Metal-Supported Solid Oxide Fuel Cells: A Review of Recent Developments and Problems
Abstract
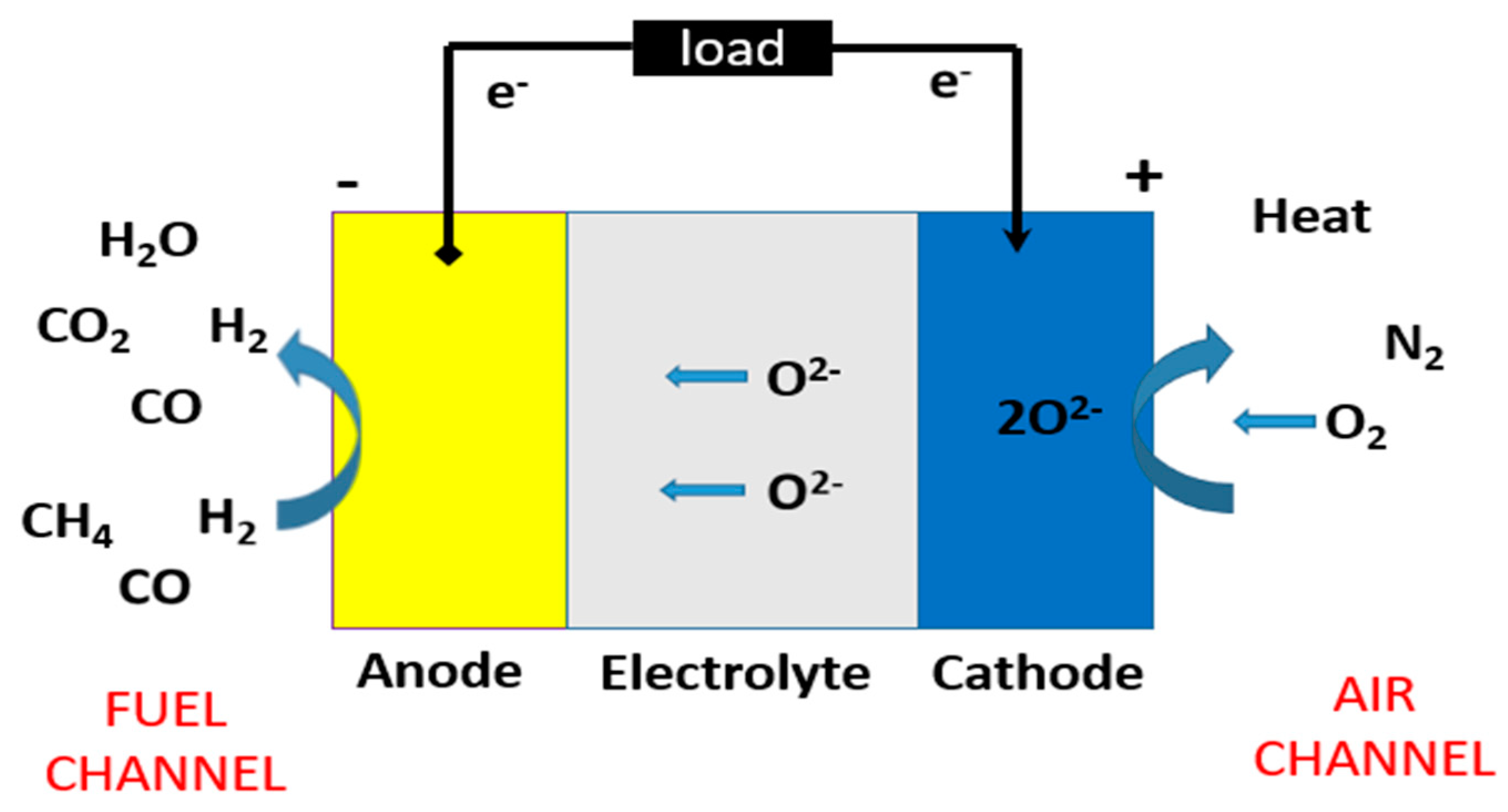
1. Introduction
- (a)
- Low cost. The use of metal substrates can lower material costs compared to ceramic-supported SOFCs, which can require expensive materials such as yttria-stabilized zirconia (YSZ).
- (b)
- Quick startup. The good thermal conductivity of the metal can make the MS-SOFC start up quickly, allowing it to be used in the mobile area.
- (c)
- Manufacturability. Stainless steel metal has good ductility, which greatly reduces the complexity of SOFC processing.
- (d)
- Ease of sealing. The use of proven technology for sealing metallic materials avoids the difficulties associated with sealing conventional SOFCs [9].
Types of MS-SOFCs
2. Materials of MS-SOFC
2.1. Substrate Materials
- Compatibility with other materials used in SOFCs, particularly during heating and cooling phases.
- High ability to resist oxidation.
- High ability to withstand thermal cycling.
- High electrical conductivity.
2.2. Anode Materials
- (1)
- High electrical conductivity: The anode material should exhibit a high level of electrical conductivity to facilitate efficient transportation of electrons from the active sites to the external circuit.
- (2)
- High activity for fuel oxidation: The anode material should have a high level of activity towards the oxidation of fuel, enabling effective electrochemical reactions.
- (3)
- Electrochemical activity: The anode material must be electrochemically active to catalyze the oxidation of the fuel. This activity is usually evaluated by measuring the anode’s electrochemical oxidation current density and comparing it with the theoretical current density calculated from the fuel’s chemical reaction kinetics.
- Gas transport: The anode material should have good gas transport properties to ensure efficient fuel supply and exhaust. This is particularly important for high-performance MS-SOFCs where the anode material should allow for rapid transport of fuel and waste gases while minimizing mass transport losses and reducing the likelihood of fuel starvation.
- Chemical stability: The anode material should have good chemical stability, meaning that it should not undergo any chemical reactions that could lead to its degradation or failure over time. This is particularly important in MS-SOFCs, where the anode is often exposed to harsh chemical and thermal environments.
- Microstructure: The anode material should have a well-defined microstructure with good porosity and tortuosity. This allows for efficient fuel distribution and diffusion while minimizing mass transport losses and ensuring high electrochemical activity.
- Adhesion to metal support: In MS-SOFCs, the anode material is deposited onto a metallic support. The anode material should have good adhesion to the metal support to prevent delamination and ensure mechanical stability during cell operation.
