Experimental Study of Oxygen Depletion Effects on Soot Morphology and Nanostructure in Coflow Diffusion Aviation Fuel (RP-3) Flames
Abstract
:1. Introduction
2. Experimental Setup
2.1. Burner, Vaporizer, and Flames
2.2. Sampling and Electron Microscope Image Analysis
2.3. Soot Volume Fraction Measurements
2.4. Temperature Measurements
3. Results and Discussion
3.1. Flame Appearance
3.2. Soot Volume Fractions
3.3. Soot Primary Particle Diameter
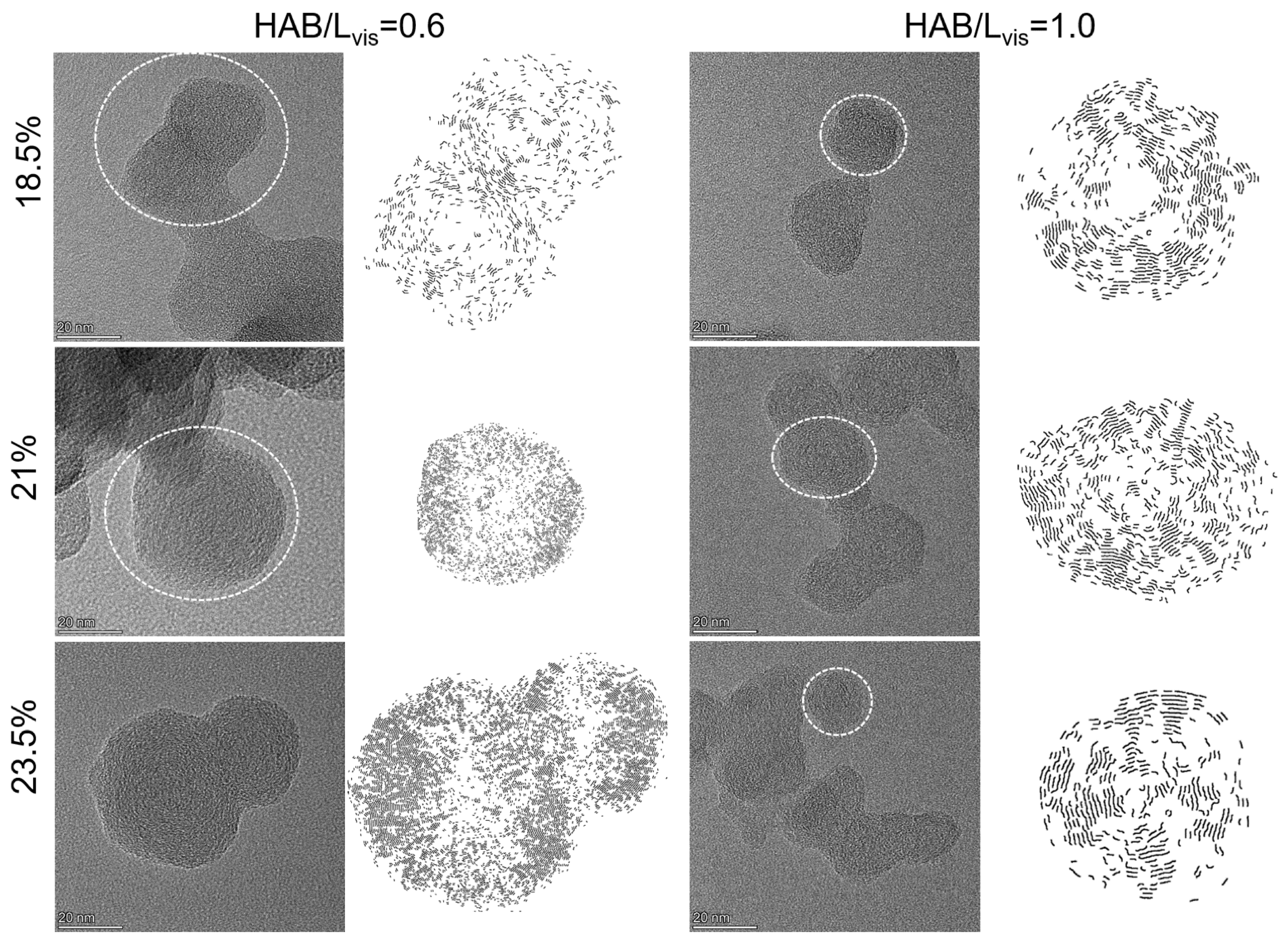
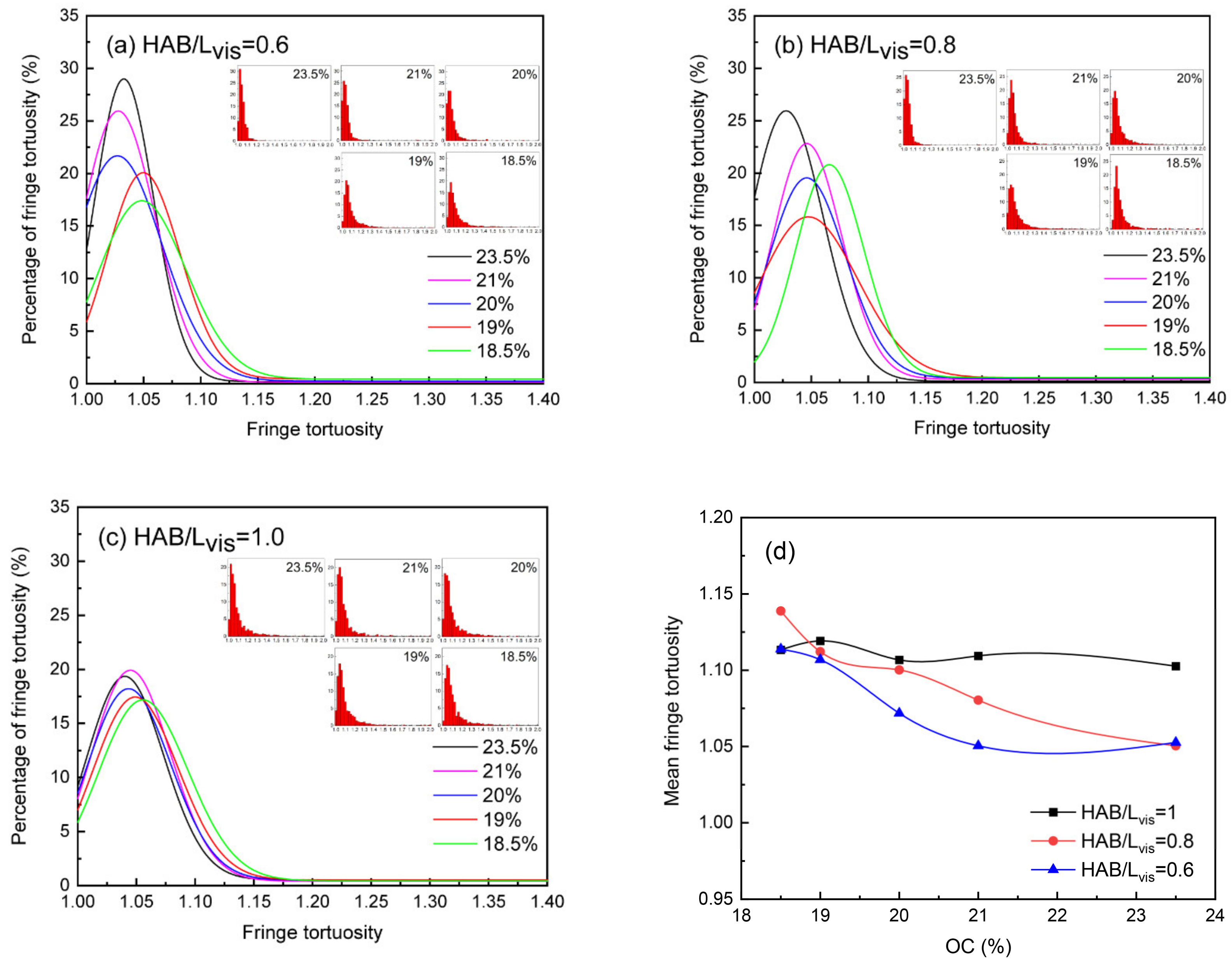
3.4. Soot Nanostructure
4. Conclusions
- (1)
- The peak value of the soot volume fraction of the RP-3 coflow flames increased by 0.73 ppm as the oxygen concentration increased from 21% to 23.5% (a variation of 2.5), and it decreased by 1.25 ppm as the oxygen concentration decreased from 21% to 18.5% (a variation of 2.5).
- (2)
- The soot primary particle diameters in all RP-3 coflow flames showed a trend of increasing and then decreasing in the axial direction. However, when the oxygen concentration decreased from 23.5% to 18.5%, the soot primary particle diameter at the same dimensionless height did not vary linearly. This nonmonotonic trend can be attributed to competition between the two mechanisms. The variation in oxygen concentration leads to the competition between these two mechanisms: one is the change in residence time, and the other is the change in the growth rate of the soot particles. The trend of these two mechanisms changing with oxygen concentration is inconsistent. Therefore, if the oxygen concentration changes by the same value, the diameter change of the primary soot particles will not be linear.
