therm. Eng. 2019, 161, 114199. [Google Scholar] [CrossRef]
- Verma, A.; Shashidhara, S.; Rakshit, D. A comparative study on battery thermal management using phase change material (PCM). Therm. Sci. Eng. Prog. 2019, 11, 74–83. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Li, C. An up-to-date review on the design improvement and optimization of the liquid-cooling battery thermal management system for electric vehicles. Appl. Therm. Eng. 2023, 219, 119626. [Google Scholar] [CrossRef]
- Weragoda, D.M.; Tian, G.; Burkitbayev, A.; Lo, K.-H.; Zhang, T. A comprehensive review on heat pipe based battery thermal management systems. Appl. Therm. Eng. 2023, 224, 120070. [Google Scholar] [CrossRef]
- Ghaeminezhad, N.; Wang, Z.; Ouyang, Q. A Review on lithium-ion battery thermal management system techniques: A control-oriented analysis. Appl. Therm. Eng. 2023, 219, 119497. [Google Scholar] [CrossRef]
- Li, A.; Weng, J.; Yuen, A.C.Y.; Wang, W.; Liu, H.; Lee, E.W.M.; Wang, J.; Kook, S.; Yeoh, G.H. Machine learning assisted advanced battery thermal management system: A state-of-the-art review. J. Energy Storage 2023, 60, 106688. [Google Scholar] [CrossRef]
- Zhang, Z.; Fouchard, D.; Rea, J.R. Differential scanning calorimetry material studies: Implications for the safety of lithium-ion cells. J. Power Sources 1998, 70, 16–20. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Steele, L.A.; Spangler, S.W. Failure propagation in multi-cell lithium ion batteries. J. Power Sources 2015, 283, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Ratnakumar, B.V.; Smart, M.C.; Surampudi, S. Effects of SEI on the kinetics of lithium intercalation. J. Power Sources 2001, 97-98, 137–139. [Google Scholar] [CrossRef]
- Senyshyn, A.; Mühlbauer, M.J.; Dolotko, O.; Ehrenberg, H. Low-temperature performance of Li-ion batteries: The behavior of lithiated graphite. J. Power Sources 2015, 282, 235–240. [Google Scholar] [CrossRef]
- Lin, H.P.; Chua, D.; Salomon, M.; Shiao, H.-C.; Hendrickson, M.; Plichta, E.; Slane, S. Low-Temperature Behavior of Li-Ion Cells. Electrochem. Solid-State Lett. 2001, 4, A71–A73. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Charge and discharge characteristics of a commercial LiCoO2-based 18650 Li-ion battery. J. Power Sources 2006, 160, 1403–1409. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, Y.; Howey, D.A.; Perez, H.; Foley, A.; Pecht, M. Battery warm-up methodologies at subzero temperatures for automotive applications: Recent advances and perspectives. Prog. Energy Combust. Sci. 2020, 77, 100806. [Google Scholar] [CrossRef]
- Lawag, R.A.; Muhammad Ali, H. Phase change materials for thermal management and energy storage: A review. J. Energy Storage 2022, 55, 105602. [Google Scholar] [CrossRef]
- Ugurlu, A.; Gokcol, C. A review on thermal energy storage systems with phase change materials in vehicles. Electron. J. Vocat. Coll. 2012, 2, 1–14. [Google Scholar]
- Al Hallaj, S.; Selman, J. A novel thermal management system for electric vehicle batteries using phase-change Material. J. Electrochem. Soc. 2000, 147, 3231–3236. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Zhang, T.; Jiang, Z.; Ding, J.; Ding, Y. A comprehensive review of composite phase change material based thermal management system for lithium-ion batteries. Renew. Sustain. Energy Rev. 2022, 167, 112667. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewit, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Sari, A.; Karaipekli, A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl. Therm. Eng. 2007, 27, 1271–1277. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Ling, Z.; Wen, X.; Zhang, Z.; Fang, X.; Gao, X. Thermal management performance of phase change materials with different thermal conductivities for Li-ion battery packs operated at low temperatures. Energy 2018, 144, 977–983. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Wu, T.; Li, Z.; Wu, C. A novel stable and flexible composite phase change materials for battery thermal management. Appl. Therm. Eng. 2022, 212, 118510. [Google Scholar] [CrossRef]
- Goli, P.; Legedza, S.; Dhar, A.; Salgado, R.; Renteria, J.; Balandin, A.A. Graphene-enhanced hybrid phase change materials for thermal management of Li-ion batteries. J. Power Sources 2014, 248, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Du, C.; Qi, R.; Wang, Y. Experimental study on thermal management of lithium-ion battery with graphite powder based composite phase change materials covering the whole climatic range. Appl. Therm. Eng. 2022, 216, 119072. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Yan, X.; Dou, B.; Zhang, D.; Cui, G.; Yang, Q. Composite phase change materials with carbon foam and fibre combination for efficient battery thermal management: Dual modulation roles of interfacial heat transfer. J. Mater. Res. Technol. 2023, 23, 551–563. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, X.; Hu, M.; Xu, L.; Ding, J.; Ding, Y. Development and investigation of form-stable and cyclable decanoic acid-based composite phase change materials for efficient battery thermal management. J. Power Sources 2023, 558, 232615. [Google Scholar] [CrossRef]
