Potential Pathway for Reliable Long-Term CO2 Storage as Clathrate Hydrates in Marine Environments
Abstract
:1. Introduction
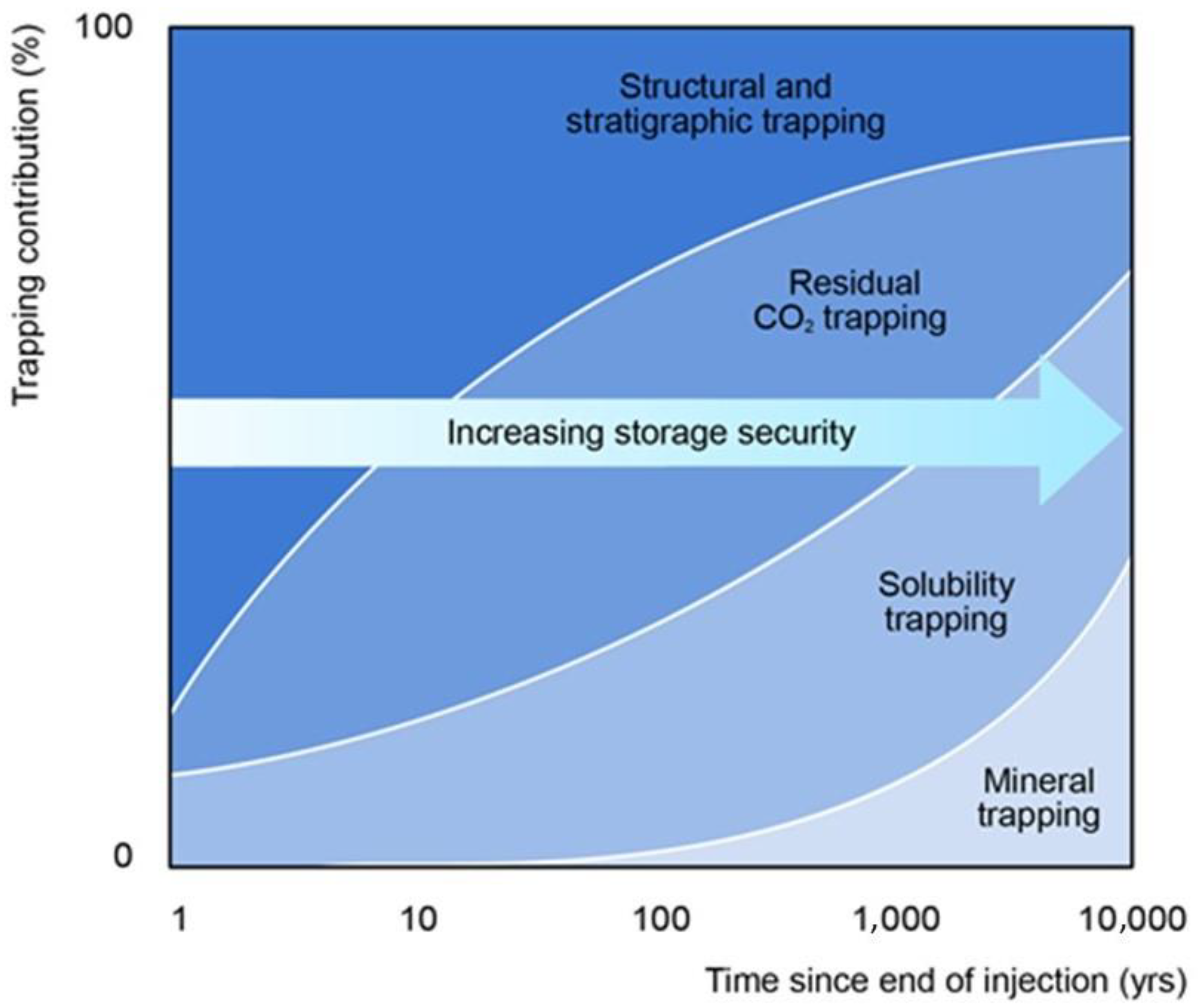
2. CO2 Storage as a Pathway against Global Warming
- Structural Trapping (physical trapping in the rock with rocks as seals)
- Residual Trapping (CO2 trapping in the pore space between the rock grains)
- Solubility Trapping (CO2 dissolves into the brine water in the pore spaces)
- Mineral Trapping (when in the reservoir, the dissolved CO2 reacts with the present minerals to form solid carbonate minerals)

3. Clathrate Hydrates for CO2 Storage
4. Potential and Challenges for Direct CO2 Storage as Clathrate Hydrates
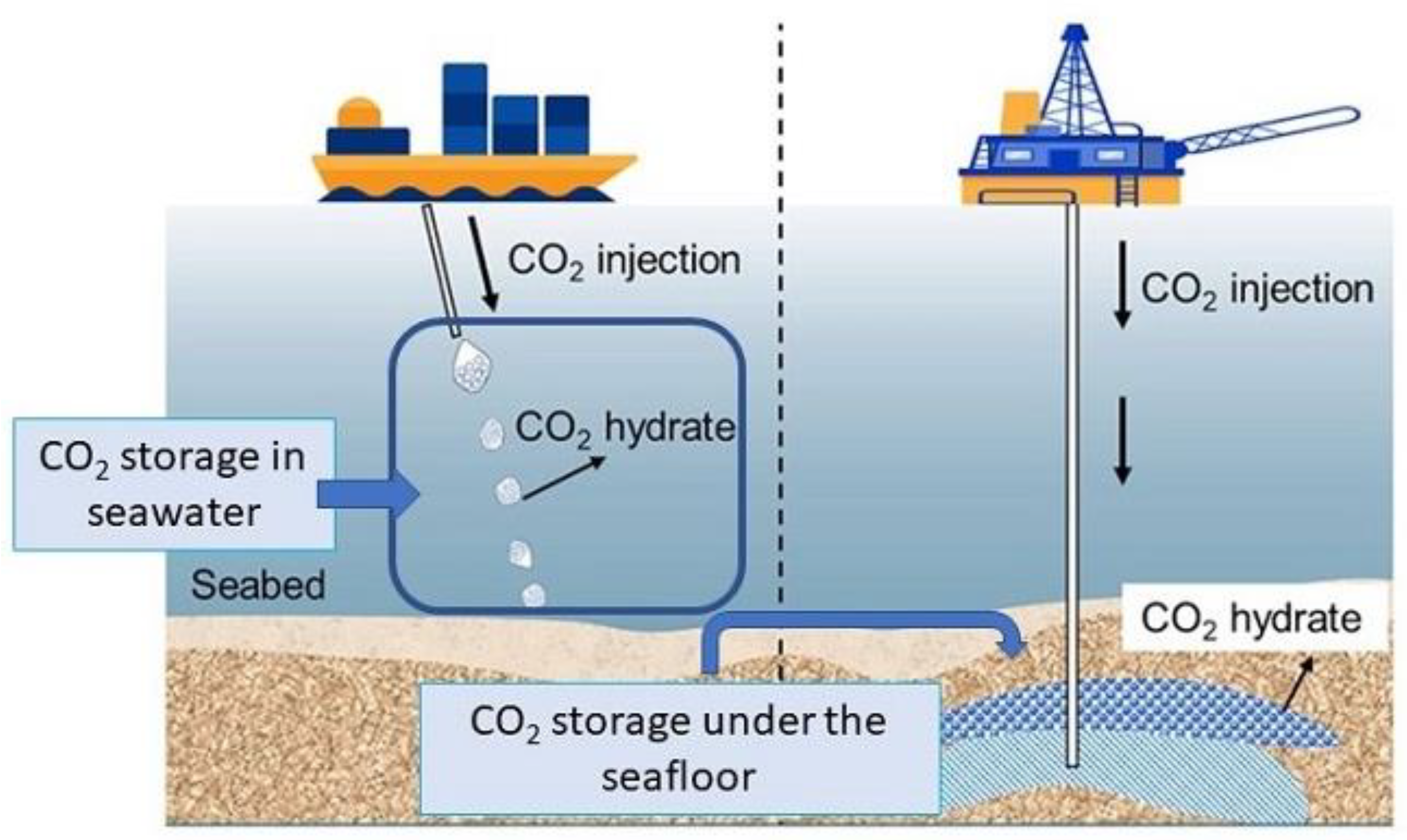
4.1. The Choice of the Approaches of CO2 Storage as CH
4.2. Laboratory Investigations on CO2 CH in Marine Environments
4.3. Identification of Major Chemical Compounds in Seawater and Pore Water in Seafloor Sediments
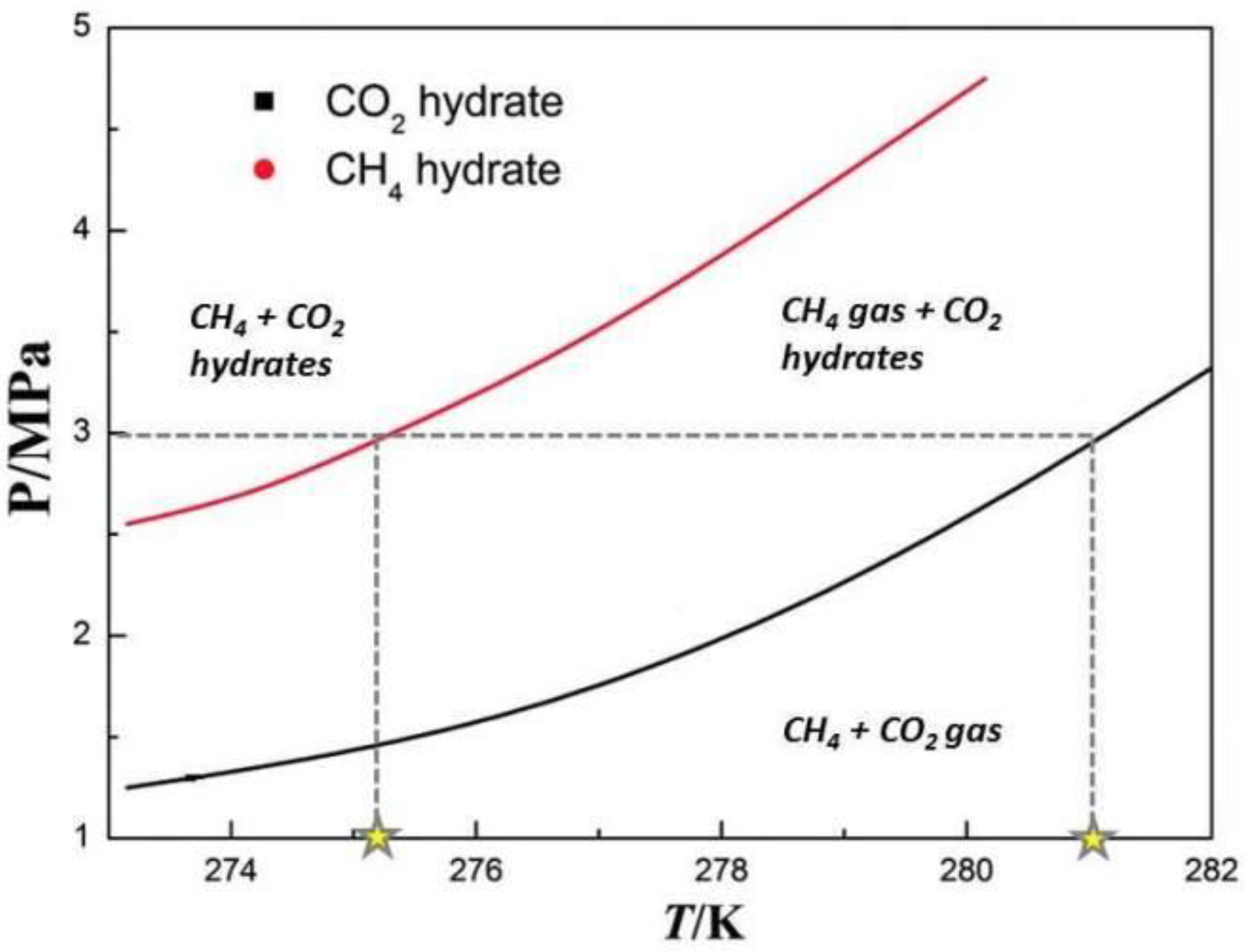
