Implications of Cation Interdiffusion between Double Perovskite Cathode and Proton-Conducting Electrolyte for Performance of Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
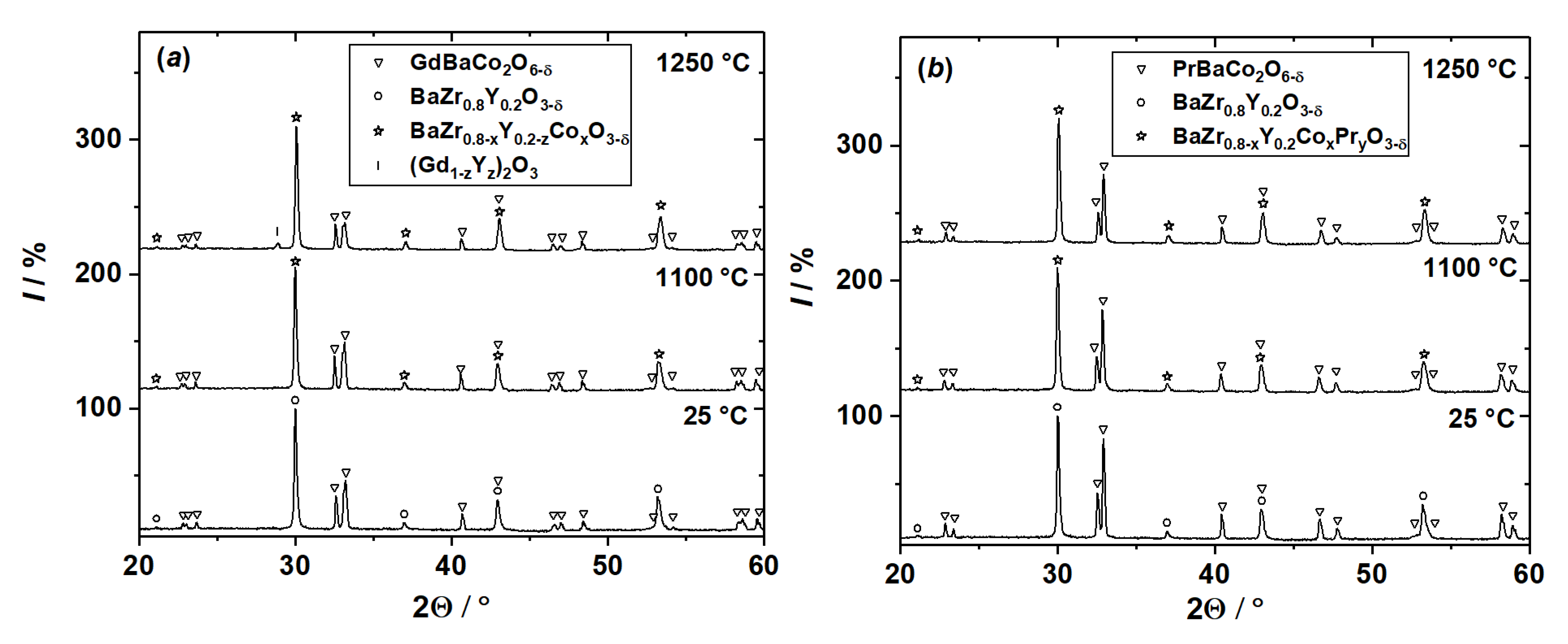
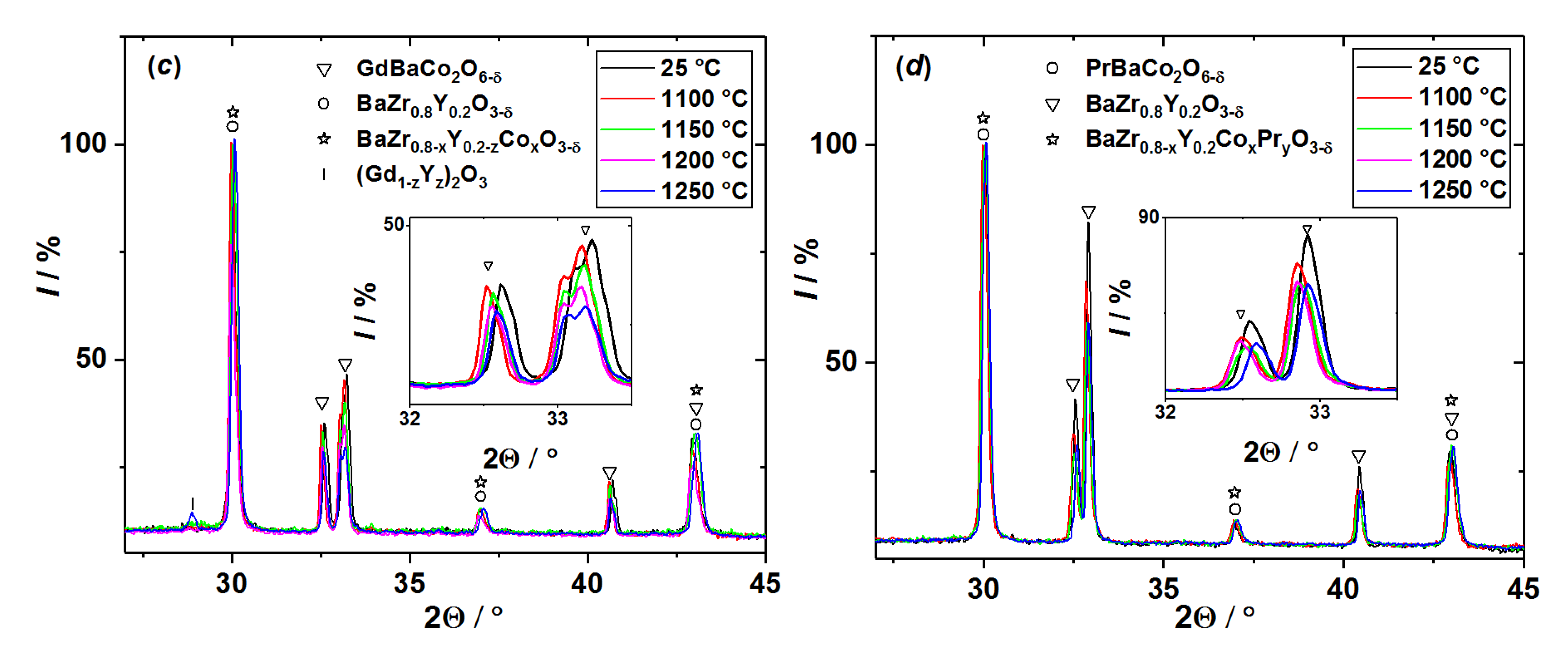
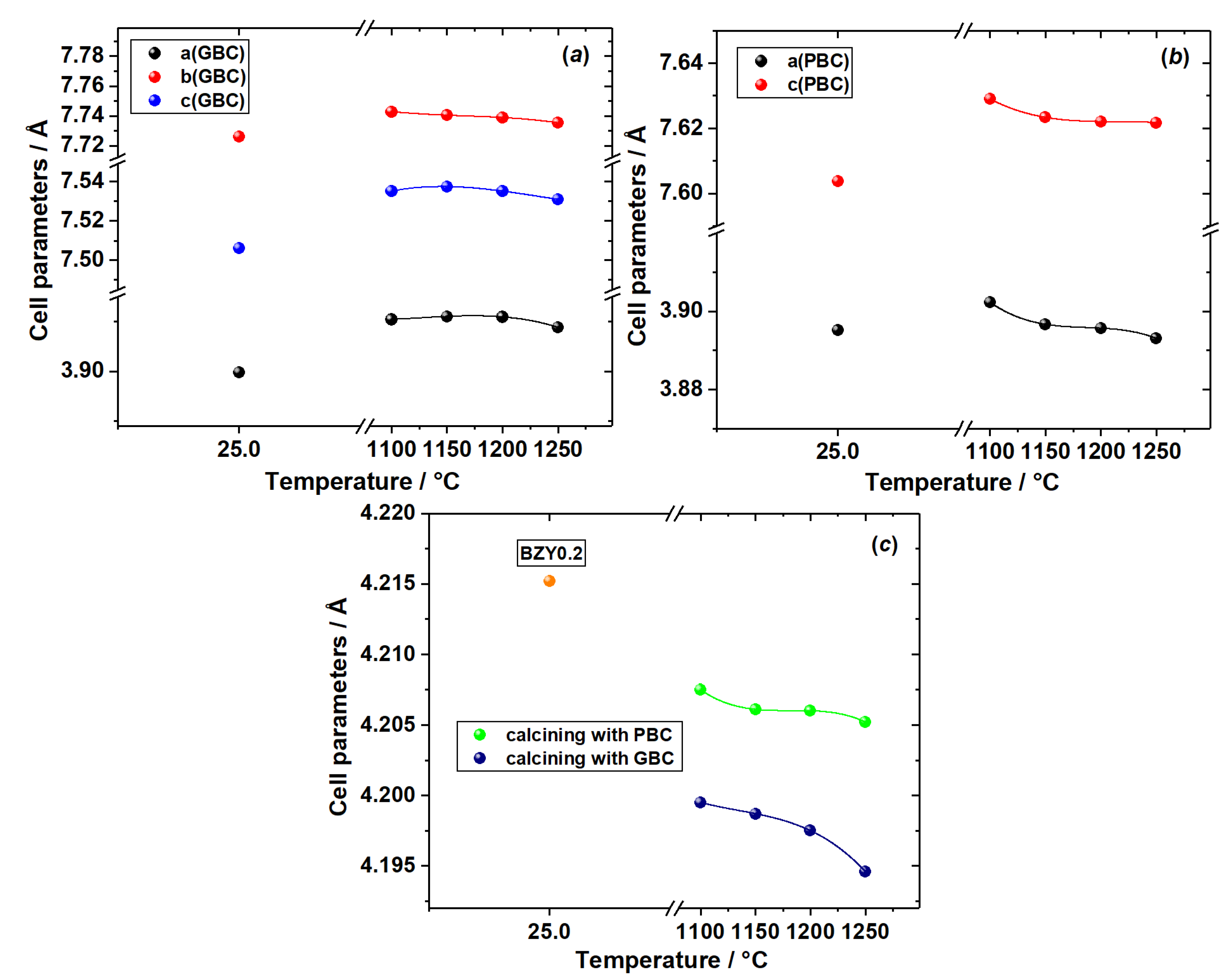
3.1. Chemical Compatibility
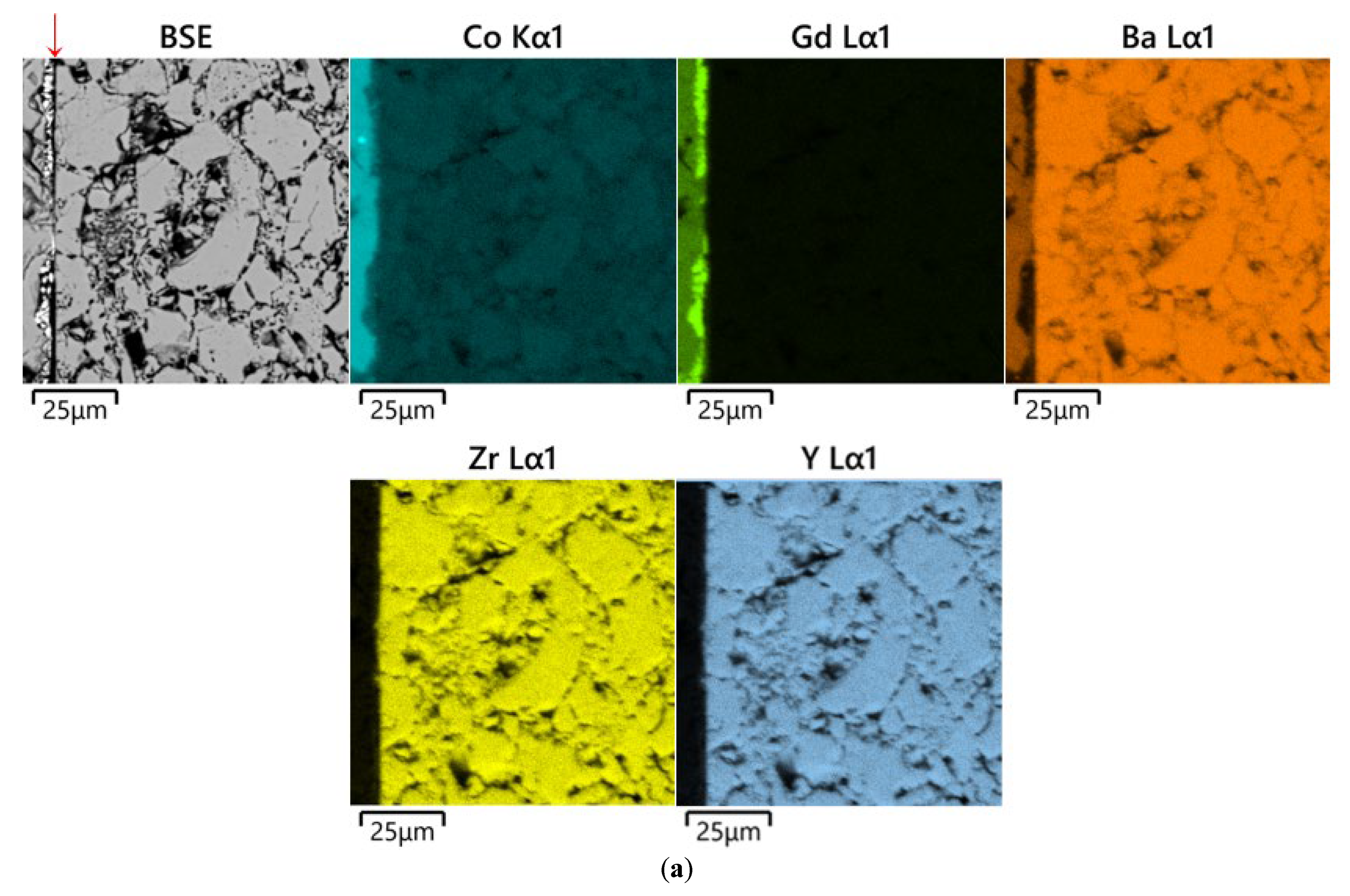
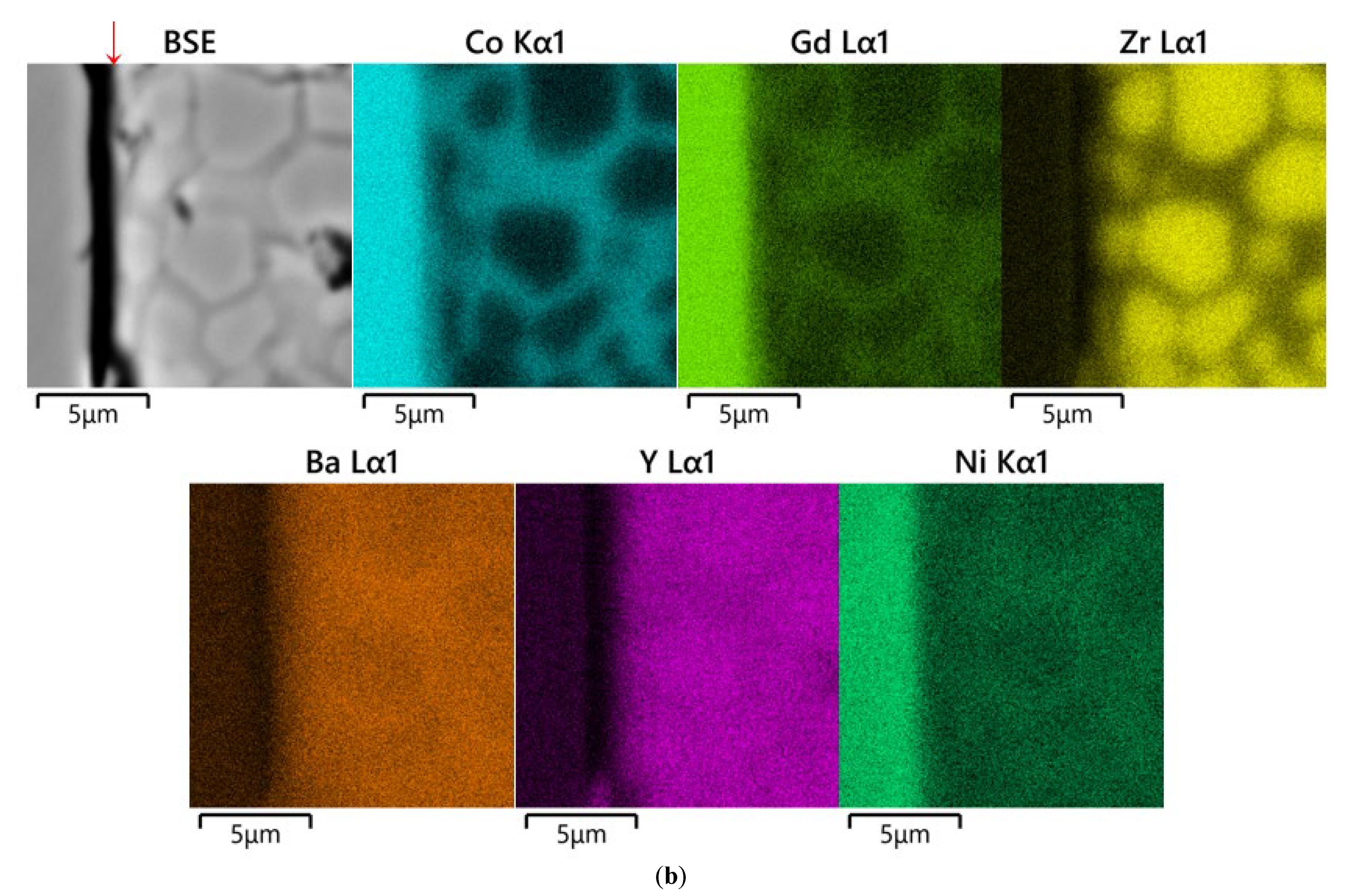
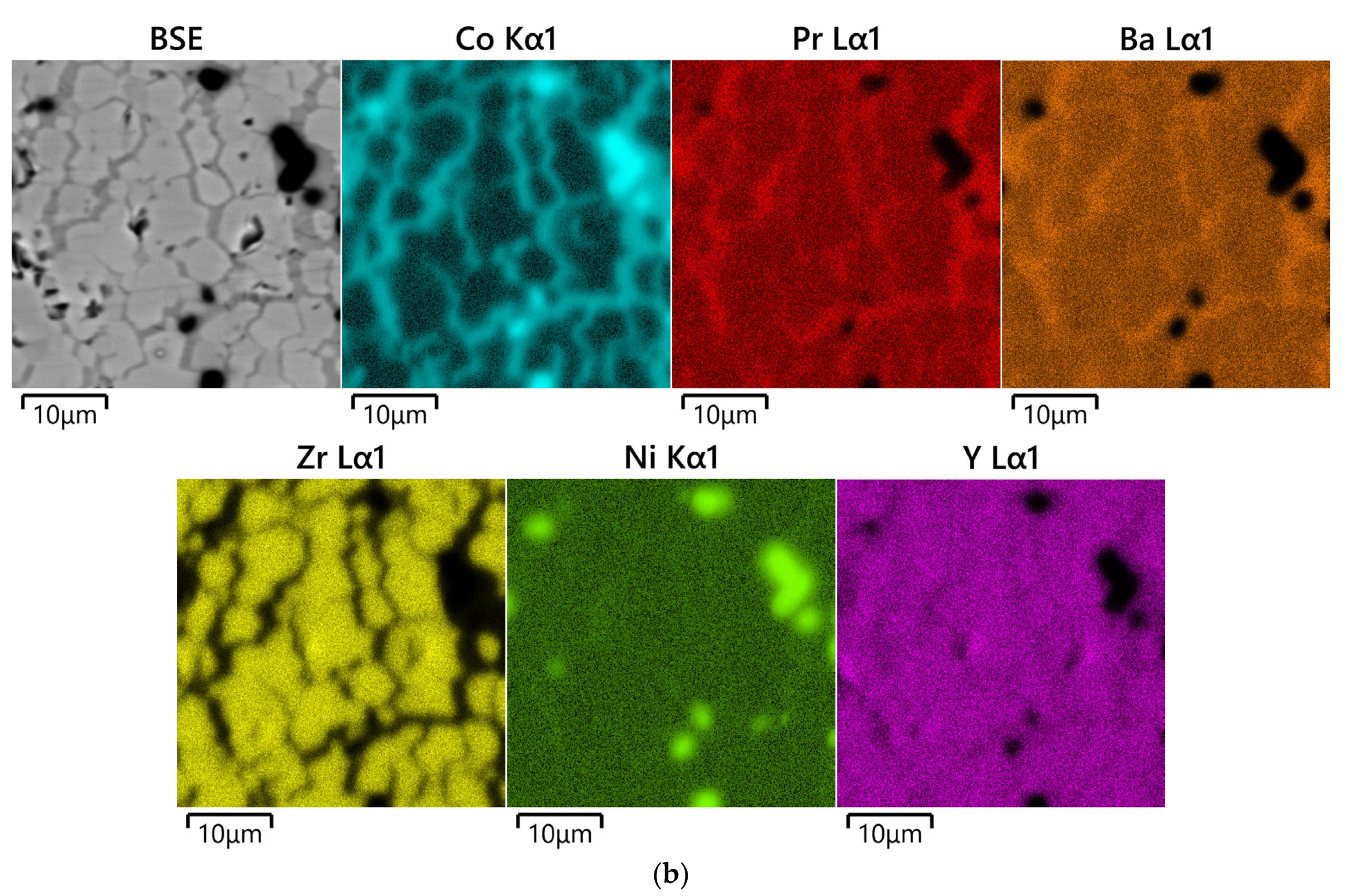
3.2. Analysis of Diffusion Couples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boudghene Stambouli, A.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Kumar, R.V.; Khandale, A.P. A review on recent progress and selection of cobalt-based materials for low temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2022, 156, 111985. [Google Scholar] [CrossRef]