- Zhou, D.; Luo, Y.; Bi, C.; Li, X.; Deng, J.; Yang, W.; Li, C. Experimental and simulative investigation on battery thermal management system with structural optimization of composite phase change material. J. Energy Storage 2023, 60, 106613. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Behi, M.; Nargesi, N.; Aminian, M.; Ghanbarpour, A.; Mirmohseni, F.; Van Mierlo, J.; Berecibar, M. An Enhanced Phase Change Material Composite for Electrical Vehicle Thermal Management. Designs 2022, 6, 70. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; He, F.; He, J.; Zhong, Z.; Zhang, G. Experimental Investigation on Thermal Management of Electric Vehicle Battery Module with Paraffin/Expanded Graphite Composite Phase Change Material. Int. J. Photoenergy 2017, 2017, 2929473. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, J.; Cao, M.; Liu, Z.; Chen, Q. A novel flexible phase change material with well thermal and mechanical properties for lithium batteries application. J. Energy Storage 2021, 44, 103433. [Google Scholar] [CrossRef]
- Taghilou, M.; Mohammadi, M.S. Thermal management of lithium-ion battery in the presence of phase change material with nanoparticles considering thermal contact resistance. J. Energy Storage 2022, 56, 106029. [Google Scholar] [CrossRef]
- Hussain, A.; Abidi, I.H.; Tso, C.Y.; Chan, K.C.; Luo, Z.; Chao, C.Y. Thermal management of lithium ion batteries using graphene coated nickel foam saturated with phase change materials. Int. J. Therm. Sci. 2018, 124, 23–35. [Google Scholar] [CrossRef]
- Karimi, G.; Azizi, M.; Babapoor, A. Experimental study of a cylindrical lithium ion battery thermal management using phase change material composites. J. Energy Storage 2016, 8, 168–174. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Youssef, R.; Suresh Patil, M.; Van Mierlo, J.; Berecibar, M. Comprehensive Passive Thermal Management Systems for Electric Vehicles. Energies 2021, 14, 3881. [Google Scholar] [CrossRef]
- Jilte, R.; Afzal, A.; Panchal, S. A novel battery thermal management system using nano-enhanced phase change materials. Energy 2021, 219, 119564. [Google Scholar] [CrossRef]
- Muhammad Azim, M.K.; Arifutzzaman, A.; Saidur, R.; Khandaker, M.U.; Bradley, D.A. Recent progress in emerging hybrid nanomaterials towards the energy storage and heat transfer applications: A review. J. Mol. Liq. 2022, 360, 119443. [Google Scholar] [CrossRef]
- Ismail, K.A.R.; Lino, F.A.M.; Machado, P.L.O.; Teggar, M.; Arıcı, M.; Alves, T.A.; Teles, M.P. New potential applications of phase change materials: A review. J. Energy Storage 2022, 53, 105202. [Google Scholar] [CrossRef]
- Tariq, S.L.; Muhammad Ali, H.; Akram, M.A.; Janjua, M.M.; Ahmadlouydarab, M. Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl. Therm. Eng. 2020, 176, 115305. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Hu, S.; Liu, X.; He, S. A copper nanoparticle enhanced phase change material with high thermal conductivity and latent heat for battery thermal management. J. Loss Prev. Process. Ind. 2022, 78, 104814. [Google Scholar] [CrossRef]
- Li, B.; Mao, Z.; Song, B.; Chen, P.; Wang, H.; Sunden, B.; Wang, Y.-F. Enhancement of phase change materials by nanoparticles to improve battery thermal management for autonomous underwater vehicles. Int. Commun. Heat Mass Transf. 2022, 137, 106301. [Google Scholar] [CrossRef]
- Fan, R.; Zheng, N.; Sun, Z. Evaluation of fin intensified phase change material systems for thermal management of Li-ion battery modules. Int. J. Heat Mass Transf. 2021, 166, 120753. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.; Li, X.; Zhang, G.; Wang, Z.; Yang, C. Experimental study on a novel battery thermal management technology based on low density polyethylene-enhanced composite phase change materials coupled with low fins. Appl. Energy 2016, 178, 376–382. [Google Scholar] [CrossRef]
- Chen, G.; Shi, Y.; Ye, H.; Kang, H. Experimental study on phase change material based thermal management design with adjustable fins for lithium-ion battery. Appl. Therm. Eng. 2023, 221, 119808. [Google Scholar] [CrossRef]
- Ping, P.; Peng, R.; Kong, D.; Chen, G.; Wen, J. Investigation on thermal management performance of PCM-fin structure for Li-ion battery module in high-temperature environment. Energy Convers. Manag. 2018, 176, 131–146. [Google Scholar] [CrossRef]
- Chen, H.; Abidi, A.; Hussein, A.K.; Younis, O.; Degani, M.; Heidarshenas, B. Investigation of the use of extended surfaces in paraffin wax phase change material in thermal management of a cylindrical lithium-ion battery: Applicable in the aerospace industry. J. Energy Storage 2022, 45, 103685. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, Y.; Gao, R.; Liu, S. Investigation on heat transfer enhancement of phase change material for battery thermal energy storage system based on composite triply periodic minimal surface. J. Energy Storage 2023, 57, 106222. [Google Scholar] [CrossRef]