- Redox stability: The anode material should have good redox stability, meaning that it should be able to tolerate repeated changes in oxidation state without undergoing any significant changes in its microstructure or electrochemical activity. This is particularly important for MS-SOFCs that operate under cyclic or transient conditions [45].
2.3. Electrolyte Materials
2.4. Cathode Materials
3. Degradation Processes in MS-SOFC Electrodes
3.1. Carbon Deposition Problems
- -
- Reduced electrochemical activity: Carbon deposited on the anode surface can block the active sites and reduce the effective surface area available for electrochemical reactions, leading to decreased anode activity and cell performance.
- -
- Increased polarization resistance: Carbon deposition can increase the polarization resistance of the anode, making it more difficult for electrons to be transferred from the anode to the cathode, resulting in decreased cell performance.
- -
- Formation of carbonates: Carbon deposition can also lead to the formation of carbonates on the anode surface, which can further reduce the electrochemical activity and lead to reduced cell performance [102].
- -
- Several factors can contribute to carbon deposition in MS-SOFCs, including the fuel composition, operating temperature, and anode material properties. Strategies to mitigate carbon deposition in MS-SOFCs include:
- -
- Increasing the operating temperature: Raising the operating temperature can promote more complete oxidation of hydrocarbons at the anode surface and reduce the likelihood of carbon deposition.
- -
- Use of reforming catalysts: Catalysts can be used to promote more complete oxidation of hydrocarbons and reduce carbon deposition on the anode surface.
- -
- Optimization of fuel composition: The fuel composition can be optimized to reduce the concentration of hydrocarbons and other species that are prone to carbon deposition.
- -
- Anode design: The anode design can be optimized to promote a more uniform distribution of fuel and oxygen and reduce the likelihood of hot spots that can promote carbon deposition [91].
3.2. Anode Problems
3.3. Agglomeration and Enlargement of Nickel Particles
4. Cathode Problems
5. Problems with the Metal Support
6. Sealing Problems of MS-SOFC
- Thermal expansion mismatch: The metal substrate and the ceramic components of the fuel cell have different coefficients of thermal expansion, which can cause stress and cracking at the interface between the two materials. This can lead to gas leaks and reduced performance.
- Oxidation and corrosion: The high operating temperatures of MS-SOFCs can cause the oxidation and corrosion of the cell components, including the sealing materials. This can lead to gas leaks and reduced performance over time.
- Sealant compatibility: The sealing materials used in MS-SOFCs must be compatible with both the metal substrate and the ceramic components of the fuel cell. If the sealant is not compatible, it can degrade and fail, leading to gas leaks and reduced performance.
7. Future Perspectives and Problems
7.1. Future Perspectives
- Enhanced Electrode and Electrolyte Stability: The development of new materials for the electrode and electrolyte layers is essential to improve the stability of MS-SOFCs. Research is currently focused on developing new cathode materials with improved catalytic activity and stability under high-temperature and corrosive conditions. Additionally, new electrolyte materials with higher ionic conductivity and improved chemical stability are being developed.
- Low-Cost Substrates: The use of low-cost metal substrates such as iron and steel can significantly reduce the cost of MS-SOFCs. Research is ongoing to optimize the manufacturing process of MS-SOFCs and reduce the cost of the metal substrates.
- Integration with Renewable Energy Sources: MS-SOFCs can be integrated with renewable energy sources such as solar and wind power to provide a reliable and sustainable source of electricity. This technology has the potential to revolutionize the energy sector by providing clean and affordable energy to remote and off-grid areas.
- Miniaturization: MS-SOFCs can be miniaturized to create portable power devices for various applications such as laptops, mobile phones, and medical devices. The miniaturization of MS-SOFCs requires the development of new materials and manufacturing processes.
7.2. Future Problems
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bove, R. Recent trends in fuel cell science and technology. In Solid Oxide Fuel Cells: Rinciples, Designs and State-of-the-Art in Industries; Springer: Berlin/Heidelberg, Germany, 2007; pp. 267–285. [Google Scholar] [CrossRef]
- Minh, N.Q. Solid oxide fuel cell technology-features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Singhal, S.C.; Kendall, K. High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design, and Applications; Elsevier Advanced Technology: New York, NY, USA, 2003; p. 405. [Google Scholar]
- Yamamoto, O. Solid oxide fuel cells: Fundamental aspects and prospects. Electrochim. Acta. 2000, 45, 2423–2435. [Google Scholar] [CrossRef]
- Manohar, A.K.; Narayanan, S.R. Efficient generation of electricity from methane using high temperature fuel cells—status, challenges and prospects. Isr. J. Chem. 2014, 54, 1443–1450. [Google Scholar] [CrossRef]