- (3)
- The soot particles in all RP-3 coflow flames showed a trend towards an increased degree of graphitization along the axial direction. In particular, when the oxygen concentration decreased from 23.5% to 18.5%, the graphitization process of soot particles located at the same dimensionless height was slowed down, which can be quantified by a decrease of 0.18 nm in the mean fringe length, an increase of 0.053 in the mean fringe tortuosity, and an increase of 0.035 nm in the mean fringe spacing.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| OC | oxygen concentration, oxygen volume fraction of the oxidant |
| TEM | transmission electron microscopy |
| HRTEM | high-resolution transmission electron microscopy |
| LRTEM | low-resolution transmission electron microscopy |
| LE | laser extinction |
| SLM | standard liters per minute, equivalent to 1 L/min under 273.15 K and 1 atm |
| sccm | standard cubic centimeter per minute |
| SVF | soot volume fraction |
| ppm | parts per million |
| λ | the wavelength of light |
| Kext | extinction coefficient |
| E(m) | soot absorption function |
| Lvis | visible height |
| HAB | height above the burner |
| M | the number of aromatic rings |
| La | fringe length |
| τ | fringe tortuosity |
| d | fringe spacing |
References
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Karcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.S.; Wagner, H.G. Soot formation. Prog. Energy Combust. Sci. 1981, 7, 229–273. [Google Scholar] [CrossRef]
- Zhigang, L.; Kim, A.K. A Review of Water Mist Fire Suppression Systems—Fundamental Studies. J. Fire Prot. Eng. 1999, 10, 32–50. [Google Scholar] [CrossRef] [Green Version]
- Palash, S.M.; Kalam, M.A.; Masjuki, H.H.; Masum, B.M.; Rizwanul Fattah, I.M.; Mofijur, M. Impacts of biodiesel combustion on Nox emissions and their reduction approaches. Renew. Sustain. Energy Rev. 2013, 23, 473–490. [Google Scholar] [CrossRef]
- Glassman; Yaccarino, P. The Effect of Oxygen Concentration on Sooting Diffusion Flames. Combust. Sci. Technol. 1980, 24, 107–114. [Google Scholar] [CrossRef]
- Lee, K.O.; Megaridis, C.M.; Zelepouga, S.; Saveliev, A.V.; Kennedy, L.A.; Charon, O.; Ammouri, F. Soot formation effects of oxygen concentration in the oxidizer stream of laminar coannular nonpremixed methane/air flames. Combust. Flame 2000, 121, 323–333. [Google Scholar] [CrossRef]
- Leusden, C.P.; Peters, N. Experimental and numerical analysis of the influence of oxygen on soot formation in laminar counterflow flames of acetylene. Proc. Combust. Inst. 2000, 28, 2619–2625. [Google Scholar] [CrossRef]