- Mei, J.; Shi, G.; Liu, H.; Wang, Z.; Chen, M. Investigation on the optimization strategy of phase change material thermal management system for lithium-ion battery. J. Energy Storage 2022, 55, 105365. [Google Scholar] [CrossRef]
- Choudhari, V.G.; Dhoble, A.S.; Panchal, S. Numerical analysis of different fin structures in phase change material module for battery thermal management system and its optimization. Int. J. Heat Mass Transf. 2020, 163, 120434. [Google Scholar] [CrossRef]
- Choudhari, V.G.; Dhoble, A.S.; Panchal, S.; Fowler, M.; Fraser, R. Numerical investigation on thermal behaviour of 5 × 5 cell configured battery pack using phase change material and fin structure layout. J. Energy Storage 2021, 43, 103234. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Wang, J. Optimization of the internal fin in a phase-change-material module for battery thermal management. Appl. Therm. Eng. 2020, 167, 114698. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Liu, Y.; Wang, F.; Yang, N.; Liu, X.; Liu, H.; Li, W.; Liu, H.; Huang, B. Performance analysis of phase change material in battery thermal management with biomimetic honeycomb fin. Appl. Therm. Eng. 2021, 196, 117296. [Google Scholar] [CrossRef]
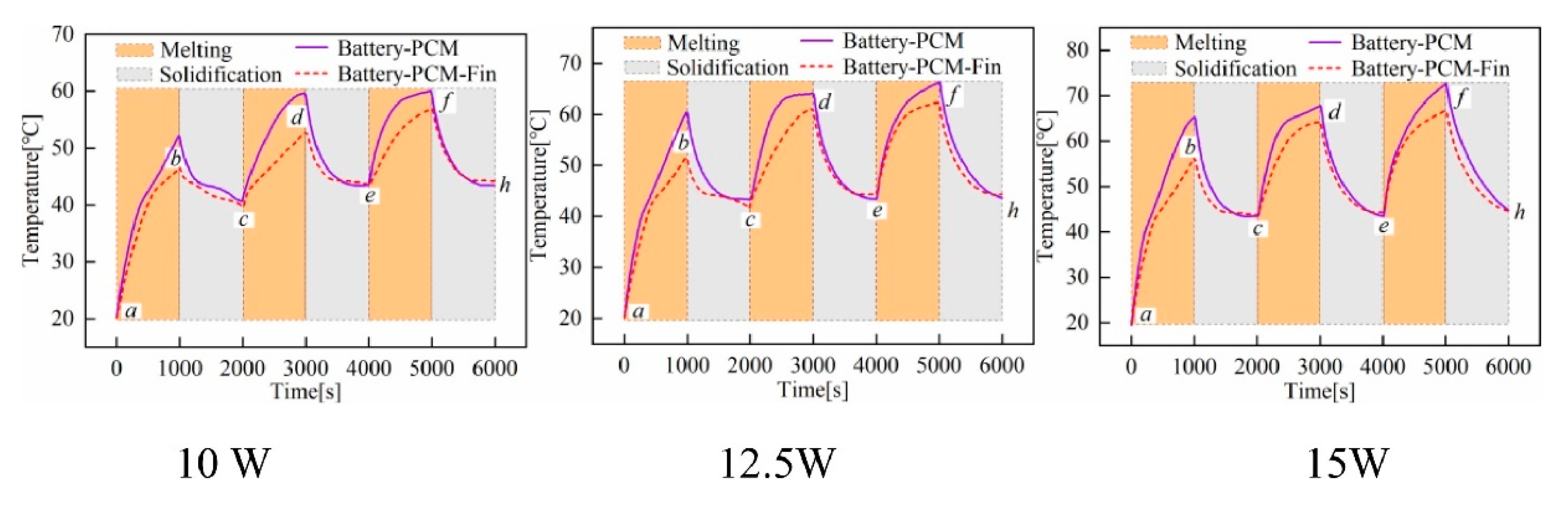
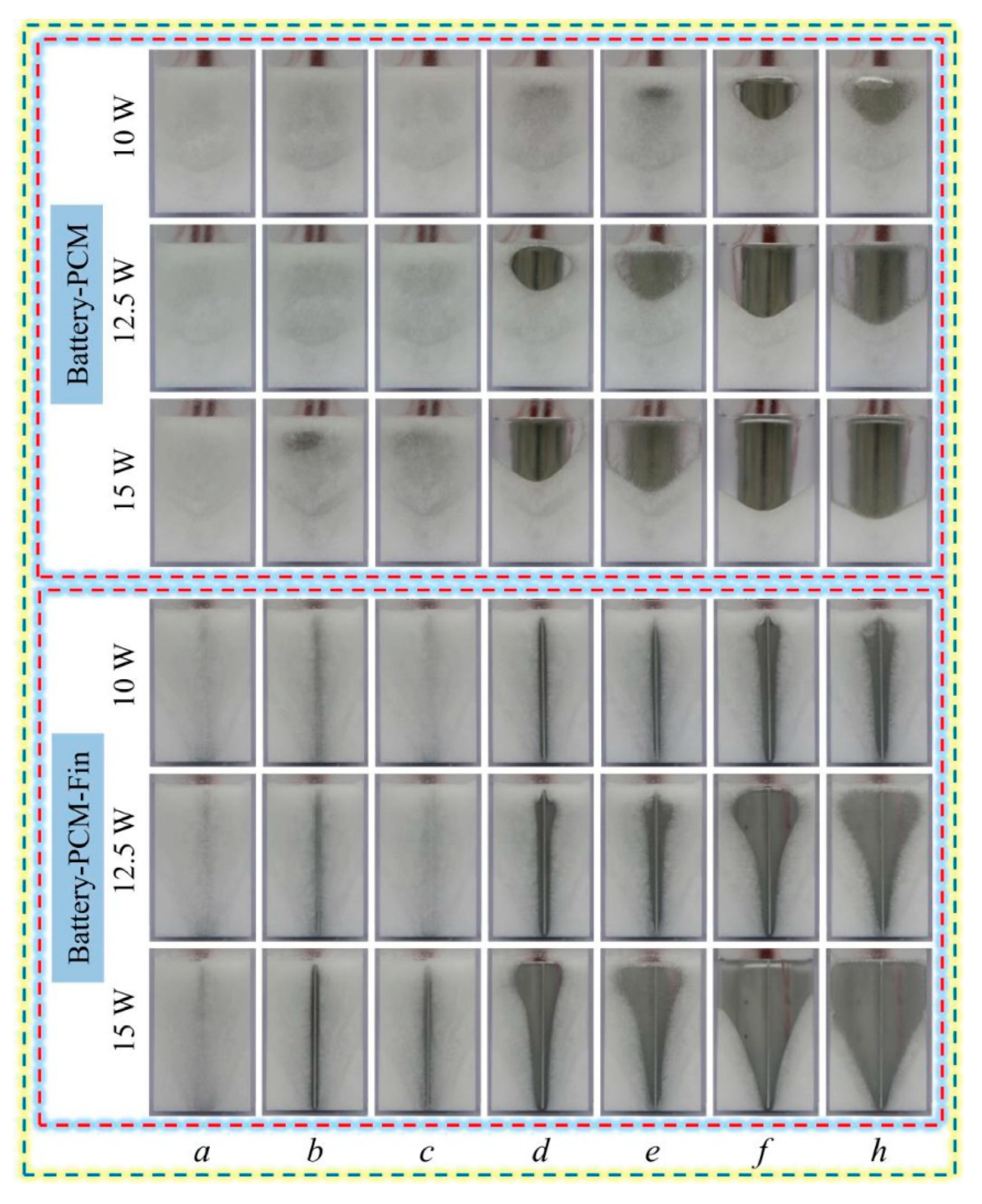
- Sun, Z.; Fan, R.; Yan, F.; Zhou, T.; Zheng, N. Thermal management of the lithium-ion battery by the composite PCM-Fin structures. Int. J. Heat Mass Transf. 2019, 145, 118739. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, R.; Zheng, N. Thermal management of a simulated battery with the compound use of phase change material and fins: Experimental and numerical investigations. Int. J. Therm. Sci. 2021, 165, 106945. [Google Scholar] [CrossRef]
- Shahsavar, A.; Goodarzi, A.; Askari, I.B.; Jamei, M.; Karbasi, M.; Afrand, M. The entropy generation analysis of the influence of using fins with tip clearance on the thermal management of the batteries with phase change material: Application a new gradient-based ensemble machine learning approach. Eng. Anal. Bound. Elem. 2022, 140, 432–446. [Google Scholar] [CrossRef]
- Qi, X.; Sidi, M.O.; Tlili, I.; Ibrahim, T.K.; Elkotb, M.A.; El-Shorbagy, M.A.; Li, Z. Optimization and sensitivity analysis of extended surfaces during melting and freezing of phase changing materials in cylindrical Lithium-ion battery cooling. J. Energy Storage 2022, 51, 104545. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Deng, Y.; Wu, F.; Wang, H. Effect of functional modification of porous medium on phase change behavior and heat storage characteristics of form-stable composite phase change materials: A critical review. J. Energy Storage 2021, 44, 103637. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Y.; Zhang, K.; Wu, S.; Chen, D.; Gao, Y. Enhanced thermal storage capacity of paraffin/diatomite composite using oleophobic modification. J. Clean. Prod. 2021, 279, 123211. [Google Scholar] [CrossRef]
- Cui, W.; Si, T.; Li, X.; Li, X.; Lu, L.; Ma, T.; Wang, Q. Heat transfer enhancement of phase change materials embedded with metal foam for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2022, 169, 112912. [Google Scholar] [CrossRef]
- Situ, W.; Zhang, G.; Li, X.; Yang, X.; Wei, C.; Rao, M.; Wang, Z.; Wang, C.; Wu, W. A thermal management system for rectangular LiFePO4 battery module using novel double copper mesh-enhanced phase change material plates. Energy 2017, 141, 613–623. [Google Scholar] [CrossRef]
- Wu, W.; Yang, X.; Zhang, G.; Ke, X.; Wang, Z.; Situ, W.; Li, X.; Zhang, J. An experimental study of thermal management system using copper mesh-enhanced composite phase change materials for power battery pack. Energy 2016, 113, 909–916. [Google Scholar] [CrossRef]
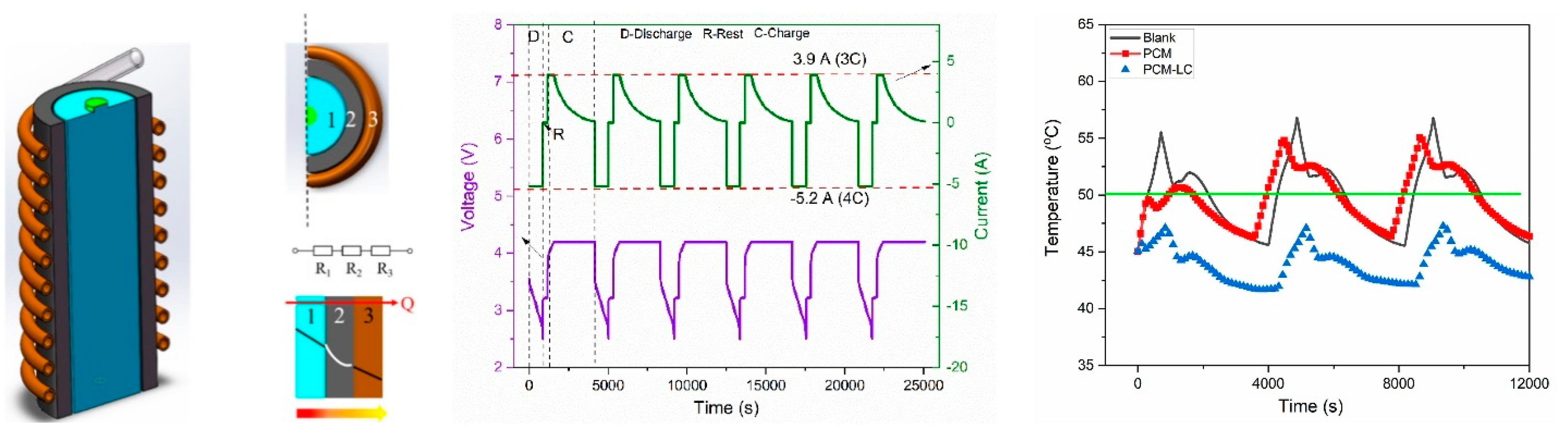
- Zhao, Y.; Zou, B.; Ding, J.; Ding, Y. Experimental and numerical investigation of a hybrid battery thermal management system based on copper foam-paraffin composite phase change material and liquid cooling. Appl. Therm. Eng. 2023, 218, 119312. [Google Scholar] [CrossRef]
- Pan, M.; Zhong, Y. Experimental and numerical investigation of a thermal management system for a Li-ion battery pack using cutting copper fiber sintered skeleton/paraffin composite phase change materials. Int. J. Heat Mass Transf. 2018, 126, 531–543. [Google Scholar] [CrossRef]
- Alipanah, M.; Li, X. Numerical studies of lithium-ion battery thermal management systems using phase change materials and metal foams. Int. J. Heat Mass Transf. 2016, 102, 1159–1168. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Jia, L.; Yang, L. Paraffin and paraffin/aluminum foam composite phase change material heat storage experimental study based on thermal management of Li-ion battery. Appl. Therm. Eng. 2015, 78, 428–436. [Google Scholar] [CrossRef]
- Heyhat, M.M.; Mousavi, S.; Siavashi, M. Battery thermal management with thermal energy storage composites of PCM, metal foam, fin and nanoparticle. J. Energy Storage 2020, 28, 101235. [Google Scholar] [CrossRef]