- Khan, M.Z.; Song, R.-H.; Mehran, M.T.; Lee, S.-B.; Lim, T.-H. Controlling cation migration and inter-diffusion across cathode/interlayer/electrolyte interfaces of solid oxide fuel cells: A review. Ceram. Int. 2021, 47, 5839–5869. [Google Scholar] [CrossRef]
- Martin, M. Materials in thermodynamic potential gradients. J. Chem. Thermodyn. 2003, 35, 1291–1308. [Google Scholar] [CrossRef] [Green Version]
- Sakai, N.; Yamaji, K.; Horita, T.; Negishi, H.; Yokokawa, H. Chromium diffusion in lanthanum chromites. Solid State Ion. 2000, 135, 469–474. [Google Scholar] [CrossRef]
- Horita, T.; Ishikawa, M.; Yamaji, K.; Sakai, N.; Yokokawa, H.; Dokiya, M. Calcium tracer diffusion in (La,Ca)CrO3 by SIMS. Solid State Ion. 1999, 124, 301–307. [Google Scholar] [CrossRef]
- Shulz, O.; Martin, M.; Argirusis, C.; Borchardt, G. Cation tracer diffusion of 138La, 84Sr and 25Mg in polycrystalline La0.9Sr0.1Ga0.9Mg0.1O2.9. Phys. Chem. Chem. Phys. 2003, 5, 2308–2313. [Google Scholar] [CrossRef]
