A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges
Abstract
:1. Introduction
2. CO2 Capture
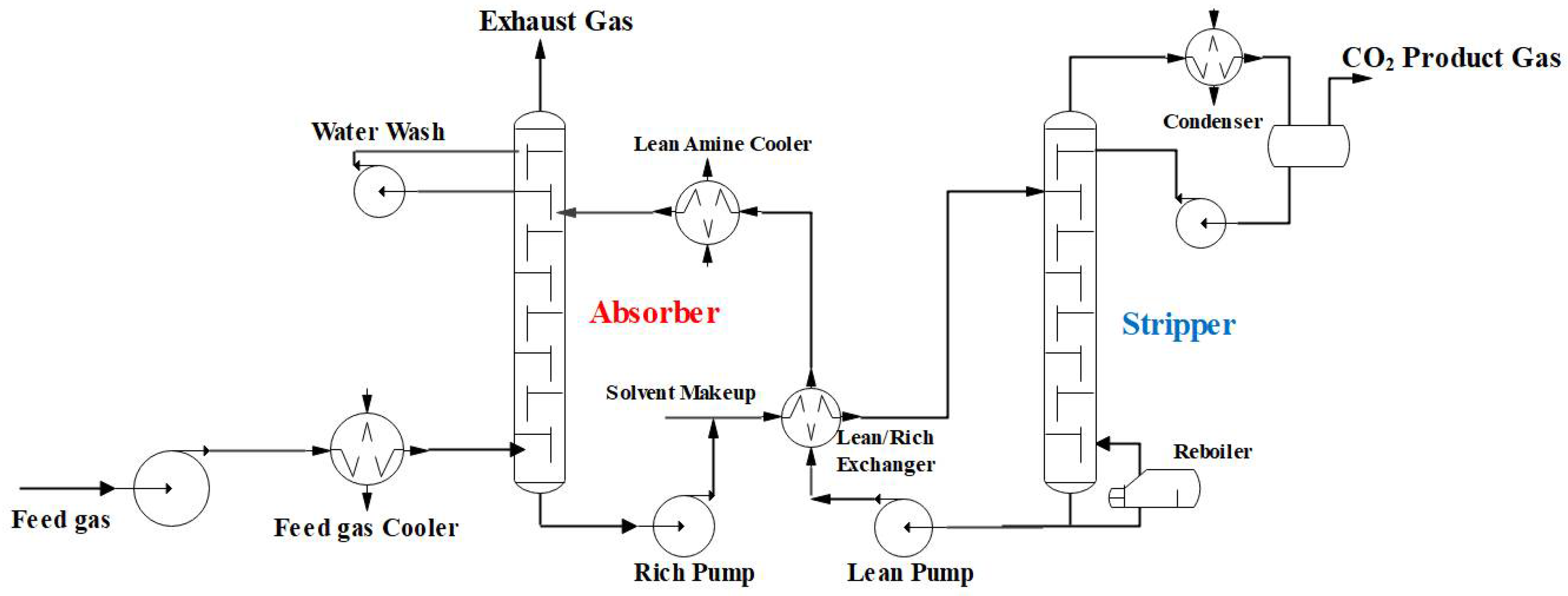
2.1. Solvent Absorption Technology
2.1.1. Chemical Absorption Technology
2.1.2. Physical Absorption Technology
2.1.3. Solvent Mixed Absorption Technology
2.1.4. Ionic Liquid Absorption Technology
2.2. Solid Adsorption Technology
2.2.1. Carbon-Based Adsorbent
2.2.2. Porous Material
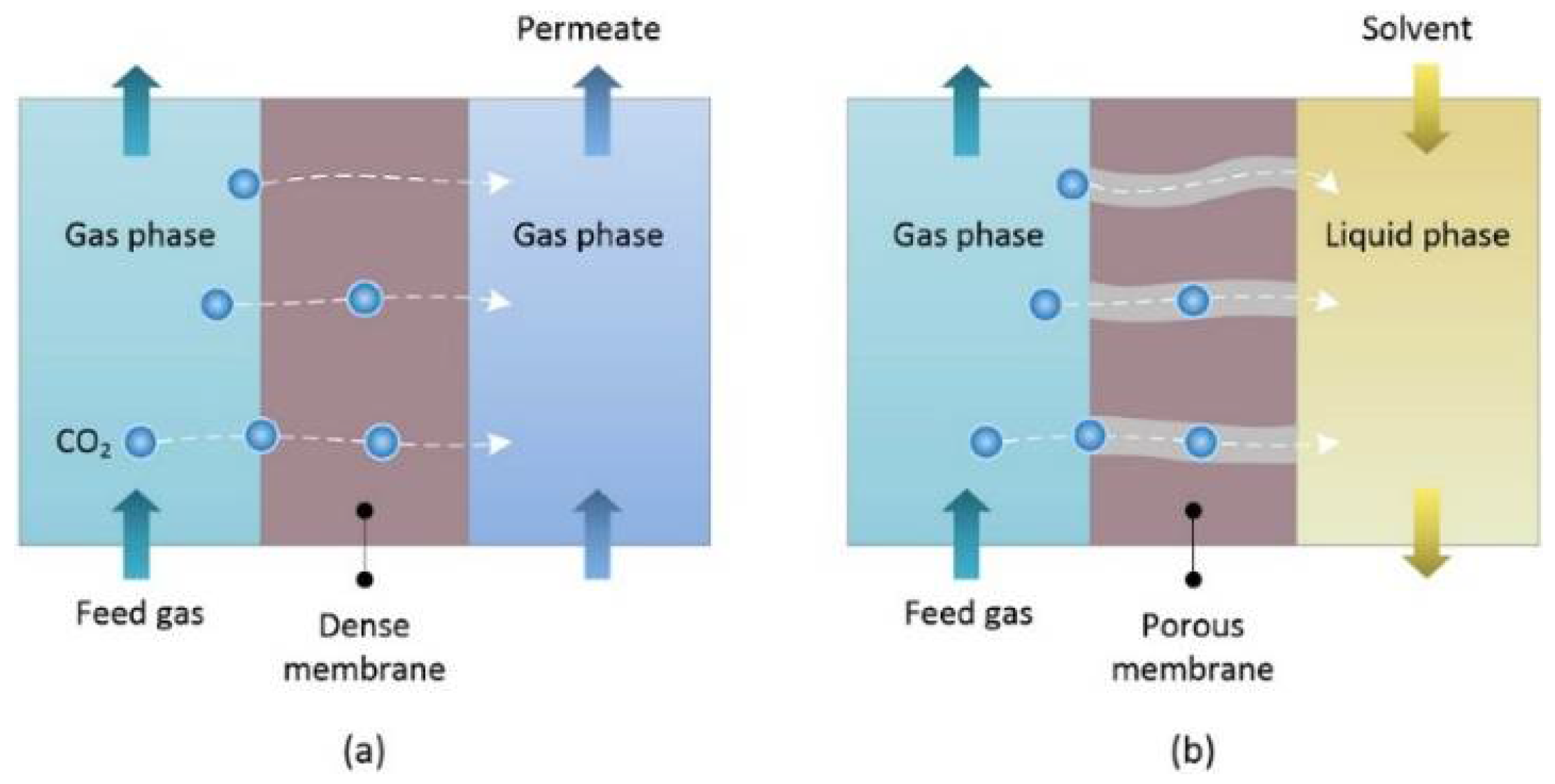
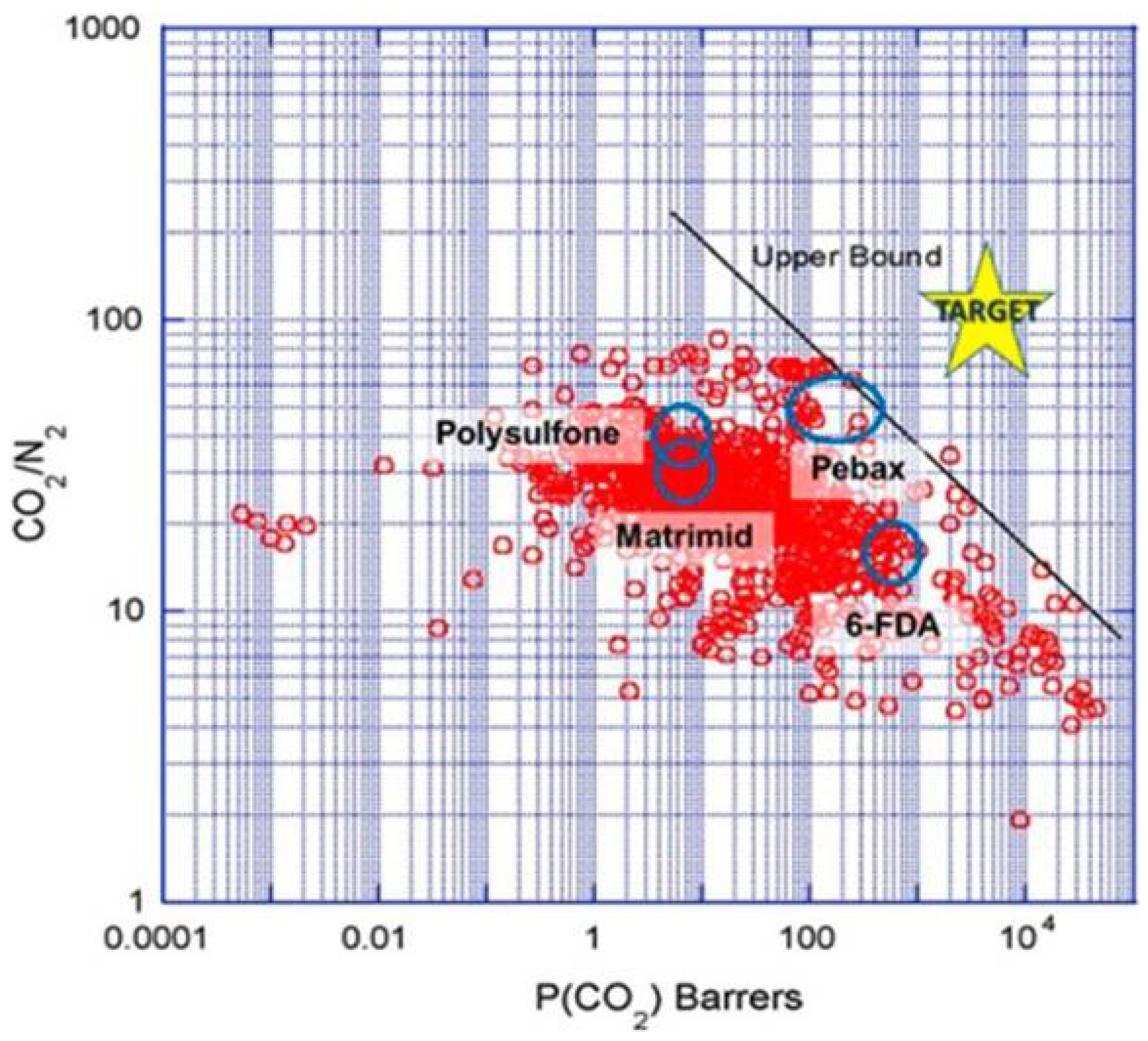
2.3. Membrane Separation Technology
2.4. Other Technologies
2.4.1. Low-Temperature Separation Technology
2.4.2. Microalgae
2.4.3. Chemical Looping Combustion Technology
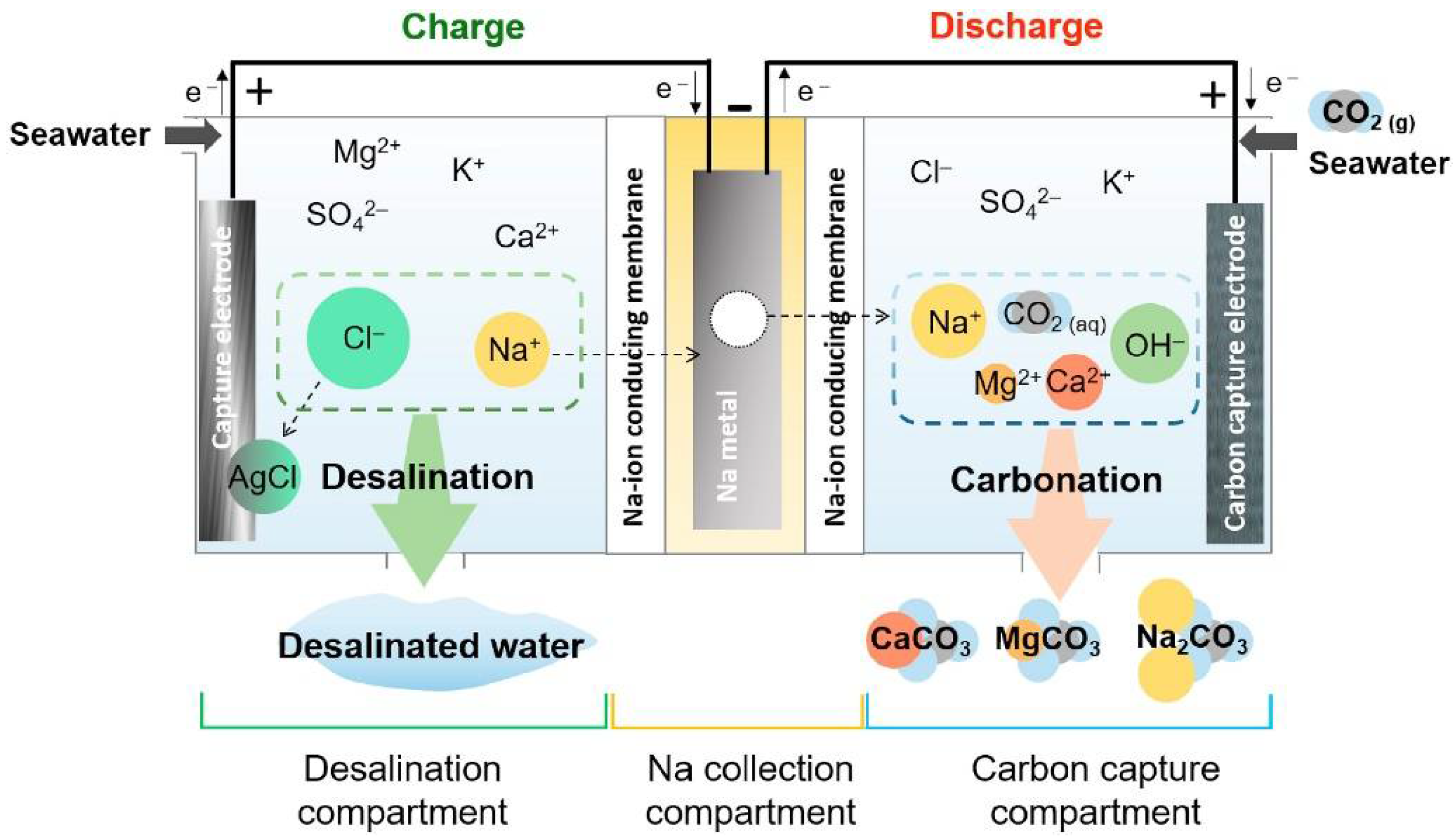
2.4.4. Electrochemical Technology
3. CO2 Transport
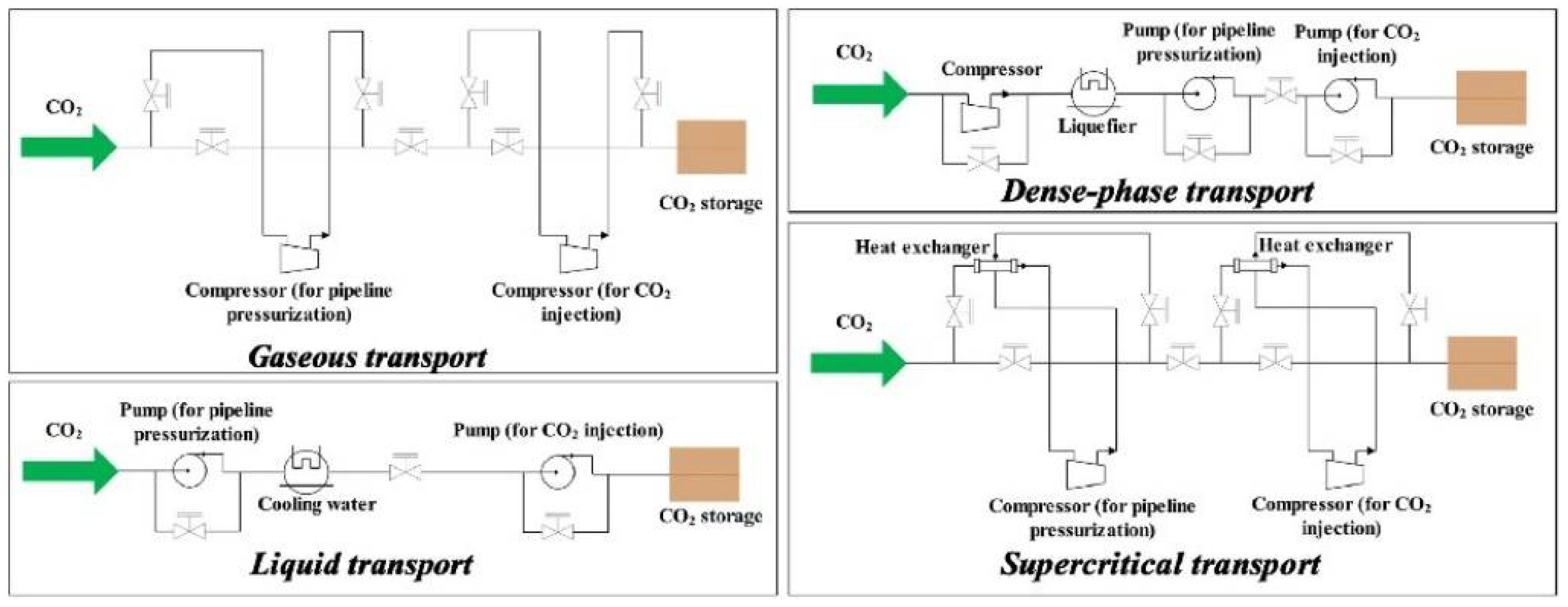
3.1. Pipeline Transportation Technology
3.1.1. Conveying Status and Process
3.1.2. Pipeline Design and Flow Assurance
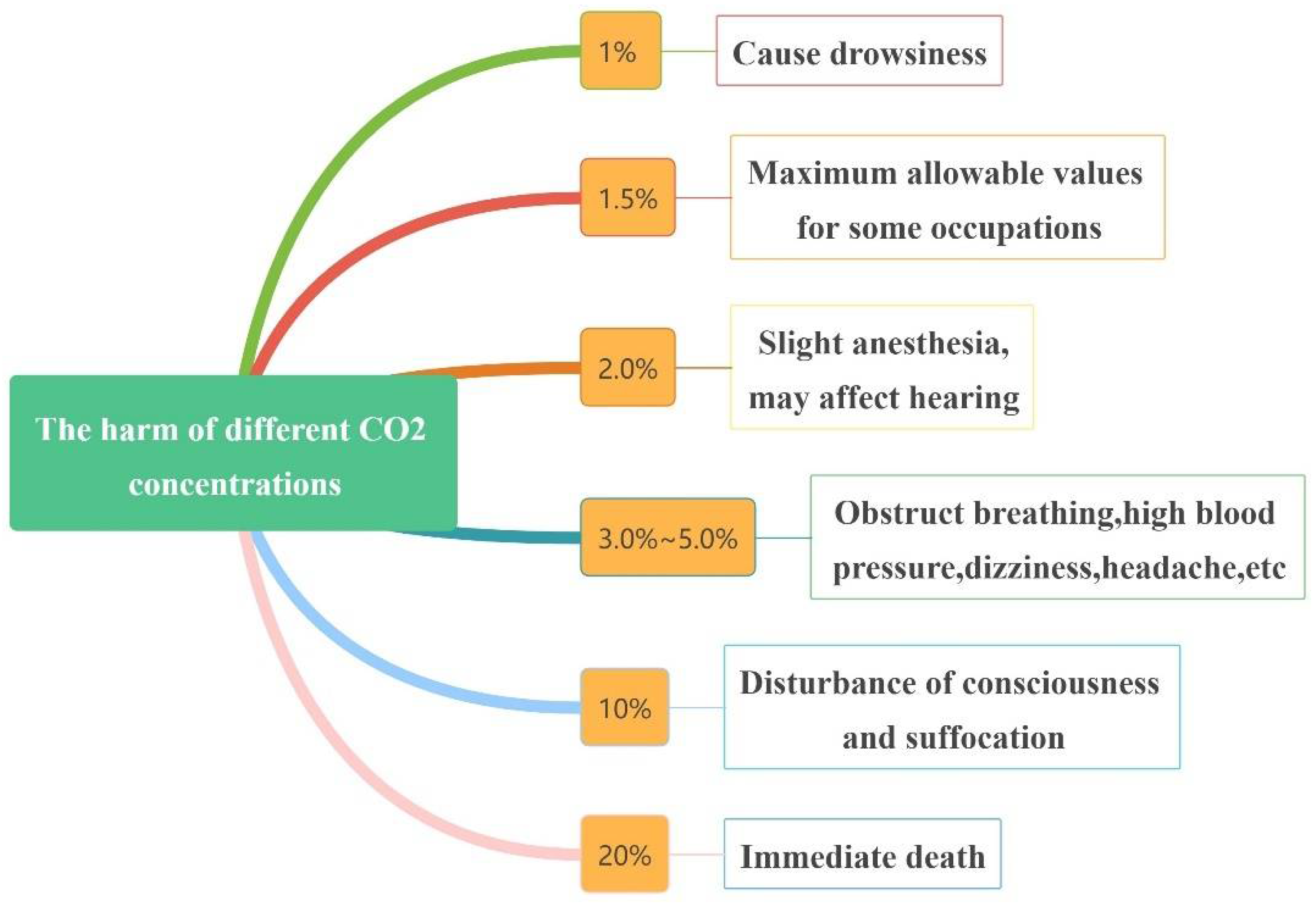
3.1.3. Transportation Safety and Risk
3.2. Ship Transportation Technology
4. CO2 Utilization
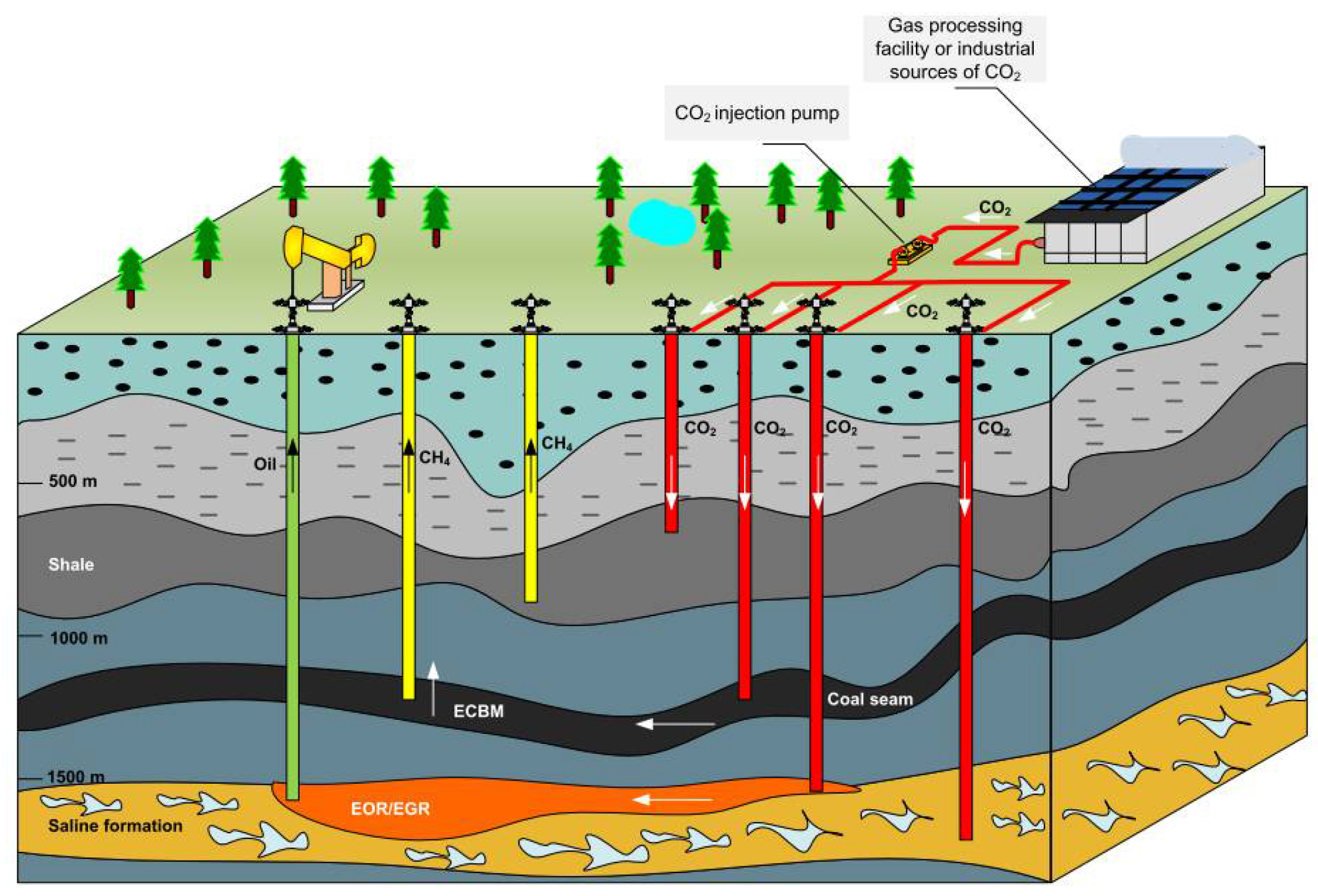
4.1. Strengthen Energy Production Technology
4.1.1. Enhanced Oil Production Technology
4.1.2. Strengthening Gas Production Technology
4.1.3. Technology of Displacing Coalbed Methane
4.1.4. Enhanced Geothermal Technology
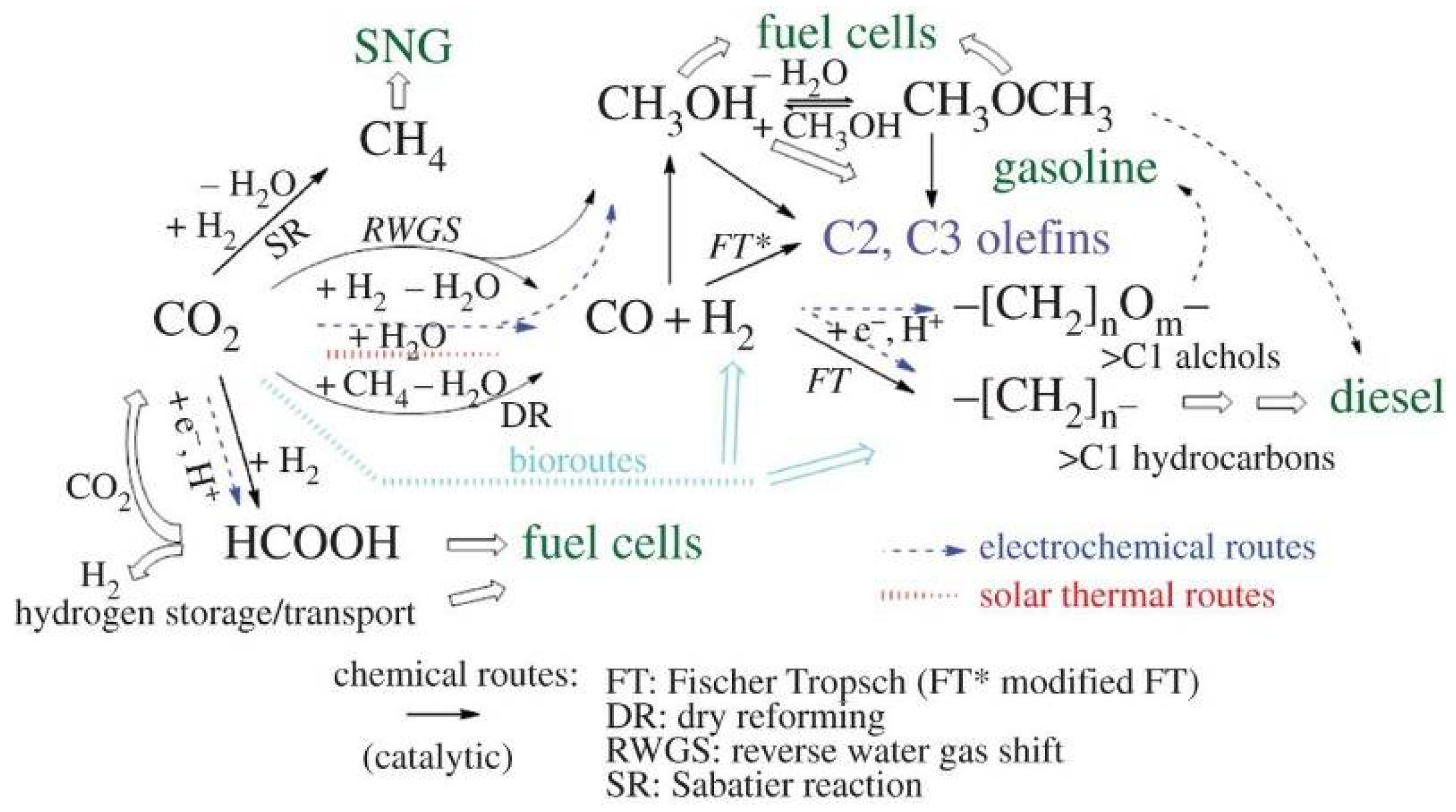
4.2. Chemical Conversion Technology
4.2.1. Raw Materials for the Chemical Industry
4.2.2. Mineralization
4.3. Biological Utilization Technology
4.4. Beverage and Food Processing
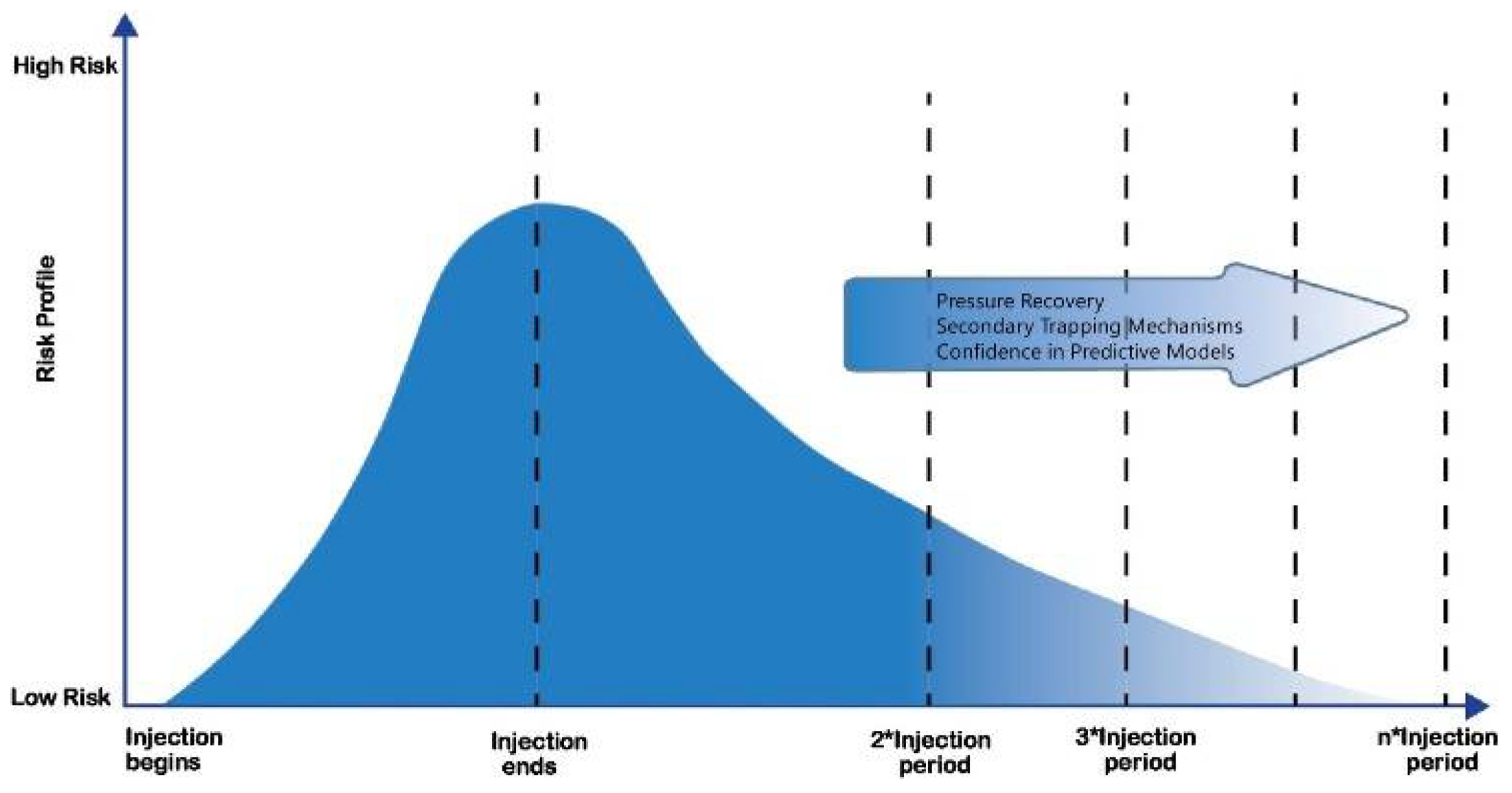
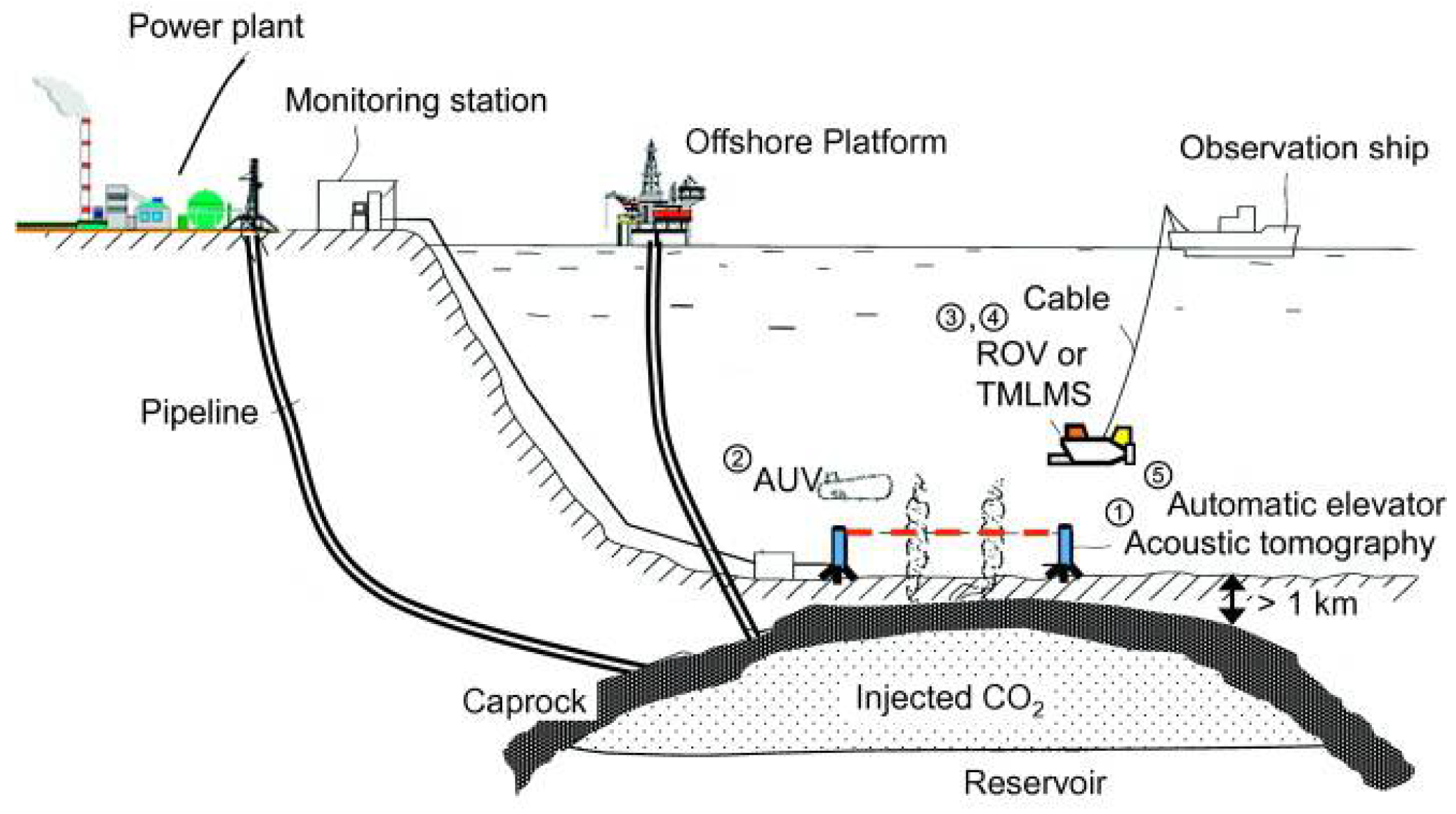
5. CO2 Storage
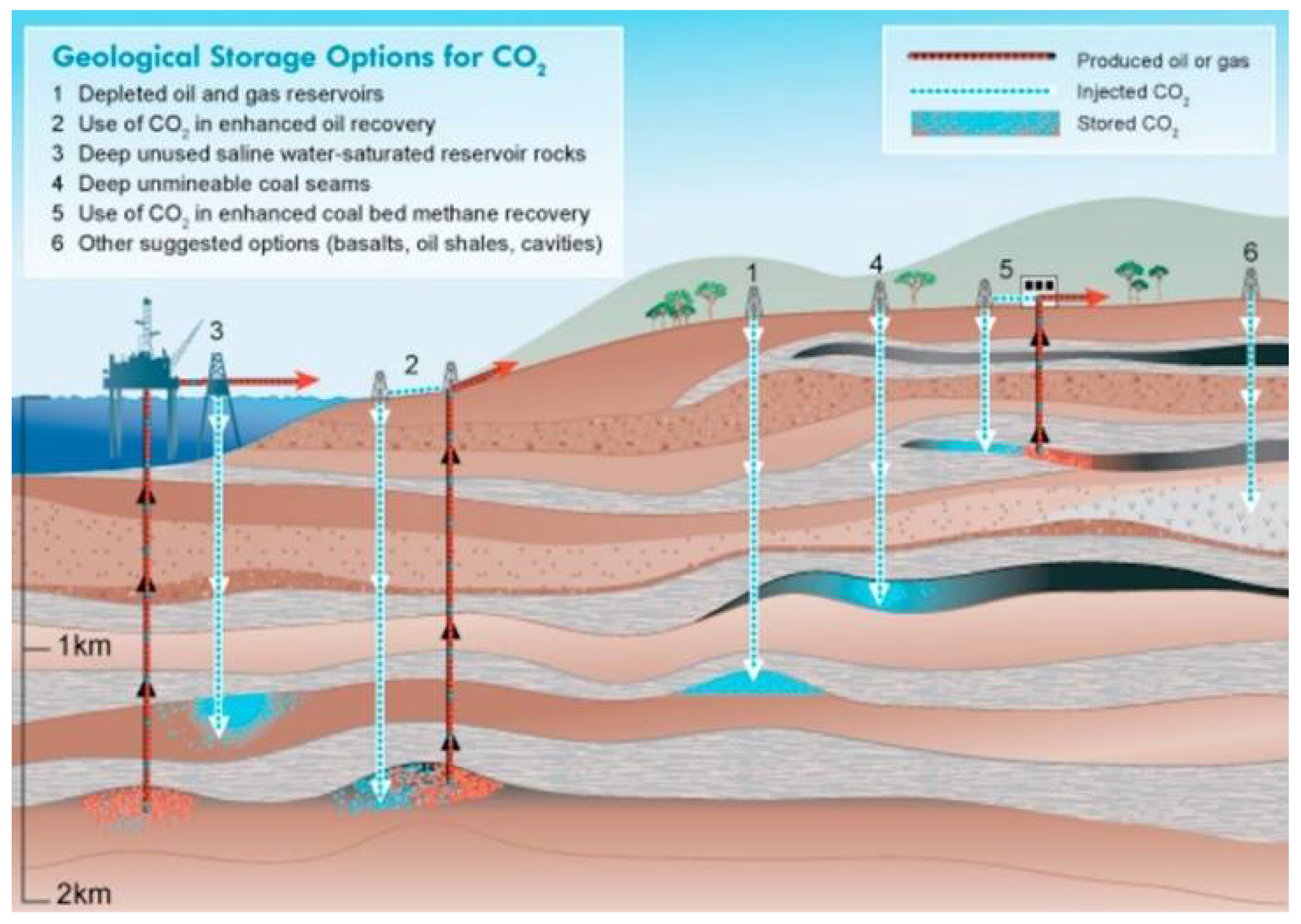
5.1. Geological Storage
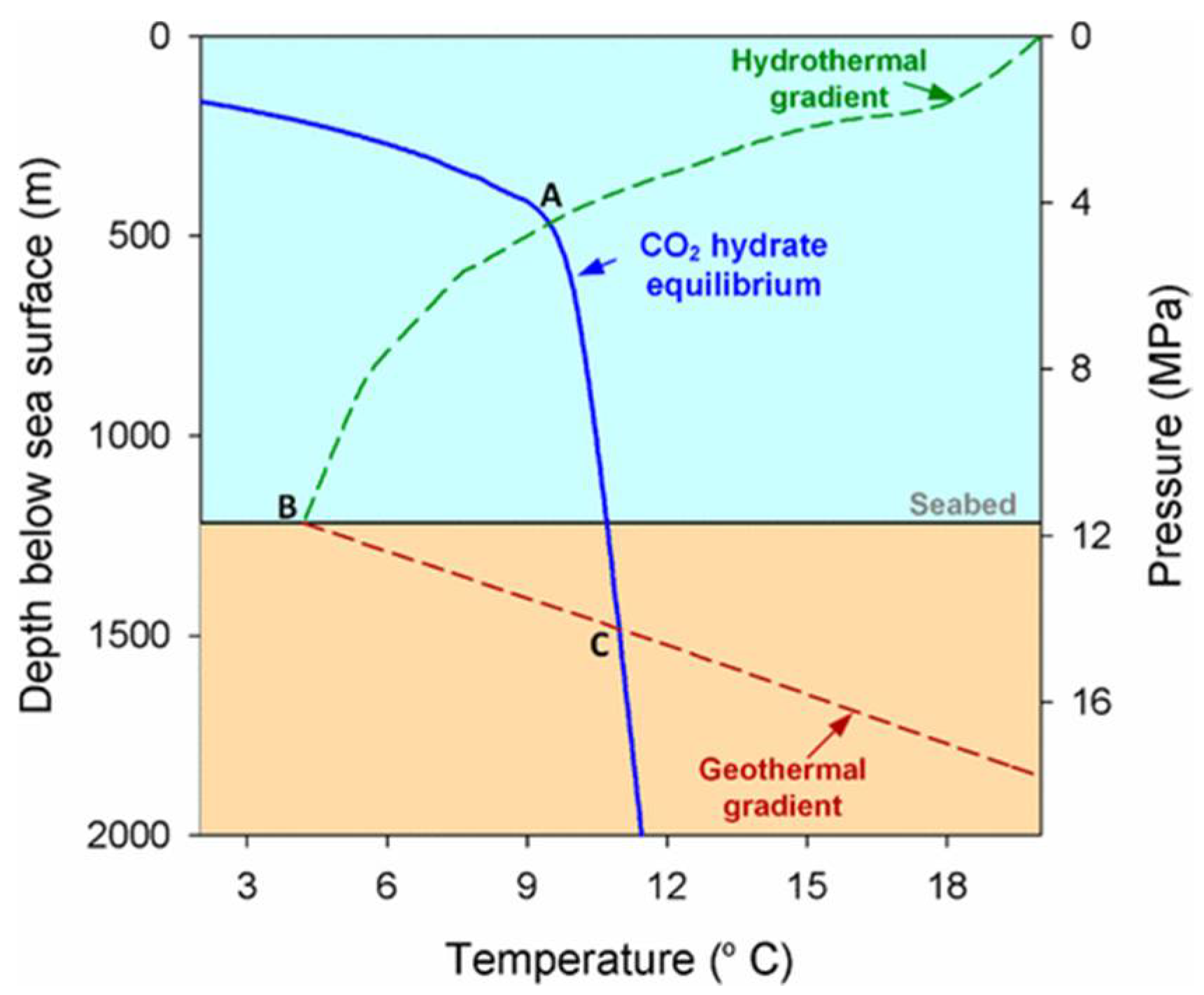
5.2. Ocean Storage
6. Outlook and Challenges
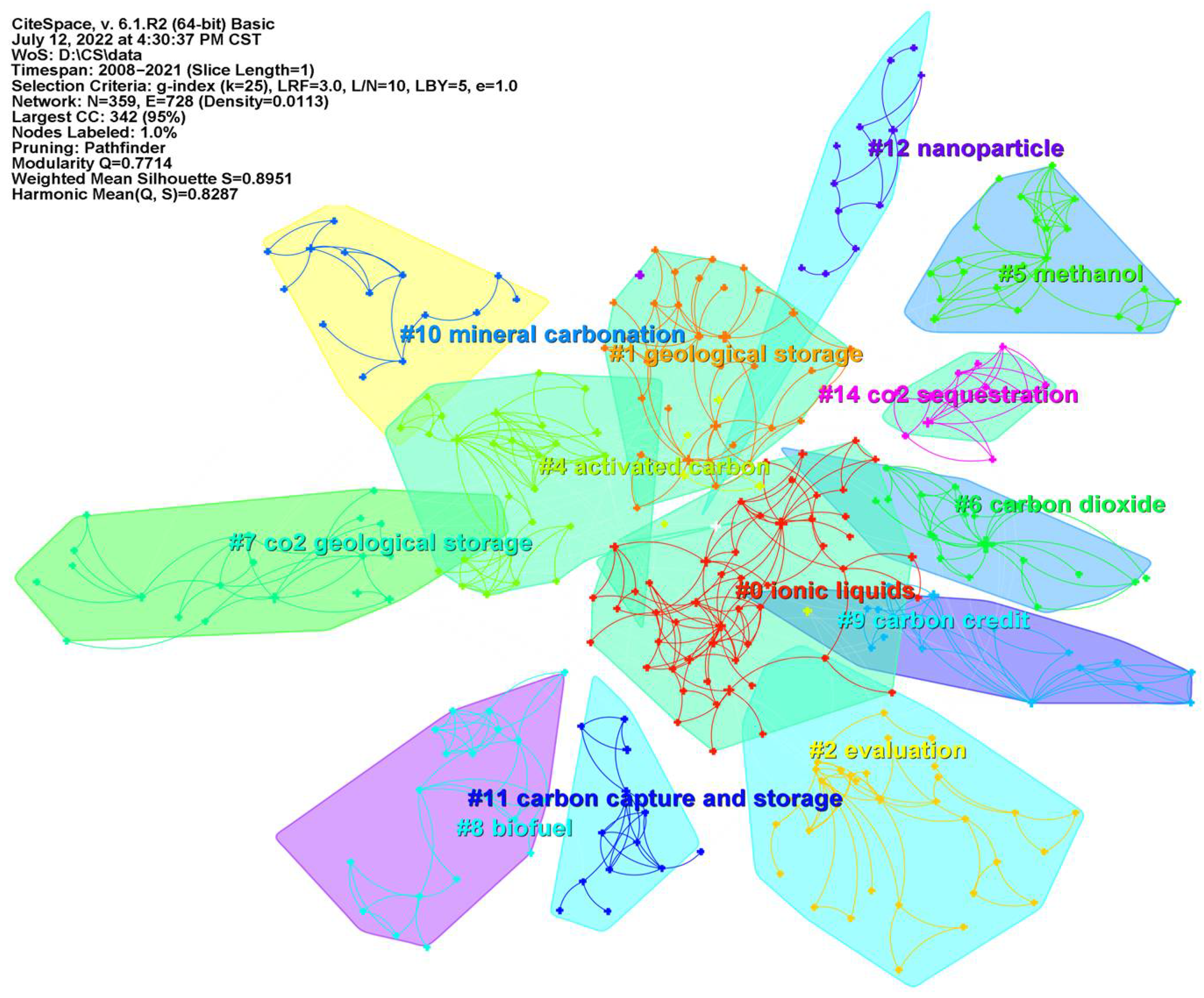
6.1. Keyword Co-Occurrence and Cluster Analysis
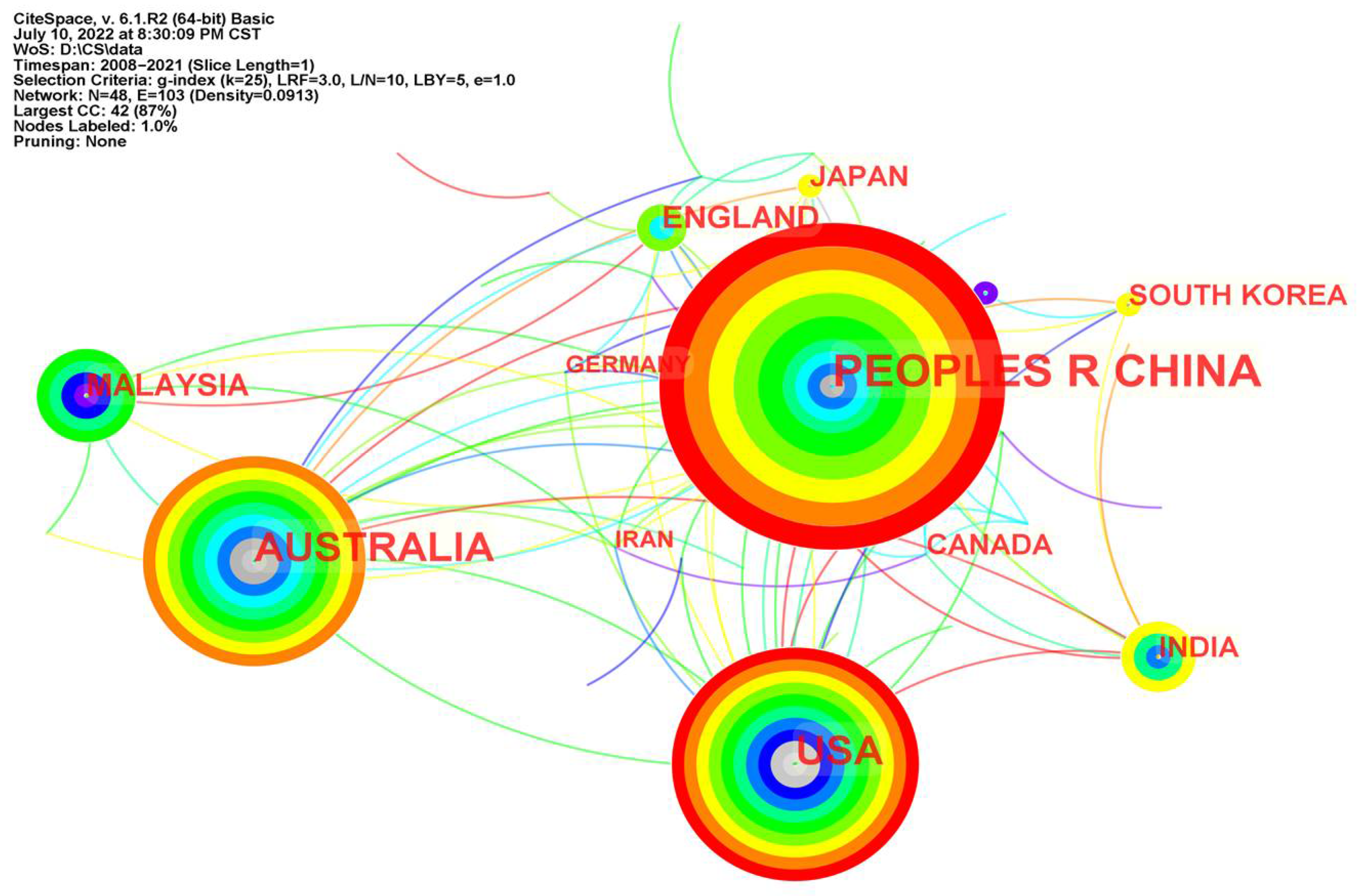
6.2. Analysis of Cooperation Networks between Countries and Institutions
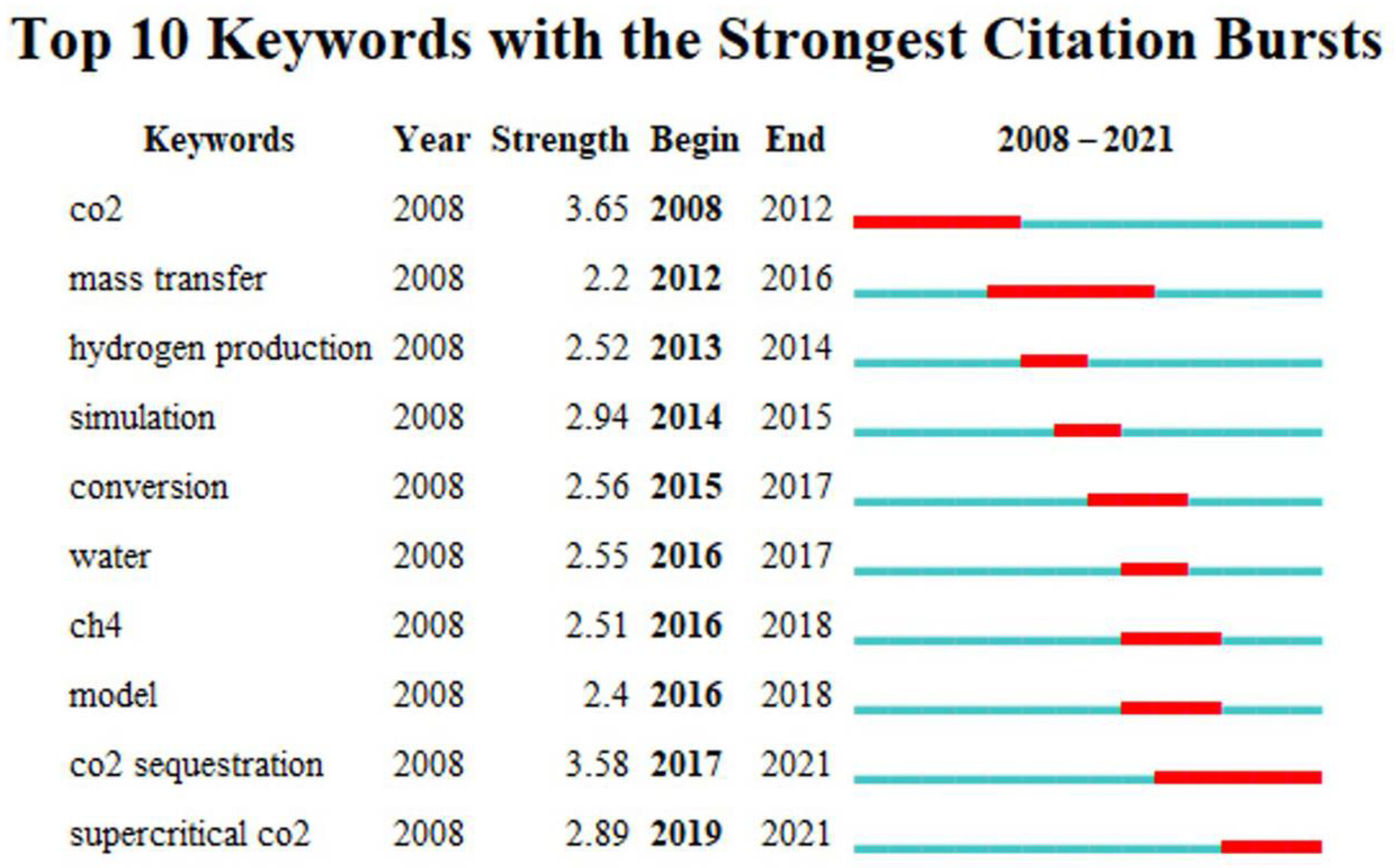
6.3. Keyword Highlighting Analysis
6.4. Challenge
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wu, Z.S.; Li, X.C. Prediction of CO2 leakage during sequestration into marine sedimentary strata. Energy Convers. Manag. 2009, 50, 503–509. [Google Scholar] [CrossRef]
- Liu, D.Q.; Li, Y.L.; Yang, S.; Agarwal, R.K. CO2 sequestration with enhanced shale gas recovery. Energy Sources Part A Recover. Util. Environ. Eff. 2021, 43, 3227–3237. [Google Scholar] [CrossRef]
- Zhan, J.; Chen, Z.X.; Zhang, Y.; Zheng, Z.G.; Deng, Q. Will the future of shale reservoirs lie in CO2 geological sequestration? Sci. China Technol. Sci. 2020, 63, 1154–1163. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.D.; Yang, Y.; Song, Y.M.; Li, G.H. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Liu, G.Z.; Cai, B.F.; Li, Q.; Zhang, X.; Ouyang, T. China’s pathways of CO2 capture, utilization and storage under carbon neutrality vision 2060. Carbon Manag. 2022, 13, 435–449. [Google Scholar] [CrossRef]
- Chen, S.Y.; Liu, J.F.; Zhang, Q.; Teng, F.; McLellan, B.C. A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Shen, M.H.; Tong, L.G.; Yin, S.W.; Liu, C.P.; Wang, L.; Feng, W.J.; Ding, Y.L. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. Sep. Purif. Technol. 2022, 299, 121734. [Google Scholar] [CrossRef]
- Zhang, Z.E.; Pan, S.Y.; Li, H.; Cai, J.C.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Kunze, C.; Spliethoff, H. Assessment of oxy-fuel, pre- and post-combustion-based carbon capture for future IGCC plants. Appl. Energy 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Omoregbe, O.; Mustapha, A.N.; Steinberger-Wilckens, R.; El-Kharouf, A.; Onyeaka, H. Carbon capture technologies for climate change mitigation: A bibliometric analysis of the scientific discourse during 1998–2018. Energy Rep. 2020, 6, 1200–1212. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Mitra, S.K.; Kozinski, J.A. The progressive routes for carbon capture and sequestration. Energy Sci. Eng. 2016, 4, 99–122. [Google Scholar] [CrossRef] [Green Version]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Liang, Z.W.; Rongwong, W.; Liu, H.L.; Fu, K.Y.; Gao, H.X.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Mumford, K.A.; Wu, Y.; Smith, K.H.; Stevens, G.W. Review of solvent based carbon-dioxide capture technologies. Front. Chem. Sci. Eng. 2015, 9, 125–141. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Single solvents, solvent blends, and advanced solvent systems in CO2 capture by absorption: A review. Int. J. Glob. Warm. 2015, 7, 184–225. [Google Scholar] [CrossRef]
- Weaver, K.D.; Kim, H.J.; Sun, J.Z.; MacFarlane, D.R.; Elliott, G.D. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010, 12, 507–513. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep Eutectic Solvents: Sustainable Media for Nanoscale and Functional Materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef]
- Carriazo, D.; Serrano, M.C.; Gutierrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef]
- Lian, S.H.; Song, C.F.; Liu, Q.L.; Duan, E.H.; Ren, H.W.; Kitamura, Y. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization. J. Environ. Sci. 2021, 99, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Sarmad, S.; Mikkola, J.P.; Ji, X.Y. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef]
- Xu, Y.J. CO2 absorption behavior of azole-based protic ionic liquids: Influence of the alkalinity and physicochemical properties. J. CO2 Util. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.P.; Vo, D.V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 18, 2031–2054. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Yokozeki, A. Separation of CO2 and H2S using room-temperature ionic liquid [bmim][PF6]. Fluid Phase Equilibria 2010, 294, 105–113. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-Based Adsorbents for Postcombustion CO2 Capture: A Critical Review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Liu, G.J.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Wang, Q.A.; Luo, J.Z.; Zhong, Z.Y.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of biomass gasification: A review. Renew. Sustain. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefin. 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Jeguirim, M.; Colin, B.; Limousy, L. Combustion characteristics and kinetics of torrefied olive pomace. Energy 2016, 107, 453–463. [Google Scholar] [CrossRef]