4.4. CO2 CH Stability
4.5. Social Acceptance
5. Conclusions
- Among the potential CO2 storage sites, the geographical assessment of hydrate stability zones in marine locations with a detailed comprehension of the advantages/disadvantages of the chosen approaches;
- New knowledge of hydrodynamics and permeability, thermodynamics/kinetics of CO2 hydrate formation/dissociation;
- New knowledge on the effect of salinity, of the dissolved organic matter, and of the presence of other gaseous components;
- New experimental data on the long-term stability of CO2 hydrates;
- New technological solutions for CO2 injection to obtain a high, continuous, and uniform formation of CO2 hydrates;
- New specific social acceptance studies considering impacts, social communities, and hydrate technology peculiarities.
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, M.R.; Dube, O.P.; Solecki, W.; Aragón-Durand, F.; Cramer, W.; Humphreys, S.; Kainuma, M.; Kala, J.; Mahowald, N.; Mulugetta, Y.; et al. Framing and Context. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 658–740. [Google Scholar]
- Smith, S.M.; Lowe, J.A.; Bowerman, N.H.; Gohar, L.K.; Huntingford, C.; Allen, M.R. Equivalence of greenhouse-gas emissions for peak temperature limits. Nat. Clim. Chang. 2012, 2, 535–538. [Google Scholar] [CrossRef]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Summary for Policymakers. In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y.E., Farahani, S., Kadner, K., Seyboth, A., Adler, I., Baum, S., Brunner, P., Eickemeier, B., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–30. [Google Scholar]
- Lal, R. Terrestrial sequestration of carbon dioxide. In Developments and Innovation in CO2 Capture and Storage Technology; Woodhead Publishing: Soston, UK, 2010; pp. 271–303. [Google Scholar]
- Hovorka, S.D.; Meckel, T.A.; Treviño, R.H. Monitoring a large-volume injection at Cranfield, Mississippi: Project design and recommendations. Int. J. Greenh. Gas Control 2013, 18, 345–360. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—A potential energy source for the 21st Century. J. Pet. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Moridis, G.J.; Regan, M.T. Estimating the upper limit of gas production from class 2 hydrate accumulations in the permafrost:1. Concepts, system description, and the production base case. J. Pet. Sci. Eng. 2011, 76, 194–204. [Google Scholar] [CrossRef]
- Dornan, P.; Alavi, S.; Woo, T.K. Free energies of carbon dioxide sequestration and methane recovery in clathrate hydrates. J. Chem. Phys. 2007, 127, 124510. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Koh, C.; Kastner, M.; Blasingame, T.; Carstens, C.; Hornbach, M.J.; Johnson, J.E.; Kaminsky, R.D.; Kleinberg, R.; Max, M.; McConnell, D.; et al. Gas Hydrates Research and Development Roadmap: 2020–2035. July 2019. Available online: https://www.energy.gov/sites/prod/files/2019/07/f65/Gas%20Hydrates%20Roadmap_MHAC.pdf (accessed on 5 February 2023).