- Adams, T.A.; Nease, J.; Tucker, D.; Barton, P.I. Energy conversion with solid oxide fuel cell systems: A review of concepts and outlooks for the short and long-term. Ind. Eng. Chem. Res. 2013, 52, 3089–3111. [Google Scholar] [CrossRef]
- Ansar, A.; Szabo, P.; Arnold, J.; Ilhan, Z.; Soysal, D.; Costa, R.; Zagst, A.; Gindrat, M.; Franco, T. Metal supported solid oxide fuel cells and stacks for auxiliary power units-progress, challenges and lessons learned. ECS Trans. 2011, 35, 147–155. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, B.H.; Kim, S.J.; Lee, Y.; Bae, H.; Choi, G.M. Micro solid oxide fuel cell fabricated on porous stainless steel: A new strategy for enhanced thermal cycling ability. Sci. Rep. 2016, 6, 22443. [Google Scholar] [CrossRef] [PubMed]
- Franco, T.; Haydn, M.; Mücke, R.; Weber, A.; Rüttinger, M.; Büchler, O.; Uhlenbruck, S.; Menzler, N.H.; Venskutonis, A.; Sigl, L.S. Development of metal-supported solid oxide fuel cells. ECS Trans. 2011, 35, 343–349. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Mougin, J.; Brevet, A.; Grenier, J.C.; Laucournet, R.; Larsson, P.O.; Montinaro, D.; Rodriguez-Martinez, L.M.; Alvarez, M.A.; Stange, M.; Trombert, S. Metal supported solid oxide fuel cells: From materials development to single cell performance and durability tests. ECS Trans. 2013, 57, 481–490. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemensø, T.; Ramousse, S.; Kromp, A.; Leonide, A.; Weber, A. Manufacturing and characterization of metal-supported solid oxide fuel cells. J. Power Sources 2011, 196, 7117–7125. [Google Scholar] [CrossRef]
- Tucker, M.C.; Carreon, B.; Charyasatit, J.; Langston, K.; Taylor, C.; Manjarrez, J.; Burton, N.; LaBarbera, M.; Jacobson, C.P. R&D and commercialization of metal-supported SOFC personal power products at point source power. ECS Trans. 2013, 57, 503–509. [Google Scholar] [CrossRef]
- Brandon, N.P.; Blake, A.; Corcoran, D. Development of metal supported solid oxide fuel cells for operation at 500–600 °C. J. Fuel Cell Sci. Technol. 2004, 1, 61–65. [Google Scholar] [CrossRef]
- McKenna, B.J.; Christiansen, N.; Schauperl, R.; Prenninger, P.; Nielsen, J.; Blennow, P.; Klemensø, T.; Ramousse, S.; Kromp, A.; Weber, A. Advances in Metal Supported Cells in the METSOFC EU Consortium. Fuel Cells 2013, 13, 592–597. [Google Scholar] [CrossRef]
- Oishi, N.; Yoo, Y. Fabrication of Cerium Oxide based SOFC having a Porous Stainless Steel Support: Cell Designs, Processing and Performance. ECS Trans. 2009, 25, 739–744. [Google Scholar] [CrossRef]
- Zhou, Y.; Xin, X.; Li, J.; Ye, X.; Xia, C.; Wang, S.; Zhan, Z. Performance and Degradation of Metal-Supported Solid Oxide Fuel Cells with Impregnated Electrodes. Int. J. Hydrogen Energy 2014, 39, 2279–2285. [Google Scholar] [CrossRef]
- Wang, R.; Byrne, C.; Tucker, M.C. Assessment of co-sintering as a fabrication approach for metal-supported proton-conducting solid oxide cells. Solid State Ion. 2019, 332, 25–33. [Google Scholar] [CrossRef]
- Tucker, M.C.; Lau, G.Y.; Jacobson, C.P. Performance of metal-supported SOFCs with infiltrated electrodes. J. Power Sources 2007, 171, 477–482. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.Y.; Tucker, M.C. Metal-supported solid oxide electrolysis cell (MS-SOEC) with significantly enhanced catalysis. Energy Technol. 2019, 7, 1801154. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.Y.; Karimaghaloo, A.; Lee, M.H.; Tucker, M.C. Progress in durability of metal-supported solid oxide fuel cells with infiltrated electrodes. J. Power Sources 2019, 437, 226935. [Google Scholar] [CrossRef]
- Kesler, O.; Cuglietta, M.; Harris, J.; Kuhn, J.; Marr, M.; Metcalfe, C. Progress in metal-supported SOFCs using hydrogen and methane fuels. ECS Trans. 2013, 57, 491–501. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kawabata, T.; Tachikawa, Y.; Matsuda, J.; Taniguchi, S.; Matsuzaki, Y.; Hayashi, A.; Sasaki, K. Preparation of Model SOFCs with Proton-conducting electrolyte on metal support using pulse laser deposition. ECS Trans. 2021, 103, 2033. [Google Scholar] [CrossRef]
- Krishnan, V.V. Recent developments in metal-supported solid oxide fuel cells. Wiley Interdiscip. Rev. 140 Energy Env. 2017, 6, 246–281. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Tucker, M.C.; Jacobson, C.P.; De Jonghe, L.C.; Visco, S.J. A braze system for sealing metal-supported solid oxide fuel cells. J. Power Sources 2006, 160, 1049–1057. [Google Scholar] [CrossRef]
- Tucker, M.C.; Sholklapper, T.Z.; Lau, G.Y.; De Jonghe, L.C.; Visco, S.J. Progress in metal-supported SOFCs. ECS Trans. 2009, 25, 673–680. [Google Scholar] [CrossRef]
- Antepara, I.; Villarreal, I.; Rodríguez-Martínez, L.M.; Lecanda, N.; Castro, U.; Laresgoiti, A. Evaluation of ferritic steels for use as interconnects and porous metal supports in IT-SOFCs. J. Power Sources 2005, 151, 103–107. [Google Scholar] [CrossRef]
- Matus, Y.B.; De Jonghe, L.C.; Jacobson, C.P.; Visco, S.J. Metal-supported solid oxide fuel cell membranes for rapid thermal cycling. Solid State Ion. 2004, 176, 443–449. [Google Scholar] [CrossRef]
- Haydn, M.; Ortner, K.; Franco, T.; Menzler, N.H.; Venskutonis, A.; Sigl, L.S. Development of Metal-Supported Solid Oxide Fuel Cells Based on a Powder-Metallurgical Manufacturing Route. Powder Metall. 2013, 56, 382–387. [Google Scholar] [CrossRef]
