Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review
Abstract
1. Introduction
2. The Main Processes for Obtaining Syngas and Hydrogen
- -
- steam methane reforming (SMR);
- -
- autothermal reforming (ATR);
- -
- partial oxidation (POX);
- -
- catalytic partial oxidation (CPOX);
- -
- dry methane reforming (DMR);
- -
- combined methane reforming (CMR);
- -
- tri-reforming of methane (TRM);
- -
- reforming using membrane reactors.
3. Results of Studies of Non-Catalytic Partial Oxidation of Hydrocarbon Gases
3.1. POX Process Thermodynamic and Kinetic Modeling
3.2. POX Process CFD Modeling
3.3. Experimental Studies and Apparatus for the Implementation of POX Processes
4. Possibilities of Obtaining Other Chemical Products by Hydrocarbon POX
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Syngas Market (2022 to 2028). Size, Forecast, Insights and Competitive Landscape Dehaze. Research and Markets. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/02/25/2392165/28124/en/Global-Syngas-Market-2022-to-2028-Size-Forecast-Insights-and-Competitive-Landscape.html (accessed on 17 November 2022).
- Rouwenhorst, K.H.R.; Travis, A.S.; Lefferts, L. 1921–2021: A Century of renewable ammonia synthesis. Sustain. Chem. 2022, 3, 149–171. [Google Scholar] [CrossRef]
- Power Technology Research. Global Hydrogen Review 2022. International Energy Agency (IEA). 2022. Available online: https://powertechresearch.com/global-hydrogen-review-2022/ (accessed on 19 January 2023).
- Hydrogen Demand is Growing, with Positive Signals in Key Applications. Executive Summary. Available online: https://www.iea.org/reports/global-hydrogen-review-2022/executive-summary (accessed on 19 January 2023).
- Hydrogen Council. Available online: https://hydrogencouncil.com/en/ (accessed on 12 October 2022).
- Makaryan, I.A.; Sedov, I.V. The state and development prospects of the global Hydrogen energy sector. Russ. J. Gen. Chem. 2021, 91, 1912–1928. [Google Scholar] [CrossRef]
- Hydrogen Scaling, Up. A Sustainable Pathway for the Global Energy Transition. Hydrogen Council. 2017. Available online: https://hydrogencouncil.com/wp-content/uploads/2017/11/Hydrogen-Scaling-up_Hydrogen-Council_2017.compressed.pdf (accessed on 11 March 2023).
- Arutyunov, V.S. On the sources of hydrogen for the global replacement of hydrocarbons. Acad. Lett. 2021, 3692. [Google Scholar] [CrossRef]
- Arutyunov, V.S. Hydrogen energy: Significance, sources, problems, and prospects (A review). Pet. Chem. 2022, 62, 583–593. [Google Scholar] [CrossRef]
- Arutyunov, V.; Belyaev, A.; Arutyunov, A.; Troshin, K.; Nikitin, A. Autoignition of methane–hydrogen mixtures below 1000 K. Processes 2022, 10, 2177. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Salgansky, E.A.; Arutyunov, A.V.; Arutyunov, V.S. A Comprehensive review on the prospects of using hydrogen–methane blends: Challenges and opportunities. Energies 2022, 15, 2265. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Maksimov, A.L. Hydrogen storage using liquid organic carriers. Russ. J. Appl. Chem. 2020, 93, 1815. [Google Scholar] [CrossRef]
- Ghoneim, S.A.; El-Salamony, R.A.; El-Temtamy, S.A. Review on innovative catalytic reforming of natural gas to syngas. World J. Eng. Technol. 2016, 4, 116–139. [Google Scholar] [CrossRef]
- Arutyunov, V.S.; Savchenko, V.I.; Sedov, I.V.; Fokin, I.G.; Nikitin, A.V.; Strekova, L.N. New concept for small-scale GTL. Chem. Eng. J. 2015, 282, 206–212. [Google Scholar] [CrossRef]
- Albrecht, B. Reactor Modeling and Process Analysis for Partial Oxidation of Natural Gas. Ph.D. Thesis, Bogdan Albrecht, Enschede, The Netherlands, 2004. Available online: https://ris.utwente.nl/ws/portalfiles/portal/6119955/thesis_Albrecht.pdf (accessed on 20 December 2022).
- SynCOR™—Autothermal Reformer (ATR). Available online: https://www.topsoe.com/our-resources/knowledge/our-products/equipment/syncortm-autothermal-reformer-atr (accessed on 27 November 2022).
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sust. Energ. Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Rui, Y.; Zhu, B.; Tang, Q.; Yang, C.; Wang, D.; Pu, W.; Tang., X. Experimental study of the feasibility of in-situ hydrogen generation from gas reservoir. Energies 2022, 15, 8185. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sust. Energ. Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Ma, R.; Xu, B.; Zhang, X. Catalytic partial oxidation (CPOX) of natural gas and renewable hydrocarbons/oxygenated hydrocarbons—A review. Catal. Today 2019, 338, 18–30. [Google Scholar] [CrossRef]
- Wright, H.A.; Allison, J.D.; Jack, D.S.; Lewis, G.H.; Landis, S.R. ConocoPhillips GTL technology: The COPox™ process as the syngas generator. In Proceedings Published 2003 by the American Chemical Society. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 2003, 48, 791–792. [Google Scholar]
- Reimert, R.; Marschner, F.; Renner, H.-J.; Boll, W.; Supp, E.; Brejc, M.; Liebner, W.; Schaub, G. Gas Production, 2. Processes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- SGP (Shell Gasification Process). Available online: https://decarbonisationtechnology.com/videos/9/shell-gasification-process#.Y5DTME1UCM8 (accessed on 23 June 2022).
- Speight, J.G. Hydrocarbons from syngas. Texaco gasification process. In Handbook of Industrial Hydrocarbon Processes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; Available online: https://www.sciencedirect.com/topics/engineering/partial-oxidation-gasification (accessed on 4 April 2022).
- Equipment EVT—Pilot Plants—Bergakademie Freiberg. Available online: https://tu-freiberg.de/en/iec/evt/groups/equipment/pilot-plants (accessed on 12 October 2022).
- Air Liquide. GAS POX. Available online: https://www.engineering-airliquide.com/ru/gas-pox-chastichnoe-okislenie-prirodnogo-gaza (accessed on 11 November 2022).
