Experimental Assessment on the Coupling Effect of Mixing Length and Methane-Ammonia Blends on Flame Stability and Emissions
Abstract
1. Introduction
2. Experimental Set-Up and Methods
3. Results and Discussion
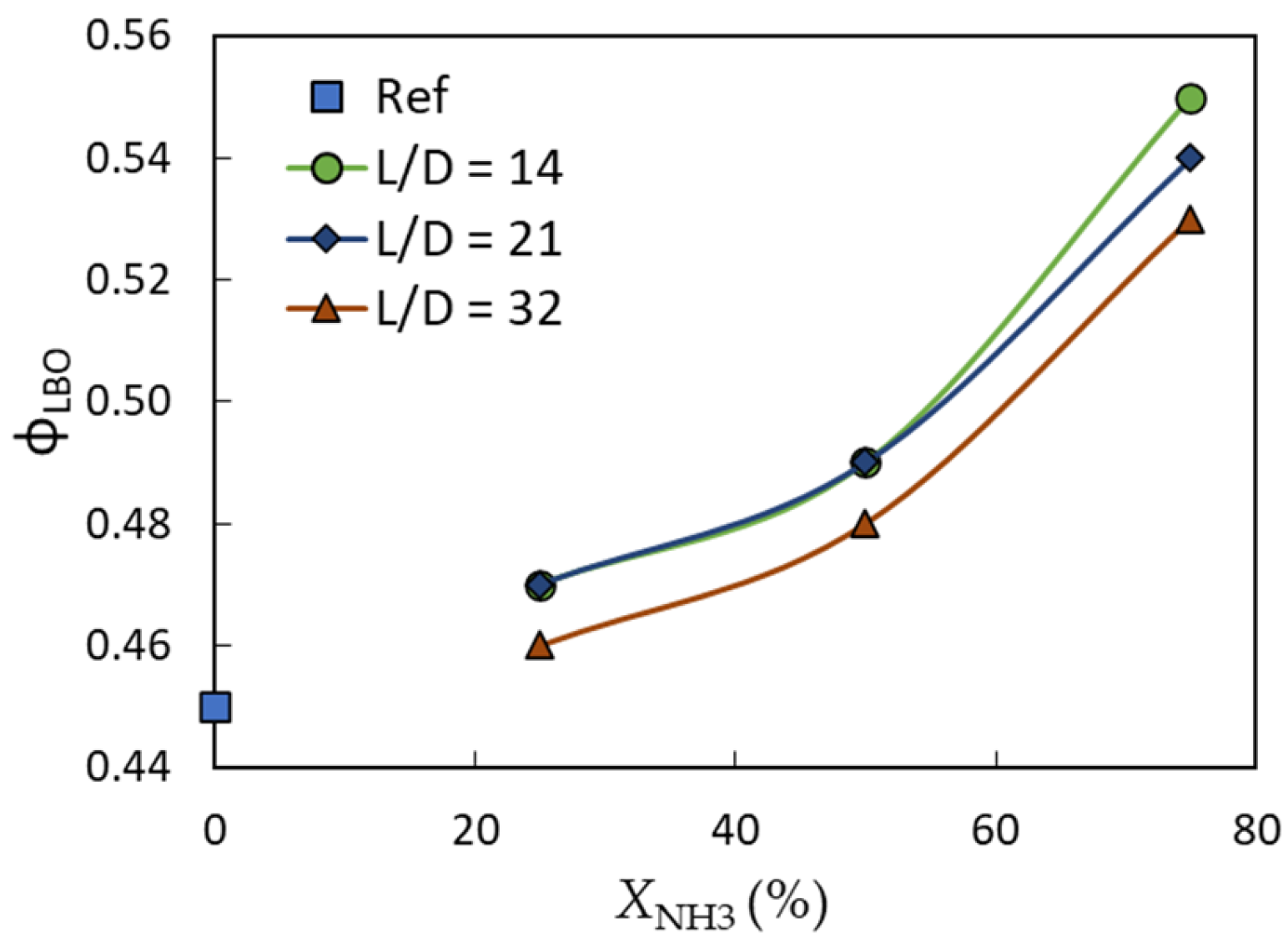
3.1. Flame Stability
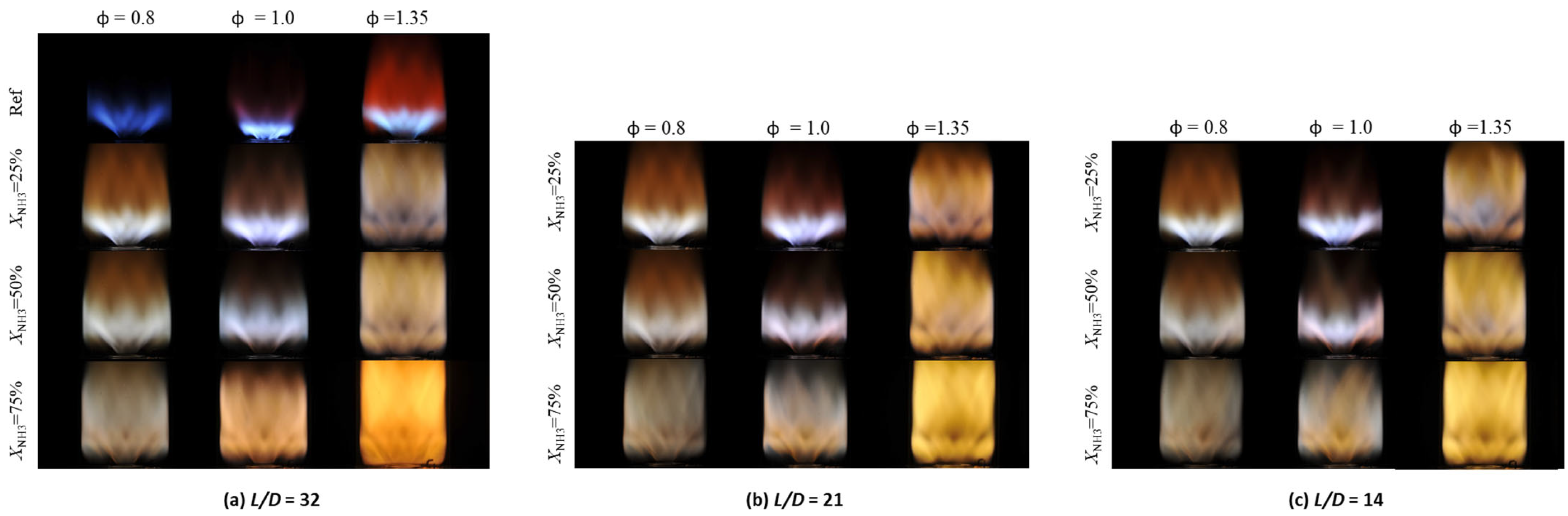
3.2. Flame Shape
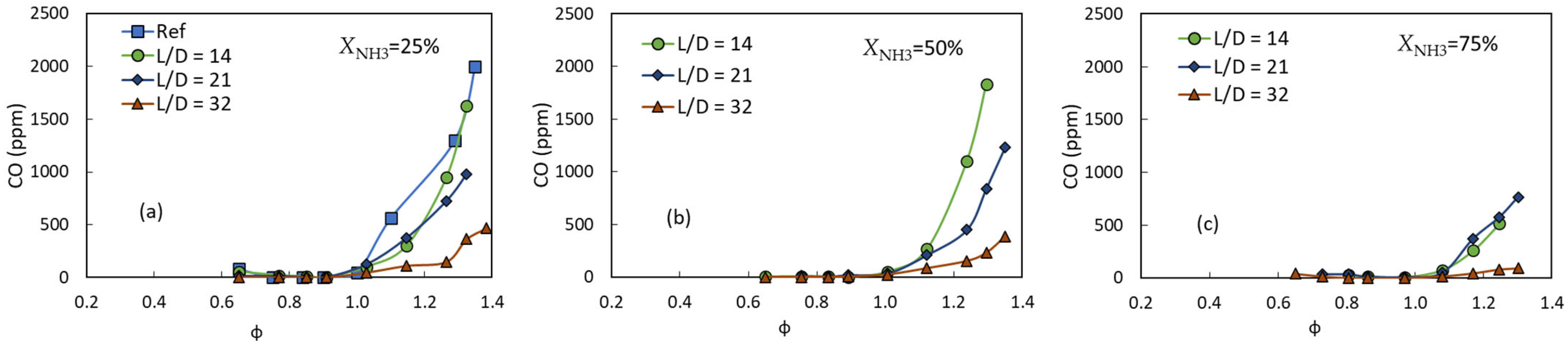
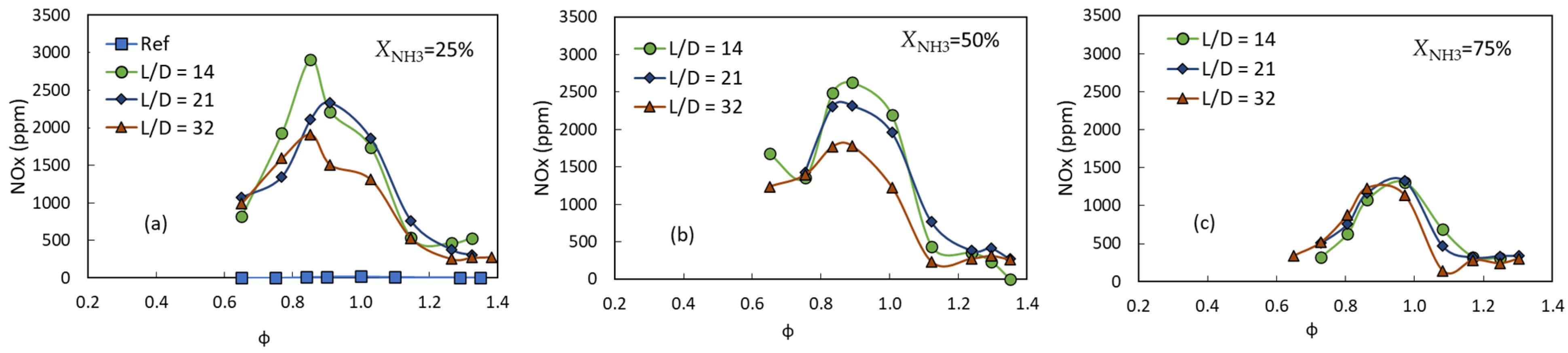
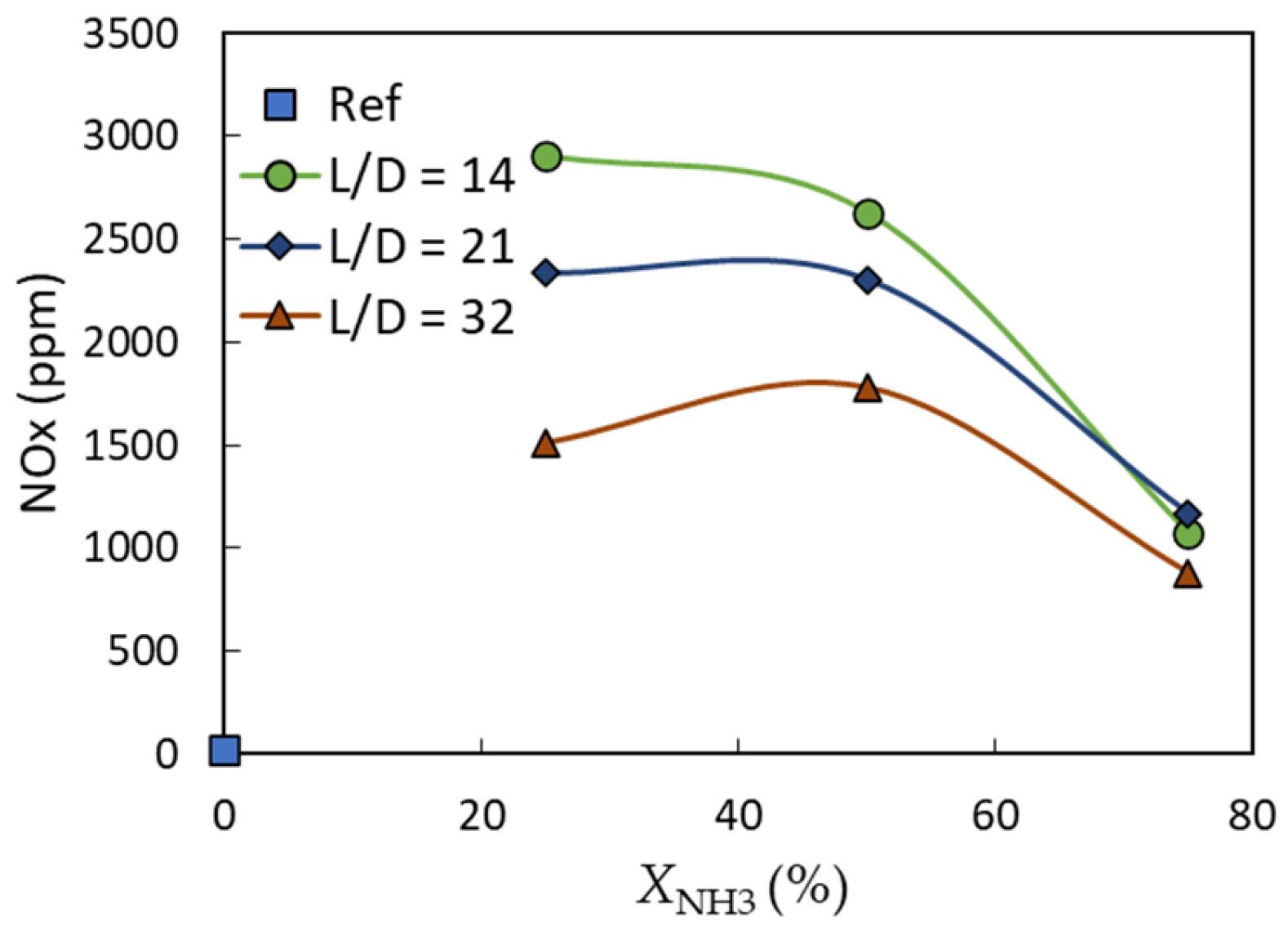
3.3. Exhaust Gas Emissions
4. Conclusions
- The lean blow-off limit for the current tested conditions was highly influenced by the ammonia fuel fraction and to