- Rose, L.; Kesler, O.; Decès-Petit, C.; Troczynski, T.; Maric, R. Characterization of porous stainless steel 430 for low and intermediate temperature solid oxide fuel cell (SOFC) substrates. Int. J. Green Energy 2009, 6, 638–645. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Z.; Liu, B.; Ding, D.; Jiang, Z. Development of three-layer intermediate temperature solid oxide fuel cells with direct stainless steel based anodes. Int. J. Hydrogen Energy 2012, 37, 4401–4405. [Google Scholar] [CrossRef]
- Molin, S.; Kusz, B.; Gazda, M.; Jasinski, P. Evaluation of porous 430L stainless steel for SOFC operation at intermediate temperatures. J. Power Sources 2008, 181, 31–37. [Google Scholar] [CrossRef]
- Jablonski, P.D.; Sears, J.S. The impact of alloy chemistry on the formation of a silicon-rich subscale on two classes of ferritic steels. J. Power Sources 2013, 228, 141–150. [Google Scholar] [CrossRef]
- Hwang, C.S.; Tsai, C.H.; Yu, J.F.; Chang, C.L.; Lin, J.M.; Shiu, Y.H.; Cheng, S.W. High Performance Metal-supported Intermediate Temperature Solid Oxide Fuel Cells Fabricated by Atmospheric Plasma Spraying. J. Power Sources 2011, 196, 1932–1939. [Google Scholar] [CrossRef]
- Sarasketa-Zabala, E.; Otaegi, L.; Rodriguez-Martinez, L.M.; Alvarez, M.A.; Burgos, N.; Castro, F. High temperature stability of porous metal substrates under highly humidified hydrogen conditions for metal supported Solid Oxide Fuel Cells. Solid State Ion. 2012, 222, 16–22. [Google Scholar] [CrossRef]
- Bischof, C.; Nenning, A.; Malleier, A.; Martetschlager, L.; Gladbach, A.; Schafbauer, W.; Opitz, A.K.; Bram, M. Microstructure optimization of nickel/gadolinium-doped ceria anodes as key to significantly increasing power density of metal supported solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 31475–31487. [Google Scholar] [CrossRef]
- Yang, S.F.; Shie, Z.J.; Hwang, C.S.; Tsai, C.H.; Chang, C.L.; Huang, T.J.; Lee, R.Y. Ni-Mo porous alloy fabricated as supporting component for metal-supported solid oxide fuel cell and cell performance. ECS Trans. 2015, 68, 1849–1855. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Jia, L.; Zhang, Q.; Jiang, S.P.; Chi, B.; Yan, D. Porous Ni-Fe alloys as anode support for intermediate temperature solid oxide fuel cells: I. Fabrication, redox and thermal behaviors. J. Power Sources 2015, 277, 474–479. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Usoltsev, V.V.; Fedorova, Y.E.; Sobyanin, V.A.; Kalinin, P.V.; Arzhannikov, A.V.; Vlasov AYu Korobeinikov, M.V.; Bryazgin, A.A.; Salanov, A.N.; Predtechenskii, M.R.; et al. Design of Medium-Temperature Solid Oxide Fuel Cells on Porous Supports of Deformation Strengthened Ni-Al Alloy. Russ. J. Electrochem. 2011, 47, 488–493. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Rabotkin, S.V.; Shipilova, A.V.; Kirdyashkin, A.I.; Ionov, I.V.; Kovalchuk, A.N.; Maznoy, A.S.; Kitler, V.D.; Borduleva, A.O. Solid Oxide Fuel Cell with Ni-Al Support. Int. J. Hydrogen Energy 2015, 40, 14077–14084. [Google Scholar] [CrossRef]
- Liu, M.; Finlayson, T.R.; Smith, T.F.; Tanner, L.E. Martensite precursor observations using thermal expansion: Ni-Al. Mater. Sci. Eng. 1992, 157, 225–232. [Google Scholar] [CrossRef]
- Johnson, J.; Qu, J. Effective modulus and coefficient of thermal expansion of Ni-YSZ porous cermets. J. Power Sources 2008, 181, 85–92. [Google Scholar] [CrossRef]
- Windes, W.E.; Zuck, L.D.; Shaber, E.L.; Erickson, A.E.; Lessing, P.A. A Low TEC Intermetallic bipolar plate. Proc. Electrochem. Soc. 2003, 7, 879–887. [Google Scholar] [CrossRef]
- Cowin, P.I.; Petit, C.T.; Lan, R.; Irvine, J.T.; Tao, S. Recent progress in the development of anode materials for solid oxide fuel cells. Adv. Energy Mater. 2011, 1, 314–332. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Nielsen, J.; Persson, Å.H.; Sudireddy, B.R.; Irvine, J.T.; Thydén, K. Infiltrated La0.4Sr0.4Fe0.03Ni0.03Ti0.94O3 based anodes for all ceramic and metal supported solid oxide fuel cells. J. Power Sources 2017, 372, 99–106. [Google Scholar] [CrossRef]
- Haydn, M.; Ortner, K.; Franco, T.; Uhlenbruck, S.; Menzler, N.H.; Stöver, D. Multi-layer thin-film electrolytes for metal supported solid oxide fuel cells. J. Power Sources 2014, 256, 52–60. [Google Scholar] [CrossRef]
- Paek, J.Y.; Chang, I.; Park, J.H.; Ji, S.; Cha, S.W. A study on properties of yttrium stabilized zirconia thin films fabricated by different deposition techniques. Renew. Energy 2014, 65, 202–206. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhou, Y.; Wang, S.; Liu, X.; Meng, X.; Wen, T. Nanostructure Electrodes for Metal-Supported Solid Oxide Fuel Cells. ECS Trans. 2013, 57, 925–931. [Google Scholar] [CrossRef]
- Liu, X.; Han, D.; Zhou, Y.; Meng, X.; Wu, H.; Li, J.; Zeng, F.; Zhan, Z. Sc-substituted Ln0.6Sr0. 4FeO3− δ mixed conducting oxides as promising electrodes for symmetrical solid oxide fuel cells. J. Power Sources 2014, 15, 457–463. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhan, Z.; Wang, S. Metal-Supported Solid Oxide Fuel Cells with Impregnated Electrodes. ECS Trans. 2013, 57, 877–883. [Google Scholar] [CrossRef]