- Smith, J.B.; Norby, T. Cation self-diffusion in LaFeO3 measured by the solid state reaction. Solid State Ion. 2006, 177, 639–646. [Google Scholar] [CrossRef]
- Smith, J.B.; Norby, T. Electron probe micro analysis of A-site inter-diffusion between LaFeO3 and NdFeO3. J. Am. Ceram. Soc. 2006, 89, 582–586. [Google Scholar] [CrossRef]
- Palcut, M.; Wiik, K.; Grande, T. cation self-diffusion in LaCoO3 and La2CoO4 studied by diffusion couple experiments. J. Phys. Chem. B 2007, 111, 2299–2308. [Google Scholar] [CrossRef]
- Čebašek, N.; Haugsrud, R.; Norby, T. Cation transport in Sr and Cu substituted La2NiO4+δ studied by inter-diffusion. Solid State Ion. 2014, 254, 32–39. [Google Scholar] [CrossRef]
- Vollestad, E.; Norby, T.; Haugsrud, R. Inter-diffusion in lanthanum tungsten oxide. Solid State Ion. 2013, 244, 57–62. [Google Scholar] [CrossRef]
- Lein, H.L.; Wiik, K.; Grande, T. Kinetic demixing and decomposition of oxygen permeable membranes. Solid State Ion. 2006, 177, 1587–1590. [Google Scholar] [CrossRef]