- Speight, J.G. Syngas and the Fischer–Tropsch Process. In The Refinery of the Future, 2nd ed.; Chapter 12; Elsevier: Amsterdam, The Netherlands, 2020; pp. 427–468. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen production technologies: Current state and future developments. Conf. Pap. Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Saeidi, S.; Fazollah, F.; Najari, S.; Iranshahi, D.; Baxter, L.L. Hydrogen production: Perspectives, separation with special emphasis on kinetics of WGS reaction: A state-of-the-art review. J. Ind. Eng. Chem. 2017, 59, 11. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sust. Energ. Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V. Cost-Effectiveness Assessment of the scale of hydrogen production by various methods. Russ. J. Gen. Chem. 2021, 91, 2743–2757. [Google Scholar] [CrossRef]
- Carbon Capture Projects See Meteoric Growth in 2022. 2022. Available online: https://e360.yale.edu/digest/carbon-capture-storage-ccs-growth (accessed on 19 November 2022).
- IEAGHG. Techno-Economic Evaluation of SMR Based Standalone (Merchant) Plant with CCS. 2017. Available online: https://ieaghg.org/exco_docs/2017-02.pdf (accessed on 19 November 2022).
- The Shell Blue Hydrogen Process. Available online: https://www.shell.com/business-customers/catalysts-technologies/licensed-technologies/refinery-technology/shell-blue-hydrogen-process.html (accessed on 12 December 2022).
- Benson, J.; Celin, A. Recovering Hydrogen—And Profits—From Hydrogen-Rich Offgas. CEP. 2018, p. 55. Available online: http:www.aiche.org/cep (accessed on 12 February 2021).
- Zhu, J.N.; Zhang, D.K.; Bromly, J.H.; Barnes, F.; King, K.D. Characteristics of methane partial oxidation at intermediate temperatures between 800 and 1100 K. In Proceedings of the Third Asia-Pacific Conference on Combustion, Seoul, Republic of Korea, 24–27 June 2001; pp. 481–484. [Google Scholar]
- Zhu, J.N.; Zhang, D.K.; King, K.D. Reforming of CH4 by partial oxidation: Thermodynamic and kinetic analyses. Fuel 2001, 80, 899–905. [Google Scholar] [CrossRef]
- Beretta, A.; Groppi, G.; Lualdi, M.; Tavazzi, I.; Forzatti, P. Experimental and modeling analysis of methane partial oxidation: Transient and steady-state behavior of Rh-coated honeycomb monoliths. Ind. Eng. Chem. Res. 2009, 48, 3825–3836. [Google Scholar] [CrossRef]
- Arutyunov, V.; Savchenko, V.; Sedov, I.; Nikitin, A. Non-catalytic gas phase oxidation of hydrocarbons. Eurasian Chem. Technol. J. 2022, 24, 13–20. [Google Scholar] [CrossRef]
- Arutyunov, V.S.; Strekova, L.N. The interplay of catalytic and gas-phase stages at oxidative conversion of methane: A review. J. Molec. Catal. A Chem. 2017, 426, 326–342. [Google Scholar] [CrossRef]
- Savchenko, V.I.; Zimin, Y.S.; Busillo, E.; Nikitin, A.V.; Sedov, I.V.; Arutyunov, V.S. Equilibrium composition of products formed by non-catalytic conversion of hydrocarbons. Pet. Chem. 2022, 62, 515. [Google Scholar] [CrossRef]
- Salgansky, E.A.; Tsvetkov, M.V.; Zaichenko, A.Y.; Podlesniy, D.N.; Sedov, I.V. Thermodynamic evaluation of noncatalytic conversion of natural gas with the production of syngas. Russ. J. Phys. Chem. B 2021, 15, 969. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, D.; Bromly, J. A study of CH4 partial oxidation reforming with detailed mechanisms. J. Therm. Sci. 2000, 9, 176. [Google Scholar] [CrossRef]
- Han, S.; Park, J.; Song, S.; Chun, K.M. Experimental and numerical study of detailed reaction mechanism optimization for syngas (H-2 + CO) production by non-catalytic partial oxidation of methane in a flow reactor. Int. J. Hydrogen Energy 2010, 35, 8762–8771. [Google Scholar] [CrossRef]
- Konnov, A.A.; Zhu, J.N.; Bromly, J.H.; Zhang, D. Non-catalytic partial oxidation of methane into syngas over a wide temperature range. Combust. Sci. Technol. 2004, 176, 1093–1116. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Wang, F. Modeling of non-catalytic partial oxidation of natural gas under conditions found in industrial reformers. Chem. Eng. Process. Process Intensif. 2010, 49, 59–64. [Google Scholar] [CrossRef]
- Savchenko, V.I.; Nikitin, A.V.; Sedov, I.V.; Ozerskii, A.A.; Arutyunov, V.S. The role of homogeneous steam reforming of acetylene in the partial oxidation of methane to syngas in matrix type converters. Chem. Eng. Sci. 2019, 207, 744–751. [Google Scholar] [CrossRef]
- Savchenko, V.I.; Nikitin, A.V.; Zimin, Y.S.; Ozerskii, A.V.; Sedov, I.V.; Arutyunov, V.S. Impact of post-flame processes on the hydrogen yield in matrix partial oxidation reformer. Chem. Eng. Res. Des. 2021, 175, 250–258. [Google Scholar] [CrossRef]
- Buzillo, E.; Savchenko, V.I.; Arutyunov, V.S. On the Mechanism of Methane Conversion in the Noncatalytic Processes of Its Thermal Pyrolysis and Steam and Carbon Dioxide Reforming. Pet. Chem. 2021, 61, 1228–1233. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, X.; Guo, W.; Dai, Z.; Gong, X.; Liu, H.; Yu, G.; Yu, Z. Process analysis of syngas production by non-catalytic POX of oven gas. Front. Energy Power Eng. China 2009, 3, 117–122. [Google Scholar] [CrossRef]
- Bilera, I.V.; Buravtsev, N.N.; Rossikhin, I.V. Effect of hydrogen addition on noncatalytic partial oxidation of natural gas with oxygen in a flow reactor with increased calorific intensity. Russ. J. Appl. Chem. 2020, 93, 456–465. [Google Scholar] [CrossRef]
- Dagde, K.; Akpa, J.G. Computer-aided design of a non-isothermal plug flow reactor for non-catalytic partial oxidation of methane to syngas. Chem. Process Eng. Res. 2014, 28, 9. [Google Scholar]
- Shi, J.; Mao, M.; Li, H.; Liu, Y. Influence of chemical kinetics on predictions of performance of syngas production from fuel-rich combustion of CO2/CH4 mixture in a two-layer burner. Front. Chem. 2020, 7, 902. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, Z.; Li, C.; Li, X.; Zhou, Z.; Yu, G.; Wang, F. Numerical simulation of natural gas non-catalytic partial oxidation reformer. Int. J. Hydrogen Energy 2014, 39, 9149–9157. [Google Scholar] [CrossRef]
- Rehm, M.; Seifert, P.; Meyer, B. Theoretical and numerical investigation on the EDC-model for turbulence-chemistry interaction at gasification conditions. Comp. Chem. Eng. 2009, 33, 402–407. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Dong, L.; Chen, C.; Wang, F. Simulation of non-catalytic partial oxidation and scale-up of natural gas reformer. Fuel Proc. Technol. 2012, 98, 45–50. [Google Scholar] [CrossRef]
- Walter, S. The HP POX Pilot Plant—A Door Opener to a New Era of Natural Gas Valorization; IEC Gasification Conference Publication: Frankfurt am Main, Germany; Lurgi Company: Frankfurt am Main, Germany, 2005; Available online: http://tu-freiberg.de/sites/default/files/media/professur-fuer-energieverfahrenstechnik-und-thermische-rueckstandsbehandlung-16460/publikationen/2005_walter.pdf (accessed on 18 October 2022).