- Seong, H.J.; Boehman, A.L. Studies of soot oxidative reactivity using a diffusion flame burner. Combust. Flame 2012, 159, 1864–1875. [Google Scholar] [CrossRef]
- Jain, A.; Das, D.D.; McEnally, C.S.; Pfefferle, L.D.; Xuan, Y. Experimental and numerical study of variable oxygen index effects on soot yield and distribution in laminar co-flow diffusion flames. Proc. Combust. Inst. 2019, 37, 859–867. [Google Scholar] [CrossRef]
- Demarco, R.; Jerez, A.; Liu, F.; Chen, L.; Fuentes, A. Modeling soot formation in laminar coflow ethylene inverse diffusion flames. Combust. Flame 2021, 232, 111513. [Google Scholar] [CrossRef]
- Bi, X.; Liu, H.; Huo, M.; Shen, C.; Qiao, X.; Lee, C.-F.F. Experimental and numerical study on soot formation and oxidation by using diesel fuel in constant volume chamber with various ambient oxygen concentrations. Energy Convers. Manag. 2014, 84, 152–163. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Wu, H.; Wang, M.Z.; Lee, C.F.; Liu, J.P.; Fu, J.Q.; Chang, W. Computational Investigation of Oxygen Concentration Effects on a Soot Mechanism with a Phenomenological Soot Model of Acetone-Butanol-Ethanol (ABE). Energy Fuels 2015, 29, 1710–1721. [Google Scholar] [CrossRef]
- Vo, C.; Charoenphonphanich, C.; Karin, P.; Susumu, S.; Hidenori, K. Effects of variable O-2 concentrations and injection pressures on the combustion and emissions characteristics of the petro-diesel and hydrotreated vegetable oil-based fuels under the simulated diesel engine condition. J. Energy Inst. 2018, 91, 1071–1084. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, H.; Zhu, Z.; Lv, D.; Pan, Y.; Wei, H.; Zhuang, J. Effect of intake oxygen concentration on diesel–n-butanol blending combustion: An experimental and numerical study at low engine load. Energy Convers. Manag. 2018, 165, 53–65. [Google Scholar] [CrossRef]
- Sun, Z.; Dally, B.; Alwahabi, Z.; Nathan, G. The effect of oxygen concentration in the co-flow of laminar ethylene diffusion flames. Combust. Flame 2020, 211, 96–111. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ahmed, H.A.; Steinmetz, S.; Dunn, M.J.; Masri, A.R. On the effects of varying coflow oxygen on soot and precursor nanoparticles in ethylene laminar diffusion flames. Fuel 2021, 300, 120913. [Google Scholar] [CrossRef]
- Fuentes, A.; Henríquez, R.; Nmira, F.; Liu, F.; Consalvi, J.-L. Experimental and numerical study of the effects of the oxygen index on the radiation characteristics of laminar coflow diffusion flames. Combust. Flame 2013, 160, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Escudero, F.; Fuentes, A.; Consalvi, J.L.; Liu, F.; Demarco, R. Unified behavior of soot production and radiative heat transfer in ethylene, propane and butane axisymmetric laminar diffusion flames at different oxygen indices. Fuel 2016, 183, 668–679. [Google Scholar] [CrossRef]
