Recent Advances in Ball-Milling-Based Silicon Anodes for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Ball-Milling Method
3. Pure Si
4. Si/C Composite
5. Si/X Composite
6. Si/C/X Composite
7. Si/C Composite Followed by Additional Treatments
8. SiO-Based Materials
9. Other Si-Based Materials
10. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhou, C.-G.; Yang, J.; Xue, S.-C.; Gao, H.-L.; Yan, X.-H.; Huo, Q.-Y.; Wang, S.-W.; Cao, Y.; Yan, J. Advances and challenges in improvement of the electrochemical performance for lead-acid batteries: A comprehensive review. J. Power Sources 2022, 520, 230800. [Google Scholar] [CrossRef]
- Islam, J.; Anwar, R.; Shareef, M.; Zabed, H.M.; Sahu, J.N.; Qi, X.; Khandaker, M.U.; Ragauskas, A.; Boukhris, I.; Rahman, M.R.; et al. Rechargeable metal-metal alkaline batteries: Recent advances, current issues and future research strategies. J. Power Sources 2023, 563, 232777. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, B.; Shen, Y.; Wu, T.; Zang, X.; Zhao, Y.; Zhong, C.; Ma, F.; Hu, W. Comparative study of intrinsically safe zinc-nickel batteries and lead-acid batteries for energy storage. J. Power Sources 2021, 510, 230393. [Google Scholar] [CrossRef]
- Wang, C.; Mu, X.W.; Yu, J.M.; Lu, Z.D.; Han, J. Scalable hierarchical lithiophilic engineering of metal foam enables stable lithium metal batteries. Chem. Eng. J. 2022, 435, 134643. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.-T.; Popovic, J.; Lim, K.; Yin, Y.-X.; Maier, J.; Guo, Y.-G.J.M.T. Towards better Li metal anodes: Challenges and strategies. Mater. Today 2020, 33, 56–74. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhuang, J.C.; Liu, M.Z.; Wang, C.; Zhong, Y.T.; Wang, H.R.; Cheng, X.Q.; Liu, S.; Cao, G.Z.; Li, W.S. Facile and scalable engineering of a heterogeneous microstructure for uniform, stable and fast lithium plating/stripping. J. Mater. Chem. A 2019, 7, 19104–19111. [Google Scholar] [CrossRef]
- Ren, Y.X.; Zhao, T.S.; Jiang, H.R.; Wu, M.C.; Liu, M. A stabilized high-energy Li-polyiodide semi -liquid battery with a dually-protected Li anode. J. Power Sources 2017, 347, 136–144. [Google Scholar] [CrossRef]
- Sun, S.; Wang, C.; Wang, Q.-C.; Liu, Y.; Xie, Q.; Zeng, Z.; Li, X.; Han, J.; Guo, R. Three-in-one oxygen-deficient titanium dioxide in a pomegranate-inspired design for improved lithium storage. J. Colloid Interface Sci. 2023, 633, 546–554. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, X.; He, S.; Huang, S.; Qin, H.; Lou, H.; Hou, X. Artificial solid electrolyte interphase coating to reduce lithium trapping in silicon anode for highly stable lithium storage. Surf. Interfaces 2022, 31, 102029. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, J.; Jang, H.; Lee, J.; Kim, M.G.; Liu, N.; Song, H.-K. Argentophilic pyridinic nitrogen for embedding lithiophilic silver nanoparticles in a three-dimensional carbon scaffold for reversible lithium plating/stripping. J. Mater. Chem. A 2022, 10, 1768–1779. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Chen, H.; Luo, L.; Zhang, Q.; Chen, J.; Chen, S.; Yang, Y. Bulk boron doping and surface carbon coating enabling fast-charging and stable Si anodes: From thin film to thick Si electrodes. J. Mater. Chem. A 2021, 9, 3628–3636. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G.J.M.T. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Li, W.D.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Liao, Y.Q.; Wu, C.; Zhong, Y.T.; Chen, M.; Cai, L.Y.; Wang, H.R.; Liu, X.; Cao, G.Z.; Li, W.S. Highly dispersed Co-Mo sulfide nanoparticles on reduced graphene oxide for lithium and sodium ion storage. Nano Res. 2020, 13, 188–195. [Google Scholar] [CrossRef]