- Pandey, H.; Gupta, N.K. A descriptive review of the thermal transport mechanisms in mono and hybrid nanofluid-filled heat pipes and current developments. Therm. Sci. Eng. Prog. 2022, 31, 101281. [Google Scholar] [CrossRef]
- Muhammad Ali, H. Applications of combined/hybrid use of heat pipe and phase change materials in energy storage and cooling systems: A recent review. J. Energy Storage 2019, 26, 100986. [Google Scholar] [CrossRef]
- Boonma, K.; Patimaporntap, N.; Mbulu, H.; Trinuruk, P.; Ruangjirakit, K.; Laoonual, Y.; Wongwises, S. A Review of the Parameters Affecting a Heat Pipe Thermal Management System for Lithium-Ion Batteries. Energies 2022, 15, 8534. [Google Scholar] [CrossRef]
- Bernagozzi, M.; Georgoulas, A.; Miche, N.; Marengo, M. Heat pipes in battery thermal management systems for electric vehicles: A critical review. Appl. Therm. Eng. 2023, 219, 119495. [Google Scholar] [CrossRef]
- Karimi, D.; Behi, H.; Van Mierlo, J.; Berecibar, M. Novel Hybrid Thermal Management System for High-Power Lithium-Ion Module for Electric Vehicles: Fast Charging Applications. World Electr. Veh. J. 2022, 13, 86. [Google Scholar] [CrossRef]
- Putra, N.; Sandi, A.F.; Ariantara, B.; Abdullah, N.; Indra Mahlia, T.M. Performance of beeswax phase change material (PCM) and heat pipe as passive battery cooling system for electric vehicles. Case Stud. Therm. Eng. 2020, 21, 100655. [Google Scholar] [CrossRef]
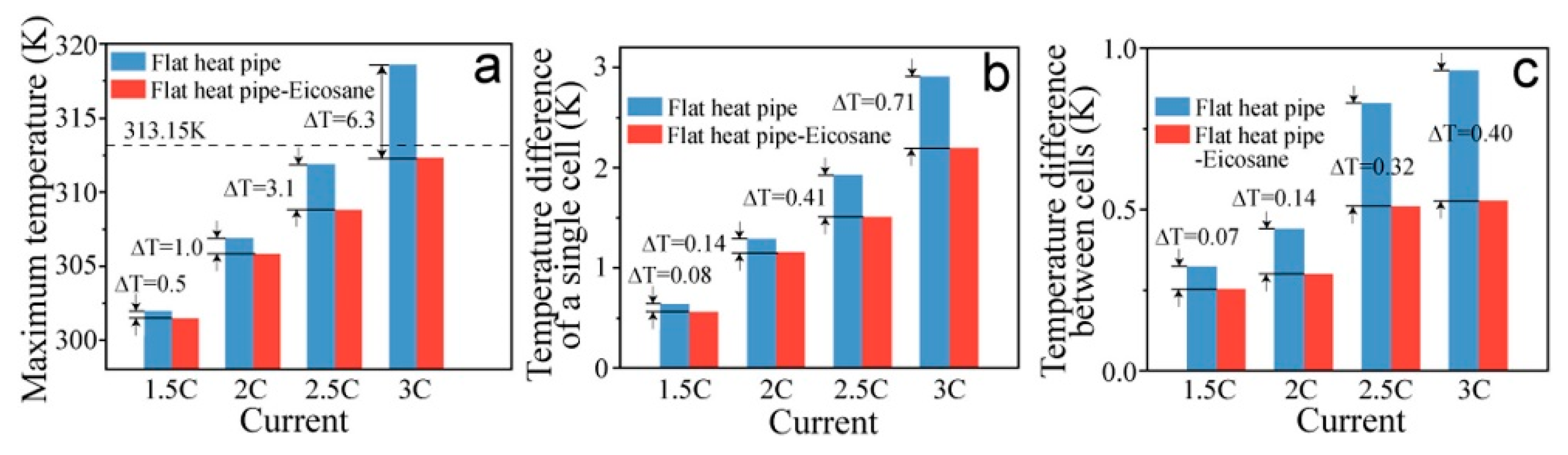
- Hu, C.; Li, H.; Wang, Y.; Hu, X.; Tang, D. Experimental and numerical investigations of lithium-ion battery thermal management using flat heat pipe and phase change material. J. Energy Storage 2022, 55, 105743. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, P.; Rao, Z. Experimental study on the thermal management performance of phase change material coupled with heat pipe for cylindrical power battery pack. Exp. Therm. Fluid Sci. 2017, 82, 182–188. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Qu, Z.G. Lithium–ion battery thermal management using heat pipe and phase change material during discharge–charge cycle: A comprehensive numerical study. Appl. Energy 2019, 242, 378–392. [Google Scholar] [CrossRef]
- Tang, Z.; Feng, R.; Huang, P.; Bai, Z.; Wang, Q. Modeling analysis on the cooling efficiency of composite phase change material-heat pipe coupling system in battery pack. J. Loss Prev. Process. Ind. 2022, 78, 104829. [Google Scholar] [CrossRef]
- Peng, P.; Wang, Y.; Jiang, F. Numerical study of PCM thermal behavior of a novel PCM-heat pipe combined system for Li-ion battery thermal management. Appl. Therm. Eng. 2022, 209, 118293. [Google Scholar] [CrossRef]
- Feng, R.; Huang, P.; Tang, Z.; He, Y.; Bai, Z. Experimental and numerical study on the cooling performance of heat pipe assisted composite phase change material-based battery thermal management system. Energy Convers. Manag. 2022, 272, 116359. [Google Scholar] [CrossRef]
- Leng, Z.; Yuan, Y.; Cao, X.; Zhong, W.; Zeng, C. Heat pipe/phase change material coupled thermal management in Li-ion battery packs: Optimization and energy-saving assessment. Appl. Therm. Eng. 2022, 208, 118211. [Google Scholar] [CrossRef]
- Leng, Z.; Yuan, Y.; Cao, X.; Zeng, C.; Zhong, W.; Gao, B. Heat pipe/phase change material thermal management of Li-ion power battery packs: A numerical study on coupled heat transfer performance. Energy 2022, 240, 122754. [Google Scholar] [CrossRef]
- Septiadi, W.N.; Alim, M.; Pradipta Adi, M.N. The application of battery thermal management system based on heat pipes and phase change materials in the electric bike. J. Energy Storage 2022, 56, 106014. [Google Scholar] [CrossRef]
- Khademi, A.; Shank, K.; Mehrjardi, S.A.A.; Tiari, S.; Sorrentino, G.; Said, Z.; Chamkha, A.J.; Ushak, S. A brief review on different hybrid methods of enhancement within latent heat storage systems. J. Energy Storage 2022, 54, 105362. [Google Scholar] [CrossRef]
- Bianco, N.; Busielo, S.; Iasiello, M.; Maria Mauro, G. Finned heat sinks with phase change materials and metal foams: Pareto optimization to address cost and operation time. Appl. Therm. Eng. 2021, 197, 117436. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Q.; Yao, F.; Zhang, C. Experimental Study on the Thermal Performance of a Finned Metal Foam Heat Sink with Phase Change Material. Heat. Transf. Eng. 2021, 42, 579–591. [Google Scholar] [CrossRef]
- Keshteli, A.N.; Iasiello, M.; Langella, G.; Bianco, N. Enhancing PCMs thermal conductivity: A comparison among porous metal foams, nanoparticles and finned surfaces in triplex tube heat exchangers. Appl. Therm. Eng. 2022, 212, 118623. [Google Scholar] [CrossRef]
- Rahmanian, S.; Moein-Jahromi, M.; Rahmanian-Koushkaki, H.; Sopian, K. Performance investigation of inclined CPV system with composites of PCM, metal foam and nanoparticles. Sol. Energy 2021, 230, 883–901. [Google Scholar] [CrossRef]