- Jerez, A.; Consalvi, J.-L.; Fuentes, A.; Liu, F.; Demarco, R. Soot production modeling in a laminar coflow ethylene diffusion flame at different Oxygen Indices using a PAH-based sectional model. Fuel 2018, 231, 404–416. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, L.; Wu, Z.; Tao, W.; Wang, S.; Ruan, C.; Zhu, L.; Lu, X. Experimental and kinetic modeling study of ignition characteristics of RP-3 kerosene over low-to-high temperature ranges in a heated rapid compression machine and a heated shock tube. Combust. Flame 2019, 203, 157–169. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, J.; Ruan, C.; Wu, Z.; Feng, Y.; Zhu, J.; Wang, S.; Yu, L.; Lu, X. An experimental and kinetic modeling study of a four-component surrogate fuel for RP-3 kerosene. Proc. Combust. Inst. 2021, 38, 555–563. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, L.; Qian, Y.; Wang, S.; Wu, Z.; Raza, M.; Zhu, L.; Hu, X.; Lu, X. Development and validation of a detailed kinetic model for RP-3 aviation fuel based on a surrogate formulated by emulating macroscopic properties and microscopic structure. Combust. Flame 2021, 229, 111401. [Google Scholar] [CrossRef]
- Xue, X.; Hui, X.; Singh, P.; Sung, C.-J. Soot formation in non-premixed counterflow flames of conventional and alternative jet fuels. Fuel 2017, 210, 343–351. [Google Scholar] [CrossRef]
- Saffaripour, M.; Veshkini, A.; Kholghy, M.; Thomson, M.J. Experimental investigation and detailed modeling of soot aggregate formation and size distribution in laminar coflow diffusion flames of Jet A-1, a synthetic kerosene, and n-decane. Combust. Flame 2014, 161, 848–863. [Google Scholar] [CrossRef]
- Li, J.; Gan, Z.; Liang, Y. An experimental investigation of soot morphology and nanostructure in high-pressure co-flow laminar methane diffusion flames. Exp. Therm. Fluid Sci. 2022, 136, 110676. [Google Scholar] [CrossRef]
- Abdalla, A.O.G.; Ying, Y.Y.; Jiang, B.; He, X.M.; Liu, D. Comparative study on characteristics of soot from n-decane and RP-3 kerosene normal/inverse diffusion flames. J. Energy Inst. 2020, 93, 62–75. [Google Scholar] [CrossRef]
- Sun, M.; Gan, Z.; Yang, Y. A Comparison Study of Soot Precursor and Aggregate Property Between Algae-Based Aviation Biofuel and Aviation Kerosene RP-3 in Laminar Flame. J. Energy Resour. Technol. 2021, 143, 112304. [Google Scholar] [CrossRef]