- Rousset, P.; Macedo, L.; Commandre, J.M.; Moreira, A. Biomass torrefaction under different oxygen concentrations and its effect on the composition of the solid by-product. J. Anal. Appl. Pyrolysis 2012, 96, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Sabouni, R.; Kazemian, H.; Rohani, S. Carbon dioxide capturing technologies: A review focusing on metal organic framework materials (MOFs). Environ. Sci. Pollut. Res. 2014, 21, 5427–5449. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Cheng, Y.; Zhong, H.; Tian, H.; Pan, J.; Pareek, V.K.; Jiang, S.P.; Lamonier, J.F.; Jaroniec, M.; Liu, J. From waste Coca Cola (R) to activated carbons with impressive capabilities for CO2 adsorption and supercapacitors. Carbon 2017, 116, 490–499. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Yang, Y.W.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Nunell, G.V.; Fernandez, M.E.; Bonelli, P.R.; Cukierman, A.L. Conversion of biomass from an invasive species into activated carbons for removal of nitrate from wastewater. Biomass Bioenergy 2012, 44, 87–95. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Yaumi, A.L.; Abu Bakar, M.Z.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Al Bahri, M.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Activated carbon from grape seeds upon chemical activation with phosphoric acid: Application to the adsorption of diuron from water. Chem. Eng. J. 2012, 203, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zeng, H.C. Hierarchical Nanocomposite by the Integration of Reduced Graphene Oxide and Amorphous Carbon with Ultrafine MgO Nanocrystallites for Enhanced CO2 Capture. Environ. Sci. Technol. 2017, 51, 12998–13007. [Google Scholar] [CrossRef]
- Low, J.X.; Yu, J.G.; Ho, W.K. Graphene-Based Photocatalysts for CO2 Reduction to Solar Fuel. J. Phys. Chem. Lett. 2015, 6, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Novacek, M.; Jankovsky, O.; Luxa, J.; Sedmidubsky, D.; Pumera, M.; Fila, V.; Lhotka, M.; Klimova, K.; Matejkova, S.; Sofer, Z. Tuning of graphene oxide composition by multiple oxidations for carbon dioxide storage and capture of toxic metals. J. Mater. Chem. A 2017, 5, 2739–2748. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Atarod, M.; Jaleh, B.; Gandomirouzbahani, M. In situ green synthesis of Ag nanoparticles on graphene oxide/TiO2 nanocomposite and their catalytic activity for the reduction of 4-nitrophenol, congo red and methylene blue. Ceram. Int. 2016, 42, 8587–8596. [Google Scholar] [CrossRef]
- Szczesniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of graphene-based materials. Adv. Colloid Interface Sci. 2017, 243, 46–59. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Nanostructured polyaniline decorated graphene sheets for reversible CO2 capture. J. Mater. Chem. 2012, 22, 3708–3712. [Google Scholar] [CrossRef]
- Ding, M.L.; Flaig, R.W.; Jiang, H.L.; Yaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Pei, X.K.; Chen, Y.F.; Li, S.Q.; Zhang, S.H.; Feng, X.; Zhou, J.W.; Wang, B. Metal-Organic Frameworks Derived Porous Carbons: Syntheses, Porosity and Gas Sorption Properties. Chin. J. Chem. 2016, 34, 157–174. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhao, Y.G.; Gong, Q.H.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in Solid Adsorbent Materials Development for CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.B.; Zhao, D.Y.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO(2)capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, Z.X.; Wang, J.X.; Elzatahry, A.A.; Zhao, D.Y. A Perspective on Mesoporous TiO2 Materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Feist, B.J.; Hill, J.M. CO2 and H2S Adsorption on gamma-Al2O3-Supported Lanthanum Oxide. Energy Fuels 2015, 29, 6049–6056. [Google Scholar] [CrossRef]
- Song, C.X.; Zhao, J.; Li, H.H.; Luo, S.Z.; Tang, Y.B.; Wang, D.B. Design, controlled synthesis, and properties of 2D CeO2/NiO heterostructure assemblies. CrystEngComm 2017, 19, 7339–7346. [Google Scholar] [CrossRef]
- Siagiana, U.W.R.; Raksajati, A.; Himma, N.F.; Khoiruddin, K.; Wenten, I.G. Membrane-based carbon capture technologies: Membrane gas separation vs. membrane contactor. J. Nat. Gas Sci. Eng. 2019, 67, 172–195. [Google Scholar] [CrossRef]
- Norahim, N.; Yaisanga, P.; Faungnawakij, K.; Charinpanitkul, T.; Klaysom, C. Recent Membrane Developments for CO2 Separation and Capture. Chem. Eng. Technol. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Sanni, E.S.; Sadiku, E.R.; Okoro, E.E. Novel Systems and Membrane Technologies for Carbon Capture. Int. J. Chem. Eng. 2021, 2021, 6642906. [Google Scholar] [CrossRef]
- Zhang, Z. Comparisons of various absorbent effects on carbon dioxide capture in membrane gas absorption (MGA) process. J. Nat. Gas Sci. Eng. 2016, 31, 589–595. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.M.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ramli, W.K.W. Hydrophobic PVDF membrane via two-stage soft coagulation bath system for Membrane Gas Absorption of CO2. Sep. Purif. Technol. 2013, 103, 230–240. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ramli, W.K.W.; Fernando, W.J.N.; Daud, W.R.W. Effect of ethanol concentration in water coagulation bath on pore geometry of PVDF membrane for Membrane Gas Absorption application in CO2 removal. Sep. Purif. Technol. 2012, 88, 11–18. [Google Scholar] [CrossRef]
- Lv, Y.X.; Yu, X.H.; Jia, J.J.; Tu, S.T.; Yan, J.Y.; Dahlquist, E. Fabrication and characterization of superhydrophobic polypropylene hollow fiber membranes for carbon dioxide absorption. Appl. Energy 2012, 90, 167–174. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.B.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Albo, J.; Tsuru, T. Thin Ionic Liquid Membranes Based on Inorganic Supports with Different Pore Sizes. Ind. Eng. Chem. Res. 2014, 53, 8045–8056. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Daniel, C.I.; Portugal, C.A.M.; Crespo, J.G.; Irabien, A. Permeability modulation of Supported Magnetic Ionic Liquid Membranes (SMILMs) by an external magnetic field. J. Membr. Sci. 2013, 430, 56–61. [Google Scholar] [CrossRef]
- Zunita, M.; Hastuti, R.; Alamsyah, A.; Khoiruddin, K.; Wenten, I.G. Ionic Liquid Membrane for Carbon Capture and Separation. Sep. Purif. Rev. 2021, 51, 261–280. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Ho, W.S.W. Steric hindrance effect on amine demonstrated in solid polymer membranes for CO2 transport. J. Membr. Sci. 2012, 415, 132–138. [Google Scholar] [CrossRef]