- Ryou, M.H.; Kim, S.H.; Kim, S.W.; Lee, S.Y. A microgrid-patterned silicon electrode as an electroactive lithium host. Energy Env. Sci. 2022, 15, 2581–2590. [Google Scholar] [CrossRef]
- Das, S.; Shamim, S.U.D.; Hossain, M.K.; Ahmed, F.; Hossain, M.A.; Rahman, M.O. A novel silicon-doped 2D Ti2C MXene monolayer as high capacity stable anode material for lithium ion batteries: Insight from density functional theory study. Appl. Surf. Sci. 2022, 600, 154173. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation-approaching the limits of, and going beyond, lithium-ion batteries. Energy Env. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Zuo, X.X.; Zhu, J.; Muller-Buschbaum, P.; Cheng, Y.J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Han, X.; Zhou, W.; Chen, M.; Chen, J.; Wang, G.; Liu, B.; Luo, L.; Chen, S.; Zhang, Q.; Shi, S.; et al. Interfacial nitrogen engineering of robust silicon/MXene anode toward high energy solid-state lithium-ion batteries. J. Energy Chem. 2022, 67, 727–735. [Google Scholar] [CrossRef]
- Shen, D.Z.; Huang, C.F.; Gan, L.H.; Liu, J.; Gong, Z.L.; Long, M.N. Rational Design of Si@SiO2/C Composites Using Sustainable Cellulose as a Carbon Resource for Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 7946–7954. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Zhu, Y.; Guo, C.; Qian, Y. Hydrothermal synthesis of nano-silicon from a silica sol and its use in lithium ion batteries. Nano Res. 2015, 8, 1497–1504. [Google Scholar] [CrossRef]
- Zong, L.; Jin, Y.; Liu, C.; Zhu, B.; Hu, X.; Lu, Z.; Zhu, J. Precise Perforation and Scalable Production of Si Particles from Low-Grade Sources for High-Performance Lithium Ion Battery Anodes. Nano Lett. 2016, 16, 7210–7215. [Google Scholar] [CrossRef]
- Huang, Y.; Hou, X.; Fan, X.; Ma, S.; Hu, S.; Lam, K.-h. Advanced Li-Rich Cathode Collaborated with Graphite/Silicon Anode for High Performance Li-Ion Batteries in Half and Full Cells. Electrochim. Acta 2015, 182, 1175–1187. [Google Scholar] [CrossRef]
- Tang, B.; He, S.; Deng, Y.; Shan, Y.; Qin, H.; Noor, H.; Hou, X. Advanced binder with ultralow-content for high performance silicon anode. J. Power Sources 2023, 556, 232237. [Google Scholar] [CrossRef]
- Liang, J.; Wei, D.; Lin, N.; Zhu, Y.; Li, X.; Zhang, J.; Fan, L.; Qian, Y. Low temperature chemical reduction of fusional sodium metasilicate nonahydrate into a honeycomb porous silicon nanostructure. Chem. Commun. 2014, 50, 6856–6859. [Google Scholar] [CrossRef]
- Zhuang, J.C.; Xu, X.; Peleckis, G.; Hao, W.C.; Dou, S.X.; Du, Y. Silicene: A Promising Anode for Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1606716. [Google Scholar] [CrossRef]
- Hong, J.E.; Lee, Y.; Mo, S.I.; Jeong, H.S.; An, J.H.; Song, H.E.; Oh, J.; Bang, J.; Oh, J.H.; Kim, K.H. Fully Bottom-Up Waste-Free Growth of Ultrathin Silicon Wafer via Self-Releasing Seed Layer. Adv. Mater. 2021, 33, e2103708. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhang, Y.M.; Lin, H.B.; Xia, P.; Cai, X.; Li, X.G.; Li, X.P.; Li, W.S. Porous ZnMn2O4 nanospheres: Facile synthesis through microemulsion method and excellent performance as anode of lithium ion battery. J. Power Sources 2016, 312, 137–145. [Google Scholar] [CrossRef]