- Sun, M.; Gan, Z.; Yang, Y. Numerical and experimental investigation of soot precursor and primary particle size of aviation fuel (RP-3) and n-dodecane in laminar flame. J. Energy Inst. 2021, 94, 49–62. [Google Scholar] [CrossRef]
- Yang, Y.; Gan, Z. In Soot Morphological Differences between Laminar Diffusion Flames of Traditional Aviation Kerosene and Bio-kerosene. In Proceedings of the Global Power and Propulsion Society, Beijing, China, 16–18 September 2019. [Google Scholar]
- Chang, D.; Li, J.; Yang, Y.; Gan, Z. Soot Morphology and Nanostructure Differences between Chinese Aviation Kerosene and Algae-Based Aviation Biofuel in Free Jet Laminar Diffusion Flames. ACS Omega 2022, 7, 11560–11569. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.M.; Gülder, Ö.L. A multi-probe thermophoretic soot sampling system for high-pressure diffusion flames. Rev. Sci. Instrum. 2016, 87, 055101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, C.D.; Botero, M.L.; Agudelo, A.F.; Agudelo, J.R. An assessment on how different collection methods impact thermal properties, surface functional groups, nanostructure and morphology of diesel particulate matter. Combust. Flame 2021, 225, 74–85. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997. Available online: https://imagej.net/ij/index.html (accessed on 1 February 2022).
- Yehliu, K.; Vander Wal, R.L.; Boehman, A.L. Development of an HRTEM image analysis method to quantify carbon nanostructure. Combust. Flame 2011, 158, 1837–1851. [Google Scholar] [CrossRef]
- Botero, M.L.; Adkins, E.M.; González-Calera, S.; Miller, H.; Kraft, M. PAH structure analysis of soot in a non-premixed flame using high-resolution transmission electron microscopy and optical band gap analysis. Combust. Flame 2016, 164, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Botero, M.L.; Sheng, Y.; Akroyd, J.; Martin, J.; Dreyer, J.A.H.; Yang, W.; Kraft, M. Internal structure of soot particles in a diffusion flame. Carbon 2019, 141, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, H.; Li, X.; Zhang, L.; Li, X. Investigation on the Characteristic of Laser Induced Plasma by Abel Inversion. Spectroscopy and Spectral Analysis. Spectrosc. Spectr. Anal. 2019, 39, 250–256. [Google Scholar]
- Simonsson, J.; Olofsson, N.-E.; Török, S.; Bengtsson, P.-E.; Bladh, H. Wavelength dependence of extinction in sooting flat premixed flames in the visible and near-infrared regimes. Appl. Phys. B 2015, 119, 657–667. [Google Scholar] [CrossRef]