- Li, F.; Muhammad, N.; Abohamzeh, E.; Hakeem, A.A.; Hajizadeh, M.R.; Li, Z.; Bach, Q.-V. Finned unit solidification with use of nanoparticles improved PCM. J. Mol. Liq. 2020, 314, 113659. [Google Scholar] [CrossRef]
- Wang, T.; Almarashi, A.; Al-Turki, Y.A.; Abu-Hamdeh, N.H.; Hajizadeh, M.R.; Chu, Y.-M. Approaches for expedition of discharging of PCM involving nanoparticles and radial fins. J. Mol. Liq. 2021, 329, 115052. [Google Scholar] [CrossRef]
- Weng, J.; Xiao, C.; Yang, X.; Ouyang, D.; Chen, M.; Zhang, G.; Waiming, E.L.; Kit Yuen, R.K.; Wang, J. An energy-saving battery thermal management strategy coupling tubular phase-change-material with dynamic liquid cooling under different ambient temperatures. Renew. Energy 2022, 195, 918–930. [Google Scholar] [CrossRef]
- Mousavi, S.; Zadehkabir, A.; Siavashi, M.; Yang, X. An improved hybrid thermal management system for prismatic Li-ion batteries integrated with mini-channel and phase change materials. Appl. Energy 2023, 334, 120643. [Google Scholar] [CrossRef]
- Hekmat, S.; Bamdezh, M.A.; Molaeimanesh, G.R. Hybrid thermal management for achieving extremely uniform temperature distribution in a lithium battery module with phase change material and liquid cooling channels. J. Energy Storage 2022, 50, 104272. [Google Scholar] [CrossRef]
- Singh, L.K.; Gupta, A.K.; Sharma, A.K. Hybrid thermal management system for a lithium-ion battery module: Effect of cell arrangement, discharge rate, phase change material thickness and air velocity. J. Energy Storage 2022, 52, 104907. [Google Scholar] [CrossRef]
- Abbas, S.; Ramadan, Z.; Park, C.W. Thermal performance analysis of compact-type simulative battery module with paraffin as phase-change material and flat plate heat pipe. Int. J. Heat Mass Transf. 2021, 173, 121269. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.-F.; Zhou, J.-G.; Xiong, X.; Wang, Y.-P. Thermal performance of battery thermal management system using fins to enhance the combination of thermoelectric Cooler and phase change Material. Appl. Energy 2022, 322, 119503. [Google Scholar] [CrossRef]
- Gharehghani, A.; Gholami, J.; Shamsizadeh, P.; Mehranfar, S. Effect analysis on performance improvement of battery thermal management in cold weather. J. Energy Storage 2022, 45, 103728. [Google Scholar] [CrossRef]
- Xie, P.; Jin, L.; Qiao, G.; Lin, C.; Barreneche, C.; Ding, Y. Thermal energy storage for electric vehicles at low temperatures: Concepts, systems, devices and materials. Renew. Sustain. Energy Rev. 2022, 160, 112263. [Google Scholar] [CrossRef]
- Henry, A.; Prasher, R.; Majumdar, A. Five thermal energy grand challenges for decarbonization. Nat. Energy 2020, 5, 635–637. [Google Scholar] [CrossRef]
- Lau, J.; Papp, J.K.; Lilley, D.; Khomein, P.; Kaur, S.; Dames, C.; Liu, G.; Prasher, R. Dynamic tunability of phase-change material transition temperatures using ions for thermal energy storage. Cell Rep. Phys. Sci. 2021, 2, 100613. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Xu, X.; Lin, X.; Liu, L. A review on the effect of external fields on solidification, melting and heat transfer enhancement of phase change materials. J. Energy Storage 2020, 31, 101567. [Google Scholar] [CrossRef]
- Dai, R.; Wu, Y.; Mostaghimi, J.; Tang, L.; Zeng, M. Characteristics and control mechanism of melting process under extra magnetic force fields. Appl. Therm. Eng. 2020, 167, 114704. [Google Scholar] [CrossRef]
- Mehryan, S.A.M.; Tahmasebi, A.; Izadi, M.; Ghalambaz, M. Melting behavior of phase change materials in the presence of a non-uniform magnetic-field due to two variable magnetic sources. Int. J. Heat Mass Transf. 2020, 149, 119184. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, S.K.; Kumar, K.R.; Rakshit, D. Study of melting of paraffin dispersed with copper nanoparticles in square cavity subjected to external magnetic field. J. Energy Storage 2022, 50, 104338. [Google Scholar] [CrossRef]
- Xu, W.; Huang, T.; Huang, S.-M.; Zhuang, Y. Regulation mechanism of magnetic field on non-Newtonian melting and energy storage performance of metal foam composite nano-enhanced phase change materials. Int. J. Heat Mass Transf. 2023, 200, 123501. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, P.; Luo, K.; Wu, J. Experimental investigation on the melting characteristics of n-octadecane with electric field inside macrocapsule. Int. J. Heat Mass Transf. 2021, 173, 121238. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Luo, K.; Perez, A.T.; Yi, H.; Wu, J. Experimental investigation on melting heat transfer of an organic material under electric field. Exp. Therm. Fluid Sci. 2022, 131, 110530. [Google Scholar] [CrossRef]
- Sun, Z.; Luo, K.; Wu, J. Experimental study on the melting characteristics of n-octadecane with passively installing fin and actively applying electric field. Int. Commun. Heat Mass Transf. 2021, 127, 105570. [Google Scholar] [CrossRef]