- Liu, N.; Wu, H.; McDowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A yolk-shell design for stabilized and scalable li-ion battery alloy anodes. Nano Lett. 2012, 12, 3315–3321. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Sui, X.Y.; Wang, A.C.; Chang, J.Q.; Wang, W.B.; Chen, Z.X.; Jiang, W.Y.; Ma, Y.H.; Zhang, J.Q.; Liu, X.F.; et al. Controlled Production of MoS2 Full-Scale Nanosheets and Their Strong Size Effects. Adv. Mater. Interfaces 2020, 7, 2001130. [Google Scholar] [CrossRef]
- Nzabahimana, J.; Liu, Z.; Guo, S.; Wang, L.; Hu, X. Top-Down Synthesis of Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries: Mechanical Milling and Etching. ChemSusChem 2020, 13, 1923–1946. [Google Scholar] [CrossRef]
- Lee, B.S. A Review of Recent Advancements in Electrospun Anode Materials to Improve Rechargeable Lithium Battery Performance. Polymers 2020, 12, 2035. [Google Scholar] [CrossRef]
- Huang, A.; Ma, Y.; Peng, J.; Li, L.; Chou, S.-l.; Ramakrishna, S.; Peng, S. Tailoring the structure of silicon-based materials for lithium-ion batteries via electrospinning technology. eScience 2021, 1, 141–162. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.K.; Kim, J.H.; Jang, J.H.; Hong, S.K.; Oh, I.H. Constitutive behavior and microstructural evolution in Ti-Al-Si ternary alloys processed by mechanical milling and spark plasma sintering. J. Mater. Res. Technol. 2020, 9, 2247–2258. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Lai, Y.; Yang, Z.; Yang, Q.; Liu, Y.; Zheng, Z.; Liu, Y.; Sun, Y.; Zhong, B.; et al. Revisiting the Preparation Progress of Nano-Structured Si Anodes toward Industrial Application from the Perspective of Cost and Scalability. Adv. Energy Mater. 2022, 12, 2102181. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Qi, G.D.; Pan, Z.F.; Zhang, X.Y.; Miao, X.D.; Xiang, W.; Gao, B. Effect of ball milling with hydrogen peroxide or ammonia hydroxide on sorption performance of volatile organic compounds by biochar from different pyrolysis temperatures. Chem. Eng. J. 2022, 450, 138027. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Caporaso, M.; Tuza, K.; Martina, K.; Calcio Gaudino, E.; Cravotto, G. Nucleophilic Substitutions of 6I-O-Monotosyl-β-cyclodextrin in a Planetary Ball Mill. ACS Sustain. Chem. Eng. 2016, 4, 919–929. [Google Scholar] [CrossRef]
- El-Sayed, T.H.; Aboelnaga, A.; El-Atawy, M.A.; Hagar, M. Ball Milling Promoted N-Heterocycles Synthesis. Molecules 2018, 23, 1348. [Google Scholar] [CrossRef] [Green Version]
- Kubota, K.; Pang, Y.D.; Miura, A.; Ito, H. Redox reactions of small organic molecules using ball milling and piezoelectric materials. Science 2019, 366, 1500–1504. [Google Scholar] [CrossRef]
- Nicholson, W.I.; Barreteau, F.; Leitch, J.A.; Payne, R.; Priestley, I.; Godineau, E.; Battilocchio, C.; Browne, D.L. Direct Amidation of Esters by Ball Milling. Angew. Chem. Int. Ed. 2021, 60, 21868–21874. [Google Scholar] [CrossRef]
- De Bondt, Y.; Liberloo, I.; Roye, C.; Windhab, E.J.; Lamothe, L.; King, R.; Courtin, C.M. The Effect of Wet Milling and Cryogenic Milling on the Structure and Physicochemical Properties of Wheat Bran. Foods 2020, 9, 1755. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying: Nanotechnology, Materials Science and Powder Metallurgy; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Austin, L.G.; Bagga, P. An analysis of fine dry grinding in ball mills. Powder Technol. 1981, 28, 83–90. [Google Scholar] [CrossRef]
- Salur, E.; Aslan, A.; Kuntoğlu, M.; Acarer, M.J.A.P.T. Effect of ball milling time on the structural characteristics and mechanical properties of nano-sized Y2O3 particle reinforced aluminum matrix composites produced by powder metallurgy route. Adv. Powder Technol. 2021, 32, 3826–3844. [Google Scholar] [CrossRef]