- Castellani, B.; Rossetti, G.; Tupsakhare, S.; Rossi, F.; Nicolini, A.; Castaldi, M.J. Simulation of CO2 storage and methane gas production from gas hydrates in a large scale laboratory reactor. J. Pet. Sci. Eng. 2016, 147, 515–527. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J. Thermal stimulation based methane production from hydrate bearing quartz sediment. Ind. Eng. Chem. Res. 2013, 52, 6571–6581. [Google Scholar] [CrossRef]
- IEA (International Energy Agency) Net Zero by 2050 A Roadmap for the Global Energy Sector 2021. Available online: https://iea.blob.core.windows.net/assets/deebef5d-0c34-4539-9d0c-10b13d840027/NetZeroby2050-ARoadmapfortheGlobalEnergySector_CORR.pdf (accessed on 5 February 2023).
- Ali, M.; Jha, N.K.; Pal, N.; Keshavarz, A.; Hoteit, H.; Sarmadivaleh, M. Recent advances in carbon dioxide geological storage, experimental procedures, influencing parameters, and future outlook. Earth Sci. Rev. 2022, 225, 103895. [Google Scholar] [CrossRef]
- IEA (International Energy Agency). CO2 Storage Resources and their Development. An IEA CCUS Handbook. December 2022. Available online: https://www.iea.org/reports/co2-storage-resources-and-their-development (accessed on 5 February 2023).
- Lokhorst, A.; Wildenborg, T. Introduction on CO2 Geological Storage. Classification of Storage Options. Oil Gas Sci. Technol. 2005, 60, 513–515. [Google Scholar] [CrossRef] [Green Version]
- Metz, B. Carbon Dioxide Capture and Storage: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Sun, J.; Chen, Z.; Wang, X.; Zhang, Y.; Qin, Y.; Chen, C.; Li, W.; Zhou, W. Displacement Characteristics of CO2 to CH4 in Heterogeneous Surface Slit Pores. Energy Fuels 2023, 37, 2926–2944. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Harrison, B.; Falcone, G. Carbon capture and sequestration versus carbon capture utilisation and storage for enhanced oil recovery. Acta Geotech. 2014, 9, 29–38. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- de Coninck, H.; Benson, S.M. Carbon dioxide capture and storage: Issues and prospects. Annu. Rev. Environ. Resour. 2014, 39, 243–270. [Google Scholar] [CrossRef]
- IPCC. Carbon Dioxide Capture and Storage: IPCC Special Report; Betz, B., Davidson, O., de Coninck, H., Loos, M., Meyer, L., Eds.; Cambridge University Press: Cambridge, UK, 2005; p. 431. [Google Scholar]
- Ranathunga, A.; Perera, M.S.A.; Ranjith, P.G. Deep coal seams as a greener energy source: A review. J. Geophys. Eng. 2014, 11, 063001. [Google Scholar] [CrossRef]
- Abdulelah, H.; Al-Yaseri, A.; Ali, M.; Giwelli, A.; Negash, B.M.; Sarmadivaleh, M. CO2/Basalt’s interfacial tension and wettability directly from gas density: Implications for Carbon Geo-sequestration. J. Pet. Sci. Eng. 2021, 204, 108683. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Wiese, F.; Fridriksson, T.; Ármansson, H.; Einarsson, G.M.; Gislason, S.R. CO2 storage potential of basaltic rocks in Iceland and the oceanic ridges. Energy Procedia 2014, 63, 4585–4600. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.M.; Fathi, E.; Ambrose, R.J.; Akkutlu, I.Y.; Sigal, R.F. Carbon dioxide storage capacity of organic-rich shales. SPE J. 2011, 16, 842–855. [Google Scholar] [CrossRef] [Green Version]
- Sloan, E.; Koh, C. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hu, W.; Chen, C.; Sun, J.; Zhang, N.; Zhao, J.; Liu, Y.; Ling, Z.; Li, W.; Liu, W.; Song, Y. Three-body aggregation of guest molecules as a key step in methane hydrate nucleation and growth. Commun. Chem. 2022, 5, 33. [Google Scholar] [CrossRef]