- Zhou, W.; Mohammed, H.I.; Chen, S.; Luo, M.; Wu, Y. Effects of mechanical vibration on the heat transfer performance of shell-and-tube latent heat thermal storage units during charging process. App. Therm. Eng. 2022, 216, 119133. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Wu, W.; Huang, J. Influence of mechanical vibration on composite phase change material based thermal management system for lithium-ion battery. J. Energy Storage 2022, 54, 105237. [Google Scholar] [CrossRef]
- Joshy, N.; Hajiyan, M.; Siddique, A.R.M.; Tasnim, S.; Simha, H.; Mahmud, S. Experimental investigation of the effect of vibration on phase change material (PCM) based battery thermal management system. J. Power Sources 2020, 450, 227717. [Google Scholar] [CrossRef]


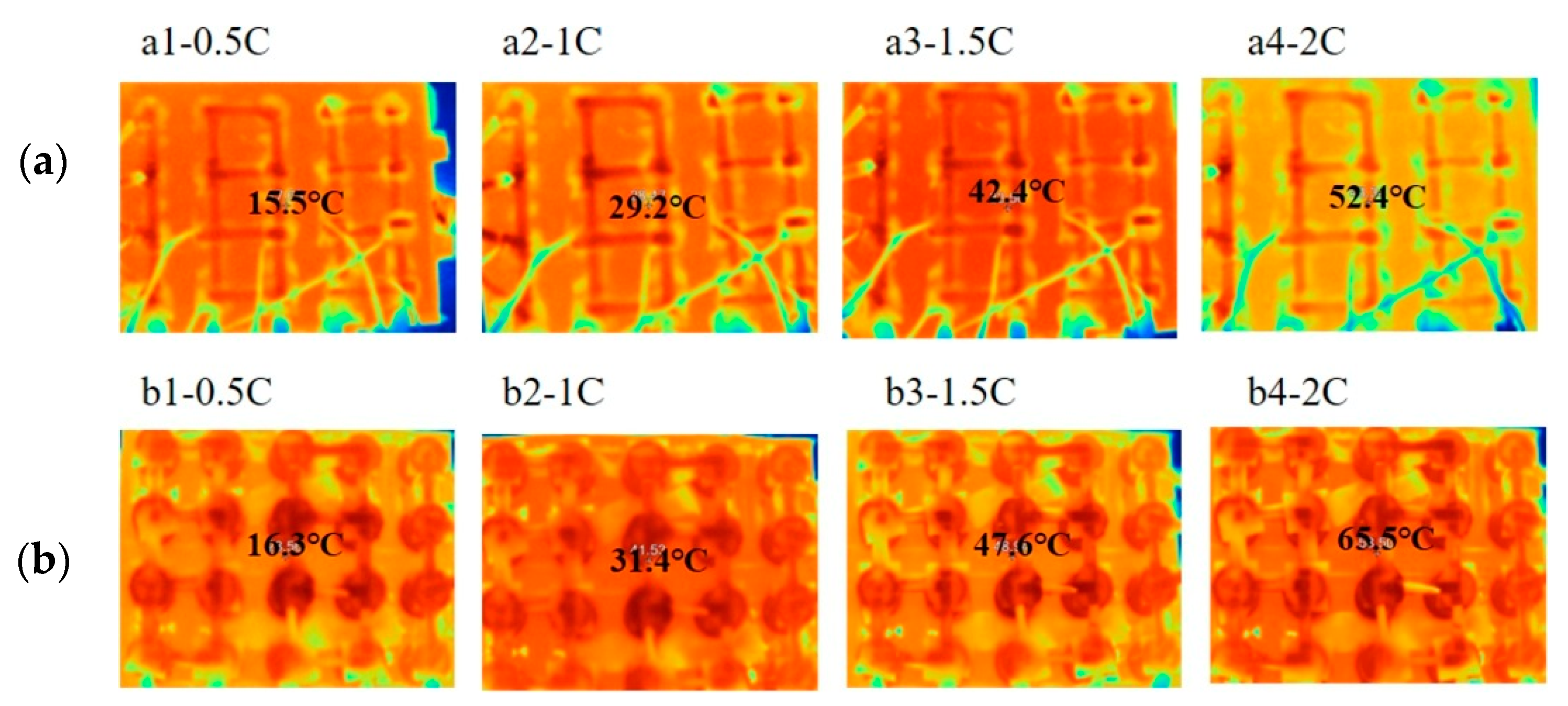
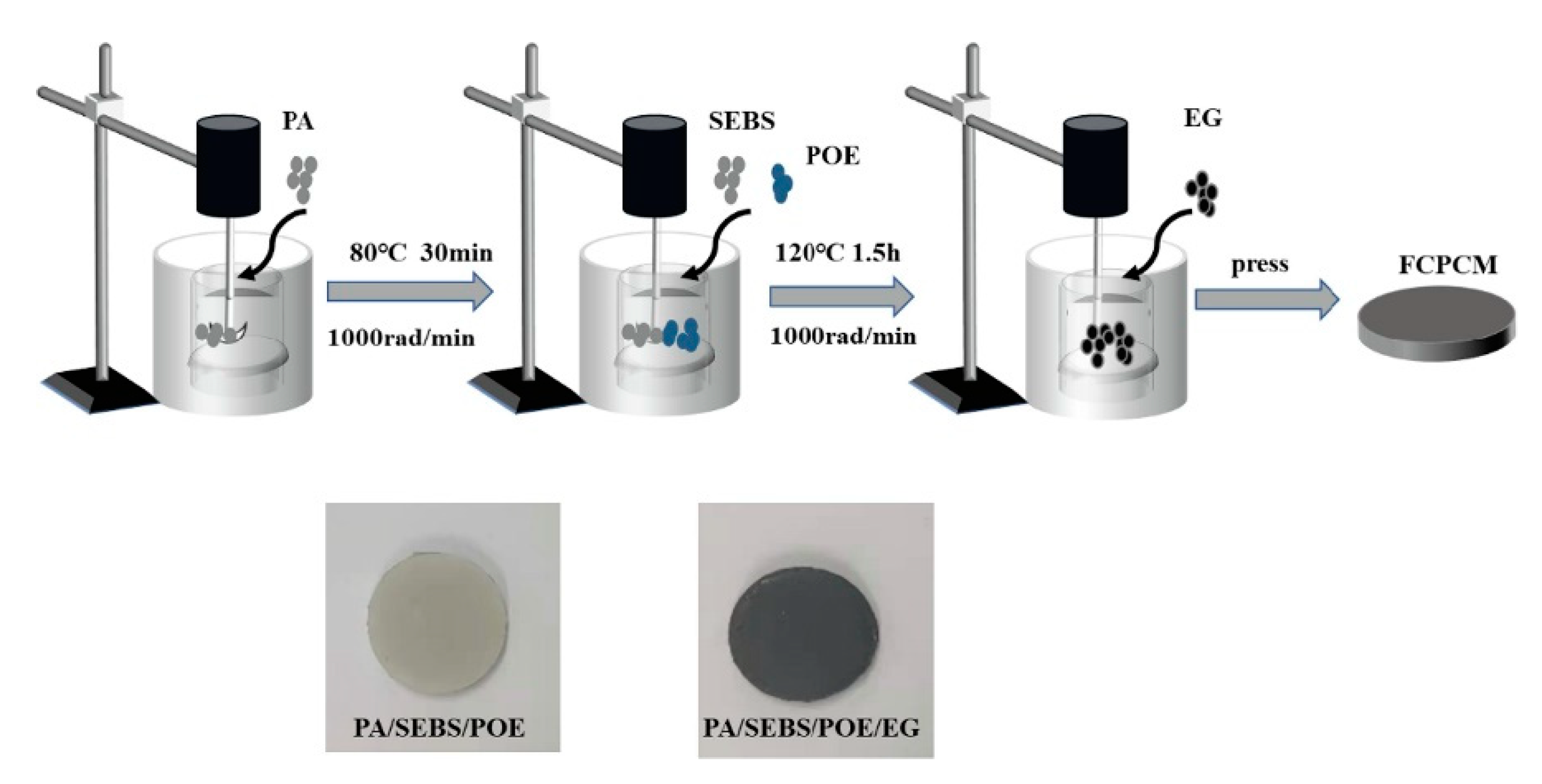

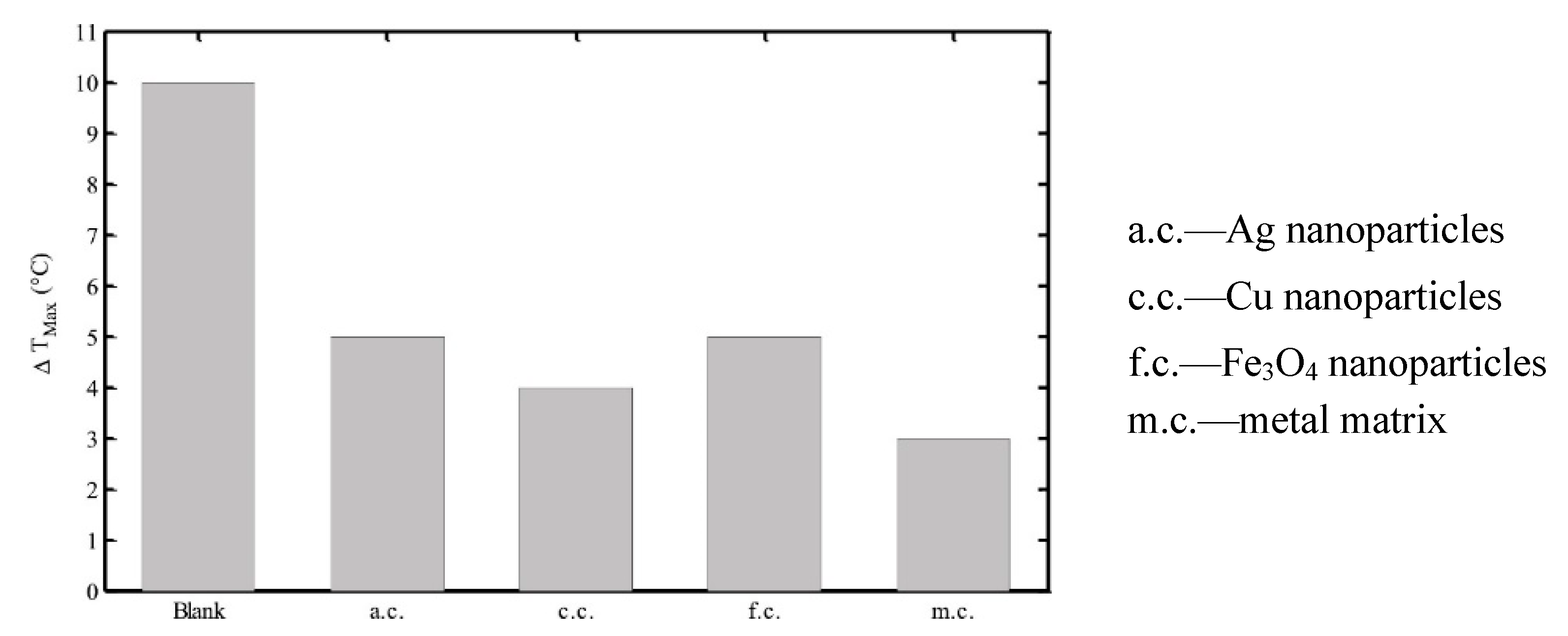





















| PCM | Advantages | Disadvantages |
|---|---|---|
| Organics | High latent heat No phase segregation Chemical stability Self-nucleate Extended MP range Compatible with most materials Recyclable Low-vapor pressure | Flammable Limited availability Limited biodegradability Significant volume change during phase transition Low thermal conductivity Low volumetric latent heat |
| Inorganics | Non-flammable High thermal conductivity Phase transition over a very narrow temperature range High volumetric latent heat Low-volume change during phase transition | Tendency to undergo supercooling Corrosive May require nucleating and thickening agents Phase segregation |
| Eutectics | High volumetric latent heat Phase transition over a very narrow temperature range Properties can be modulated to match the application requirements | High cost Limited data on thermos-physical properties |
| Ref | PCM | Additive | Battery system | Methods/Preparation | Results |
|---|---|---|---|---|---|
| Goli et al. [36] | Paraffin IGI-1260 | Graphene with flake thickness 0.35, 1 and 8 nm | Six 4-V Li-ion cells with the capacity 3000 mAh each | Dispersing a solution of the liquid-phase exfoliated graphene and few-layer graphene (FLG) in the paraffin wax at 70 °C followed by the high-shear mixing on a hot plate | The maximum temperature increase during charging/discharging (at 16 and 5 A) cycle dropped from ~37 °C (no PCM) to 23 °C (pure PCM), 17 °C (PCM and Graphene, 1 nm) and 14 °C (PCM and Graphene 0.35 nm) |
| Wang et al. [37] | Paraffin with MP 48–50 °C | Graphite powder with mass fraction 2.5%, 5%, 10%, 20% Nickel foam | LiFePO4, capacity 80 Ah, nominal voltage 3.2 V | The graphite powder was added when the PCM was completely melted into liquid. Then, the mixture was stirred continuously for 1 h by magnetic stirrer. | The charging and discharging performance of LiFePO4 battery was less sensitive to high-temperature environment, but more sensitive to low-temperature environment. Both binary and ternary composites ensured that the lithium-ion battery with the initial temperature of 28℃ can charge and discharge normally without preheating after standing in the environment of −20℃ for less than 40 min. |
| Yang et al. [38] | SAT with MP 58.4 °C | Carbon foam Polyurethane Copper foam Nano Al2O3 | Li-ion, 3.85 V, 4590 mAh | SAT was added to a flask held in an oil bath maintained at 110 °C. The flask was stirred and heated for 10 min. Next, DHPD was added to the flask, and the mixture was stirred and heated for. Al nanoparticles and CMC were added. Stirring was performed at 1100 r/min. | The composite PCM achieved a dual modulation of the high enthalpy in the CPCM and carbon material-enhanced heat transfer for the intelligent and stable long-term operation of the BTMS. |
| Zhao et al. [39] | OBC DA | Graphite powder | LiFePO4, 50 Ah, 3.2 V | DA and OBC were milled into fine powders. The mixtures were placed into a customized mold and heated up in an oven at 200 °C for 1 h. The molds and mixtures were removed from the oven and cooled in the air. The CPCM plates were cut into cuboids with a dimension of 240 × 160 × 3 mm (which has the same size as the battery in length and width). | Thermal conductivity increased 8–18 times compared to pure PCM. Tested in a pack level, a maximum of 15 °C temperature reduction was achieved. compared to a high ambient temperature of 55 °C. Thermal conductivity increased 8 to 18 times. |
| Zhou et al. [40] | Paraffin with MP 50 °C | EG Melamine | LiFePO4, 20 Ah, 3.2 V | 95 wt% paraffin and 5 wt% EG were put into an oil bath at 80 °C. Molten paraffin and EG were stirred uniformly at 800 rad/min. | Thermal conductivity increased from 0.21 W/m-K (pure PCM) to 0.85 (95% PCM + 5%EG) and 0.73 W/m-K (92% PCM + 5% EG + 3% Melamine). Latent heat decreased when EG and Melamine were added. |
| Behi et al. [41] | Paraffin with MP 35–42 °C | Graphite | Li-ion, 18,650 cells, 2200 mAh | Battery modules (24) were immersed in the molten PCM contained in a PVC rectangular box. The arrangement was 4 rows each with six modules, 2 mm spacing between modules | Adding graphite increased the thermal conductivity of the PCM from 0.25–0.4 to 0.5–1 W/m-K. The maximum temperature without PCM was 64 °C. Pure PCM and PCM-graphite reduced the battery temperature to 40 and 39 °C. PCM-graphite increased the uniformity of the temperature distribution. |