- Dai, Z.D.; Deng, L.Y. Membrane absorption using ionic liquid for pre-combustion CO2 capture at elevated pressure and temperature. Int. J. Greenh. Gas Control 2016, 54, 59–69. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Gayatri, D.V.; Sreedhar, I.; Raghavan, K.V. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Willson, D.; Favre, E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Membr. Sci. 2012, 415, 424–434. [Google Scholar] [CrossRef]
- Xu, J.X.; Lin, W.S. A CO2 cryogenic capture system for flue gas of an LNG-fired power plant. Int. J. Hydrogen Energy 2017, 42, 18674–18680. [Google Scholar] [CrossRef]
- Song, C.F.; Liu, Q.L.; Deng, S.; Li, H.L.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The Potential for Carbon Capture. Bioscience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Song, C.F.; Liu, Q.L.; Qi, Y.; Chen, G.Y.; Song, Y.J.; Kansha, Y.; Kitamura, Y. Absorption-microalgae hybrid CO2 capture and biotransformation strategy-A review. Int. J. Greenh. Gas Control 2019, 88, 109–117. [Google Scholar] [CrossRef]
- Thomas, D.M.; Mechery, J.; Paulose, S.V. Carbon dioxide capture strategies from flue gas using microalgae: A review. Environ. Sci. Pollut. Res. 2016, 23, 16926–16940. [Google Scholar] [CrossRef]
- Douskova, I.; Doucha, J.; Livansky, K.; Machat, J.; Novak, P.; Umysova, D.; Zachleder, V.; Vitova, M. Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl. Microbiol. Biotechnol. 2009, 82, 179–185. [Google Scholar] [CrossRef]
- Zhou, W.G.; Wang, J.H.; Chen, P.; Ji, C.C.; Kang, Q.Y.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Galinsky, N.L.; Huang, Y.; Shafiefarhood, A.; Li, F.X. Iron Oxide with Facilitated O2− Transport for Facile Fuel Oxidation and CO2 Capture in a Chemical Looping Scheme. ACS Sustain. Chem. Eng. 2013, 1, 364–373. [Google Scholar] [CrossRef]
- Lisi, L.; Mancino, G.; Cimino, S. Chemical looping oxygen transfer properties of Cu-doped lanthanum oxysulphate. Int. J. Hydrogen Energy 2015, 40, 2047–2054. [Google Scholar] [CrossRef]
- Sajen, S.; Singh, S.K.; Mungse, P.; Rayalu, S.; Watanabe, K.; Saravanan, G.; Labhasetwar, N. Mechanically Stable Mixed Metal Oxide of Cu and Mn as Oxygen Carrier for Chemical Looping Syngas Combustion. Energy Fuels 2016, 30, 7596–7603. [Google Scholar] [CrossRef]
- Adanez, J.; Gayan, P.; Adanez-Rubio, I.; Cuadrat, A.; Mendiara, T.; Abad, A.; Garcia-Labiano, F.; de Diego, L.F. Use of Chemical-Looping processes for coal combustion with CO2 capture. Energy Procedia 2013, 37, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.W.; Galvita, V.V.; Poelman, H.; Marin, G.B. Advanced Chemical Looping Materials for CO2 Utilization: A Review. Materials 2018, 11, 1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alalwan, H.A.; Alminshid, A.H. CO2 capturing methods: Chemical looping combustion (CLC) as a promising technique. Sci. Total Environ. 2021, 788, 147850. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, R.; Wagterveld, R.M.; Digdaya, I.A.; Xiang, C.; Vermaas, D.A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 2021, 14, 781–814. [Google Scholar] [CrossRef]
- Muroyama, A.P.; Patru, A.; Gubler, L. Review-CO(2)Separation and Transport via Electrochemical Methods. J. Electrochem. Soc. 2020, 167, 133504. [Google Scholar] [CrossRef]
- Wang, F.; Deng, S.; Zhang, H.C.; Wang, J.T.; Zhao, J.P.; Miao, H.; Yuan, J.L.; Yan, J.Y. A comprehensive review on high-temperature fuel cells with carbon capture. Appl. Energy 2020, 275, 115342. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, J.J.; Huang, K.; Zhu, X.F.; Yang, W.S. The current status of high temperature electrochemistry-based CO2 transport membranes and reactors for direct CO2 capture and conversion. Prog. Energy Combust. Sci. 2021, 82, 100888. [Google Scholar] [CrossRef]
- Bae, H.; Park, J.S.; Senthilkumar, S.T.; Hwang, S.M.; Kim, Y. Hybrid seawater desalination-carbon capture using modified seawater battery system. J. Power Sources 2019, 410, 99–105. [Google Scholar] [CrossRef]
- Liu, E.B.; Peng, Y.; Peng, S.B.; Yu, B.; Chen, Q.K. Research on low carbon emission optimization operation technology of natural gas pipeline under multi-energy structure. Pet. Sci. 2022, 19, 3046–3058. [Google Scholar] [CrossRef]
- Liu, E.B.; Peng, Y.; Ji, Y.Q.; Azimi, M.; Shi, L.M. Energy Consumption Optimization Model of Large Parallel Natural Gas Pipeline Network: Using Compressors with Multiple Operating Modes. Energy Fuels 2022, 37, 774–784. [Google Scholar] [CrossRef]
- Lu, H.F.; Ma, X.; Huang, K.; Fu, L.D.; Azimi, M. Carbon dioxide transport via pipelines: A systematic review. J. Clean. Prod. 2020, 266, 121994. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Yang, X.X.; Li, Z.; Ni, W.D. The upper limit of moisture content for supercritical CO2 pipeline transport. J. Supercrit. Fluids 2012, 67, 14–21. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, G.X.; Massarotto, P.; Rudolph, V. Optimization of pipeline transport for CO2 sequestration. Energy Convers. Manag. 2006, 47, 702–715. [Google Scholar] [CrossRef]
- Lu, W.; Hu, H.; Qi, G.S. Effect of Pipe Diameter and Inlet Parameters on Liquid CO2 Flow in Transportation by Pipeline with Large Height Difference. Processes 2019, 7, 756. [Google Scholar] [CrossRef] [Green Version]
- Drescher, M.; Fahmi, A.; Aursand, P.; Hammer, M.; Lund, H.; Stang, J.; Austegard, A. Towards a thorough Validation of Simulation Tools for CO2 Pipeline Transport. Energy Procedia 2017, 114, 6730–6740. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Rahmanian, N.; Mujtaba, I.M. CO2 Pipeline Design: A Review. Energies 2018, 11, 2184. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, X.R. Simulation of Heat Transfer and System Behavior in a Supercritical CO2 Based Thermosyphon: Effect of Pipe Diameter. J. Heat Transf. 2011, 133, 122505. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hourfar, F.; Elkamel, A.; Leonenko, Y. Economic Optimization Design of CO2 Pipeline Transportation with Booster Stations. Ind. Eng. Chem. Res. 2019, 58, 16730–16742. [Google Scholar] [CrossRef]
- Teh, C.; Barifcani, A.; Pack, D.; Tade, M.O. The importance of ground temperature to a liquid carbon dioxide pipeline. Int. J. Greenh. Gas Control 2015, 39, 463–469. [Google Scholar] [CrossRef]