a lesser extent by the mixing length. A maximum lean blow-off enhancement of ~4% was noticed when the mixing length ratio increased from L/D = 14 to 32 for the 75% ammonia fraction, while a maximum flame stability reduction of ~17% was observed when the ammonia volume fraction increased from 25% to 75% in the shorter mixing length case (i.e., L/D = 14).
- Averaged flame images showed that increasing ammonia fuel fraction, for a constant equivalence ratio, led to a larger and brighter flame. The flame size shrank when the mixing length ratio was reduced for a fixed ammonia fraction and equivalence ratio, and also became more compact near the swirler outlet.
- Increasing the mixing ratio and/or ammonia volume fraction decreased the CO concentration due to the relatively complete combustion of a more homogeneous mixture and/or the presence of less carbon caused by the increasing amount of carbon-free (ammonia) fuel, respectively.
- Regardless of the equivalence ratio, the NOx concentration was negatively influenced by the mixing length ratio and the ammonia fraction. Generally, for a fixed equivalence ratio and L/D = 32, the NOx concentration increased from ~20 ppm to 1780 ppm when the ammonia fraction was increased from 0 (pure methane) to XNH3 = 50%, before it dropped again to ~880 ppm at XNH3 = 75%.
- Delaying the injection (down to L/D = 14, which is the lower mixing limit used in current study) of the less reactive carbon-free fuel (ammonia), showed negative impacts on flame performance and emissions, leading to flame extinguishing at a larger equivalence ratio and increased CO and NOx emissions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Capuano, L. US Energy Information Administration’s International Energy Outlook 2020 (Ieo2020); US Department of Energy: Washington DC, USA, 2020; Volume 7.