- Sudireddy, B.R.; Nielsen, J.; Thydén KT, S.; Persson, A.H.; Brodersen, K. Investigation of Novel Electrocatalysts for Metal Supported Solid Oxide Fuel Cells–Ru:GDC. ECS Trans. 2015, 68, 1417–1426. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, S.J.; Choi, G.M. Y0.08Sr0.88TiO3-CeO2 composite as a diffusion barrier layer for stainless-steel supported solid oxide fuel cell. J. Power Sources 2016, 307, 385–390. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrogen Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Thaler, F.; Nenning, A.; Bischof, C.; Udomsilp, D.; de Haart, L.G.J.; Opitz, A.K.; Bram, M. Optimized Cell Processing as the Key of High Electrochemical Performance of Metal Supported Solid Oxide Fuel Cells. ECS Trans. 2019, 91, 887–900. [Google Scholar] [CrossRef]
- Liu, J.; Briss, V.; Hill, J. Electrochemical performance and microstructure characterization of nickel yttrium-stabilized zirconia anode. AIChE J. 2010, 56, 1651–1656. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Huang, Y.H. Alternative anode materials for solid oxide fuel cells. J. Power Sources 2007, 173, 1–10. [Google Scholar] [CrossRef]
- Koide, H.; Someya, Y.; Yoshida, T.; Maruyama, T. Properties of Ni/YSZ cermet as anode for SOFC. Solid State Ion. 2001, 132, 253–260. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Yuan, C.; Li, J.; Xia, C.; Zhan, Z.; Wang, S. Metal-supported solid oxide fuel cells with in-situ sintered (Bi2O3)0.7(Er2O3)0.3-Ag composite cathode. Int. J. Hydrogen Energy 2013, 38, 16579–16583. [Google Scholar] [CrossRef]
- Udomsilp, D.; Thaler, F.; Menzler, N.H.; Bischof, C.; de Haart, L.G.J.; Opitz, A.K.; Guillon, O.; Bram, M. Dual-Phase Cathodes for Metal-Supported Solid Oxide Fuel Cells: Processing, Performance, Durability. J. Electrochem. Soc. 2019, 166, F506–F510. [Google Scholar] [CrossRef]
- Jordan, N.; Assenmacher, W.; Uhlenbruck, S.; Haanappel, V.A.C.; Buchkremer, H.P.; Stöver, D.; Mader, W. Ce0.8Gd0.2O2−δ protecting layers manufactured by physical vapor deposition for IT-SOFC. Solid State Ionics. 2008, 179, 919–923. [Google Scholar] [CrossRef]
- Fergus, J.W. Electrolytes for solid oxide fuel cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Badwal, S.P. Oxygen-ion conducting electrolyte materials for solid oxide fuel cells. Ionics 2000, 6, 1–21. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, C.; Chen, T.; Meng, X.; Ye, X.; Li, J. Evaluation of Ni and Ni-Ce0.8Sm0.2O2- δ (SDC) impregnated 430L anodes for metal-supported solid oxide fuel cells. J. Power Sources 2014, 267, 117–122. [Google Scholar] [CrossRef]
- Ralph, J.M. Materials for lower temperature solid oxide fuel cell. J. Mater. Sci. 2001, 36, 1161–1172. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Kharton, V.V. Electrode materials and reaction mechanisms in solid oxide fuel cells: A brief review. III. Recent trends and selected methodological aspects. J. Solid State Electrochem. 2011, 15, 1007–1040. [Google Scholar] [CrossRef]
- Figueiredo, F.M.; Marques, M.B. Electrolytes for Solid Oxide Fuel Cellls. WREs Energy Environ. 2013, 2, 52–72. [Google Scholar] [CrossRef]
- Molin, S.; Tolczyk, M.; Gazda, M.; Jasinski, P. Stainless steel yttria stabilized zirconia composite supported solid oxide fuel cell. J. Fuel Cell Sci. Technol. 2011, 8, 1–5. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Zvonareva, I.A.; Tarasova, N.A.; Bi, L.; Medvedev, D.A.; Shao, Z. Electrolyte materials for protonic ceramic electrochemical cells: Main limitations and potential solutions. Mater. Rep. Energy 2022, 2, 100158. [Google Scholar] [CrossRef]
- Cervera, R.B.; Oyama, Y.; Miyoshi, S.; Oikawa, I.; Takamura, H.; Yamaguchi, S. Nanograined Sc-doped BaZrO3 as a proton conducting solid electrolyte for intermediate temperature solid oxide fuel cells (IT-SOFCs). Solid State Ion. 2014, 264, 1–6. [Google Scholar] [CrossRef]
- Zając, W.; Rusinek, D.; Zheng, K.; Molenda, J. Applicability of Gd-doped BaZrO3, SrZrO3, BaCeO3 and SrCeO3 proton conducting perovskites as electrolytes for solid oxide fuel cells. Open Chem. 2013, 11, 471–484. [Google Scholar] [CrossRef]
- Qu, E.; Hao, X.; Xiao, M.; Han, D.; Huang, S.; Huang, Z.; Meng, Y. Proton exchange membranes for high temperature proton exchange membrane fuel cells: Challenges and perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]
- Selim, A.; Szijjártó, G.P.; Románszki, L.; Tompos, A. Development of WO3–Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation. Polymers 2022, 14, 2492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Meng, X.; Liu, X.; Pan, X.; Li, J.; Ye, X. Novel architectured metal-supported solid oxide fuel cells with Mo-doped SrFeO3-δ electrocatalysts. J. Power Sources 2014, 267, 128–135. [Google Scholar] [CrossRef]
- Klemensø, T.; Nielsen, J.; Blennow, P.; Persson, A.H.; Stegk, T.; Christensen, B.H. High performance metal-supported solid oxide fuel cells with Gd-doped ceria barrier layers. J. Power Sources 2011, 196, 9459–9466. [Google Scholar] [CrossRef]
- Franco, T.; Schibinger, K.; Ilhan, Z.; Schiller, G.; Venskutonis, A. Ceramic Diffusion Barrier Layers for Metal Supported SOFCs. ECS Trans. 2007, 7, 771–780. [Google Scholar] [CrossRef]
- Rüttinger, M.; Mücke, R.; Franco, T.; Büchler, O.; Menzler, N.H.; Venskutonis, A. Metal-Supported Cells with Comparable Performance to Anode-Supported Cells in Short-Term Stack Environment. ECS Trans. 2011, 35, 259–268. [Google Scholar] [CrossRef]
- Macwan, A.; Chen, D.L.; Marr, M.; Kesler, O. Residual stresses in suspension plasma sprayed electrolytes in metal-supported solid oxide fuel half cells. J. Power Sources 2013, 221, 397–405. [Google Scholar] [CrossRef]