- Wang, Z.; Weihs, G.A.F.; Neal, P.R.; Wiley, D.E. Effects of pipeline distance, injectivity and capacity on CO2 pipeline and storage site selection. Int. J. Greenh. Gas Control 2016, 51, 95–105. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Rulik, S.; Stolecka, K. Comprehensive analysis of pipeline transportation systems for CO2 sequestration. Thermodynamics and safety problems. Energy Convers. Manag. 2013, 76, 665–673. [Google Scholar] [CrossRef]
- Onyebuchi, V.E.; Kolios, A.; Hanak, D.P.; Biliyok, C.; Manovic, V. A systematic review of key challenges of CO2 transport via pipelines. Renew. Sustain. Energy Rev. 2018, 81, 2563–2583. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Yun, R. Prediction of heat transfer coefficient and compressor power consumption for CO2 with impurities under pipeline transporting condition. J. Mech. Sci. Technol. 2018, 32, 2339–2346. [Google Scholar] [CrossRef]
- Baik, W.; Lee, W.; Yun, R. Heat transfer and pressure drop characteristics of CO2 mixtures in a pipeline under the seawater condition. Int. J. Heat Mass Transf. 2019, 136, 627–634. [Google Scholar] [CrossRef]
- Vitali, M.; Zuliani, C.; Corvaro, F.; Marchetti, B.; Terenzi, A.; Tallone, F. Risks and Safety of CO2 Transport via Pipeline: A Review of Risk Analysis and Modeling Approaches for Accidental Releases. Energies 2021, 14, 4601. [Google Scholar] [CrossRef]
- Liu, E.-B.; Tang, H.; Zhang, Y.-H.; Li, D.-J.; Kou, B.; Liu, N.; Azimi, M. Experiment and numerical simulation of distribution law of water-based corrosion inhibitor in natural gas gathering and transportation pipeline. Pet. Sci. 2023; in press. [Google Scholar]
- Kather, A.; Kownatzki, S. Assessment of the different parameters affecting the CO2 purity from coal fired oxyfuel process. Int. J. Greenh. Gas Control 2011, 5, S204–S209. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Mujtaba, I.M.; Rahmanian, N. Process simulation of impurity impacts on CO2 fluids flowing in pipelines. J. Clean. Prod. 2019, 240, 118145. [Google Scholar] [CrossRef]
- Bilio, M.; Brown, S.; Fairweather, M.; Mahgerefteh, H. CO2 Pipelines Material and Safety Considerations. In Hazards XXI: Process Safety and Environmental Protection in a Changing World; Institution of Chemical Engineers: Rugby, UK, 2009; pp. 423–429. [Google Scholar]
- Zhao, Q.; Li, Y.X. The influence of impurities on the transportation safety of an anthropogenic CO2 pipeline. Process. Saf. Environ. Prot. 2014, 92, 80–92. [Google Scholar] [CrossRef]
- Wetenhall, B.; Race, J.M.; Downie, M.J. The Effect of CO2 Purity on the Development of Pipeline Networks for Carbon Capture and Storage Schemes. Int. J. Greenh. Gas Control 2014, 30, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Zhu, C.Y.; Cui, G.; Xing, X.; Mu, J.; Li, Z.L. Analysis of internal corrosion of supercritical CO2 pipeline. Corros. Rev. 2021, 39, 219–241. [Google Scholar] [CrossRef]
- Cui, G.; Yang, Z.Q.; Liu, J.G.; Li, Z.L. A comprehensive review of metal corrosion in a supercritical CO2 environment. Int. J. Greenh. Gas Control 2019, 90, 102814. [Google Scholar] [CrossRef]
- Sim, S.; Bocher, F.; Cole, I.S.; Chen, X.B.; Birbilis, N. Investigating the Effect of Water Content in Supercritical CO2 as Relevant to the Corrosion of Carbon Capture and Storage Pipelines. Corrosion 2014, 70, 185–195. [Google Scholar] [CrossRef]
- Cao, Q.; Yan, X.Q.; Guo, X.L.; Zhu, H.L.; Liu, S.R.; Yu, J.L. Temperature evolution and heat transfer during the release of CO2 from a large-scale pipeline. Int. J. Greenh. Gas Control 2018, 74, 40–48. [Google Scholar] [CrossRef]
- Herzog, N.; Gorenz, P.; Egbers, C. CFD modeling of high-pressurized CO2 released from onshore pipeline leakages. Environ. Earth Sci. 2013, 70, 3749–3759. [Google Scholar] [CrossRef]
- Joshi, P.; Bikkina, P.; Wang, Q.S. Consequence analysis of accidental release of supercritical carbon dioxide from high pressure pipelines. Int. J. Greenh. Gas Control 2016, 55, 166–176. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Tu, R.; Jiang, X.; Li, K.; Zhou, X.J. The leakage behavior of supercritical CO2 flow in an experimental pipeline system. Appl. Energy 2014, 130, 574–580. [Google Scholar] [CrossRef]
- Jung, J.Y.; Huh, C.; Kang, S.G.; Seo, Y.; Chang, D. CO2 transport strategy and its cost estimation for the offshore CCS in Korea. Appl. Energy 2013, 111, 1054–1060. [Google Scholar] [CrossRef]
- Decarre, S.; Berthiaud, J.; Butin, N.; Guillaume-Combecave, J.L. CO2 maritime transportation. Int. J. Greenh. Gas Control 2010, 4, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Roussanaly, S.; Han, D.; Skaugen, G.; Gundersen, T. At what Pressure Shall CO2 Be Transported by Ship? An in-Depth Cost Comparison of 7 and 15 Barg Shipping. Energies 2021, 14, 5635. [Google Scholar] [CrossRef]
- Al Baroudi, H.; Awoyomi, A.; Patchigolla, K.; Jonnalagadda, K.; Anthony, E.J. A review of large-scale CO2 shipping and marine emissions management for carbon capture, utilisation and storage. Appl. Energy 2021, 287, 116510. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Maksoud, M.I.A.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Baena-Moreno, F.M.; Rodriguez-Galan, M.; Vega, F.; Alonso-Farinas, B.; Arenas, L.F.V.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.M.; Kumar, G.; Yang, Y.H. Carbon dioxide capture and bioenergy production using biological system—A review. Renew. Sustain. Energy Rev. 2019, 110, 143–158. [Google Scholar] [CrossRef]
- Mavar, K.N.; Gaurina-Medimurec, N.; Hrncevic, L. Significance of Enhanced Oil Recovery in Carbon Dioxide Emission Reduction. Sustainability 2021, 13, 1800. [Google Scholar] [CrossRef]
- Stewart, R.J.; Johnson, G.; Heinemann, N.; Wilkinson, M.; Haszeldine, R.S. Low carbon oil production: Enhanced oil recovery with CO2 from North Sea residual oil zones. Int. J. Greenh. Gas Control 2018, 75, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.L.; Wang, G.; Lu, C.J. Research Progress in Carbon Dioxide Storage and Enhanced Oil Recovery. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012054. [Google Scholar] [CrossRef]
- Cao, M.; Gu, Y.G. Oil recovery mechanisms and asphaltene precipitation phenomenon in immiscible and miscible CO2 flooding processes. Fuel 2013, 109, 157–166. [Google Scholar] [CrossRef]