- Salam, M.A.; Khan, S.A. Transition towards Sustainable Energy Production—A Review of the Progress for Solar Energy in Saudi Arabia. Energy Explor. Exploit. 2018, 36, 3–27. [Google Scholar] [CrossRef]
- Amran, Y.H.A.; Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H. Renewable and Sustainable Energy Production in Saudi Arabia According to Saudi Vision 2030; Current Status and Future Prospects. J. Clean. Prod. 2020, 247, 119602. [Google Scholar] [CrossRef]
- Taamallah, S.; Vogiatzaki, K.; Alzahrani, F.M.; Mokheimer, E.M.A.; Habib, M.A.; Ghoniem, A.F. Fuel Flexibility, Stability and Emissions in Premixed Hydrogen-Rich Gas Turbine Combustion: Technology, Fundamentals, and Numerical Simulations. Appl. Energy 2015, 154, 1020–1047. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, V. Dynamics and Stability of Lean-Premixed Swirl-Stabilized Combustion. Prog. Energy Combust. Sci. 2009, 35, 293–364. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Mansour, M.S.; Akoush, B.M.; Juddoo, M.; Khedr, A.M.; Al-Bulqini, H.M.; Zayed, M.F.; Ahmed, M.M.A.; Roberts, W.L.; Masri, A.R. Detailed Investigation of the Mixing Field and Stability of Natural Gas and Propane in Highly Turbulent Planar Flames. Fuel 2022, 309, 122222. [Google Scholar] [CrossRef]
- Souflas, K.; Koutmos, P. On the Non-Reacting Flow and Mixing Fields of an Axisymmetric Disk Stabilizer, under Inlet Mixture Stratification and Preheat. Exp. Therm. Fluid Sci. 2018, 99, 357–366. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Mansour, M.S. A Concentric Flow Conical Nozzle Burner for Highly Stabilized Partially Premixed Flames. Combust. Sci. Technol. 2000, 152, 115–145. [Google Scholar] [CrossRef]
- Meares, S.; Masri, A.R. A Modified Piloted Burner for Stabilizing Turbulent Flames of Inhomogeneous Mixtures. Combust. Flame 2014, 161, 484–495. [Google Scholar] [CrossRef]
- Barlow, R.S.; Meares, S.; Magnotti, G.; Cutcher, H.; Masri, A.R. Local Extinction and Near-Field Structure in Piloted Turbulent CH4/Air Jet Flames with Inhomogeneous Inlets. Combust. Flame 2015, 162, 3516–3540. [Google Scholar] [CrossRef]
- Ahmed, M.M.A.; Birouk, M. Mixing Tube Length Effect on the Stability of Confined Swirling Partially Premixed Methane Flames off a Concentric Flow Conical Nozzle Burner. Combust. Sci. Technol. 2021, 193, 2833–2855. [Google Scholar] [CrossRef]
- Badawy, T.; Hamza, M.; Mansour, M.S.; Elbaz, A.M.; Turner, J.W.G.; Fayad, M.A.; Al Jubori, A.M.; Daabo, A.M.; Wang, Z.; Wang, C. Flame Stability and Equivalence Ratio Assessment of Turbulent Partially Premixed Flames. Fuel 2022, 326, 125107. [Google Scholar] [CrossRef]