- Richter, A.; Seifert, P.; Compart, F.; Tischer, P.; Meyer, B. A large-scale benchmark for the CFD modeling of non-catalytic reforming of natural gas based on the Freiberg test plant HP POX. Fuel 2015, 152, 110–121. [Google Scholar] [CrossRef]
- Voloshchuk, Y.; Vascellari, M.; Hasse, C.; Meyer, B.; Richter, A. Numerical study of natural gas reforming by non-catalytic partial oxidation based on the VIRTUHCON Benchmark. Chem. Eng. J. 2017, 327, 307–319. [Google Scholar] [CrossRef]
- Voloshchuk, Y.; Richter, A. Reduced order modeling and large-scale validation for non-catalytic partial oxidation of natural gas. Chem. Eng. Sci. 2022, 255, 117620. [Google Scholar] [CrossRef]
- Jeon, S.; Kwon, M.; Kim, Y. Multi-environment PDF modeling for non-catalytic partial oxidation process under MILD oxy-combustion condition. Int. J. Hydrogen Energy 2018, 43, 5486–5500. [Google Scholar] [CrossRef]
- Haworth, D.C. Progress in probability density function methods for turbulent reacting flows. Prog. Energy Combust. Sci. 2010, 36, 168–259. [Google Scholar] [CrossRef]
- Jaehyeon, K.; Namsu, K.; Yongmo, K. Multi-environment PDF modeling for turbulent piloted premixed jet flames. Proc. Combust. Inst. 2019, 37, 2573–2581. [Google Scholar] [CrossRef]
- Caballero, J.J.B.; Zaini, I.N.; Yang, W. Reforming processes for syngas production: A mini-review on the current status, challenges, and prospects for biomass conversion to fuels. Appl. Energy Combust. Sci. 2022, 10, 100064. [Google Scholar] [CrossRef]