- Gelfond, N.V.; Bobrenok, O.F.; Predtechensky, M.R.; Morozova, N.B.; Zherikova, K.V.; Igumenov, I.K. Chemical vapor deposition of thin films of electrolytes based on zirconium oxide stabilized with yttrium oxide. Inorg. Mater. 2009, 45, 718–725. [Google Scholar] [CrossRef]
- Szabo, P.; Arnold, J.; Franco, T.; Gindrat, M.; Refke, A.; Zagst, A.; Ansar, A. Progress in the Metal Supported Solid Oxide Fuel Cells and Stacks for APU. ECS Trans. 2009, 25, 175–185. [Google Scholar] [CrossRef]
- Kurokawa, H.; Lau, G.Y.; Jacobson, C.P.; De Jonghe, L.C.; Visco, S.J. Water-based binder system for SOFC porous steel substrate. J. Mater. Porc. Technol. 2007, 182, 469–4760. [Google Scholar] [CrossRef]
- Yoon, S.P. Performance of anode-supported solid oxide fuel cell with La0.85Sr0.15MnO3 cathode modified by sol-gel coating technique. J. Power Sources 2002, 106, 160–166. [Google Scholar] [CrossRef]
- Sønderby, S.; Aijaz, A.; Helmersson, U.; Sarakinos, K.; Eklund, P. Deposition of yttria-stabilized zirconia thin films by high power impulse magnetron sputtering and pulsed magnetron sputtering. Surf. Coat. Technol. 2014, 240, 1–6. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, L.M.; Otaegi, L.; Alvarez, M.; Rivas, M.; Gomez, N.; Zabala, A.; Arizmendiarrieta, I.; Antepara, A.; Urriolabeitia, M.; Olave, I.; et al. Degradation Studies on Tubular Metal Supported SOFC Cell Designs, Processing and Performance. ECS Trans. 2009, 25, 745–752. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Richter, J.; Holtappels, P.; Graule, T.; Nakamura, T.; Gauckler, L.J. Materials design for perovskite SOFC cathodes. Mon. Chem. Chem. Mon. 2009, 140, 985–999. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Li, J.; Nie, H.; Ye, X.; Wang, S.; Zhan, Z. Novel metal-supported solid oxide fuel cells with impregnated symmetric La0.6Sr0.4Fe0.9Sc0.1O3-d electrodes. J. Power Sources 2014, 252, 164–168. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, D.; Yuan, C.; Liu, M.; Chen, T.; Wang, S. Infiltrated SmBa0.5Sr0.5Co2O5+δ cathodes for metal-supported solid oxide fuel cells. Electrochim. Acta 2014, 149, 231–236. [Google Scholar] [CrossRef]
- Yu, R. Quantitative assessment of anode contribution to cell degradation under various polarization conditions using industrial size planar solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 2429–2435. [Google Scholar] [CrossRef]
- Kim, S.H. Effect of Water Vapor and SOx in Air on the Cathodes of Solid Oxide Fuel Cells. Symposium R–Life-Cycle Analysis for New Energy Conversion and Storage Systems. MRS Online Proc. Libr. 2007, 1041, R03–R10. [Google Scholar] [CrossRef]
- Baek, S.W.; Jeong, J.; Kim, Y.M.; Kim, J.H.; Shin, S.; Bae, J. Metal-supported solid oxide fuel cells with barium-containing in-situ cathodes. Solid State Ion. 2011, 192, 387–393. [Google Scholar] [CrossRef]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental understanding and modeling of spin coating process: A review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim-Lohsoontorn, P.; Bae, J. Effect of unsintered gadolinium-doped ceria buffer layer on performance of metal-supported solid oxide fuel cells using unsintered barium strontium cobalt ferrite cathode. J. Power Sources 2010, 195, 64207. [Google Scholar] [CrossRef]
- Han, D.; Wu, H.; Li, J.; Wang, S.; Zhan, Z. Nanostructuring of SmBa0.5Sr0.5Co2O5+δ cathodes for reduced-temperature solid oxide fuel cells. J. Power Sources 2014, 246, 409–416. [Google Scholar] [CrossRef]
- Ni, D.W.; Esposito, V. Densification of Ce0.9Gd0.1O1.95 barrier layer by in-situ solid state reaction. J. Power Sources 2014, 266, 393–400. [Google Scholar] [CrossRef]
- Sønderby, S.; Klemensø, T.; Christensen, B.H.; Almtoft, K.P.; Lu, J.; Nielsen, L.P.; Eklund, P. Magnetron sputtered gadolinia-doped ceria diffusion barriers for metal supported solid oxide fuel cells. J. Power Sources 2014, 267, 452–458. [Google Scholar] [CrossRef]
- Shen, Y. Preparation of Nanocomposite GDC/LSCF Cathode Material for IT-SOFC by Induction Plasma Spraying. J. Therm. Spray Technol. 2011, 20, 145–153. [Google Scholar] [CrossRef]
- Blennow, P.; Klemensø, T.; Nielsen, J.; Persson, A.H.; Stegk, T.; Hjalmarsson, P. Development of Long-term Stable and High-performing Metal-supported SOFCs. ECS Trans. 2011, 35, 369–378. [Google Scholar] [CrossRef]
- Bae, J.; Lee, C. Fabrication and characterization of metal-supported solid oxide fuel cells. J. Power Sources 2008, 176, 62–69. [Google Scholar] [CrossRef]
- Li, J. High-performance and stable La0.8Sr02Fe0.9Nb0.1O3-δ anode for direct carbon solid oxide fuel cells fueled by activated carbon and corn straw derived carbon. Int. J. Hydrogen Energy 2018, 43, 12358–12367. [Google Scholar] [CrossRef]
- Hagen, A. Effect of Humidity in Air on Performance and Long-Term Durability of SOFCs. ECS Trans. 2009, 25, 439–446. [Google Scholar] [CrossRef]
- Nielsen, J. Effect of cathode gas humidification on performance and durability of Solid Oxide Fuel Cells. Solid State Ion. 2010, 181, 517–524. [Google Scholar] [CrossRef]
- Yang, Z. A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Y.; Gong, J.; Chen, Y.; Zhang, Z. Preparation of Ni/YSZ materials for SOFC anodes by buffer-solution method. Mater. Sci. Eng. B 2001, 86, 119–122. [Google Scholar] [CrossRef]
- Bronin, D. Nature of Electrode Degradation in SOFCs. In Proceedings of the 8th European Solid Oxide Fuel Cell Forum, Lucerne, Switzerland, 30 June–3 July 2008; p. B1001. [Google Scholar]