- Zhuman, B.; Saepurahman; Anis, S.F.; Hashaikeh, R. Obtaining high crystalline ball milled H-Y zeolite particles with carbon nanostructures as a damping material. Microporous Mesoporous Mater. 2019, 273, 19–25. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D. Ball Milled Nano-Structured Stainless Steel Powders: Fabrication and Characterization; Educreation Publishing: New Delhi, India, 2017. [Google Scholar]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Cevher, O.; Tocoglu, U.; Guler, M.O.; Akbulut, H. Electrochemical Characterization of the Powder Silicon Anodes Reinforced with Graphite Using Planetary Ball Milling. Acta. Phys. Pol. A 2013, 123, 393–395. [Google Scholar] [CrossRef]
- Gauthier, M.; Mazouzi, D.; Reyter, D.; Lestriez, B.; Moreau, P.; Guyomard, D.; Roue, L. A low-cost and high performance ball-milled Si-based negative electrode for high-energy Li-ion batteries. Energy Env. Sci. 2013, 6, 2145–2155. [Google Scholar] [CrossRef]
- Chen, M.; Duan, P.; Zhong, Y.; Wu, Z.; Zhang, Z.; Wang, Y.; Guo, X.; Wang, X. Constructing a Sheet-Stacked Si/C Composite by Recycling Photovoltaic Si Waste for Li-Ion Batteries. Ind. Eng. Chem. Res. 2022, 61, 2809–2816. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Yu, C.; Feng, X. Si nanoplates prepared by ball milling photovoltaic silicon sawdust waste as lithium-ion batteries anode material. Mater. Lett. 2023, 331, 133469. [Google Scholar] [CrossRef]
- Koraag, P.Y.E.; Firdaus, A.M.; Hawari, N.H.; Refino, A.D.; Dempwolf, W.; Iskandar, F.; Peiner, E.; Wasisto, H.S.; Sumboja, A. Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material. Batteries 2022, 8, 165. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-Based Anode Materials for Li-Ion Batteries: A Mini Review. Nanomicro. Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-W.; Kil, H.-S.; Kim, J.; Mochida, I.; Nakabayashi, K.; Rhee, C.K.; Miyawaki, J.; Yoon, S.-H. Highly graphitized carbon from non-graphitizable raw material and its formation mechanism based on domain theory. Carbon 2017, 121, 301–308. [Google Scholar] [CrossRef]
- Wang, C.S.; Wu, G.T.; Zhang, X.B.; Qi, Z.F.; Li, W.Z. Lithium insertion in carbon-silicon composite materials produced by mechanical milling. J. Electrochem. Soc. 1998, 145, 2751–2758. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.; Dahn, J. Lithium insertion in carbons containing nanodispersed silicon. J. Electrochem. Soc. 1995, 142, 326. [Google Scholar] [CrossRef]
- Cabello, M.; Gucciardi, E.; Herran, A.; Carriazo, D.; Villaverde, A.; Rojo, T. Towards a High-Power Si@graphite Anode for Lithium Ion Batteries through a Wet Ball Milling Process. Molecules 2020, 25, 2494. [Google Scholar] [CrossRef]
- Yoshio, M.; Tsumura, T.; Dimov, N. Silicon/graphite composites as an anode material for lithium ion batteries. J. Power Sources 2006, 163, 215–218. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Guler, M.O.; Akbulut, H. Enhancing electrochemical performance of silicon anodes by dispersing MWCNTs using planetary ball milling. Microelectron. Eng. 2013, 108, 169–176. [Google Scholar] [CrossRef]
- Cameán, I.; Garcia, A.B. Graphite materials prepared by HTT of unburned carbon from coal combustion fly ashes: Performance as anodes in lithium-ion batteries. J. Power Sources 2011, 196, 4816–4820. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.Q.; Song, J.H.; Zhang, Y.J.; Shi, Q.; Wang, J.X.; Tian, F.H.; Yuan, S.; Su, Z.; Zhou, C.; et al. Functionalization-assistant ball milling towards Si/graphene anodes in high performance Li-ion batteries. Carbon 2021, 181, 300–309. [Google Scholar] [CrossRef]