- Doorn van, R.H.E.; Bouwmeester, H.J.M.; Burggraaf, A.J. Kinetic decomposition of La0.3Sr0.7CoO3−δ perovskite membranes during oxygen permeation. Solid State Ion. 1998, 111, 263–272. [Google Scholar] [CrossRef]
- Čebašek, N.; Haugsrud, R.; Milošević, J.; Li, Z.; Smith, J.B.; Magrasó, A.; Norby, T. Determination of the self-diffusion coefficient of Ni2+ in La2NiO4+d by the solid state reaction method. J. Electrochem. Soc. 2012, 159, B702–B708. [Google Scholar] [CrossRef]
- Čebašek, N.; Haugsrud, R.; Li, Z.; Norby, T. Determination of chemical tracer diffusion coefficient for the La- and Ni-site in La2NiO4+d studied by SIMS. J. Am. Ceram. Soc. 2013, 96, 598–605. [Google Scholar] [CrossRef]
- Čebašek, N.; Haugsrud, R.; Norby, T. Determination of inter-diffusion coefficient for the A- and B-site in the A2BO4+d (A = La, Nd and B = Ni, Cu) system. Solid State Ion. 2013, 231, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Čebašek, N.; Norby, T.; Li, Z. Kinetic Decomposition of a La2NiO4+d membrane under an oxygen potential gradient. J. Electrochem. Soc. 2012, 159, F461–F467. [Google Scholar] [CrossRef]
- Li, Z.-P.; Mori, T.; Auchterlonie, G.J.; Zou, J.; Drennan, J. Two types of diffusions at the cathode/electrolyte interface in It-SOFCs. J. Solid State Chem. 2011, 184, 2458–2461. [Google Scholar] [CrossRef]
- Matsui, T.; Li, S.; Yu, I.; Yoshida, N.; Muroyama, H.; Eguchi, K. Degradation analysis of solid oxide fuel cells with (La,Sr)(Co,Fe)O3−δ cathode/Gd2O3-CeO2 interlayer/Y2O3-ZrO2 electrolyte system: The influences of microstructrural change and solid solution formation. J. Electrochem. Soc. 2019, 166, F295–F300. [Google Scholar] [CrossRef] [Green Version]
- Tsvetkov, D.; Tsvetkova, N.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. PrBaCo2O6−δ-Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Stability and Cation Interdiffusion. Energies 2019, 12, 417. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://www.icdd.com/products/pdf2.htm (accessed on 30 January 2023).