- Hagen, A. Degradation of anode supported SOFCs as a function of temperature and current load. J. Electrochem. Soc. 2006, 153, A1165–A1171. [Google Scholar] [CrossRef]
- He, Z. Cyclic polarization enhances the operating stability of La0.57Sr0.38Co0.18Fe0.72Nb0.1O3-δ oxygen electrode of reversible solid oxide cells. J. Power Sources 2018, 404, 73–80. [Google Scholar] [CrossRef]
- Hilpert, K.; Das, D.; Miller, M.; Peck, D.H.; Weiß, R. Chromium vapor species over solid oxide fuel cell interconnect materials and their potential for degradation processes. J. Electrochem. Soc. 1996, 143, 3642–3647. [Google Scholar] [CrossRef]
- Williams, M.C. Solid Oxide Fuel Cells: Fundamentals to Systems. Fuel Cells 2007, 1, 78–85. [Google Scholar] [CrossRef]
- Simwonis, D. Nickel coarsening in annealed Ni/8YSZ anode substrates for solid oxide fuel cells. Solid State Ion. 2000, 132, 241–251. [Google Scholar] [CrossRef]
- Tanasini, P. Experimental and theoretical investigation of degradation mechanisms by particle coarsening in SOFC Electrodes. Fuel Cells 2009, 9, 740–752. [Google Scholar] [CrossRef]
- Jiang, S.P. Sintering behavior of Ni/Y2O3-ZrO2 cermet electrodes of solid oxide fuel. J. Mater. Sci. 2003, 38, 3775–3782. [Google Scholar] [CrossRef]
- Matsui, T. Comparative study on performance stability of Ni–oxide cermet anodes under humidified atmospheres in solid oxide fuel cells. J. Electrochem. Soc. 2012, 159, F456–F460. [Google Scholar] [CrossRef]
- Faes, A. Nickel–zirconia anode degradation and triple phase boundary quantification from microstructural analysis. Fuel Cells 2009, 9, 841–851. [Google Scholar] [CrossRef]
- Pihlatie, M.H. Electrical conductivity of Ni–YSZ composites: Degradation due to Ni particle growth. Solid State Ion. 2011, 189, 82–90. [Google Scholar] [CrossRef]
- De Angelis, S.; Jørgensen, P.S.; Tsai, E.H.; Holler, M.; Kreka, K.; Bowen, J.R. Three dimensional characterization of nickel coarsening in solid oxide cells via ex-situ ptychographic nano-tomography. J. Power Sources 2018, 383, 72–79. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Bae, J. Investigation of mixed conducting cathode for metal-supported SOFC. In Proceedings of the ASME 2009 7th International Conference on Fuel Cell Science, Engineering and Technology, Newport Beach, CA, USA, 8–10 June 2009; pp. 847–849. [Google Scholar] [CrossRef]
- Joo, J.H.; Choi, G.M. Simple fabrication of micro-solid oxide fuel cell supported on metal substrate. J. Power Sources 2008, 182, 589–593. [Google Scholar] [CrossRef]
- Park, Y.M.; Kim, J.H.; Kim, H. Effects of a current treatment for an in-situ sintered cathode in a Ni-supported solid oxide fuel cell. Int. J. Hydrogen Energy 2012, 37, 555–565. [Google Scholar] [CrossRef]
- Esposito, V.; Traversa, E.; Wachsman, E.D. Pb2Ru2O6.5 as a low-temperature cathode for bismuth oxide electrolytes. J. Electrochem. Soc. 2005, 152, A2300–A2305. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hwang, C.S.; Chang, C.L.; Wu, S.H.; Lin, H.H.; Shiu, W.H.; Lin, J.K.; Yang, S.F.; Fu, C.Y.; Yang, C.S. Performances of plasma sprayed metal-supported solid oxide fuel cell and stack. Fuel Cells 2018, 18, 800–808. [Google Scholar] [CrossRef]
- Baek, S.W.; Jeong, J.; Kim, J.H.; Lee, C.; Bae, J. Interconnect-integrated solid oxide fuel cell with high temperature sinter-joining process. Int. J. Hydrogen Energy 2010, 35, 11878–11889. [Google Scholar] [CrossRef]
- Shen, F.; Wang, R.; Tucker, M.C. Long term durability test and post mortem for metal-supported solid oxide electrolysis cells. J. Power Sources 2020, 474, 228618. [Google Scholar] [CrossRef]
- Lee, K.; Kang, J.; Lee, J.; Lee, S.; Bae, J. Evaluation of metal-supported solid oxide fuel cells (MS-SOFCs) fabricated at low temperature (∼1000 °C) using wet chemical coating processes and a catalyst wet impregnation method. Int. J. Hydrogen Energy 2018, 43, 3786–3796. [Google Scholar] [CrossRef]
- Choi, J.-J.; Lee, J.-H.; Park, D.-S.; Hahn, B.-D.; Yoon, W.-H.; Lin, H.-T. Oxidation Resistance Coating of LSM and LSCF on SOFC Metallic Interconnects by the Aerosol Deposition Process. J. Am. Ceram. Soc. 2007, 90, 1926–1929. [Google Scholar] [CrossRef]
- Konysheva, E.; Penkalla, H.; Wessel, E.; Mertens, J.; Seeling, U.; Singheiser, L.; Hilpert, K. Chromium poisoning of perovskite cathodes by the ODS alloy Cr5Fe1Y2O3 and the high chromium ferritic steel Crofer22APU. J. Electrochem. Soc. 2006, 153, A765–A773. [Google Scholar] [CrossRef]
- Talic, B.; Falk-Windisch, H.; Venkatachalam, V.; Hendriksen, P.V.; Wiik, K.; Lein, H.L. Effect of coating density on oxidation resistance and Cr vaporization from solid oxide fuel cell interconnects. J. Power Sources 2017, 354, 57–67. [Google Scholar] [CrossRef]
- Roehrens, D.; Packbier, U.; Fang, Q.; Blum, L.; Sebold, D.; Bram, M.; Menzler, N. Operation of Thin-Film Electrolyte Metal-Supported Solid Oxide Fuel Cells in Lightweight and Stationary Stacks: Material and Microstructural Aspects. Materials 2016, 9, 762. [Google Scholar] [CrossRef]
- Brandner, M.; Bram, M.; Froitzheim, J.; Buchkremer, H.P.; Stöver, D. Electrically Conductive diffusion barrier layers for metal-supported SOFC. Solid State Ion. 2008, 179, 1501–1504. [Google Scholar] [CrossRef]
- Brylewski, T.; Nanko, M.; Maruyama, T.; Przybylski, K. Application of Fe-16Cr ferritic alloy to interconnector for a solid oxide fuel cell. Solid State Ion. 2001, 143, 131–150. [Google Scholar] [CrossRef]