- Shi, W.Y.; Wu, H.B.; Baucom, J.; Li, X.Y.; Ma, S.X.; Chen, G.; Lu, Y.F. Covalently Bonded Si-Polymer Nanocomposites Enabled by Mechanochemical Synthesis as Durable Anode Materials. ACS Appl. Mater. Interfaces 2020, 12, 39127–39134. [Google Scholar] [CrossRef]
- Dong, Z.; Gu, H.; Du, W.; Feng, Z.; Zhang, C.; Jiang, Y.; Zhu, T.; Chen, G.; Chen, J.; Liu, Y.; et al. Si/Ti3SiC2 composite anode with enhanced elastic modulus and high electronic conductivity for lithium-ion batteries. J. Power Sources 2019, 431, 55–62. [Google Scholar] [CrossRef]
- Cao, Y.; Scott, B.; Dunlap, R.A.; Wang, J.; Obrovac, M.N. An Investigation of the Fe-Mn-Si System for Li-Ion Battery Negative Electrodes. J. Electrochem. Soc. 2019, 166, A21–A26. [Google Scholar] [CrossRef]
- Liu, X.; Dai, Y.; Xie, J.Y.; Zhao, H.L.; Lv, P.P.; Wang, K.; Swierczek, K. Improvement of silicon-based electrode for Li-ion batteries by formation of Si-TiB2-C nanocomposites. Solid State Ion. 2015, 281, 60–67. [Google Scholar] [CrossRef]
- Pan, W.; Cai, X.; Yang, C.; Zhou, L. Amorphous Si/TiC/Graphite Composite Fabricated by High-Energy Ball-Milling as an Anode for Lithium-Ion Batteries. J. Electron. Mater. 2021, 50, 2584–2593. [Google Scholar] [CrossRef]
- Sbrascini, L.; Staffolani, A.; Bottoni, L.; Darjazi, H.; Minnetti, L.; Minicucci, M.; Nobili, F. Structural and Interfacial Characterization of a Sustainable Si/Hard Carbon Composite Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 33257–33273. [Google Scholar] [CrossRef]
- Liu, S.; Tao, W.; Yu, Y.; Fakudze, S.; Wang, C.; Wang, J.; Han, J.; Chen, J. Ball milling synthesis of robust sandwich-structured C/Si@SnO2 anode with porous silicon buffer layer for fast charging lithium-ion battery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130088. [Google Scholar] [CrossRef]
- He, Y.; Ye, Z.; Chamas, M.; Sougrati, M.T.; Lippens, P.-E. Si/Cu-Zn(ox)/C composite as anode material for Li-ion batteries. Solid State Ion. 2021, 372, 115774. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Wang, R.; Tan, G.; Su, Y.; Wu, F. Preparation and electrochemical performance of porous Si/SiOx/G composite anode for lithium ion batteries. IOP Conf. Ser. Mater. Sci. Eng. 2020, 735, 012015. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, M.; Dong, Y.; Ma, L.; Zhang, Y.; Niu, S.; Wei, L. Facile preparation of micron-sized silicon-graphite-carbon composite as anode material for high-performance lithium-ion batteries. Powder Technol. 2022, 404, 117455. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, I.T.; Hur, J. Core-shell Si@c-PAN particles deposited on graphite as promising anode for lithium-ion batteries. Electrochim. Acta 2019, 297, 355–364. [Google Scholar] [CrossRef]
- Nulu, A.; Nulu, V.; Sohn, K.Y. Silicon and porous MWCNT composite as high capacity anode for lithium-ion batteries. Korean J. Chem. Eng. 2020, 37, 1795–1802. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Y.; Ru, Q.; Yan, H.; Chen, F.; Ling, F.C.-C. Scalable preparation of porous nano-silicon/TiN@carbon anode for lithium-ion batteries. Appl. Surf. Sci. 2019, 498, 143829. [Google Scholar] [CrossRef]
- Chung, W.-Y.; Brahma, S.; Hou, S.-C.; Chang, C.-C.; Huang, J.-L. Petroleum waste hydrocarbon resin as a carbon source modified on a Si composite as a superior anode material in lithium ion batteries. Mater. Chem. Phys. 2021, 259, 124011. [Google Scholar] [CrossRef]
- Miao, R.; Zhu, J.; Kang, S.; Yang, J.; Wang, J.; Fu, J.; Li, M.; Shi, C. In-situ mechanochemical synthesis of sub-micro Si/Sn@SiOx-C composite as high-rate anode material for lithium-ion batteries. Electrochim. Acta 2021, 384, 138413. [Google Scholar] [CrossRef]