- Salgansky, E.A.; Tsvetkov, M.V.; Tsvetkova, Y.Y.; Zaichenko, A.Y.; Podlesniy, D.N.; Sedov, I.V. Thermodynamic evaluation of biogas conversion with the production of hydrogen and syngas. Russ. J. Phys. Chem. B 2022, 16, 1085–1091. [Google Scholar] [CrossRef]
- Lugvishchuk, D.S.; Kulchakovsky, P.I.; Mitberg, E.B.; Mordkovich, V.Z. Soot formation in the methane partial oxidation process under conditions of partial saturation with water vapor. Pet. Chem. 2018, 58, 427–433. [Google Scholar] [CrossRef]
- Tindall, B.M.; Crews, M.A. Alternative technologies to steam-methane reforming. Hydrocarb. Process. 1995, 74, 75–82. [Google Scholar]
- Liu, K.; Deluga, G.D.; Bitsch-Larsen, A.; Schmidt, L.D.; Zhang, L. Catalytic partial oxidation and autothermal reforming. In Hydrogen and Syngas Production and Purification Technologies; Liu, K., Song, C., Subramani, V., Eds.; Wiley: New York, NY, USA, 2010; pp. 127–155. [Google Scholar]
- Oortwijn, P.; Wentinck, H.M.; Martens, F.J.A. A Process for Preparing. Syngas. Patent WO 97/22547, 26 June 1997. [Google Scholar]
- Li, C.; Burke, N.; Gerdes, K.; Patel, J. The undiluted, non-catalytic partial oxidation of methane in a flow tube reactor—An experimental study using indirect induction heating. Fuel 2013, 109, 409–416. [Google Scholar] [CrossRef]
- Li, C.; Kuan, B.; Lee, W.J.; Burke, N.; Patel, J. The non-catalytic partial oxidation of methane in a flow tube reactor using indirect induction heating—An experimental and kinetic modelling study. Chem. Eng. Sci. 2018, 187, 189–199. [Google Scholar] [CrossRef]