- Li, X.-S.; Xu, C.-G.; Zhang, Y.; Ruan, X.-K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Tupsakhare, S.S.; Castaldi, M.J. Efficiency enhancements in methane recovery from natural gas hydrates using injection of CO2/N2 gas mixture simulating in-situ combustion. Appl. Energy 2019, 236, 825–836. [Google Scholar] [CrossRef]
- Komatsu, H.; Ota, M.; Smith, R.L.; Inomata, H. Review of CO2-CH4 clathrate hydrate replacement reaction laboratory studies-Properties and kinetics. J. Taiwan Instig. Chem. Eng. 2013, 44, 517–537. [Google Scholar] [CrossRef]
- Almenningen, S.; Graue, A.; Ersland, G. Experimental Investigation of critical parameters controlling CH4-CO2 exchange in sedimentary CH4 hydrates. Energy Fuels 2021, 35, 2468–2477. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, K.; Song, Y.; Liu, W.; Lam, W.; Liu, Y.; Xue, K.; Zhu, Y.; Yu, X.; Li, Q. A Review on Research on Replacement of CH4 in Natural Gas Hydrates by Use of CO2. Energies 2012, 5, 399–419. [Google Scholar] [CrossRef] [Green Version]
- Ndlovu, P.; Babaee, S.; Naidoo, P. Review on CH4-CO2 replacement for CO2 sequestration and CH4/CO2 hydrate formation in porous media. Fuel 2022, 320, 123795. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. The effect of grainsize of sediments in the CO2/CH4 replacement process within a hydrate lattice: An experimental report. Chem. Eng. Process. Process Intensif. 2022, 181, 109149. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Water Salinity as Potential Aid for Improving the Carbon Dioxide Replacement Process’ Effectiveness in Natural Gas Hydrate Reservoirs. Processes 2020, 8, 1298. [Google Scholar] [CrossRef]
- Castellani, B.; Gambelli, A.M.; Minelli, G.; Rossi, F. The effect of different sediment conditions on CO2-CH4 replacement in natural gas hydrates. In Proceedings of the 39th Heat Transfer Conference, Gaeta, Italy, 20–22 June 2022. [Google Scholar]
- Castellani, B.; Gambelli, A.M.; Nicolini, A.; Rossi, F. Energy and Environmental Analysis of Membrane-Based CH4-CO2 Replacement Processes in Natural Gas Hydrates. Energies 2019, 12, 850. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Gambelli, A.M.; Sharma, D.K.; Castellani, B.; Nicolini, A.; Marco, J.; Castaldi, M.J. Experiments on methane hydrates formation in seabed deposits and gas recovery adopting carbon dioxide replacement strategies. Appl. Therm. Eng. 2019, 148, 371–381. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Y.; Zhao, Y.; Zhu, J. Experimental Studies on Gas Hydrate-Based CO2 Storage: State-of-the-Art and Future Research Directions. Energy Technol. 2021, 9, 2100004. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Brewer, P.G.; Friederich, G.; Peltzer, E.T.; Orr, F.M. Direct experiments on the ocean disposal of fossil fuel CO2. Science 1999, 284, 943–945. [Google Scholar] [CrossRef] [Green Version]
- Warzinski, R.P.; Lynn, R.J.; Holder, G.D. The Impact of CO2 Clathrate Hydrate on Deep Ocean Sequestration of CO2: Experimental Observations and Modeling Results. Ann. N. Y. Acad. Sci. 2000, 912, 226. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, H.; Lee, J.; Kang, J.M. CO2 hydrate behavior in the deep ocean sediments; phase equilibrium, formation kinetics, and solubility. Geophys. Res. Lett. 2002, 29, 301–304. [Google Scholar] [CrossRef]