- Wu, Y.S.; Yang, C.C.; Wu, S.H.; Wu, Z.H.; Wei, C.N.; Yang, M.Y.; Lue, S.J. Preparation of ternary hierarchical silicon/reduced graphene oxide/carbon composites as anodes for lithium–ion batteries. J. Alloys Compd. 2019, 793, 433–445. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Z.; Yang, Y.; Yang, Y.; Jin, H.; Hou, G.; Yuan, F. Mussel-pearl-inspired design of Si/C composite for ultrastable lithium storage anodes. J. Alloys Compd. 2021, 872, 159717. [Google Scholar] [CrossRef]
- Wang, X.; Wen, K.; Chen, T.; Chen, S.; Zhang, S. Supercritical fluid-assisted preparation of Si/CNTs@FG composites with hierarchical conductive networks as a high-performance anode material. Appl. Surf. Sci. 2020, 522, 146507. [Google Scholar] [CrossRef]
- Dong, H.; Fu, X.; Wang, J.; Wang, P.; Ding, H.; Song, R.; Wang, S.; Li, R.; Li, S. In-situ construction of porous Si@C composites with LiCl template to provide silicon anode expansion buffer. Carbon 2021, 173, 687–695. [Google Scholar] [CrossRef]
- Li, J.Y.; Li, G.; Zhang, J.; Yin, Y.X.; Yue, F.S.; Xu, Q.; Guo, Y.G. Rational Design of Robust Si/C Microspheres for High-Tap-Density Anode Materials. ACS Appl. Mater. Interfaces 2019, 11, 4057–4064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, S.; Zhang, W.; Xu, J.; Wang, X.; Fang, C.; Li, Q.; Han, J. Internally inflated core-buffer-shell structural Si/EG/C composites as high-performance anodes for lithium-ion batteries. Sci. China Mater. 2022, 65, 2949–2957. [Google Scholar] [CrossRef]
- Miyachi, M.; Yamamoto, H.; Kawai, H.; Ohta, T.; Shirakata, M. Analysis of SiO Anodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2089. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhang, Q.; Su, X. Recent advancement of SiOx based anodes for lithium-ion batteries. J. Power Sources 2017, 363, 126–144. [Google Scholar] [CrossRef]
- Ouyang, P.H.; Jin, C.X.; Xu, G.J.; Yang, X.X.; Kong, K.J.; Liu, B.B.; Dan, J.L.; Chen, J.; Yue, Z.H.; Li, X.M.; et al. Lithium ion batteries with enhanced electrochemical performance by using carbon-coated SiOx/Ag composites as anode material. Ceram. Int. 2021, 47, 1086–1094. [Google Scholar] [CrossRef]
- Yuan, T.; Tang, R.; Xiao, F.; Zuo, S.; Wang, Y.; Liu, J. Modifying SiO as a ternary composite anode material((SiOx/G/SnO2)@C) for Lithium battery with high Li-ion diffusion and lower volume expansion. Electrochim. Acta 2023, 439, 141655. [Google Scholar] [CrossRef]
- Wang, P.; Hou, S.; Pang, F.; Liu, M.; Li, Y.X.; Liu, T.Z.; Luo, Y.Z.; Fan, Y.M.; Zhao, L.Z. SiOx/C-Decorated CoO Nanosheets as a Long-life Anode for Lithium-Ion Batteries. Chemelectrochem 2019, 6, 1574–1581. [Google Scholar] [CrossRef]
- Zhang, K.; Du, W.; Qian, Z.; Lin, L.; Gu, X.; Yang, J.; Qian, Y. SiOx embedded in N-doped carbon nanoslices: A scalable synthesis of high-performance anode material for lithium-ion batteries. Carbon 2021, 178, 202–210. [Google Scholar] [CrossRef]
- Si, Q.; Hanai, K.; Ichikawa, T.; Phillipps, M.B.; Hirano, A.; Imanishi, N.; Yamamoto, O.; Takeda, Y. Improvement of cyclic behavior of a ball-milled SiO and carbon nanofiber composite anode for lithium-ion batteries. J. Power Sources 2011, 196, 9774–9779. [Google Scholar] [CrossRef]
- Zheng, C.-H.; Zhang, G.-P.; Wang, S.-S.; Mao, A.-Q.; Fang, D.-L. Efficient transformation of rice husk to a high-performance Si@SiO2@C anode material by a mechanical milling and molten salt coactivated magnesiothermic reduction. J. Alloy. Compd. 2021, 875, 159974. [Google Scholar] [CrossRef]