- Rasmussen, C.L.; Jakobsen, J.G.; Glarborg, P. Experimental measurements and kinetic modeling of CH4/O2 and CH4/C2H6/O2 conversion at high pressure. Int. J. Chem. Kinet. 2008, 40, 778–807. [Google Scholar] [CrossRef]
- Buravtsev, N.N.; Kolbanovskii, Y.A.; Rossikhina, I.V.; Bilera, I.V. Effect of the calorific intensity of combustion chamber on production of syngas in partial oxidation of methane–oxygen mixtures in the combustion mode. Russ. J. Appl. Chem. 2018, 91, 1588–1596. [Google Scholar] [CrossRef]
- Dorofeenko, S.O.; Polianczyk, E.V. Conversion of hydrocarbon gases to syngas in a reversed-flow filtration combustion reactor. Chem. Eng. J. 2016, 292, 183–189. [Google Scholar] [CrossRef]
- Toledo, M.; Ripoll, N.; Cespedes, J.; Zbogar-Rasic, A.; Fedorova, N.; Jovicic, V.; Delgado, A. Syngas production from waste tires using a hybrid filtration reactor under different gasifier agents. Int. J. Hydrogen Energy 2018, 172, 381–390. [Google Scholar] [CrossRef]
- Polianczyk, E.V.; Dorofeenko, S.O. Conversion of hydrocarbons to syngas in a counterflow moving bed filtration combustion reactor. Int. J. Hydrogen Energy 2019, 44, 4079–4089. [Google Scholar] [CrossRef]
- Dorofeenko, S.O.; Polianczyk, E.V. Enhancing efficiency of hydrocarbons to syngas conversion in a counter flow moving bed filtration combustion reactor. Int. J. Hydrogen Energy 2019, 44, 30039–30052. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Voß, S.; Trimis, D. Hydrogen production by thermal partial oxidation of hydrocarbon fuels in porous media based reforme. Int. J. Hydrogen Energy 2008, 34, 827–832. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, H.; Dai, H.; Song, Z.; Wang, Z.; He, S.; Wang, X. Syngas production by methane-rich combustion in a divergent burner of porous media. Int. J. Hydrogen Energy 2021, 46, 23279–23291. [Google Scholar] [CrossRef]
- Nikitin, A.; Ozersky, A.; Savchenko, V.; Sedov, I.; Shmelev, V.; Arutyunov, V. Matrix conversion of natural gas to syngas: The main parameters of the process and possible applications. Chem. Eng. J. 2019, 377, 120883. [Google Scholar] [CrossRef]
- Förster, T.; Voloshchuk, Y.; Richter, A.; Meyer, B. 3D numerical study of the performance of different burner concepts for the high-pressure non-catalytic natural gas reforming based on the Freiberg semi-industrial test facility HP POX. Fuel 2017, 203, 954–963. [Google Scholar] [CrossRef]
- Espinoza, L.; Guerrero, F.; Ripoll, N.; Toledo, M.; Guerrero, L.; Carvajal, A.; Barahona, A. Syngas production by non-catalytic reforming of biogas with steam addition under filtration combustion mode. Int. J. Hydrogen Energy 2018, 43, 15693–15702. [Google Scholar] [CrossRef]
- Kertthong, T.; Schmid, M.; Scheffknecht, G. Non-catalytic partial oxidation of methane in biomass-derived syngas with high steam and hydrogen content optimal for subsequent synthesis process. J. Energy Inst. 2022, 105, 251–261. [Google Scholar] [CrossRef]
- Shapovalova, O.V.; Chun, Y.N.; Lim, S.M.; Arutyunov, V.S.; Shmelev, V.M. Syngas and hydrogen production from biogas in volumetric (3D) matrix reformers. Int. J. Hydrogen Energy 2012, 37, 14040–14046. [Google Scholar] [CrossRef]