- El-Mahallawy, F.; Abdelhafez, A.; Mansour, M.S. Mixing and Nozzle Geometry Effects on Flame Structure and Stability. Combust. Sci. Technol. 2007, 179, 249–263. [Google Scholar] [CrossRef]
- Kwak, S.; Joo, S.; Chul Lee, M.; Yoon, Y. Difference in Thermo-Acoustic Instability Frequency Between Partially and Fully Premixed Flames. J. Propuls. Power 2022, 38, 726–735. [Google Scholar] [CrossRef]
- Scarinci, T.; Freeman, C.; Day, I. Passive Control of Combustion Instability in a Low Emissions Aeroderivative Gas Turbine. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Vienna, Austria, 14–17 June 2004; Volume 41669, pp. 487–499. [Google Scholar]
- Meares, S.; Prasad, V.N.; Magnotti, G.; Barlow, R.S.; Masri, A.R. Stabilization of Piloted Turbulent Flames with Inhomogeneous Inlets. Proc. Combust. Inst. 2015, 35, 1477–1484. [Google Scholar] [CrossRef]
- Alsulami, R.A. Study on Lean Premixed Flame Stability Enhancement by Altering Fuel-Air Mixture Homogeneity. In Proceedings of the AIAA SCITECH 2023 Forum, National Harbor, MD, USA, 23–27 January 2023; p. 922. [Google Scholar]
- Zamfirescu, C.; Dincer, I. Using Ammonia as a Sustainable Fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Banares-Alcantara, R. Techno-Economic Challenges of Green Ammonia as an Energy Vector; Academic Press: Cambridge, MA, USA, 2020; ISBN 0128208864. [Google Scholar]
- Hayakawa, A.; Arakawa, Y.; Mimoto, R.; Somarathne, K.D.K.A.; Kudo, T.; Kobayashi, H. Experimental Investigation of Stabilization and Emission Characteristics of Ammonia/Air Premixed Flames in a Swirl Combustor. Int. J. Hydrog. Energy 2017, 42, 14010–14018. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.D.K.A.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Towards the Development of an Efficient Low-NOx Ammonia Combustor for a Micro Gas Turbine. Proc. Combust. Inst. 2019, 37, 4597–4606. [Google Scholar] [CrossRef]
- Ariemma, G.B.; Sorrentino, G.; Ragucci, R.; de Joannon, M.; Sabia, P. Ammonia/Methane Combustion: Stability and NOx Emissions. Combust. Flame 2022, 241, 112071. [Google Scholar] [CrossRef]
- Vigueras-Zúñiga, M.O.; Tejeda-del-Cueto, M.E.; Mashruk, S.; Kovaleva, M.; Ordóñez-Romero, C.L.; Valera-Medina, A. Methane/Ammonia Radical Formation during High Temperature Reactions in Swirl Burners. Energies 2021, 14, 6624. [Google Scholar] [CrossRef]
- Kim, N.; Lee, M.; Park, J.; Park, J.; Lee, T. A Comparative Study of NOx Emission Characteristics in a Fuel Staging and Air Staging Combustor Fueled with Partially Cracked Ammonia. Energies 2022, 15, 9617. [Google Scholar] [CrossRef]