- Bikkina, P.; Wan, J.M.; Kim, Y.; Kneafsey, T.J.; Tokunaga, T.K. Influence of wettability and permeability heterogeneity on miscible CO2 flooding efficiency. Fuel 2016, 166, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, H.; Haddad, A.S.; Mohammadian, E.; Rafati, R.; Azdarpour, A.; Ghahri, P.; Ombewa, P.; Neuert, T.; Zink, A. Ultrasound-assisted CO2 flooding to improve oil recovery. Ultrason. Sonochem. 2017, 35, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Polak, S.; Grimstad, A.A. Reservoir simulation study of CO(2) storage and CO(2)-EGR in the Atzbach-Schwanenstadt gas field in Austria. Energy Procedia 2009, 1, 2961–2968. [Google Scholar] [CrossRef] [Green Version]
- Honari, A.; Bijeljic, B.; Johns, M.L.; May, E.F. Enhanced gas recovery with CO2 sequestration: The effect of medium heterogeneity on the dispersion of supercritical CO2-CH4. Int. J. Greenh. Gas Control 2015, 39, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Hussen, C.; Amin, R.; Madden, G.; Evans, B. Reservoir simulation for enhanced gas recovery: An economic evaluation. J. Nat. Gas Sci. Eng. 2012, 5, 42–50. [Google Scholar] [CrossRef]
- Qi, R.R.; Ning, Z.F.; Wang, Q.; Zeng, Y.; Huang, L.; Zhang, S.; Du, H.M. Sorption of Methane, Carbon Dioxide, and Their Mixtures on Shales from Sichuan Basin, China. Energy Fuels 2018, 32, 2926–2940. [Google Scholar] [CrossRef]
- Huo, P.L.; Zhang, D.F.; Yang, Z.; Li, W.; Zhang, J.; Jia, S.Q. CO2 geological sequestration: Displacement behavior of shale gas methane by carbon dioxide injection. Int. J. Greenh. Gas Control 2017, 66, 48–59. [Google Scholar] [CrossRef]
- Eliebid, M.; Mahmoud, M.; Shawabkeh, R.; Elkatatny, S.; Hussein, I.A. Effect of CO2 adsorption on enhanced natural gas recovery and sequestration in carbonate reservoirs. J. Nat. Gas Sci. Eng. 2018, 55, 575–584. [Google Scholar] [CrossRef]
- Zhou, J.P.; Xie, S.; Jiang, Y.D.; Xian, X.F.; Liu, Q.L.; Lu, Z.H.; Lyu, Q. Influence of Supercritical CO2 Exposure on CH4 and CO2 Adsorption Behaviors of Shale: Implications for CO2 Sequestration. Energy Fuels 2018, 32, 6073–6089. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Luo, Y.H.; Lu, Y.Y.; Qin, C.; Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. [Google Scholar] [CrossRef]
- Song, R.; Cui, M.M. Molecular simulation on competitive adsorption mechanism of CH4/CO2 on shale kerogen. Arab. J. Geosci. 2018, 11, 403. [Google Scholar] [CrossRef]
- Chen, T.Y.; Feng, X.T.; Pan, Z.J. Experimental study on kinetic swelling of organic-rich shale in CO2, CH4 and N-2. J. Nat. Gas Sci. Eng. 2018, 55, 406–417. [Google Scholar] [CrossRef]
- Chen, T.Y.; Feng, X.T.; Pan, Z.J. Experimental study of swelling of organic rich shale in methane. Int. J. Coal Geol. 2015, 150, 64–73. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Ao, X.; Tang, J.R.; Jia, Y.Z.; Zhang, X.W.; Chen, Y.T. Swelling of shale in supercritical carbon dioxide. J. Nat. Gas Sci. Eng. 2016, 30, 268–275. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Ma, D.S.; Li, J.S.; Zhou, T.Y.; Ji, Z.M.; Han, H.S. Progress and prospects of carbon dioxide capture, EOR-utilization and storage industrialization. Pet. Explor. Dev. 2022, 49, 955–962. [Google Scholar] [CrossRef]
- Zhang, X.G.; Ranjith, P.G.; Perera, M.S.A.; Ranathunga, A.S.; Haque, K. Gas Transportation and Enhanced Coalbed Methane Recovery Processes in Deep Coal Seams: A Review. Energy Fuels 2016, 30, 8832–8849. [Google Scholar] [CrossRef]
- Pan, Z.J.; Ye, J.P.; Zhou, F.B.; Tan, Y.L.; Connell, L.D.; Fan, J.J. CO2 storage in coal to enhance coalbed methane recovery: A review of field experiments in China. Int. Geol. Rev. 2018, 60, 754–776. [Google Scholar] [CrossRef]
- Hu, Z.C.; Li, C.; Zhang, D.F. Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals: Implications for CO2 sequestration in coal seams. Chin. J. Chem. Eng. 2021, 35, 288–301. [Google Scholar] [CrossRef]
- Zhang, X.G.; Ranjith, P.G. Experimental investigation of effects of CO2 injection on enhanced methane recovery in coal seam reservoirs. J. CO2 Util. 2019, 33, 394–404. [Google Scholar] [CrossRef]
- Hou, Y.D.; Huang, S.P.; Han, J.; Liu, X.B.; Han, L.F.; Fu, C.F. Numerical Simulation of the Effect of Injected CO2 Temperature and Pressure on CO2-Enhanced Coalbed Methane. Appl. Sci. 2020, 10, 1385. [Google Scholar]
- Perera, M.S.A. Influences of CO2 Injection into Deep Coal Seams: A Review. Energy Fuels 2017, 31, 10324–10334. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G. Deep coal seams as a greener energy source: A review. J. Geophys. Eng. 2014, 11, 063001. [Google Scholar] [CrossRef]
- Perera, M.S.A. A Comprehensive Overview of CO2 Flow Behaviour in Deep Coal Seams. Energies 2018, 11, 063001. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Liu, S.L.; Wang, H.T.; Lun, Z.M.; Zhou, X.; Zhao, C.P.; Min, C.G.; Zhang, H.; Xu, Y.; Zhang, D.F. Influence of H2O on Adsorbed CH4 on Coal Displaced by CO2 Injection: Implication for CO2 Sequestration in Coal Seam with Enhanced CH4 Recovery (CO2-ECBM). Ind. Eng. Chem. Res. 2021, 60, 15817–15833. [Google Scholar] [CrossRef]
- Mazumder, S.; Wolf, K.H. Differential swelling and permeability change of coal in response to CO2 injection for ECBM. Int. J. Coal Geol. 2008, 74, 123–138. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, H.J.; Shi, Y.; Keffer, D.; Lee, C.H. Competitive adsorption of CO2/CH4 mixture on dry and wet coal from subcritical to supercritical conditions. Chem. Eng. J. 2013, 230, 93–101. [Google Scholar] [CrossRef]
- Charriere, D.; Pokryszka, Z.; Behra, P. Effect of pressure and temperature on diffusion of CO2 and CH4 into coal from the Lorraine basin (France). Int. J. Coal Geol. 2010, 81, 373–380. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Airey, D.W.; Choi, S.K. Sub- and super-critical carbon dioxide flow behavior in naturally fractured black coal: An experimental study. Fuel 2011, 90, 3390–3397. [Google Scholar] [CrossRef]
- Fan, Z.L.; Fan, G.W.; Zhang, D.S.; Zhang, L.; Zhang, S.; Liang, S.S.; Yu, W. Optimal injection timing and gas mixture proportion for enhancing coalbed methane recovery. Energy 2021, 222, 119880. [Google Scholar] [CrossRef]
- Fan, C.J.; Elsworth, D.; Li, S.; Chen, Z.W.; Luo, M.K.; Song, Y.; Zhang, H.H. Modelling and optimization of enhanced coalbed methane recovery using CO2/N-2 mixtures. Fuel 2019, 253, 1114–1129. [Google Scholar] [CrossRef]
- Talapatra, A.; Halder, S.; Chowdhury, A.I. Enhancing coal bed methane recovery: Using injection of nitrogen and carbon dioxide mixture. Pet. Sci. Technol. 2021, 39, 49–62. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Airey, D. The effects of sub-critical and super-critical carbon dioxide adsorption-induced coal matrix swelling on the permeability of naturally fractured black coal. Energy 2011, 36, 6442–6450. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Wei, C.H. An experimental investigation of applicability of CO2 enhanced coal bed methane recovery to low rank coal. Fuel 2017, 189, 391–399. [Google Scholar] [CrossRef]