- Arutyunov, V.; Nikitin, A.; Strekova, L.; Savchenko, V.; Sedov, I. Utilization of renewable sources of biogas for small-scale production of liquid fuels. Catal. Today 2021, 379, 23–27. [Google Scholar] [CrossRef]
- Wang, H.; Shao, C.; Gascon, J.; Takanabe, K.; Sarathy, S.M. Noncatalytic oxidative coupling of methane (OCM): Gas-phase reactions in a jet stirred reactor (JSR). ACS Omega 2021, 6, 33757. [Google Scholar] [CrossRef]
- Pässler, P.; Hefner, W.; Buckl, K.; Meinass, H.; Meiswinkel, A.; Wernicke, H.; Ebersberg, G.; Müller, R.; Bässler, J.; Behringer, H.; et al. Acetylene. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 11–12. [Google Scholar]
- Zhang, Q.; Wang, J.; Wang, T. Enhancing the acetylene yield from methane by decoupling oxidation and pyrolysis reactions: A comparison with the partial oxidation process. Ind. Eng. Chem. Res. 2016, 55, 8383. [Google Scholar] [CrossRef]
- Bryukov, M.G.; Palankoeva, A.S.; Belyaev, A.A.; Arutyunov, V.S. Partial oxidation of ethane in the temperature range 773–1023 K. Kinet. Catal. 2021, 62, 703–711. [Google Scholar] [CrossRef]
- Palankoeva, A.S.; Belyaev, A.A.; Arutyunov, V.S. Oxidative cracking of propane in a plug-flow laboratory reactor. Russ. J. Phys. Chem. B 2022, 16, 399–406. [Google Scholar] [CrossRef]
- Bryukov, M.G.; Belyaev, A.A.; Zakharov, A.A.; Arutyunov, V.S. Oxidation of rich propane mixtures in a temperature range of 773–1023 K at pressures of 1–3 atm. Kinet. Catal. 2022, 63, 653–659. [Google Scholar] [CrossRef]
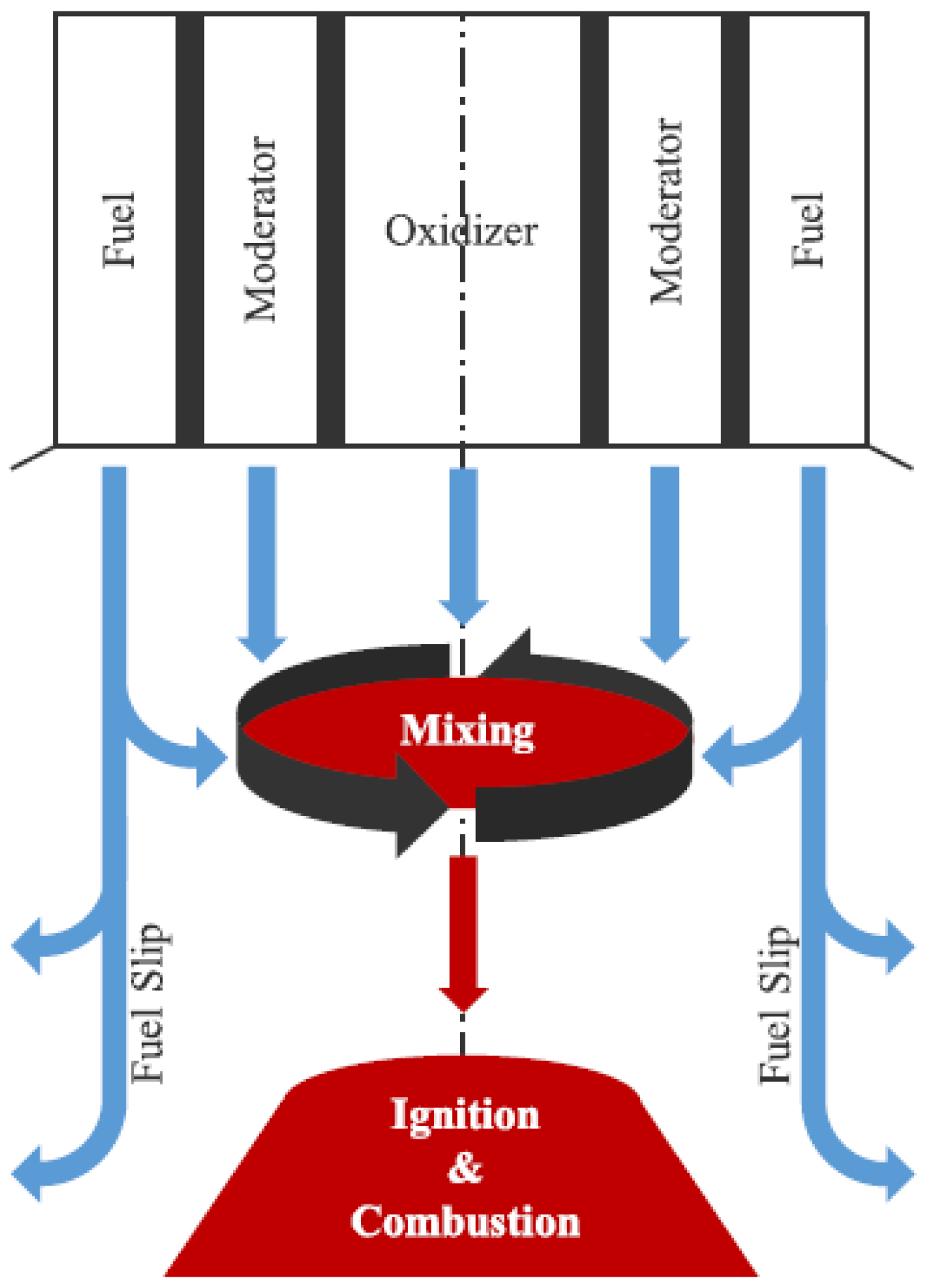