- Vijrumbana, Y.; Singh, A.S.; Lee, B.J.; Reddy, V.M. Chemical Kinetic Analysis to Study the Potential of Fuel Staging in Reducing the Emissions from NH3/CH4-Air Combustion at Different Pressures. Fuel 2023, 339, 127404. [Google Scholar] [CrossRef]
- Avila Jimenez, C.D.; Cardona, S.; Juaied, M.A.; Younes, M.; Jamal, A.; Guiberti, T.F.; Roberts, W.L. Influence of the Pilot Flame on the Morphology and Exhaust Emissions of NH3-CH4-Air Swirl Flames Using a Reduced-Scale Burner at Atmospheric Pressure. Energies 2022, 16, 231. [Google Scholar] [CrossRef]
- Jojka, J.; Ślefarski, R. Dimensionally Reduced Modeling of Nitric Oxide Formation for Premixed Methane-Air Flames with Ammonia Content. Fuel 2018, 217, 98–105. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–Methane Combustion in Tangential Swirl Burners for Gas Turbine Power Generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Zhou, H.; Ren, Z. Analysis of Air-Staged Combustion of NH3/CH4 Mixture with Low NOx Emission at Gas Turbine Conditions in Model Combustors. Fuel 2019, 237, 50–59. [Google Scholar] [CrossRef]
- Ito, S.; Kato, S.; Saito, T.; Fujimori, T.; Kobayashi, H. Development of Ammonia/Natural Gas Dual Fuel Gas Turbine Combustor. In Proceedings of the NH3 Fuel Conference, Los Angeles, CA, USA, 19 September 2016; pp. 18–21. [Google Scholar]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar Burning Velocity and Markstein Length of Ammonia/Air Premixed Flames at Various Pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability Limits and Exhaust NO Performances of Ammonia-Methane-Air Swirl Flames. Exp. Therm. Fluid Sci. 2020, 114, 110058. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Wang, G.; Boyette, W.R.; Younes, M.; Jamal, A.; Roberts, W.L. Stability Limits and NO Emissions of Premixed Swirl Ammonia-Air Flames Enriched with Hydrogen or Methane at Elevated Pressures. Int. J. Hydrog. Energy 2021, 46, 11969–11981. [Google Scholar] [CrossRef]
- Avila, C.; Wang, G.; Zhu, X.; Es-Sebbar, E.-T.; Abdullah, M.; Younes, M.; Jamal, A.; Guiberti, T.; Roberts, W.L. Lean Stability Limits and Exhaust Emissions of Ammonia-Methane-Air Swirl Flames at Micro Gas Turbine Relevant Pressure. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, American Society of Mechanical Engineers, Rotterdam, The Netherlands, 13–17 June 2022; Volume 85994, p. V03AT04A004. [Google Scholar]
- Laera, D.; Agostinelli, P.W.; Selle, L.; Cazères, Q.; Oztarlik, G.; Schuller, T.; Gicquel, L.; Poinsot, T. Stabilization Mechanisms of CH4 Premixed Swirled Flame Enriched with a Non-Premixed Hydrogen Injection. Proc. Combust. Inst. 2021, 38, 6355–6363. [Google Scholar] [CrossRef]