| Zhang et al. [42] | Paraffin with MP 35–40 °C | EG with mean particle size 150 mm | Li-ion 42110, 10 Ah, 3.2 V, connected 15S1P | Paraffin was heated to 80°C. EG was added to molten paraffin at a mass ratio of 4:1 with continuous mechanical stirring. A composite PCM module was fabricated through the hot-compaction process in a rectangular mold. | The TC of the composite increased 12 times compared to pure PCM. In extreme 10.0C pulse discharge rate, peak temperature is always controlled within 50 °C. The latent heat of the PA/EG composite was 147.61 kJ/kg, which was 35.9% lower than that of pure PCM. The MP of the composite decreased. |
| Ref | Battery | Effects/Type of Study | Fin Geometry/Layout |
|---|---|---|---|
| Ping et al. [57] | LiFePO4, 10 Ah | The PCM-fin structure resulted in the lowest temperature and the best temperature uniformity than other cooling cases. Decreasing fin thickness reduced the maximum temperature and temperature difference of battery module by increasing the heat exchange area with PCM. Fins with excessively small spacing have a negative influence by decreasing the PCM volume, which could be more significant than the positive effect of increasing the heat transfer area. Numerical study |  |
| Chen et al. [58] | LiPF6 | Annular parallel fins, cylindrical battery cell with vertical axis. The whole assembly immersed in the PCM. The fin number was varied to identify the optimum number. The best performance in reducing the temperature was found for 9 fins. Beyond this number, the fins fail to reduce further the battery temperature. This effect was attributed to inhibiting the buoyancy-driven circulation during the PCM molten phase. Numerical study |  |
| Fan et al. [59] | 18,650 Li-ion, 2.6 Ah | Triply periodic minimal surface (TPMS) P type and IWP type were used. (a)—PCM only; (b)—Kelvin type lattice; (c)—P type TPMS; (d)—P-IWP type TPMS. The melting time of the PCM in the case of P-IWP type TPMS reduced by 30.8% of that in the case of P type TPMS. At 1C discharge rate, the temperature in the case of P-IWP type TPMS decreases by 12.2% of that in the case of PCM-only. At 2C, the PCM completely melted in the case of P-IWP type TPMS due to the local heat transfer enhancement of the IWP type TPMS sheet structure with smaller lattice. Numerical study | 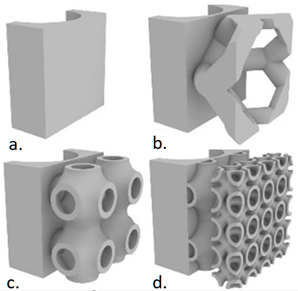 |
| Mei et al. [60] | 18,650 Li-ion, 2.6 Ah | By using EG-enhanced PCM and fins, the battery temperature was reduced by 35.5%. This effect was less significant as the environment temperature increased. Experimental study | 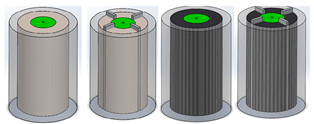 |
| Choudhari et al. [61] | 18,650 Li-ion, 2.4 Ah | PCM module containing 4 fins exhibit optimum performance. This optimum fin structure resulted in a temperature drop of 2 °C and 6.1 °C (at 2C and 3C, respectively). No significant difference in heat transfer enhancement was observed. Numerical study |  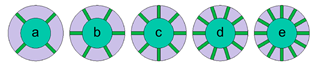
|
| Choudhari et al. [62] | 18,650 Li-ion, 2.2 Ah. Connection 5S5P | Fin structure layout Type III, was the most effective in dissipating the heat stored at the inner cells of the battery pack without affecting significantly the outer cell temperature and melting time. Type III layout produced a more uniform temperature distribution. At lower values of the natural convection heat transfer coefficient, fins are ineffective and the thermal performance is comparable to that of the no-fin case. The battery average temperature was reduced by 19.79 °C and the melting fraction by 66% when the heat transfer coefficient increased from 5 to 25 W/m2K. Numerical study | 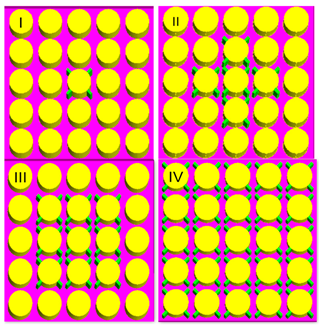 |
| Weng et al. [63] | INR18650–25R, 2500 mAh | Longitudinal fins are more effective on heat dissipation by air convection, while circular fins show a stronger heat conduction ability inside the PCM medium due to their larger heat transfer area. The heat dissipation effect was more significant when the air convection coefficient increased. Increasing the longitudinal fin number, heat dissipation, was more intense; however, in a limited-space module, increasing fins does not necessarily mean an increase in fin efficiency. The optimum number of longitudinal fins was 4, resulting in a temperature drop from 36.9 to 34.2 °C in the rectangular finned module. Circular fins providing stronger heat conduction capability were inserted in the lower section, while rectangular fins were applied in the upper section. Considering both the limited space and efficiency, the number of rectangular and circular fins was set as 4 and 2. Numerical study |  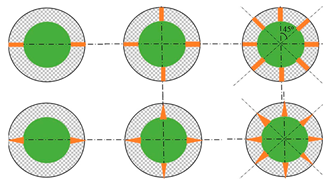 |