- Yang, M.; Jin, L.; He, M.; Yi, Z.; Duan, T.; Yao, W. SiOx@C composites obtained by facile synthesis as anodes for lithium- and potassium-ion batteries with excellent electrochemical performance. Appl. Surf. Sci. 2021, 542, 148712. [Google Scholar] [CrossRef]
- Xu, D.; Chen, W.; Luo, Y.; Wei, H.; Yang, C.; Cai, X.; Fang, Y.; Yu, X. Amorphous TiO2 layer on silicon monoxide nanoparticles as stable and scalable core-shell anode materials for high performance lithium ion batteries. Appl. Surf. Sci. 2019, 479, 980–988. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, K.; Jung, W.-S.; Choi, H.; Kim, J.-H. Facile and scalable synthesis of SiOx materials for Li-ion negative electrodes. J. Power Sources 2019, 436, 226883. [Google Scholar] [CrossRef]
- Park, D.; Kim, H.-S.; Seo, H.; Kim, K.; Kim, J.-H. Porous SiO composite tailored by scalable mechanochemical oxidation of Si for Li-ion anodes. Electrochim. Acta 2020, 357, 136862. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, X.; Liu, L.; Zhang, Z.; Teng, Y.; Yu, D.; Sui, J.; Wang, X.J.C. SiO2/C Composite Derived from Rice Husks with Enhanced Capacity as Anodes for Lithium-Ion Batteries. ChemistrySelect 2018, 3, 10338–10344. [Google Scholar] [CrossRef]
- Park, B.H.; Haghighat-Shishavan, S.; Nazarian-Samani, M.; Kim, K.-B. High-performance silicon diphosphide/nanocarbon composite anode for Li-ion batteries: Role of chemical bonding and interfaces in the establishment of cycling stability. J. Power Sources 2019, 434, 226759. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Shen, P.; Zhou, Y.; Soule, L.; Li, Y.; Wu, Y.; Zhang, H.; Liu, M. Yolk-shell structured CuSi2P3@Graphene nanocomposite anode for long-life and high-rate lithium-ion batteries. Nano Energy 2021, 80, 105506. [Google Scholar] [CrossRef]
- Li, W.W.; Liao, J.; Li, X.W.; Zhang, L.; Zhao, B.T.; Chen, Y.; Zhou, Y.C.; Cuo, Z.P.; Liu, M.L. Zn(Cu)Si2+xP3 Solid Solution Anodes for High-Performance Li-Ion Batteries with Tunable Working Potentials. Adv. Funct. Mater. 2019, 29, 1903638. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Li, Y.; Li, W. AlSixP: A new family of ternary Si-based anodes for Li-ion batteries with superior Li-storage properties. Ceram. Int. 2023, 49, 1535–1539. [Google Scholar] [CrossRef]
- Chen, D.; Yi, R.; Chen, S.; Xu, T.; Gordin, M.L.; Wang, D. Facile synthesis of graphene–silicon nanocomposites with an advanced binder for high-performance lithium-ion battery anodes. Solid State Ion. 2014, 254, 65–71. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Xi, B.J.; Xiong, S.L.; Qian, Y.T. Carbon coated SiO nanoparticles embedded in hierarchical porous N-doped carbon nanosheets for enhanced lithium storage. Inorg.Chem. Front. 2021, 8, 4282–4290. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Liu, C.; Li, D.; Kim, H.-K.; Harris, C.; Lao, C.-Y.; Abdelkader, A.; Xi, K. Facile mechanochemical synthesis of non-stoichiometric silica-carbon composite for enhanced lithium storage properties. J. Alloys Compd. 2019, 801, 658–665. [Google Scholar] [CrossRef]
- Ruttert, M.; Siozios, V.; Winter, M.; Placke, T. Mechanochemical Synthesis of Fe–Si-Based Anode Materials for High-Energy Lithium Ion Full-Cells. ACS Appl. Energy Mater. 2019, 3, 743–758. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-Y.; Wu, C.-Y.; Duh, J.-G. Facile synthesis of boron-doped graphene-silicon conductive network composite from recycling silicon for Lithium-ion batteries anodes materials. Mater. Lett. 2021, 296, 129875. [Google Scholar] [CrossRef]
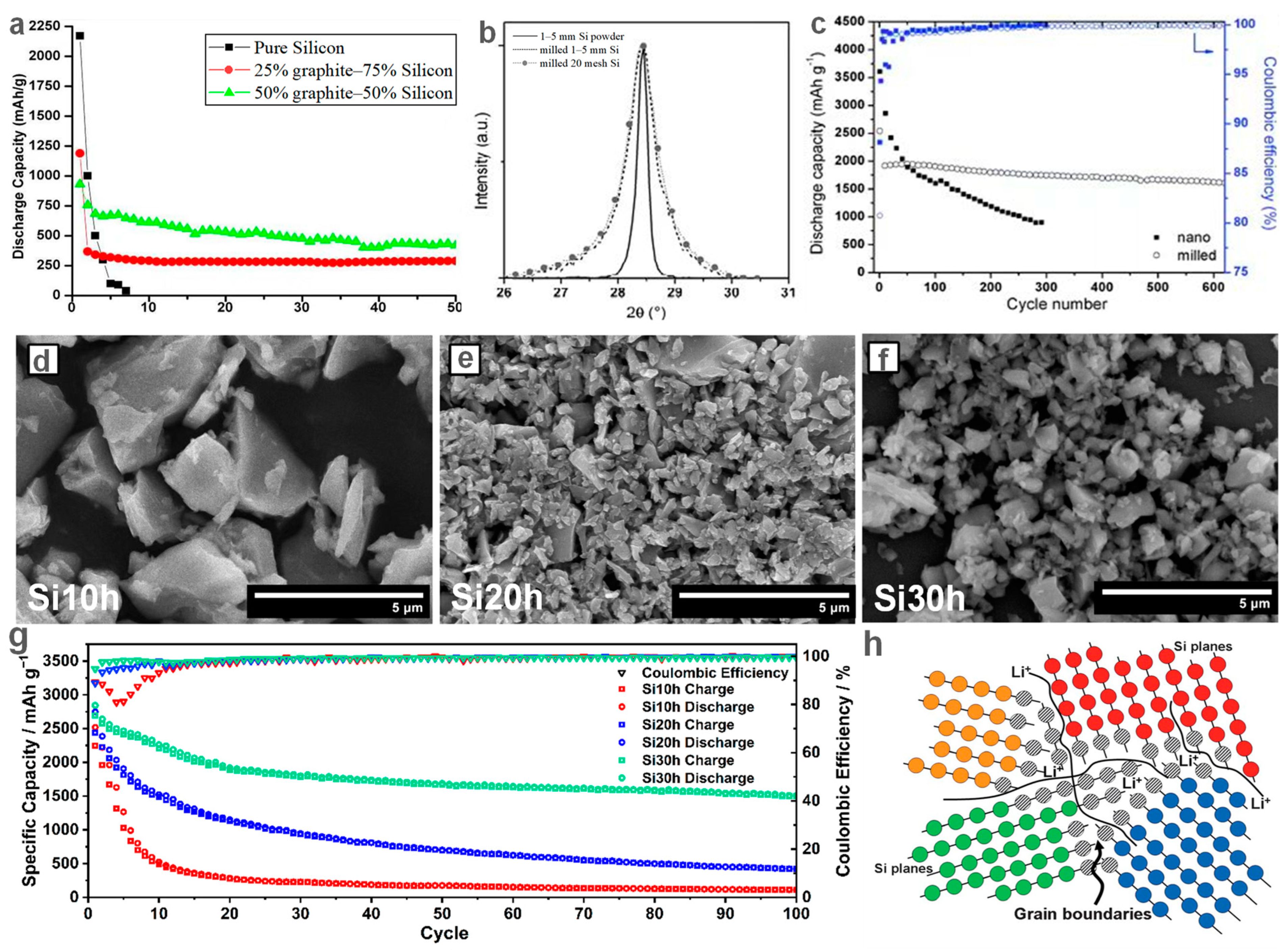

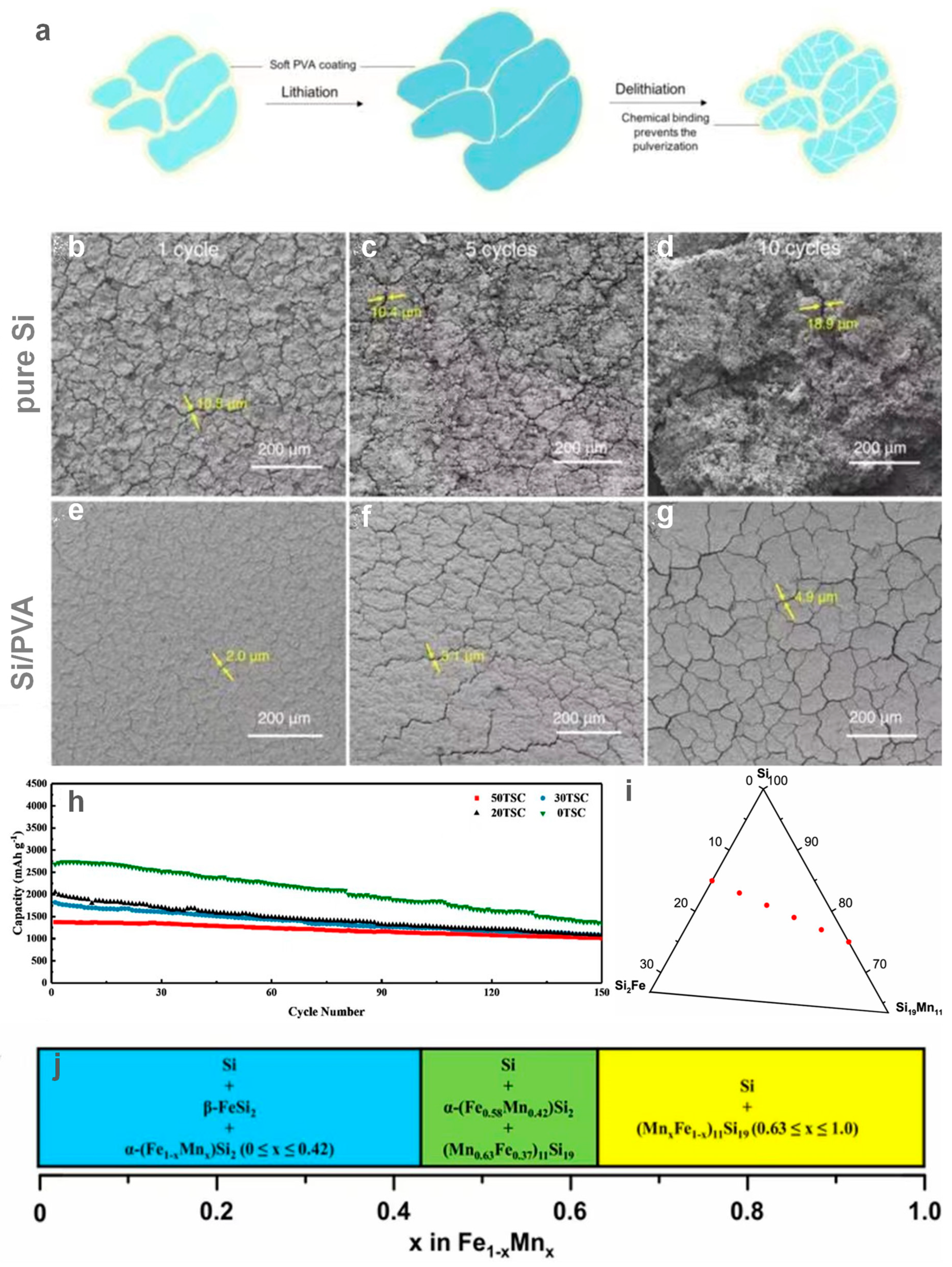