- Cheng, T.-S.; Wu, C.-Y.; Li, Y.-H.; Chao, Y.-C. Chemiluminescence Measurements of Local Equivalence Ratio in a Partially Premixed Flame. Combust. Sci. Technol. 2006, 178, 1821–1841. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and Modelling of the Laminar Burning Velocity of Methane-Ammonia-Air Flames at High Pressures Using a Reduced Reaction Mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Lefebvre, A.H.; Ballal, D.R. Gas Turbine Combustion: Alternative Fuels and Emissions; CRC Press: Boca Raton, FL, USA, 2010; ISBN 0429141041. [Google Scholar]
- Rowley, R.L.; Wilding, W.V.; Oscarson, J.L.; Yang, Y.; Zundel, N.A.; Daubert, T.E.; Danner, R.P. DIPPR Information and Data Evaluation Manager for the Design Institute for Physical Properties; AIChE: New York, NY, USA, 2011; Volume 5. [Google Scholar]
- Wargadalam, V.J.; Löffler, G.; Winter, F.; Hofbauer, H. Homogeneous Formation of NO and N2O from the Oxidation of HCN and NH3 at 600–1000 °C. Combust. Flame 2000, 120, 465–478. [Google Scholar] [CrossRef]
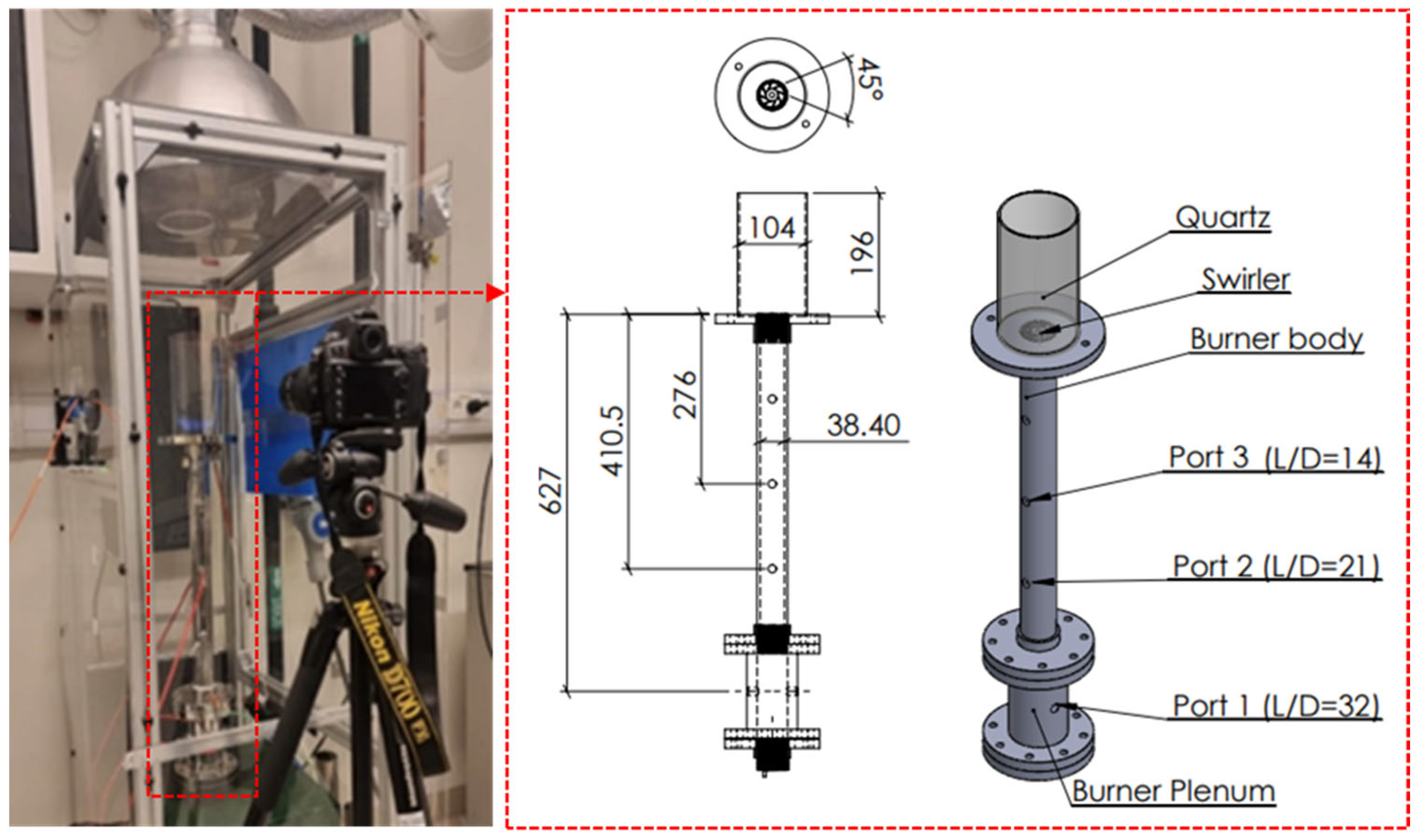


| Properties | Methane (CH4) | Ammonia (NH3) |
|---|---|---|
| Density [kg/m3] | 0.657 | 0.704 |
| Viscosity [kg/m.s] | 1.11 × 10−5 | 1.01 × 10−5 |
| Laminar burning velocity [m/s] | 0.37 | 0.07 |
| Adiabatic flame temperature [K] | 2223 | 2073 |
| Heat of combustion [MJ/kg] | 50 | 18.6 |
| Parameter | Test Point |
|---|---|
| Air flowrate (L/min) | 100 |
| Equivalence ratio (ϕ) | 0.45–1.35 |
| Reynold number (Re) | 6200–7200 |
| Swirl number (SN) | 0.78 |
| Thermal Power (kW) | ~2.1–6.1 |
| L/D ratio | 14, 21, & 32 |
| XNH3 (volume%) | 0, 25, 50, & 75 |
| Number of ports | 3 |
| Methane-air (Ref.) injection | Port 1 |
| Ammonia injection | All ports |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, M.; Guiberti, T.F.; Alsulami, R.A. Experimental Assessment on the Coupling Effect of Mixing Length and Methane-Ammonia Blends on Flame Stability and Emissions. Energies 2023, 16, 2955. https://doi.org/10.3390/en16072955
Abdullah M, Guiberti TF, Alsulami RA. Experimental Assessment on the Coupling Effect of Mixing Length and Methane-Ammonia Blends on Flame Stability and Emissions. Energies. 2023; 16(7):2955. https://doi.org/10.3390/en16072955
Chicago/Turabian StyleAbdullah, Marwan, Thibault F. Guiberti, and Radi A. Alsulami. 2023. "Experimental Assessment on the Coupling Effect of Mixing Length and Methane-Ammonia Blends on Flame Stability and Emissions" Energies 16, no. 7: 2955. https://doi.org/10.3390/en16072955
APA StyleAbdullah, M., Guiberti, T. F., & Alsulami, R. A. (2023). Experimental Assessment on the Coupling Effect of Mixing Length and Methane-Ammonia Blends on Flame Stability and Emissions. Energies, 16(7), 2955. https://doi.org/10.3390/en16072955