| Liu et al. [64] | LiCoO2 7000 mAh 3.8 V | The PCM cooling system with a honeycomb fin could maintain the battery temperature below 50 °C. Compared with the non-fin cooling system, the temperature drop of the system with honeycomb fin increased by 61%. The non-fin PCM cooling system melted completely at the bottom, and the energy was concentrated adjacent to the heat source. The honeycomb fin could distribute evenly the heat of the PCM in the vertical direction, and the PCM with different thicknesses began melting almost simultaneously, thereby optimizing the heat absorption effect of the PCM. Experimental study | 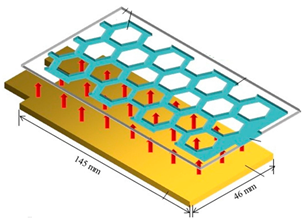 |
| Reference | BTMS Description | HP/PCM Layout |
|---|---|---|
| Zhao et al. [86] | Cylindrical battery cells in an inline arrangement with equal gaps between. The cells assembly was immersed in a composite PCM (paraffin/EG with mass ratio 5, 10, 15 and 20%) filled container. The composite PCM MP was 39 °C. Circular HP (start-up temperature 30 °C) with annular parallel fins attached to the condenser section. HPs immersed vertically in the PCM. In horizontal plane, the HPs arrangement layout was with each HP at equal distance from the four neighboring battery cells. |  |
| Jiang et al. [87] | Layered structure consisting of a (1) prismatic battery cell 3.2 V, 8 Ah, (2) a Cu plate with two horizontal flat HP attached (3) an EG-PCM layer. Two EG-PCM composites were developed with the MP 48 and 30 °C and thermal conductivity 2.4 and 2.5 W/m-K. Flat HPs with the evaporator section attached continuously to the Cu plate over the whole plate length. Fins attached to the HP condenser section (HP axis perpendicular on the fins planes). |  |
| Tang et al. [88] | Six cylindrical battery cells (4 Ah) equally spaced forming an array. Cells immersed in a PCM. Three cell arrays placed next to each other with flat HPs separating them along the whole array length. Flat HPs with the evaporator section separating the cell arrays. Condenser section extended vertically and cooling naturally. |  |
| Peng et al. [89] | Cylindrical 18650 battery cells (40) in a 4 × 10 matrix arrangement with no gap between them. The voids were filled alternately with paraffin wax and circular HPs with annular parallel fins attached to the condenser section. | 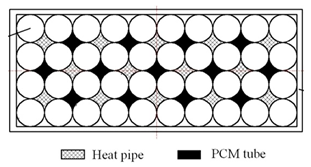  |
| Feng et al. [90] | 3P × 6S INR21700 battery cells (4000 mAh). The battery cells in a group ox six were embedded in a composite PCM (paraffin—Cu MF). Each group of six cells was separated from the next by a flat heat pipe (operating at 32 °C). Fins were applied to the upper section of the flat HP. | 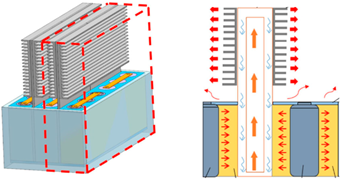 |
| Leng et al. [91] | 55 Ah battery pack consisting of rectangular cells arranged parallelly. All spaces between cells were filled with a composite PCM (paraffin/EG). Flat HPs were used with the width equal to the distance between two cells, oriented vertically with finned condenser section. HP evaporator section fully immersed in the composite PCM. Four HPs were inserted in each space. | 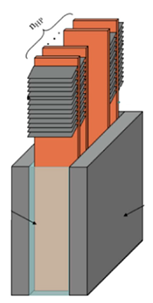 |
| Leng et al. [92] | Rectangular cells arranged parallelly, equally distanced. The space between cells was filled with PCM. Flat HPs were used with the width equal to the distance between two cells, oriented vertically with finned condenser section. Two HPs were inserted in each inter-cell space with a common fin system. | 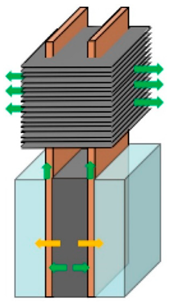 |
| Septiadi et al. [93] | 13 × 4 18,650 Li-ion batteries embedded in a composite PCM (paraffin with MP 42 °C + EG 10%, 20% and 30% mass fraction values). Evaporator section of the HPs embedded in the CPCM. Finned heat sink on the condenser HP section. | 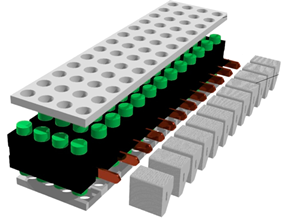 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconu, B.; Cruceru, M.; Anghelescu, L.; Racoceanu, C.; Popescu, C.; Ionescu, M.; Tudorache, A. Latent Heat Storage Systems for Thermal Management of Electric Vehicle Batteries: Thermal Performance Enhancement and Modulation of the Phase Transition Process Dynamics: A Literature Review. Energies 2023, 16, 2745. https://doi.org/10.3390/en16062745
Diaconu B, Cruceru M, Anghelescu L, Racoceanu C, Popescu C, Ionescu M, Tudorache A. Latent Heat Storage Systems for Thermal Management of Electric Vehicle Batteries: Thermal Performance Enhancement and Modulation of the Phase Transition Process Dynamics: A Literature Review. Energies. 2023; 16(6):2745. https://doi.org/10.3390/en16062745
Chicago/Turabian StyleDiaconu, Bogdan, Mihai Cruceru, Lucica Anghelescu, Cristinel Racoceanu, Cristinel Popescu, Marian Ionescu, and Adriana Tudorache. 2023. "Latent Heat Storage Systems for Thermal Management of Electric Vehicle Batteries: Thermal Performance Enhancement and Modulation of the Phase Transition Process Dynamics: A Literature Review" Energies 16, no. 6: 2745. https://doi.org/10.3390/en16062745
APA StyleDiaconu, B., Cruceru, M., Anghelescu, L., Racoceanu, C., Popescu, C., Ionescu, M., & Tudorache, A. (2023). Latent Heat Storage Systems for Thermal Management of Electric Vehicle Batteries: Thermal Performance Enhancement and Modulation of the Phase Transition Process Dynamics: A Literature Review. Energies, 16(6), 2745. https://doi.org/10.3390/en16062745