- Liu, F.; Kang, Y.; Hu, Y.; Chen, H.; Wang, X.; Pan, H.; Xie, J. Comparative investigation on the heat extraction performance of an enhanced geothermal system with N2O, CO2 and H2O as working fluids. Appl. Therm. Eng. 2022, 200, 117594. [Google Scholar] [CrossRef]
- Luo, F.; Xu, R.N.; Jiang, P.X. Numerical investigation of fluid flow and heat transfer in a doublet enhanced geothermal system with CO2 as the working fluid (CO2-EGS). Energy 2014, 64, 307–322. [Google Scholar] [CrossRef]
- Wang, C.L.; Cheng, W.L.; Nian, Y.L.; Yang, L.; Han, B.B.; Liu, M.H. Simulation of heat extraction from CO2-based enhanced geothermal systems considering CO2 sequestration. Energy 2018, 142, 157–167. [Google Scholar] [CrossRef]
- Isaka, B.L.A.; Ranjith, P.G.; Rathnaweera, T.D. The use of super-critical carbon dioxide as the working fluid in enhanced geothermal systems (EGSs): A review study. Sustain. Energy Technol. Assess. 2019, 36, 100547. [Google Scholar]
- Abanades, J.C.; Rubin, E.S.; Mazzotti, M.; Herzog, H.J. On the climate change mitigation potential of CO2 conversion to fuels. Energy Environ. Sci. 2017, 10, 2491–2499. [Google Scholar] [CrossRef]
- Bao, J.; Yang, G.H.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef] [Green Version]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G.O.; Perez-Ramirez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.S.; Aziz, M.A.; Yamani, Z.H. Recent Progress in Carbonaceous and Redox-active Nanoarchitectures for Hybrid Supercapacitors: Performance Evaluation, Challenges, and Future Prospects. Chem. Rec. 2022, 22, e202200018. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K. Recent advances in low-temperature electrochemical conversion of carbon dioxide. Rev. Chem. Eng. 2021, 37, 863–884. [Google Scholar] [CrossRef]
- Park, S.; Wijaya, D.T.; Na, J.; Lee, C.W. Towards the Large-Scale Electrochemical Reduction of Carbon Dioxide. Catalysts 2021, 11, 253. [Google Scholar] [CrossRef]
- Feng, G.; Chen, W.; Wang, B.; Song, Y.; Li, G.; Fang, J.; Wei, W.; Sun, Y. Oxygenates from the electrochemical reduction of carbon dioxide. Chem. Asian J. 2018, 13, 1992–2008. [Google Scholar] [CrossRef]
- Ampelli, C.; Perathoner, S.; Centi, G. CO2 utilization: An enabling element to move to a resource- and energy-efficient chemical and fuel production. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangeneh, F.T.; Sahebdelfar, S.; Ravanchi, M.T. Conversion of carbon dioxide to valuable petrochemicals: An approach to clean development mechanism. J. Nat. Gas Chem. 2011, 20, 219–231. [Google Scholar] [CrossRef]
- Poliakoff, M.; Leitner, W.; Streng, E.S. The Twelve Principles of CO2 Chemistry. Faraday Discuss. 2015, 183, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, A.; Shah, S.S.; Mazumder, M.A.J.; Oyama, M.; Al-Betar, A.R. Carbon Nanofiber and Poly [2-(methacryloyloxy) ethyl] Trimethylammonium Chloride Composite as a New Benchmark Carbon-based Electrocatalyst for Sulfide Oxidation. Chem.-Asian J. 2021, 16, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
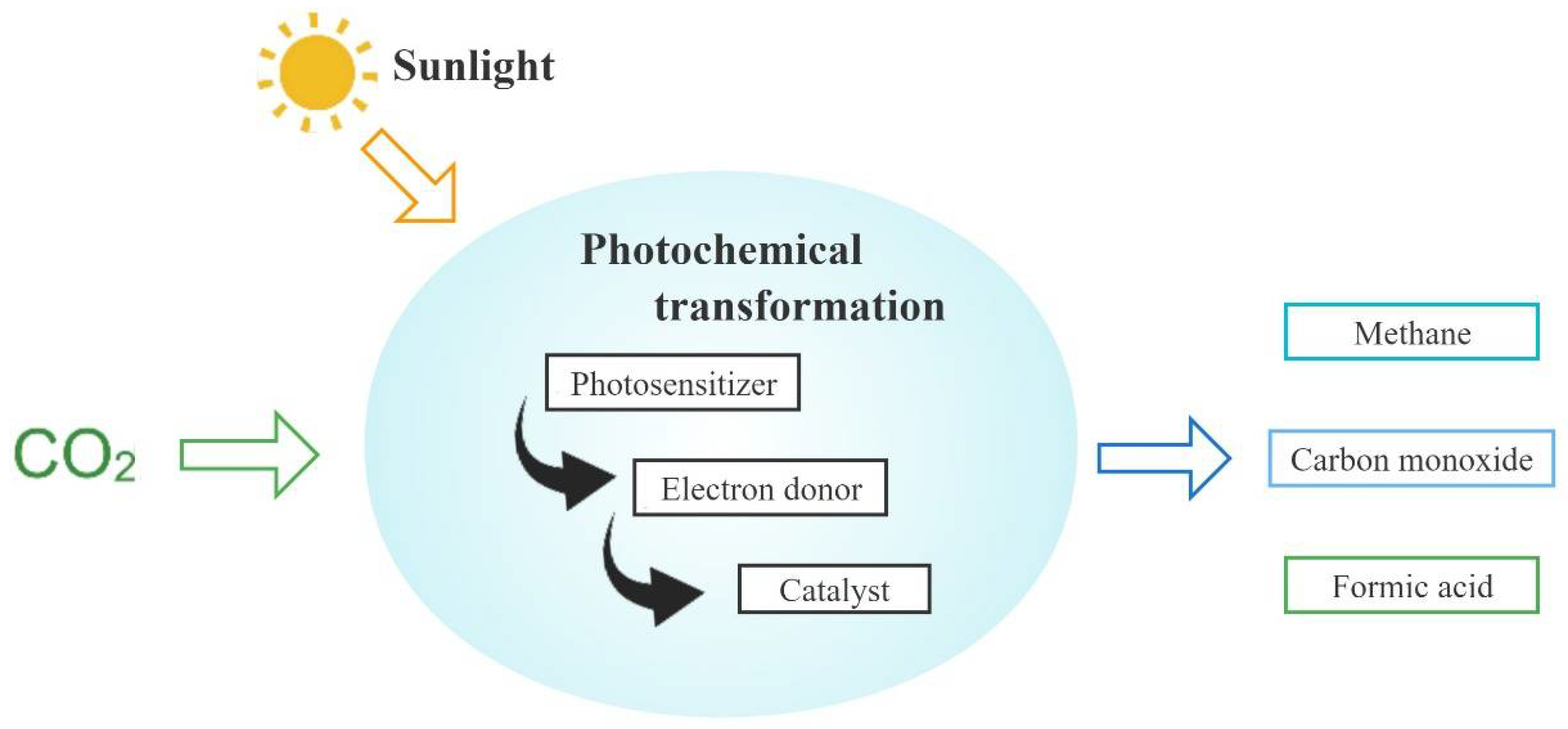
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Su, T.M.; Qin, Z.Z.; Ji, H.B.; Jiang, Y.X.; Huang, G. Recent advances in the photocatalytic reduction of carbon dioxide. Environ. Chem. Lett. 2016, 14, 99–112. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.A.S. Photo-induced CO2 reduction by hydrogen for selective CO evolution in a dynamic monolith photoreactor loaded with Ag-modified TiO2 nanocatalyst. Int. J. Hydrogen Energy 2017, 42, 15507–15522. [Google Scholar] [CrossRef]
- Egawa, C. Methane dry reforming reaction on Ru(001) surfaces. J. Catal. 2018, 358, 35–42. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Aramouni, N.A.K.; Touma, J.G.; Abu Tarboush, B.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Syngas production from CO2 reforming of methane over ceria supported cobalt catalyst: Effects of reactants partial pressure. J. Nat. Gas Sci. Eng. 2015, 27, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Rad, S.J.H.; Haghighi, M.; Eslami, A.A.; Rahmani, F.; Rahemi, N. Sol-gel vs. impregnation preparation of MgO and CeO2 doped Ni/Al2O3 nanocatalysts used in dry reforming of methane: Effect of process conditions, synthesis method and support composition. Int. J. Hydrogen Energy 2016, 41, 5335–5350. [Google Scholar]
- Zhang, X.B.; Zhang, G.H.; Song, C.S.; Guo, X.W. Catalytic Conversion of Carbon Dioxide to Methanol: Current Status and Future Perspective. Front. Energy Res. 2021, 8, 621119. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Heterogeneous catalysts for production of chemicals using carbon dioxide as raw material: A review. Renew. Sustain. Energy Rev. 2012, 16, 4951–4964. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH. Appl. Catal. A Gen. 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Kattel, S.; Ramirez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Catalysis Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. How oxide carriers control the catalytic functionality of the Cu-ZnO system in the hydrogenation of CO2 to methanol. Catal. Today 2013, 210, 39–46. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.S.; Alotaibi, M.A.; Alharthi, A.I.; Naeem, A. Recent developments on heterogeneous catalytic CO2 reduction to methanol. J. CO2 Util. 2019, 34, 20–33. [Google Scholar] [CrossRef]
- Guil-Lopez, R.; Mota, N.; Llorente, J.; Millan, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
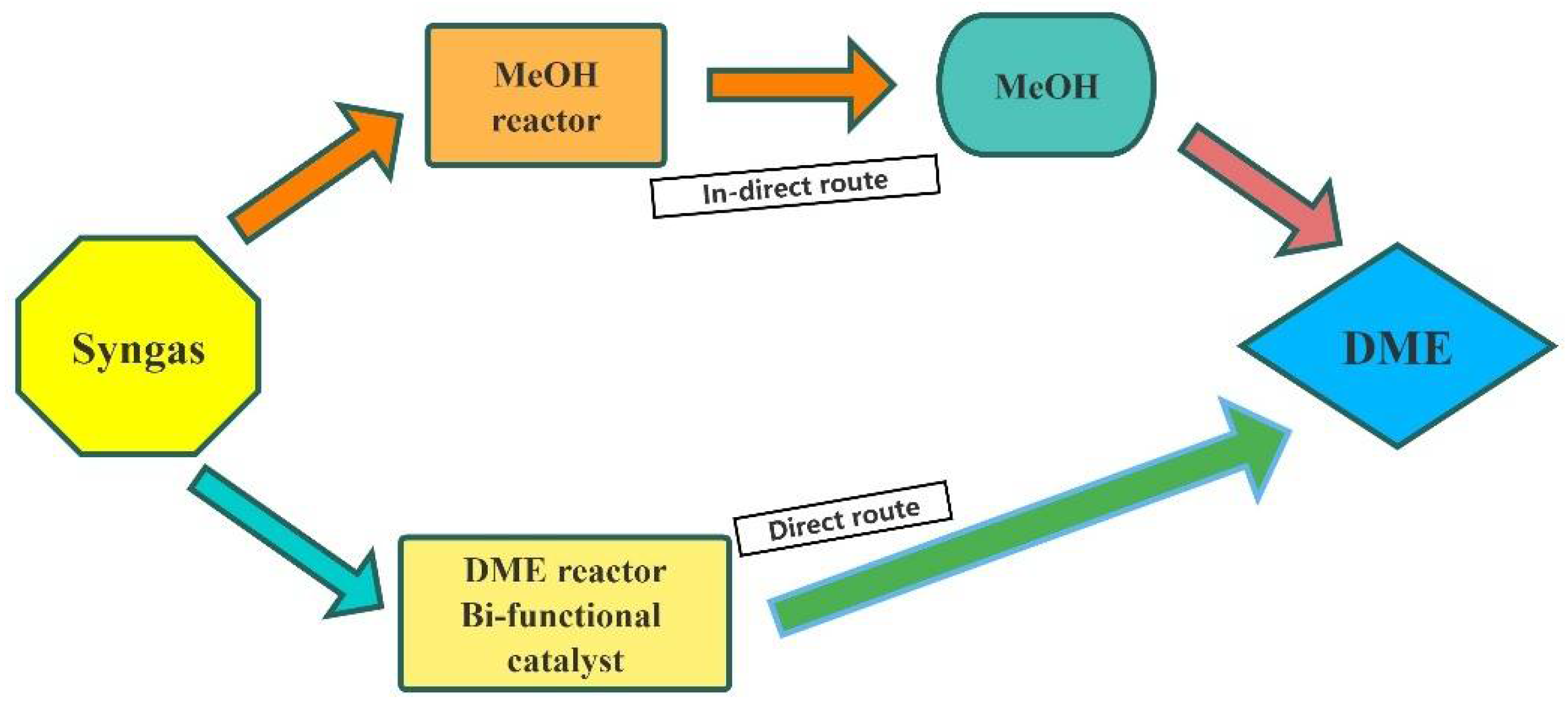
- Chen, W.H.; Lin, B.J.; Lee, H.M.; Huang, M.H. One-step synthesis of dimethyl ether from the gas mixture containing CO2 with high space velocity. Appl. Energy 2012, 98, 92–101. [Google Scholar] [CrossRef]
- Samimi, F.; Bayat, M.; Rahimpour, M.R.; Keshavarz, P. Mathematical modeling and optimization of DME synthesis in two spherical reactors connected in series. J. Nat. Gas Sci. Eng. 2014, 17, 33–41. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Perspective of dimethyl ether as fuel: Part I. Catalysis. J. CO2 Util. 2019, 32, 299–320. [Google Scholar] [CrossRef]