- Yan, F.; Zhou, M.; Xu, L.; Wang, Y.; Chung, S.H. An experimental study on the spectral dependence of light extinction in sooting ethylene counterflow diffusion flames. Exp. Therm. Fluid Sci. 2019, 100, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Hui, X.; Sung, C.-J. Soot formation in non-premixed counterflow flames of butane and butanol isomers. Combust. Flame 2016, 164, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Kholghy, M.R.; Afarin, Y.; Sediako, A.D.; Barba, J.; Lapuerta, M.; Chu, C.; Weingarten, J.; Borshanpour, B.; Chernov, V.; Thomson, M.J. Comparison of multiple diagnostic techniques to study soot formation and morphology in a diffusion flame. Combust. Flame 2017, 176, 567–583. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.H. Soot formation in laminar counterflow flames. Prog. Energy Combust. Sci. 2019, 74, 152–238. [Google Scholar] [CrossRef]
- De, S.; De, A.; Jaiswal, A.; Dash, A. Stabilization of lifted hydrogen jet diffusion flame in a vitiated co-flow: Effects of jet and coflow velocities, coflow temperature and mixing. Int. J. Hydrog. Energy 2016, 41, 15026–15042. [Google Scholar] [CrossRef]
- Mehta, R.S.; Haworth, D.C.; Modest, M.F. Composition PDF/photon Monte Carlo modeling of moderately sooting turbulent jet flames. Combust. Flame 2010, 157, 982–994. [Google Scholar] [CrossRef]
- Du, D.X.; Axelbaum, R.L.; Law, C.K. The influence of carbon dioxide and oxygen as additives on soot formation in diffusion flames. Symp. (Int.) Combust. 1991, 23, 1501–1507. [Google Scholar] [CrossRef]
- Apicella, B.; Pre, P.; Alfe, M.; Ciajolo, A.; Gargiulo, V.; Russo, C.; Tregrossi, A.; Deldique, D.; Rouzaud, J.N. Soot nanostructure evolution in premixed flames by High Resolution Electron Transmission Microscopy (HRTEM). Proc. Combust. Inst. 2015, 35, 1895–1902. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes: Preparation and Properties; CRC Press: Boca Raton, FL, USA, 1996; pp. 42–49. [Google Scholar]
- Vander Wal, R.L.; Tomasek, A.J. Soot oxidation: Dependence upon initial nanostructure. Combust. Flame 2003, 134, 1–9. [Google Scholar] [CrossRef]






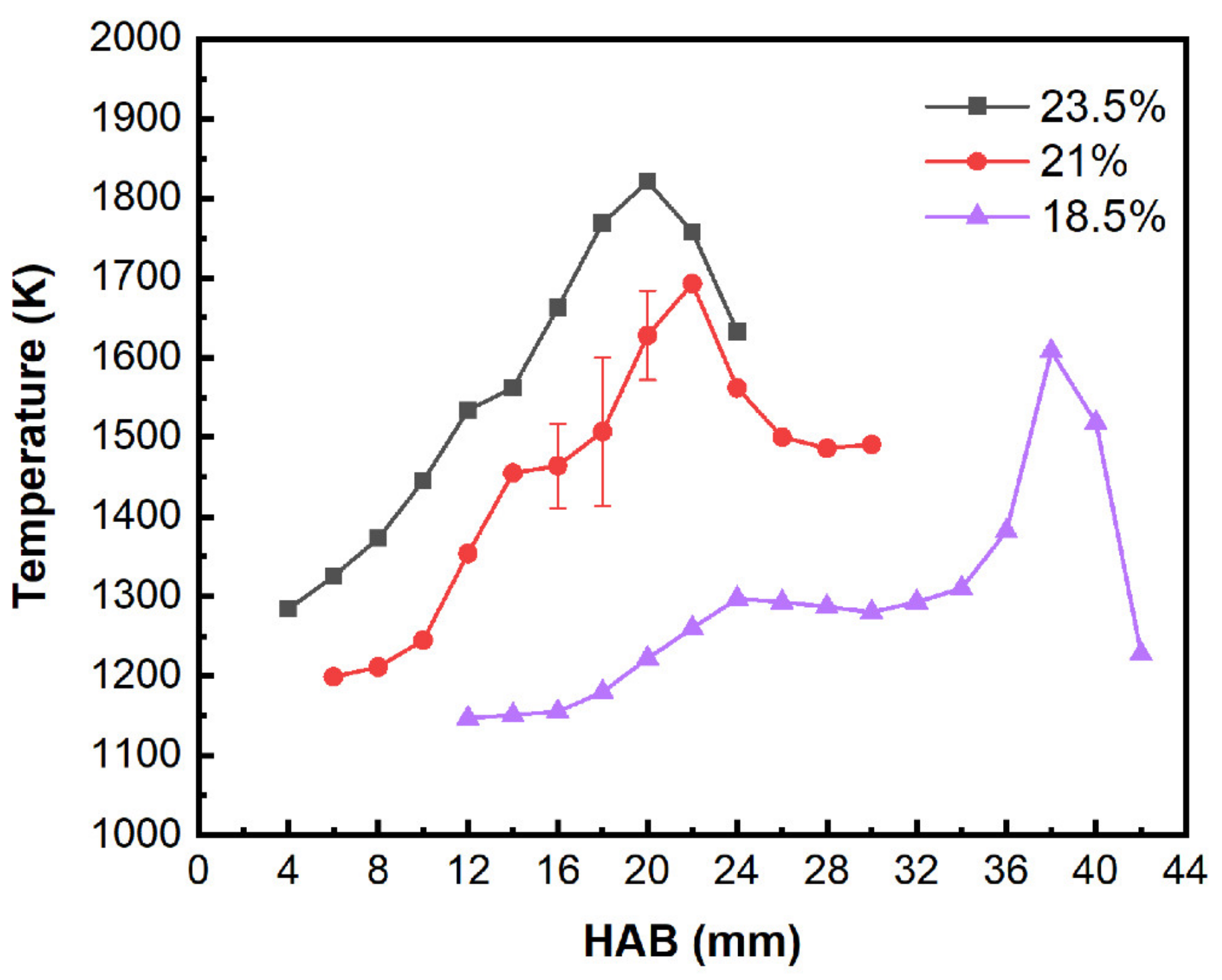
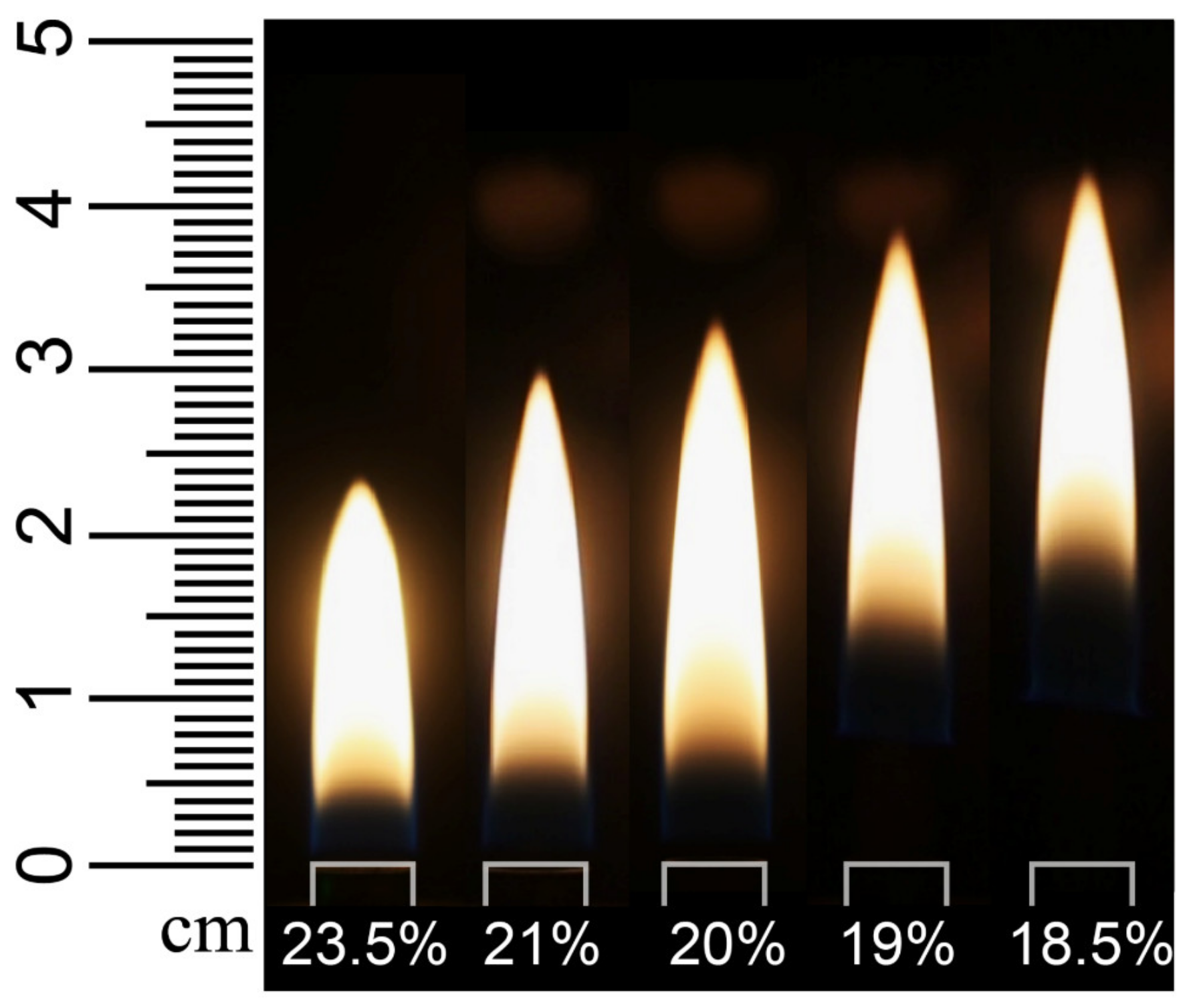
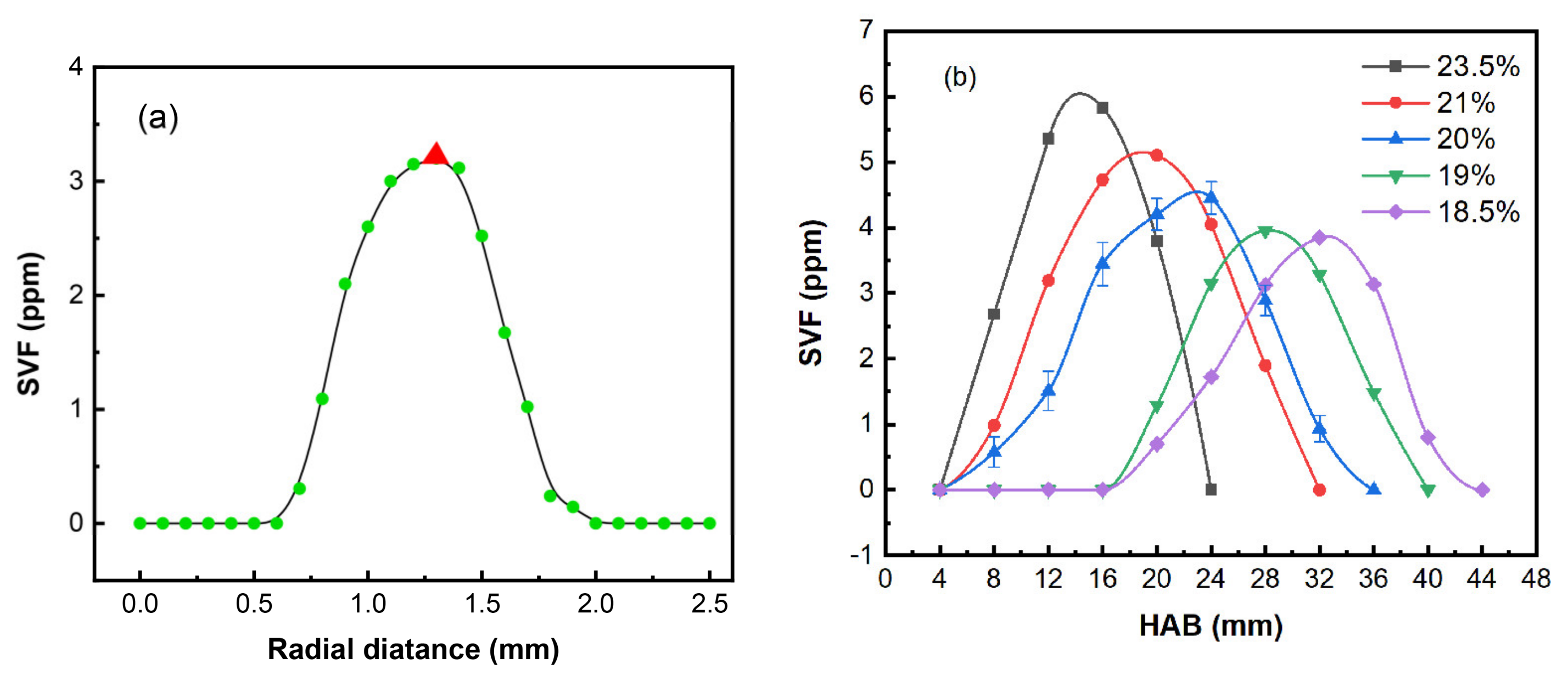
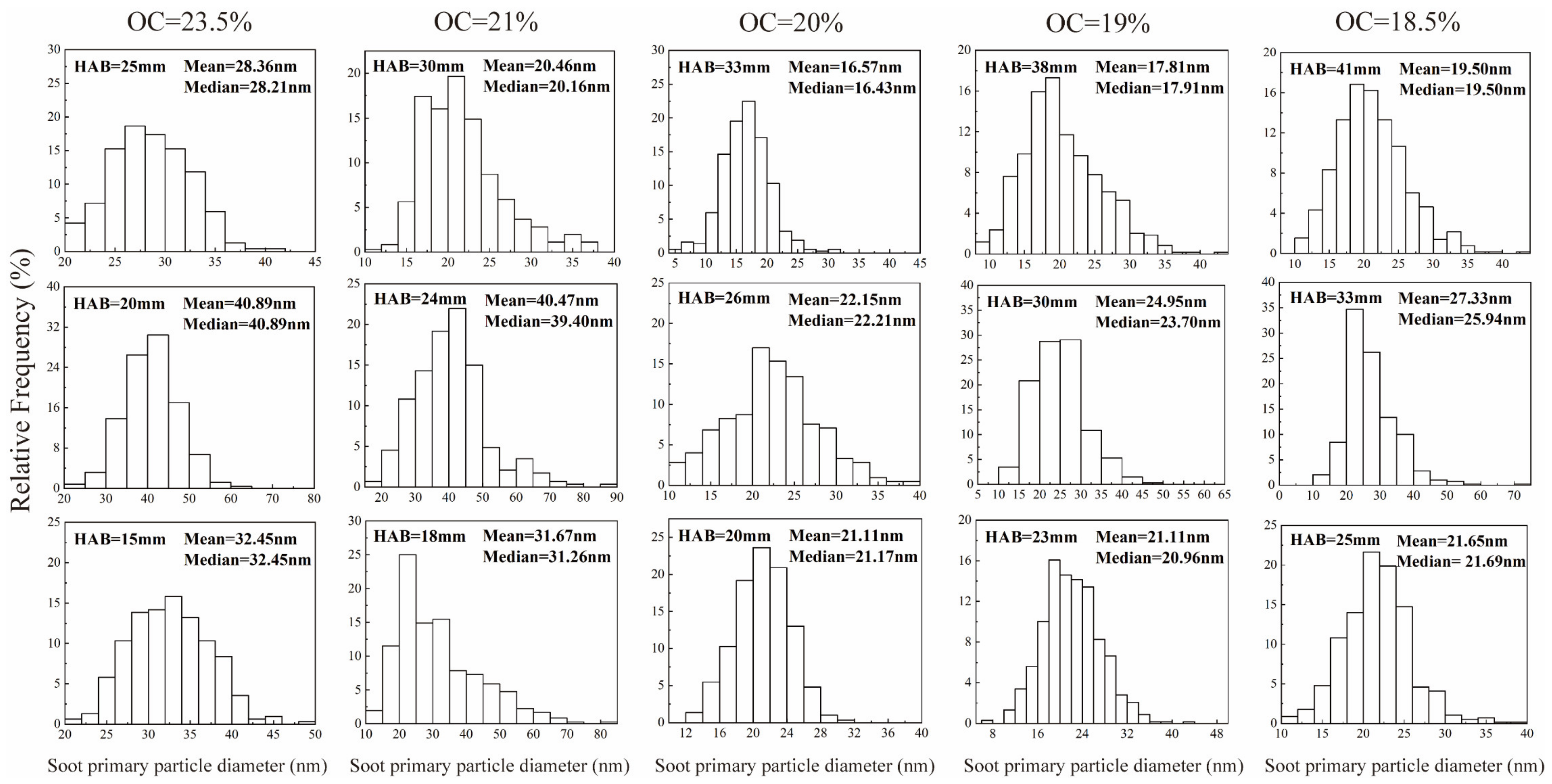



| Year/Author/Ref | Oxygen Concentration Range | Fuel/Flame Type | Object of Study |
|---|---|---|---|
| 2000, Lee et al. [8] | 21%, 50%, 100% | Methane coflow | soot yield, fractal dimensions |
| 2012, Seong and Boehman [10] | 21%, 27%, 30%, 35% | N-heptane coflow | soot oxidative reactivity |
| 2019, Jain et al. [11] | 21%~76.3% | Methane coflow | flame height, soot yield, and soot distribution |
| 2014, Bi et al. [13] | 15%, 18%, 21% (134 Mpa) | Diesel fuel constant volume chamber | soot mass concentration |