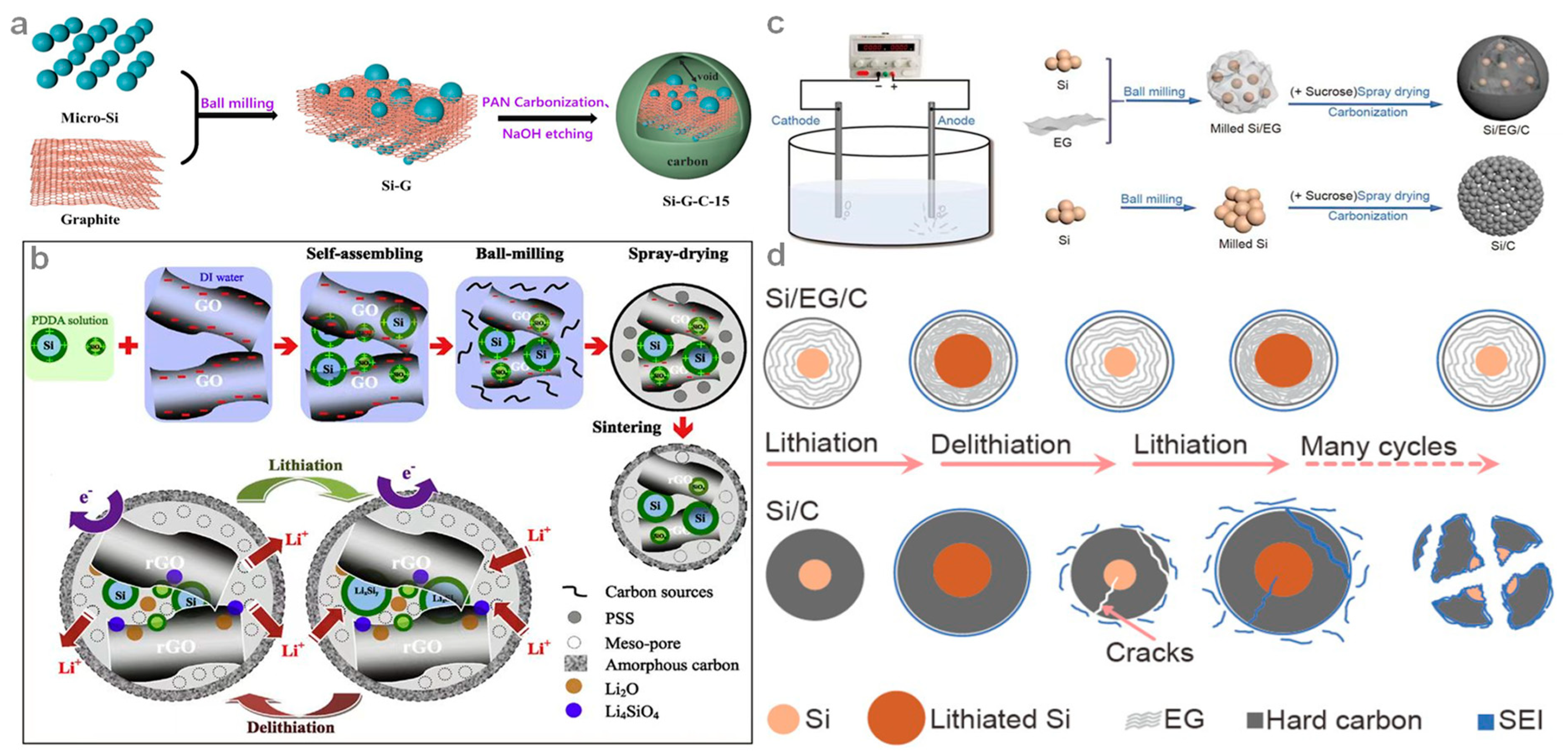
| Material | Current Density (mA g−1) | ICE (%) | Reversible Capacity (mAh g−1) | Cycle Number | Year | Ref. |
|---|---|---|---|---|---|---|
| 50:50 wt% Gr/Si | 300 | / | 418.5 | 50 | 2012 | [50] |
| Milled Si | / | 81 | 1600 | 600 | 2013 | [51] |
| Si/CNT | 400 | 98.06 | 1761 | 100 | 2022 | [54] |
| Si/C | 45 | / | 1039 | 20 | 1998 | [57] |
| Wet Si@Graphite | 250 | 77 | 850 | 100 | 2020 | [59] |
| Si/MWCNT | 200 | 66 | 750 | 30 | 2013 | [61] |
| Si@APTES/f-Gr | 1000 | 61.23 | 1151.5 | 1000 | 2021 | [63] |
| Si-5% PVA | 200 | 86 | 1526 | 100 | 2020 | [64] |
| Si/TiB2/C | 100 | 71.7 | 662 | 60 | 2015 | [67] |
| Si/TiC/Graphite | 1500 | / | 501.5 | 300 | 2021 | [68] |
| Si/Corn Cob-carbon | 1000 | 53.24 | 800 | 1800 | 2022 | [69] |
| C/Si@SnO2 | 100 | 77.96 | 919.21 | 200 | 2022 | [70] |
| Si/Cu-Zn(ox)/Cu | 200 | 50 | 800 | 100 | 2021 | [71] |
| Si/SiOx/Graphite | 100 | 62.2 | 804.2 | 100 | 2020 | [72] |
| Si-Graphite-Carbon | 200 | / | 965 | 100 | 2022 | [73] |
| Si@c-PAN@G | 200 | 83 | 1422 | 50 | 2019 | [74] |
| SiCNT | 1 C | 83.7 | 1685 | 80 | 2020 | [75] |
| nano-Si/TiN@carbon | 500 | 71.4 (100 mA g−1) | 852.82 | 800 | 2019 | [76] |
| CPC/Si | 0.1 C | 80.5 | 1402 | 100 | 2021 | [77] |
| Si/CNTs@FG | 200 | 62.3 | 1204 | 100 | 2020 | [81] |
| Si/rGO/C | 400 | / | 602 | 500 | 2019 | [79] |
| Si/C | 200 | 89.4 | 670 | 850 | 2021 | [80] |
| Porous Si@C | 0.2 C | 76.4 | 680 | 50 | 2021 | [82] |
| Si/EG/C | 500 | 85 | 834 | 500 | 2022 | [84] |
| SiOx/Ag/C | 0.5 C | 71.5 | 1102.7 | 150 | 2021 | [87] |
| SiOx/G/SnO2 | 100 | 62.2 | 424.6 | 110 | 2022 | [88] |
| SiOx/C@CoO | 1000 | 68.1 | 1079 | 250 | 2019 | [89] |
| C nanoslices-SiOx | 1000 | 66.1 | 900 | 600 | 2021 | [90] |
| SiO/C-nanofiber | 0.1 C | 35.7 | 700 | 200 | 2011 | [91] |
| Si@SiO2@C | 1000 | 54 | 973 | 200 | 2021 | [92] |
| SiOx@C(SC) | 200 | / | 729.5 | 200 | 2021 | [93] |
| SiO@TiO2 | 200 | 79.4 | 901 | 200 | 2019 | [94] |
| SiOx | 100 | 71.7 | 1270 | 100 | 2019 | [95] |
| SiO | 100 | 48.9 | 783 | 200 | 2020 | [96] |
| SiP2/nanocarbon | 500 | 88 (100 mA g−1) | 1515 | 300 | 2019 | [98] |
| CuSi2P3@G | 2000 | / | 1394 | 1500 | 2021 | [99] |
| ZnSi2P3/C | 300 | 92 | 1955 | 500 | 2019 | [100] |
| AlSiP | 100 | / | 1580 | 30 | 2023 | [101] |
| Si/Graphene | 400 | 84.6 | 484 | 50 | 2014 | [102] |
| SiO@C/CNS | 1000 | 51 (100 mA g−1) | 690 | 400 | 2021 | [103] |
| Si/Sn@SiOx-C | 2000 | 71.8 | 1073 | 500 | 2021 | [78] |
| SiOx/C | 100 | / | 550 | 180 | 2019 | [104] |
| Fe14Si86 | 0.5 C | 92 (0.1 C) | / | 100 | 2020 | [105] |
| BG/Si | 500 | 82.5 | 1110 | 100 | 2021 | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Lin, S.; Cheng, A.; He, F.; Wang, Z.; Wu, Y.; Zhang, Y.; Liu, X. Recent Advances in Ball-Milling-Based Silicon Anodes for Lithium-Ion Batteries. Energies 2023, 16, 3099. https://doi.org/10.3390/en16073099
Yang H, Lin S, Cheng A, He F, Wang Z, Wu Y, Zhang Y, Liu X. Recent Advances in Ball-Milling-Based Silicon Anodes for Lithium-Ion Batteries. Energies. 2023; 16(7):3099. https://doi.org/10.3390/en16073099
Chicago/Turabian StyleYang, Han, Shiyu Lin, Alex Cheng, Fangbo He, Zhoulu Wang, Yutong Wu, Yi Zhang, and Xiang Liu. 2023. "Recent Advances in Ball-Milling-Based Silicon Anodes for Lithium-Ion Batteries" Energies 16, no. 7: 3099. https://doi.org/10.3390/en16073099
APA StyleYang, H., Lin, S., Cheng, A., He, F., Wang, Z., Wu, Y., Zhang, Y., & Liu, X. (2023). Recent Advances in Ball-Milling-Based Silicon Anodes for Lithium-Ion Batteries. Energies, 16(7), 3099. https://doi.org/10.3390/en16073099