- Chen, W.H.; Hsu, C.L.; Wang, X.D. Thermodynamic approach and comparison of two-step and single step DME (dimethyl ether) syntheses with carbon dioxide utilization. Energy 2016, 109, 326–340. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhang, S.J.; Benson, T. A conceptual design by integrating dimethyl ether (DME) production with tri-reforming process for CO2 emission reduction. Fuel Process. Technol. 2015, 131, 7–13. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Ravanchi, M.T. Carbon dioxide utilization for methane production: A thermodynamic analysis. J. Pet. Sci. Eng. 2015, 134, 14–22. [Google Scholar] [CrossRef]
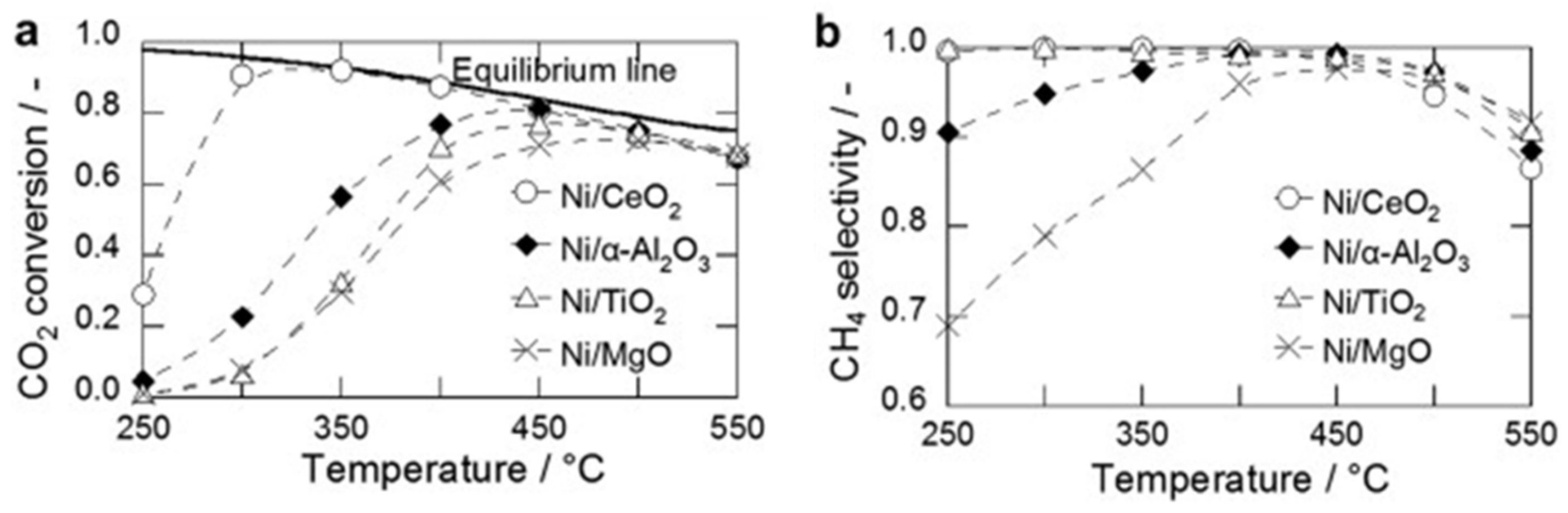
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Graca, I.; Gonzalez, L.V.; Bacariza, M.C.; Fernandes, A.; Henriques, C.; Lopes, J.M.; Ribeiro, M.F. CO2 hydrogenation into CH4 on NiHNaUSY zeolites. Appl. Catal. B Environ. 2014, 147, 101–110. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. The Role of Alkali and Alkaline Earth Metals in the CO(2)Methanation Reaction and the Combined Capture and Methanation of CO2. Catalysts 2020, 10, 812. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
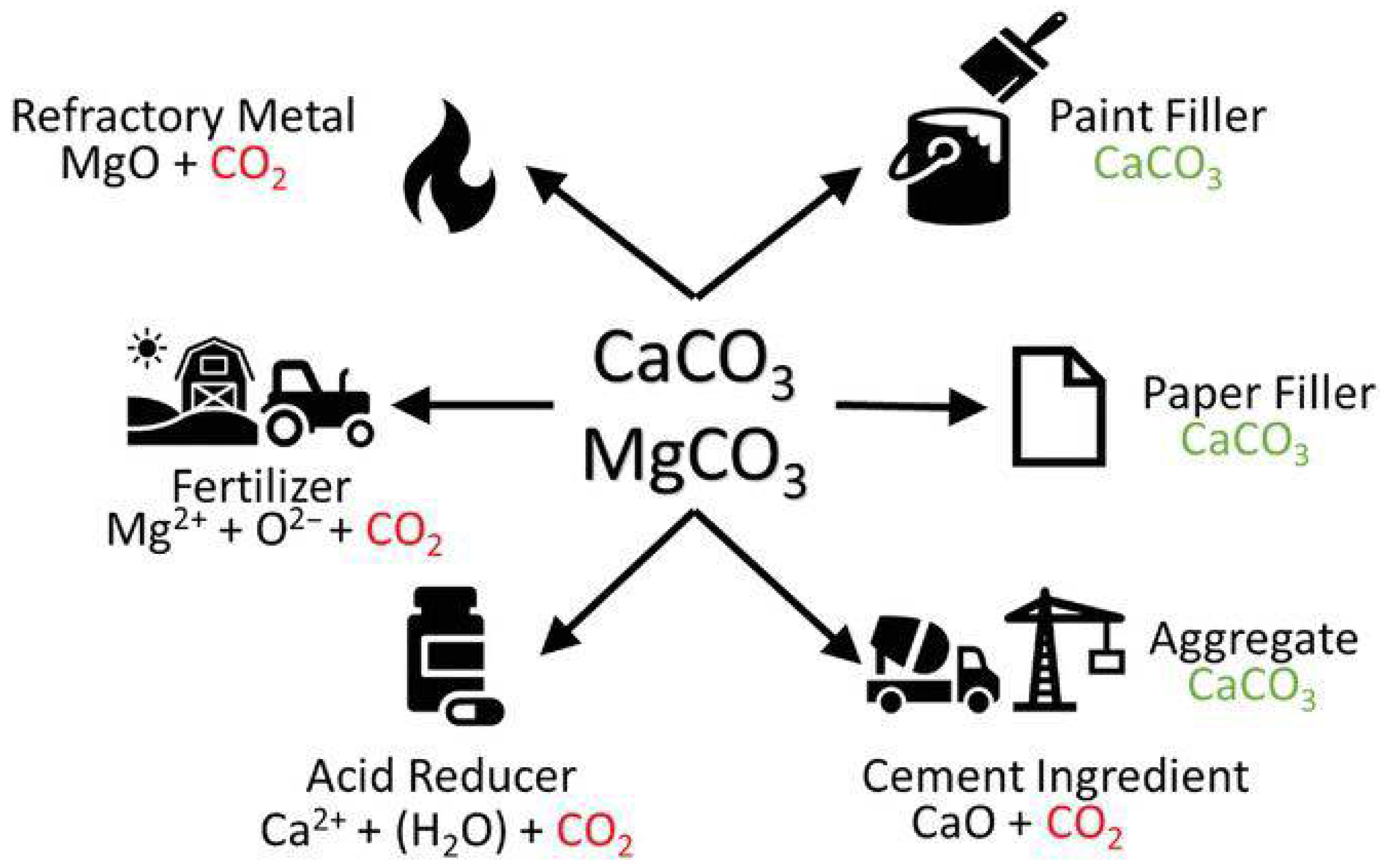
- La Plante, E.C.; Mehdipour, I.; Shortt, I.; Yang, K.; Simonetti, D.; Bauchy, M.; Sant, G.N. Controls on CO2 Mineralization Using Natural and Industrial Alkaline Solids under Ambient Conditions. ACS Sustain. Chem. Eng. 2021, 9, 10727–10739. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chen, Y.H.; Fan, L.S.; Kim, H.; Gao, X.; Ling, T.C.; Chiang, P.C.; Pei, S.L.; Gu, G.W. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- Ragipani, R.; Bhattacharya, S.; Suresh, A.K. A review on steel slag valorisation via mineral carbonation. React. Chem. Eng. 2021, 6, 1152–1178. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Li, X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement 2017, 97, 15–22. [Google Scholar] [CrossRef]
- Woodall, C.M.; McQueen, N.; Pilorge, H.; Wilcox, J. Utilization of mineral carbonation products: Current state and potential. Greenh. Gases Sci. Technol. 2019, 9, 1096–1113. [Google Scholar] [CrossRef]
- Li, P.; Pan, S.Y.; Pei, S.; Lin, Y.P.J.; Chiang, P.C. Challenges and Perspectives on Carbon Fixation and Utilization Technologies: An Overview. Aerosol Air Qual. Res. 2016, 16, 1327–1344. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Tan, C.S. A Review: CO2 Utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef] [Green Version]
- Vuppaladadiyam, A.K.; Yao, J.G.; Florin, N.; George, A.; Wang, X.X.; Labeeuw, L.; Jiang, Y.L.; Davis, R.W.; Abbas, A.; Ralph, P.; et al. Impact of Flue Gas Compounds on Microalgae and Mechanisms for Carbon Assimilation and Utilization. ChemSusChem 2018, 11, 334–355. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Song, Y.; Zheng, S.J.; Zhen, G.Y.; Lu, X.Q.; Takuro, K.; Xu, K.Q.; Bakonyi, P. Electro-conversion of carbon dioxide (CO2) to low-carbon methane by bioelectromethanogenesis process in microbial electrolysis cells: The current status and future perspective. Bioresour. Technol. 2019, 279, 339–349. [Google Scholar] [CrossRef]
- Conde-Hernandez, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltran, J.A. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Zuknik, M.H.; Norulaini, N.A.N.; Omar, A.K.M. Supercritical carbon dioxide extraction of lycopene: A review. J. Food Eng. 2012, 112, 253–262. [Google Scholar] [CrossRef]
- Yu, T.H.; Niu, L.Y.; Iwahashi, H. High-Pressure Carbon Dioxide Used for Pasteurization in Food Industry. Food Eng. Rev. 2020, 12, 364–380. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Ranjith, P.G. Review of application of molecular dynamics simulations in geological sequestration of carbon dioxide. Fuel 2019, 255, 115644. [Google Scholar] [CrossRef]
- Lubon, K. Influence of Injection Well Location on CO2 Geological Storage Efficiency. Energies 2021, 14, 8604. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.; Carpenter, M.; Flach, T.A. Intermediate storage of carbon dioxide in geological formations: A technical perspective. Int. J. Greenh. Gas Control 2008, 2, 502–510. [Google Scholar] [CrossRef]
- Lu, P.; Hao, Y.Y.; Bai, Y.; Liu, W.G.; Chen, X.; Zheng, H.A.; Liu, J.; Chen, Y.Z.; Gao, J.P. Optimal selection of favorable areas for CO2 geological storage in the Majiagou Formation in the Ordos Basin. Int. J. Greenh. Gas Control 2021, 109, 103360. [Google Scholar] [CrossRef]
- Hsu, C.W.; Chen, L.T.; Hu, A.H.; Chang, Y.M. Site selection for carbon dioxide geological storage using analytic network process. Sep. Purif. Technol. 2012, 94, 146–153. [Google Scholar] [CrossRef]
- Mi, Z.X.; Wang, F.G.; Yang, Y.Z.; Wang, F.; Hu, T.; Tian, H.L. Evaluation of the potentiality and suitability for CO2 geological storage in the Junggar Basin, northwestern China. Int. J. Greenh. Gas Control 2018, 78, 62–72. [Google Scholar] [CrossRef]
- Jiang, X. A review of physical modelling and numerical simulation of long-term geological storage of CO2. Appl. Energy 2011, 88, 3557–3566. [Google Scholar] [CrossRef]
- De Silva, G.P.D.; Ranjith, P.G.; Perera, M.S.A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Kumar, S.; Foroozesh, J.; Edlmann, K.; Rezk, M.G.; Lim, C.Y. A comprehensive review of value-added CO2 sequestration in subsurface saline aquifers. J. Nat. Gas Sci. Eng. 2020, 81, 103437. [Google Scholar] [CrossRef]
- Wildenschild, D.; Armstrong, R.T.; Herring, A.L.; Young, I.M.; Carey, J.W. Exploring capillary trapping efficiency as a function of interfacial tension, viscosity, and flow rate. Energy Procedia 2011, 4, 4945–4952. [Google Scholar] [CrossRef] [Green Version]
- Jing, J.; Yang, Y.L.; Tang, Z.H. Effects of formation dip angle and salinity on the safety of CO2 geological storage—A case study of Shiclianfeng strata with low porosity and low permeability in the Ordos Basin, China. J. Clean. Prod. 2019, 226, 874–891. [Google Scholar] [CrossRef]
- De Silva, P.N.K.; Ranjith, P.G. A study of methodologies for CO2 storage capacity estimation of saline aquifers. Fuel 2012, 93, 13–27. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Keith, D.W. Accelerating CO2 dissolution in saline aquifers for geological storage Mechanistic and sensitivity studies. Energy Fuels 2009, 23, 3328–3336. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Li, X.C.; Jiang, Z.B.; Wei, N. Impacts of CO2 leakage into shallow formations on groundwater chemistry. Fuel Process. Technol. 2015, 135, 162–167. [Google Scholar] [CrossRef]