- Available online: http://www.crystalimpact.com/match/Default.htm (accessed on 30 January 2023).
- Hunter, B.A. RIETICA, version 1.7.7. IUCR Powder Diffr. 1997, 22, 21–26. [Google Scholar]
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Oxygen nonstoichiometry and defect structure of the double perovskite GdBaCo2O6−δ. Solid State Ion. 2010, 180, 1620–1625. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Zuev, A.Y. Oxygen content, cobalt oxide exsolution and defect structure of the double perovskite PrBaCo2O6−δ. J. Mater. Chem. A 2016, 4, 1962–1969. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rao, Y.; Zhong, S.; He, F.; Wang, Z.; Peng, R.; Lu, Y. Cobalt-doped BaZrO3: A single phase air electrode material for reversible solid oxide cells. Int. J. Hydrog. Energy 2012, 37, 12522–12527. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsvetkova, N.S.; Malyshkin, D.A.; Ivanov, I.L.; Tsvetkov, D.S.; Zuev, A.Y. Implications of Cation Interdiffusion between Double Perovskite Cathode and Proton-Conducting Electrolyte for Performance of Solid Oxide Fuel Cells. Energies 2023, 16, 2980. https://doi.org/10.3390/en16072980
Tsvetkova NS, Malyshkin DA, Ivanov IL, Tsvetkov DS, Zuev AY. Implications of Cation Interdiffusion between Double Perovskite Cathode and Proton-Conducting Electrolyte for Performance of Solid Oxide Fuel Cells. Energies. 2023; 16(7):2980. https://doi.org/10.3390/en16072980
Chicago/Turabian StyleTsvetkova, Nadezhda S., Dmitry A. Malyshkin, Ivan L. Ivanov, Dmitry S. Tsvetkov, and Andrey Yu. Zuev. 2023. "Implications of Cation Interdiffusion between Double Perovskite Cathode and Proton-Conducting Electrolyte for Performance of Solid Oxide Fuel Cells" Energies 16, no. 7: 2980. https://doi.org/10.3390/en16072980