| 2020, Sun et al. [17] | 16.8%~36.8% | Ethylene coflow | soot volume fraction, soot primary particle diameter |
| 2021, Ashraf et al. [18] | 19%~40% | Ethylene coflow | soot volume fraction, soot primary particle diameter, and precursor nanoparticle concentration |
| 2019, Mao et al. [22] | 6.96%~20.88% | RP-3 shock tube | kinetic model |
| Chemical Formula | Lower Heating Value (MJ/kg) | Alkane (wt%) | Aromatics (wt%) | Cycloalkanes (wt%) | Olefin (wt%) | Naphthalenes (wt%) | Other Compounds (wt%) |
|---|---|---|---|---|---|---|---|
| C15.06H30.32 | 43.14 | 52.44% | 18.53% | 15.54% | 7.64% | 4.39% | 1.46% |
| Flame No. | Oxidant Stream Total Flow Rate = 6.00 SLM 1 | |||||
|---|---|---|---|---|---|---|
| The Flow Rate of Air (SLM 1) | The Flow Rate of N2 (SLM 1) | The Flow Rate of O2 (SLM 1) | Oxygen Concentration (OC) in the Oxidant Stream (Vol. Frac.) | Visible Flame Height (mm) | Sampling Points (Central Line) (mm) | |
| 1 | 5.81 | 0 | 0.19 | 23.5% | 23 | 14, 18, 23 |
| 2 | 6.00 | 0 | 0 | 21.0% | 30 | 18, 24, 30 |
| 3 | 5.72 | 0.28 | 0 | 20.0% | 33 | 20, 26, 33 |
| 4 | 5.44 | 0.56 | 0 | 19.0% | 38 | 23, 30, 38 |
| 5 | 5.28 | 0.72 | 0 | 18.5% | 41 | 25, 33, 41 |
| Refs | Oxygen Concentration | Fuel Type | Maximum Soot Volume Fraction (ppm) |
|---|---|---|---|
| [11] | 24% | CH4 | 0.17 |
| [20] | 23% | C3H8 | 1.90 |
| This work | 23.5% | RP-3(C15.06H30.32) | 5.83 |
| [11] | 21% | CH4 | 0.12 |
| [20] | 21% | C3H8 | 1.79 |
| This work | 21% | RP-3(C15.06H30.32) | 5.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Gan, Z.; Li, J.; Li, H.; Feng, B.; Xing, X. Experimental Study of Oxygen Depletion Effects on Soot Morphology and Nanostructure in Coflow Diffusion Aviation Fuel (RP-3) Flames. Energies 2023, 16, 3166. https://doi.org/10.3390/en16073166
Guo J, Gan Z, Li J, Li H, Feng B, Xing X. Experimental Study of Oxygen Depletion Effects on Soot Morphology and Nanostructure in Coflow Diffusion Aviation Fuel (RP-3) Flames. Energies. 2023; 16(7):3166. https://doi.org/10.3390/en16073166
Chicago/Turabian StyleGuo, Jiaqi, Zhiwen Gan, Jiacheng Li, Hanjing Li, Bin Feng, and Xinyu Xing. 2023. "Experimental Study of Oxygen Depletion Effects on Soot Morphology and Nanostructure in Coflow Diffusion Aviation Fuel (RP-3) Flames" Energies 16, no. 7: 3166. https://doi.org/10.3390/en16073166
APA StyleGuo, J., Gan, Z., Li, J., Li, H., Feng, B., & Xing, X. (2023). Experimental Study of Oxygen Depletion Effects on Soot Morphology and Nanostructure in Coflow Diffusion Aviation Fuel (RP-3) Flames. Energies, 16(7), 3166. https://doi.org/10.3390/en16073166