- Kvamme, B.; Graue, A.; Buanes, T.; Kuznetsova, T.; Ersland, G. Storage of CO2 in natural gas hydrate reservoirs and the effect of hydrate as an extra sealing in cold aquifers. Int. J. Greenh. Gas Control 2007, 1, 236–246. [Google Scholar] [CrossRef]
- Giovannetti, R.; Gambelli, A.M.; Castellani, B.; Rossi, A.; Zannotti, M.; Li, Y.; Rossi, F. May sediments affect the inhibiting properties of NaCl on CH4 and CO2 hydrates formation? An experimental report. J. Mol. Liquids 2022, 359, 119300. [Google Scholar] [CrossRef]
- Yin, W.; Li, X.; Suwarno, R.S.; Cornelissen, E.R.; Chong, T.H. Fouling behavior of isolated dissolved organic fractions from seawater in reversed osmosis(RO) desalination process. Water Res. 2019, 159, 385–396. [Google Scholar] [CrossRef]
- Park, T.; Kyung, D.; Lee, W. Effect of Organic Matter on CO2 hydrate Phase Equilibrium in Phyllosilicate Suspensions. Environ. Sci. Technol. 2014, 48, 6597–6603. [Google Scholar] [CrossRef]
- Lamorena, R.B.; Kyung, D.; Lee, W. Effect of organic matter on CO2 hydrate formation in Ulleung Basin sediment suspensions. Environ. Sci. Technol. 2011, 45, 6196–6203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Yang, L.; Dong, H.; Zhao, J.; Song, Y. Behaviors of CO2 hydrate formation in the presence of Acid-Dissolvable Organic Matters. Environ. Sci. Technol. 2021, 5, 6206–6213. [Google Scholar] [CrossRef] [PubMed]
- Rutqvist, J. The geomechanics of CO2 storage in deep sedimentary formations. Geotech. Geol. Eng. 2012, 30, 525–551. [Google Scholar] [CrossRef] [Green Version]
- Fahed, Q.M.; Zheng, J.; Khandelwal, H.; Venkataraman, P.; Usadi, A.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. Laboratory demonstration of the stability of CO2 hydrates in deep-oceanic sediments. Chem. Eng. J. 2021, 432, 134290. [Google Scholar] [CrossRef]
- Fahed, Q.M.; Khandelwal, H.; Usadi, A.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. CO2 hydrate stability in oceanic sediments under brine conditions. Energy 2022, 256, 124625. [Google Scholar]
- Huijts, N.M.A.; Midden, C.J.H.; Anneloes, L.; Meijnders, A.L. Social acceptance of carbon dioxide storage. Energy Policy 2007, 35, 2780–2789. [Google Scholar] [CrossRef]
- Koukouzas, N.; Christopoulou, M.; Giannakopoulou, P.P.; Rogkala, A.; Gianni, E.; Karkalis, C.; Pyrgaki, K.; Krassakis, P.; Koutsovitis, P.; Panagiotaras, D.; et al. Current CO2 Capture and Storage Trends in Europe in a View of Social Knowledge and Acceptance. A Short Review. Energies 2022, 15, 5716. [Google Scholar] [CrossRef]
- Nielsen, J.A.E.; Stavrianakis, K.; Morrison, Z. Community acceptance and social impacts of carbon capture, utilization and storage projects: A systematic meta-narrative literature review. PLoS ONE 2022, 17, e0272409. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellani, B. Potential Pathway for Reliable Long-Term CO2 Storage as Clathrate Hydrates in Marine Environments. Energies 2023, 16, 2856. https://doi.org/10.3390/en16062856
Castellani B. Potential Pathway for Reliable Long-Term CO2 Storage as Clathrate Hydrates in Marine Environments. Energies. 2023; 16(6):2856. https://doi.org/10.3390/en16062856
Chicago/Turabian StyleCastellani, Beatrice. 2023. "Potential Pathway for Reliable Long-Term CO2 Storage as Clathrate Hydrates in Marine Environments" Energies 16, no. 6: 2856. https://doi.org/10.3390/en16062856
APA StyleCastellani, B. (2023). Potential Pathway for Reliable Long-Term CO2 Storage as Clathrate Hydrates in Marine Environments. Energies, 16(6), 2856. https://doi.org/10.3390/en16062856