- Yang, Z.; Hardy, J.S.; Walker, M.S.; Xia, G.; Simner, S.P.; Stevenson, J.W. Structure and conductivity of thermally grown scales on ferritic Fe-Cr-Mn steel for SOFC interconnect application. J. Electrochem. Soc. 2004, 151, A1825. [Google Scholar] [CrossRef]
- Bertoldi, M.; Zandonella, T.; Montinaro, D.; Sglavo, V.M.; Fossati, A.; Lavacchi, A.; Giolli, C.; Bardi, U. Protective coatings of metallic interconnects for IT-SOFC application. J. Fuel Cell Sci. Technol. 2008, 5, 011001. [Google Scholar] [CrossRef]
- Mineshige, A.; Fukushima, K.; Okada, S.; Kikuchi, T.; Kobune, M.; Yazawa, T.; Kikuchi, K.; Inaba, M.; Ogumi, Z. Porous Metal Tubular Support for Solid Oxide Fuel Cell Design. Electrochem. Solid-State Lett. 2006, 9, A427–A429. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Jia, L.; Yan, D.; Pu, J.; Chi, B.; Jian, L. High performance Ni–Fe alloy supported SOFCs fabricated by low cost tape casting-screen printing-cofiring process. Int. J. Hydrogen Energy 2014, 39, 19747–19752. [Google Scholar] [CrossRef]
- Wei, P.; Sofie, S.; Zhang, Q.; Petric, A. Metal Supported Solid Oxide Fuel Cell by Freeze Tape Casting. ECS Trans. 2011, 35, 379–383. [Google Scholar] [CrossRef]
- Grabke, H.J. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, W.; Li, X. Oxidation behavior of NiAl nanoparticles prepared by hydrogen plasma-metal reaction. Mater. Chem. Phys. 2008, 107, 381–384. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, M.H.; Kishida, K.; Hirano, T.; Wee, D.M. Deposition of NiAl coating for improvement of oxidation resistance of cold-rolled Ni3Al foils. Intermetallics 2005, 13, 129–136. [Google Scholar] [CrossRef]
- Windes, W.E.; Smith, C.; Wendt, D.; Erickson, A.; Walraven, J.; Lessing, P.A. Electrode coatings for high temperature hydrogen electrolysis. J. Mater. Sci. 2007, 42, 2717–2723. [Google Scholar] [CrossRef]
- Promsen, M.; Komatsu, Y.; Sciazko, A.; Kaneko, S.; Shikazono, N. Feasibility study on saturated water cooled solid oxide fuel cell stack. Appl. Energy 2020, 279, 115803. [Google Scholar] [CrossRef]
- Zeng, Z.; Qian, Y.; Zhang, Y.; Hao, C.; Dan, D.; Zhuge, W. A review of heat transfer and thermal management methods for temperature gradient reduction in solid oxide fuel cell (SOFC) stacks. Appl. Energy 2020, 280, 115899. [Google Scholar] [CrossRef]
- Thaler, F.; Udomsilp, D.; Schafbauer, W.; Bischof, C.; Fukuyama, Y.; Miura, Y.; Kawabuchi, M.; Taniguchi, S.; Takemiya, A.; Nenning, A.K. Redox Stability of Metal Supported Fuel Cells with Nickel/Gadolinium-Doped Ceria Anode. J. Power Sources 2019, 434, 226751. [Google Scholar] [CrossRef]
- Sudireddy, B.R.; Nielsen, J.; Persson, Å.H.; Thydén, K.; Brodersen, K.; Ramousse, S.; Neagu, D.; Stefan, E.; Irvine, J.T.S.; Geisler, H.; et al. Development of robust metal-supported SOFCs and stack components in EU METSAPP Consortium. Fuel Cells 2017, 17, 508–516. [Google Scholar] [CrossRef]
- Leah, R.T.; Bone, P.A.; Selcuk, A.; Rahman, M.; Clare, A.; Lankin, M.; Felix, F.; Mukerjee, S.; Selby, M.A. Latest results and commercialization of the ceres power steelCell® technology platform. ECS Trans. 2019, 91, 51–61. [Google Scholar] [CrossRef]
- Gao, J.T.; Li, J.H.; Wang, Y.P.; Li, C.J.; Li, C.X. Self-Sealing Metal-Supported SOFC Fabricated by Plasma Spraying and Its Performance under Unbalanced Gas Pressure. J. Therm. Spray Technol. 2020, 29, 2001–2011. [Google Scholar] [CrossRef]




| Cell | Materials | TEC (ppm/K) |
|---|---|---|
| Cathode | LSM, LNF, LSCF, SSC, LSC | 12, 12, 18, 18.4, 22 |
| Diffusion barrier layer | GDC | 12.7 |
| Electrolyte | YSZ, SCSZ, LSGM, GDC | 10, 10.5, 12.7, 12.7 |
| Anode | Ni/YSZ, Cu/YSZ, Titanates | 12 |
| Diffusion barrier layer | GDC | 12.7 |
| Metal support | Ni, Ni-Fe (1:1), Ferritic stainless steel | 16.5, 13.7, 10–12 |
| Electrolyte Material | Electrolyte Thickness | OCV, Temperature | Polarization Resistance | Maximum Power Density, Temperature | Sealing Method | Ref. |
|---|---|---|---|---|---|---|
| BaZrO3-based electrolytes | 30–50 μm | 1.1 V at 500 °C | 0.4 Ω cm2 | 66 mW/cm2 at 550 °C | Ag paste | [72] |
| SrZrO3-based electrolytes | 10–30 μm | 1.0 V at 450 °C | 0.3 Ω cm2 | 300 mW/cm2 at 700 °C | glass-ceramic sealant | [73] |
| H3PO4-doped SrZrO3-based electrolytes | 10–30 μm | 0.9 V at 400 °C | 0.1 Ω cm2 | 17 mW/cm2 at 500 °C | glass-ceramic sealant | [74] |
| Nafion-based electrolytes | 5–10 μm | 0.4 V at room temperature | 0.5 Ω cm2 | 2 mW/cm2 at 80 °C | glass-ceramic sealant | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opakhai, S.; Kuterbekov, K. Metal-Supported Solid Oxide Fuel Cells: A Review of Recent Developments and Problems. Energies 2023, 16, 4700. https://doi.org/10.3390/en16124700
Opakhai S, Kuterbekov K. Metal-Supported Solid Oxide Fuel Cells: A Review of Recent Developments and Problems. Energies. 2023; 16(12):4700. https://doi.org/10.3390/en16124700
Chicago/Turabian StyleOpakhai, Serikzhan, and Kairat Kuterbekov. 2023. "Metal-Supported Solid Oxide Fuel Cells: A Review of Recent Developments and Problems" Energies 16, no. 12: 4700. https://doi.org/10.3390/en16124700
APA StyleOpakhai, S., & Kuterbekov, K. (2023). Metal-Supported Solid Oxide Fuel Cells: A Review of Recent Developments and Problems. Energies, 16(12), 4700. https://doi.org/10.3390/en16124700