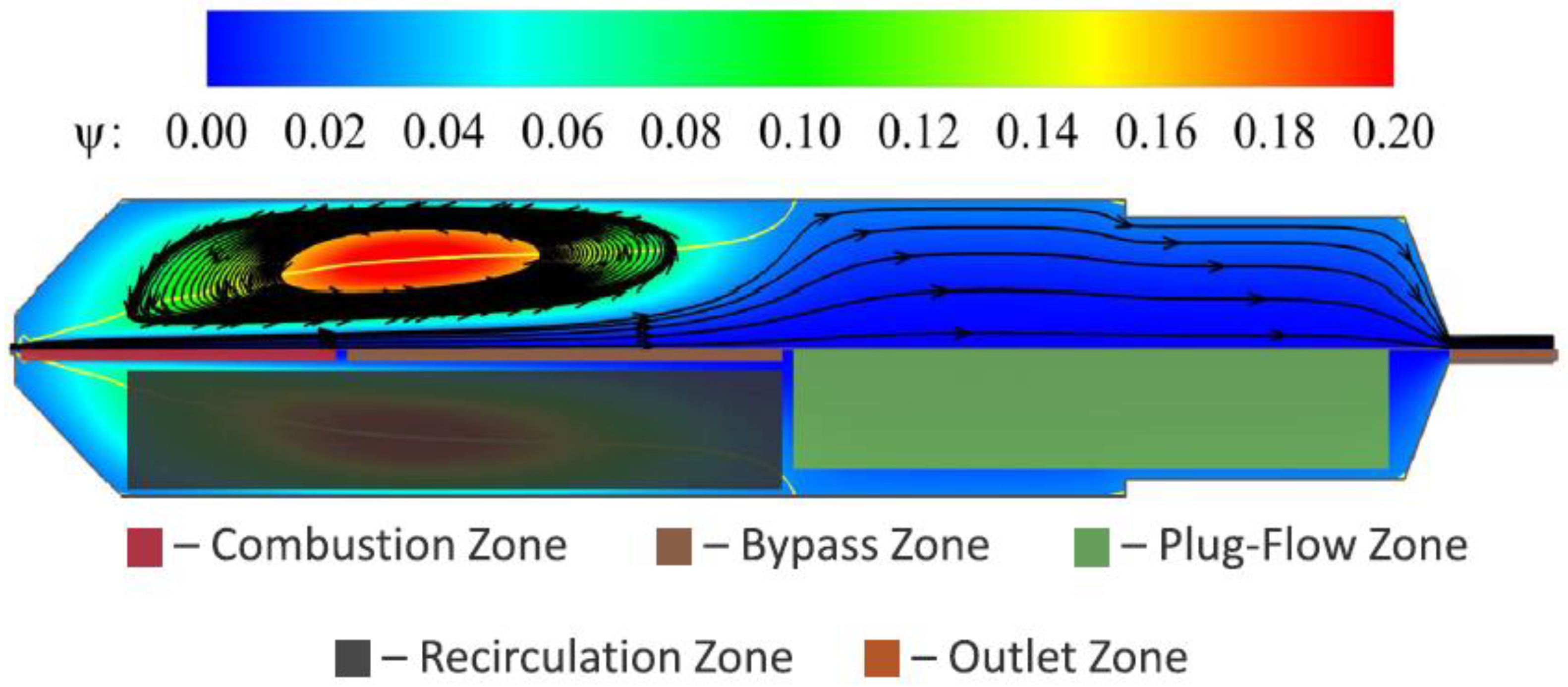
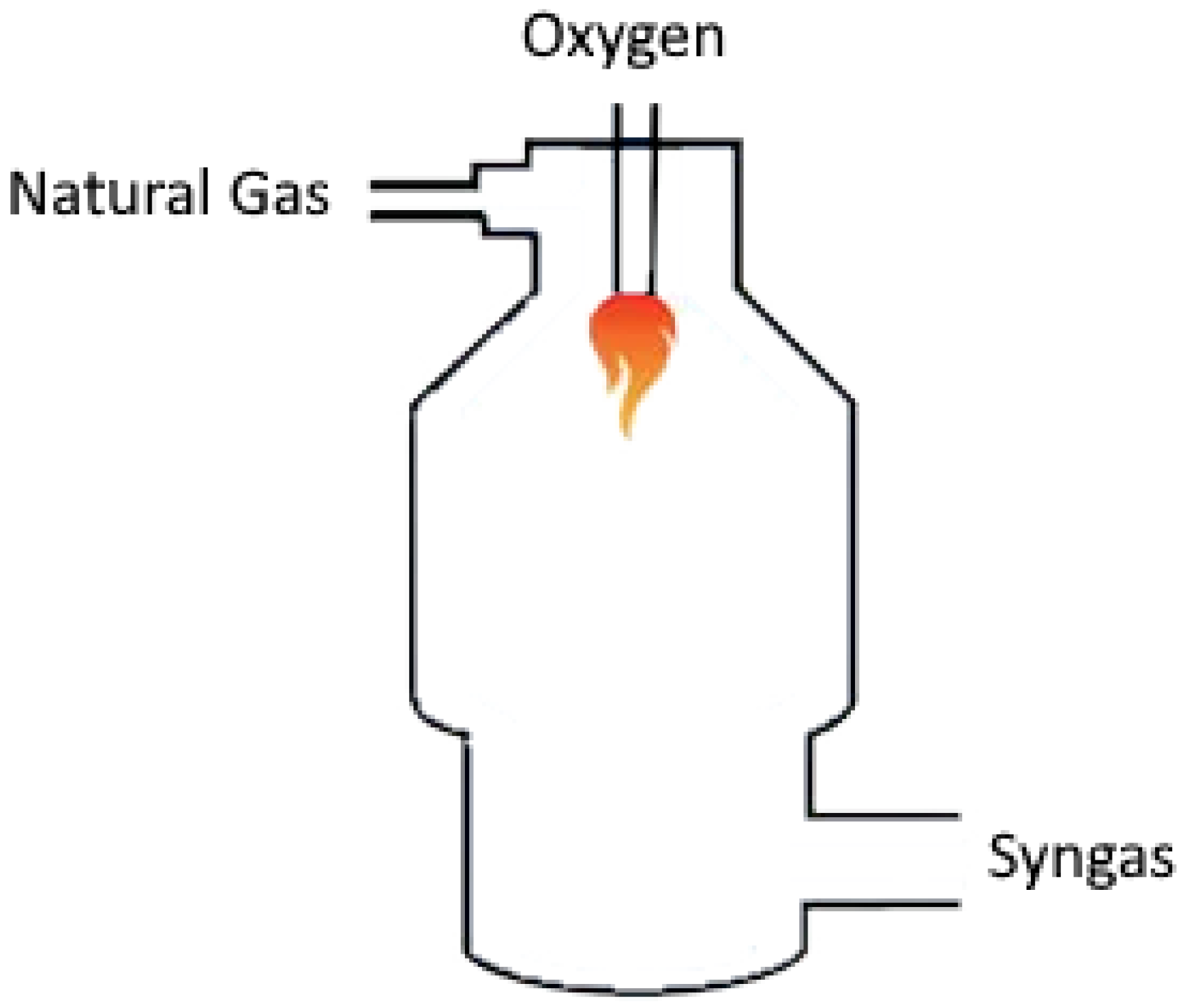
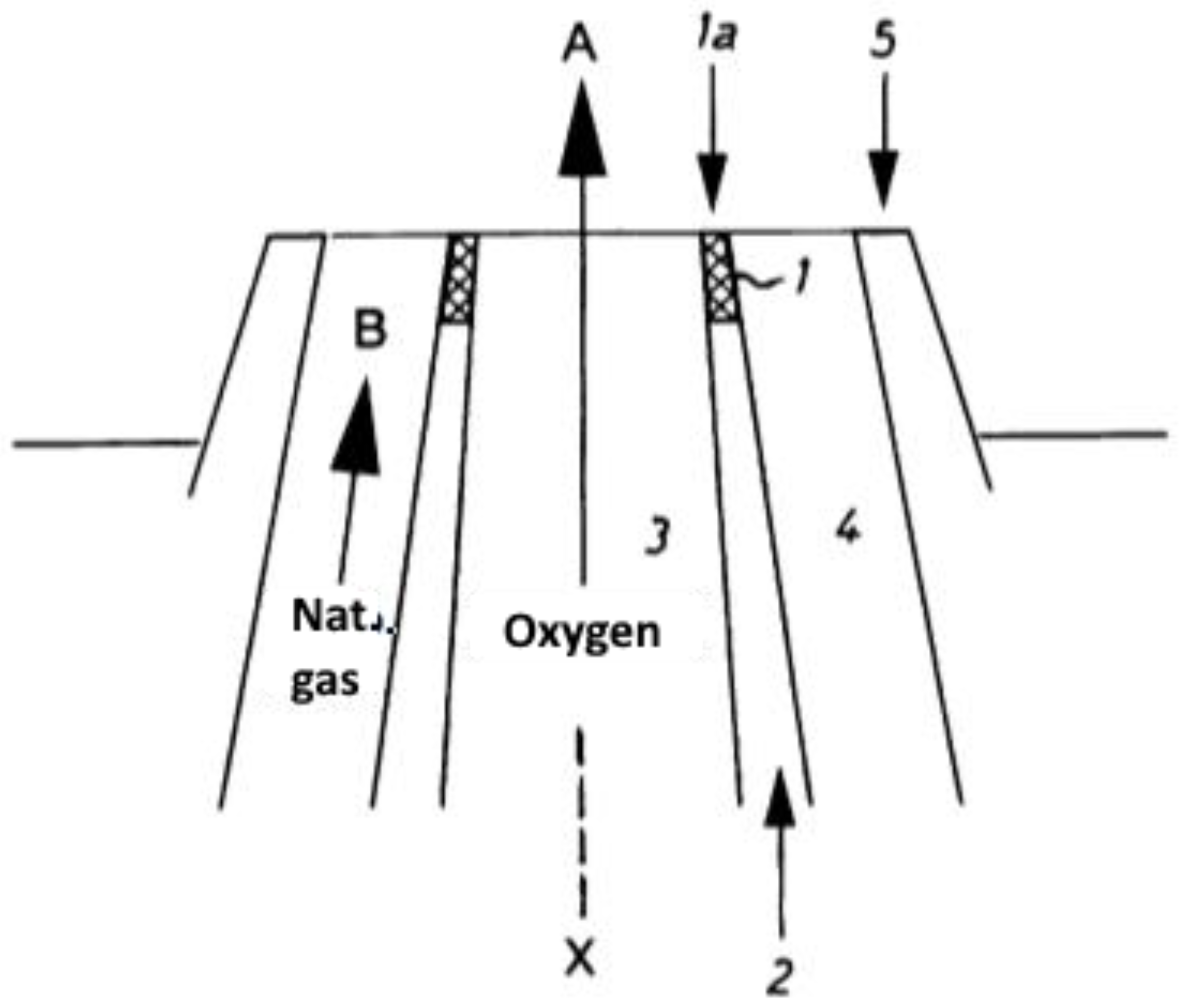
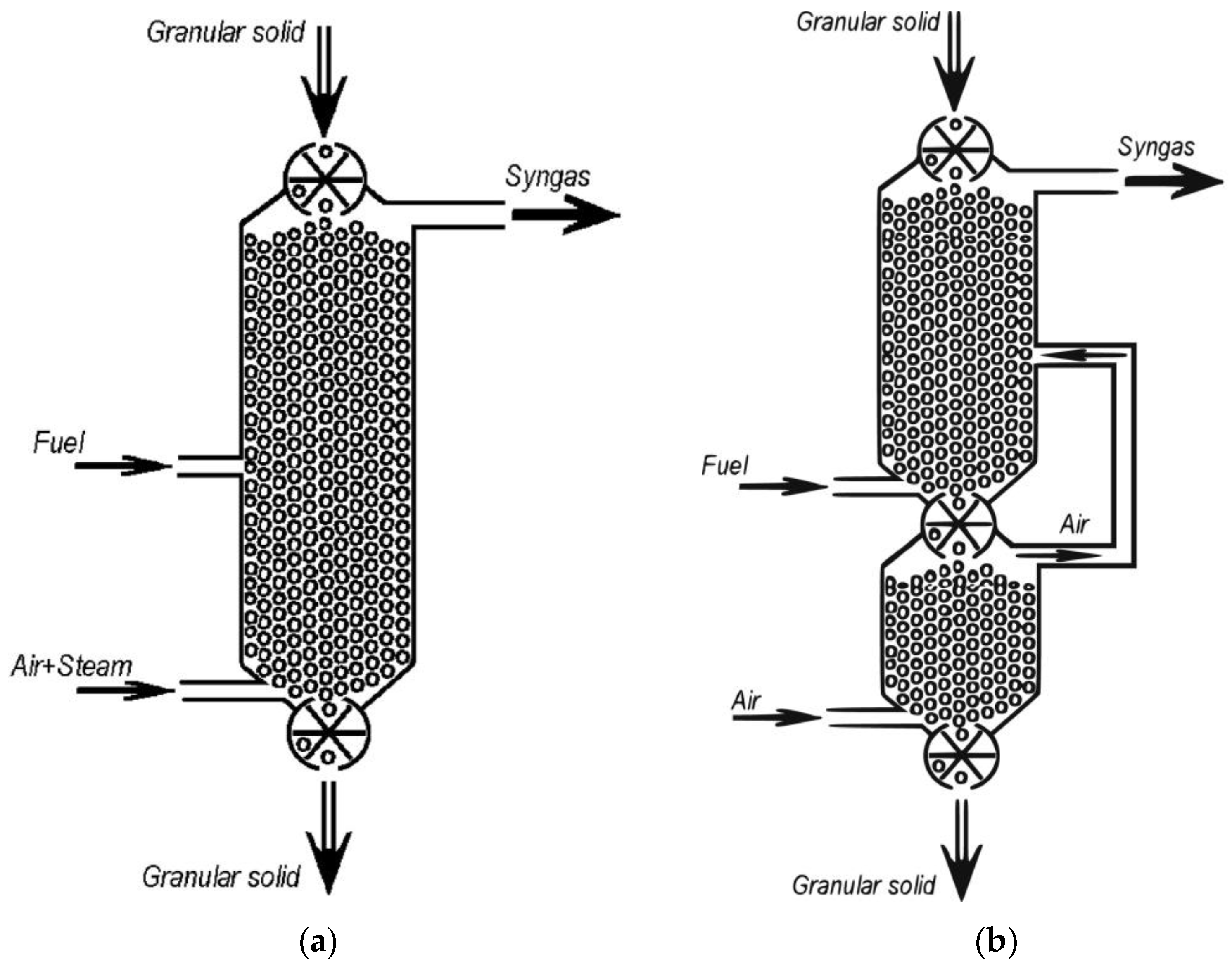
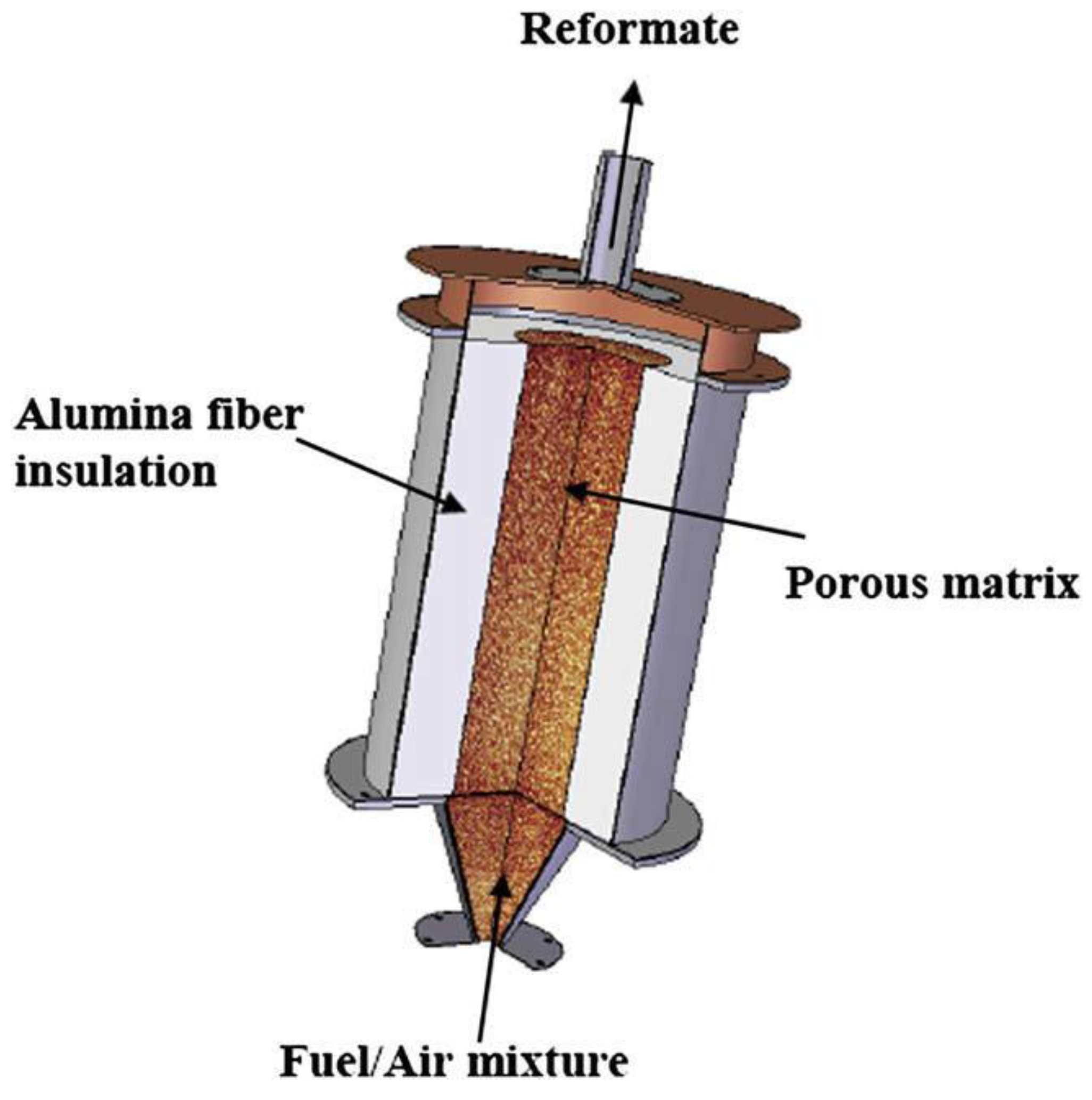

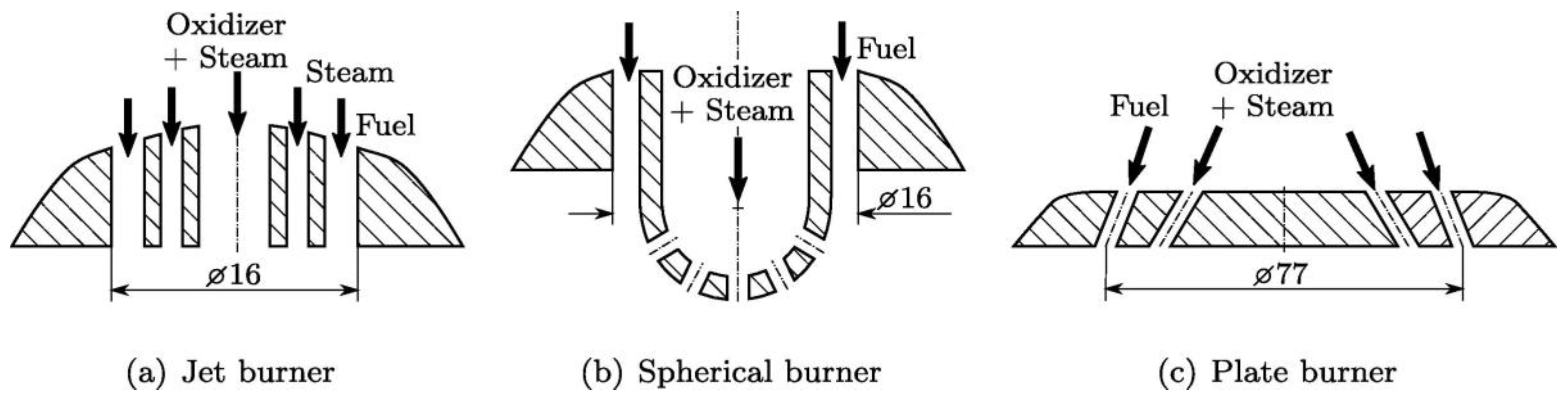
| Production Method | Temperature (°C) | Pressure (atm) | Molar Ratio H2/CO |
|---|---|---|---|
| SMR | 750–900 | 15–40 | 3–5 |
| ATR | 850–1000 | 20–40 | 1.6–2.65 |
| POX | 1200–1500 | 20–150 | 1.6–1.8 |
| Process | Feedstock | Thermal Efficiency |
|---|---|---|
| Steam reforming | Hydrocarbons | 70–85% |
| Partial oxidation | Hydrocarbons | 60–75% |
| Autothermal reforming | Hydrocarbons | 60–75% |
| Plasma reforming | Hydrocarbons | 9–85% |
| Electrolysis | H2O + electricity | 50–70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makaryan, I.A.; Salgansky, E.A.; Arutyunov, V.S.; Sedov, I.V. Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies 2023, 16, 2916. https://doi.org/10.3390/en16062916
Makaryan IA, Salgansky EA, Arutyunov VS, Sedov IV. Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies. 2023; 16(6):2916. https://doi.org/10.3390/en16062916
Chicago/Turabian StyleMakaryan, Iren A., Eugene A. Salgansky, Vladimir S. Arutyunov, and Igor V. Sedov. 2023. "Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review" Energies 16, no. 6: 2916. https://doi.org/10.3390/en16062916
APA StyleMakaryan, I. A., Salgansky, E. A., Arutyunov, V. S., & Sedov, I. V. (2023). Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies, 16(6), 2916. https://doi.org/10.3390/en16062916