- Jayasekara, D.W.; Ranjith, P.G.; Wanniarachchi, W.A.M.; Rathnaweera, T.D. Understanding the chemico-mineralogical changes of caprock sealing in deep saline CO2 sequestration environments: A review study. J. Supercrit. Fluids 2020, 161, 104819. [Google Scholar] [CrossRef]
- Gholami, R.; Raza, A.; Iglauer, S. Leakage risk assessment of a CO2 storage site: A review. Earth-Sci. Rev. 2021, 223, 103849. [Google Scholar] [CrossRef]
- Lee, K.K.; Lee, S.H.; Yun, S.T.; Jeen, S.W. Shallow groundwater system monitoring on controlled CO2 release sites: A review on field experimental methods and efforts for CO2 leakage detection. Geosci. J. 2016, 20, 569–583. [Google Scholar] [CrossRef]
- Caesary, D.; Song, S.Y.; Yu, H.; Kim, B.; Nam, M.J. A review on CO2 leakage detection in shallow subsurface using geophysical surveys. Int. J. Greenh. Gas Control 2020, 102, 103165. [Google Scholar] [CrossRef]
- Zeidouni, M.; Pooladi-Darvish, M.; Keith, D.W. Leakage Detection and Characterization through Pressure Monitoring. Energy Procedia 2011, 4, 3534–3541. [Google Scholar] [CrossRef] [Green Version]
- Zeidouni, M.; Nicot, J.P.; Hovorka, S.D. Monitoring above-zone temperature variations associated with CO2 and brine leakage from a storage aquifer. Environ. Earth Sci. 2014, 72, 1733–1747. [Google Scholar] [CrossRef]
- Moni, C.; Rasse, D.P. Detection of simulated leaks from geologically stored CO2 with C-13 monitoring. Int. J. Greenh. Gas Control 2014, 26, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Magnier, C.; Rouchon, V.; Bandeira, C.; Goncalves, R.; Miller, D.; Dino, R. Surface and Subsurface Geochemical Monitoring of an EOR-CO2 Field: Buracica, Brazil. Oil Gas Sci. Technol. 2012, 67, 355–372. [Google Scholar] [CrossRef] [Green Version]
- Mortezaei, K.; Amirlatifi, A.; Ghazanfari, E.; Vahedifard, F. Potential CO2 leakage from geological storage sites: Advances and challenges. Environ. Geotech. 2021, 8, 3–27. [Google Scholar] [CrossRef]
- Adams, E.E.; Caldeira, K. Ocean Storage of CO2. Elements 2008, 4, 319–324. [Google Scholar] [CrossRef]
- Zheng, J.J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Sheps, K.M.; Max, M.D.; Osegovic, J.P.; Tatro, S.R.; Brazel, L.A. A case for deep-ocean CO2 sequestration. Energy Procedia 2009, 1, 4961–4968. [Google Scholar] [CrossRef] [Green Version]
- Widdicombe, S.; McNeill, C.L.; Stahl, H.; Taylor, P.; Queiros, A.M.; Nunes, J.; Tait, K. Impact of sub-seabed CO2 leakage on macrobenthic community structure and diversity. Int. J. Greenh. Gas Control 2015, 38, 182–192. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qanbari, F.; Pooladi-Darvish, M.; Tabatabaie, S.H.; Gerami, S. CO2 disposal as hydrate in ocean sediments. J. Nat. Gas Sci. Eng. 2012, 8, 139–149. [Google Scholar] [CrossRef]
- Shitashima, K.; Maeda, Y.; Ohsumi, T. Development of detection and monitoring techniques of CO2 leakage from seafloor in sub-seabed CO2 storage. Appl. Geochem. 2013, 30, 114–124. [Google Scholar] [CrossRef]






| Authors | Content | Year | References |
|---|---|---|---|
| Yao et al. | This paper mainly introduces the latest progress of China’s CCUS after 2019 and finds that China’s CCUS technology has made significant progress in recent years. | 2023 | [6] |
| Hong et al. | This paper summarizes the CCUS system from the technical and economic perspectives, introduces the global CCUS projects, and introduces the progress of various technologies in detail, focusing on the economic comparison of various technologies. | 2022 | [7] |
| Liu et al. | The CCUS path in China was studied, mainly introducing the current situation of CCUS, emission reduction demand and potential cost. | 2022 | [8] |
| Shiyi et al. | Combed the technological development process and industrialization progress of CCUS-EOR, and analyzed the problems and challenges of CCUS-EOR in China. | 2022 | |
| Chen et al. | The risks of CCUS technology in finance, technology, environment, health and safety are introduced. | 2022 | [9] |
| Shen et al. | The low-temperature separation technology is introduced to capture CO2, and the existing problems are summarized. | 2022 | [10] |
| Zhan et al. | Various utilization modes of CO2 are introduced, the progress of CO2 utilization projects is discussed, and the key challenges and problems are studied. | 2020 | [11] |
| Method | Propylene Carbonate Method | Polyethylene Glycol Dimethyl Ether Method | Low-Temperature Methanol Washing Method |
|---|---|---|---|
| Applicable conditions | 3~7 Mpa >25 °C | 1.5~14 Mpa 1~25 °C | 3~8.1 Mpa −10~−70 °C |
| Characteristic | the solvent is cheap and non-toxic, suitable for absorbing CO2 from natural gas and crude hydrogen | the process flow is simple, the operation flexibility is large, the one-time purification degree is high, and the total energy consumption is low | methanol is cheap and easy to obtain, with good selectivity, thermal stability and chemical selectivity, and low operating cost |
| Transporation Method | Conditions | Phase | Capacity | REMARKS |
|---|---|---|---|---|
| Pipelines | 4.8–20 Mpa 283–307 K | gas, liquid, supercritical, dense phase | ~150 Mt CO2/year 8000 km of pipeline transport in operation |
|
| Ships | 0.65–4.5 Mpa 221–283 K | liquid | >70 Mt CO2/year |
|
| Chemical | Company (Location) | Scale Per Year b |
|---|---|---|
| Carbon monoxide (via SOEC) c | Haldor-Topsoe (Lyngby, Denmark)/ Gas Innovations (La Porte, TX, USA) | 12 Nm3/h |
| Methanol | Carbon Recycling International (Reykjavík, Iceland) Mitsui Chemical Company (Tokyo, Japan) | 4000 tons 100 tons |
| Methane | Audi (Ingolstadt, Germany) | 1000 tons |
| Fuels (via CO2-based Fischer-Tropsch) | Sunfire (Dresden, Germany) INERATEC (Espoo, Finland) | 3 tons 200 L |
| Oxalic acid | Liquid Light/Avantium (Amsterdam, The Netherlands) | 2.4 tons |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, E.; Lu, X.; Wang, D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies 2023, 16, 2865. https://doi.org/10.3390/en16062865
Liu E, Lu X, Wang D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies. 2023; 16(6):2865. https://doi.org/10.3390/en16062865
Chicago/Turabian StyleLiu, Enbin, Xudong Lu, and Daocheng Wang. 2023. "A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges" Energies 16, no. 6: 2865. https://doi.org/10.3390/en16062865
APA StyleLiu, E., Lu, X., & Wang, D. (2023). A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies, 16(6), 2865. https://doi.org/10.3390/en16062865








