Enhancement of Electrochemical Performance of Aqueous Zinc Ion Batteries by Structural and Interfacial Design of MnO2 Cathodes: The Metal Ion Doping and Introduction of Conducting Polymers †
Abstract
1. Introduction
2. Metal-Ions-Doped Manganese-Dioxide-Based Cathode Materials
2.1. Alkali and Alkaline-Earth Metal Ions
2.2. Transition and Rare-Earth-Metal Ions
2.2.1. Light Transition Metal Ions
2.2.2. Heavy Transition Metal Ions
2.2.3. Rare-Earth-Metal Ions
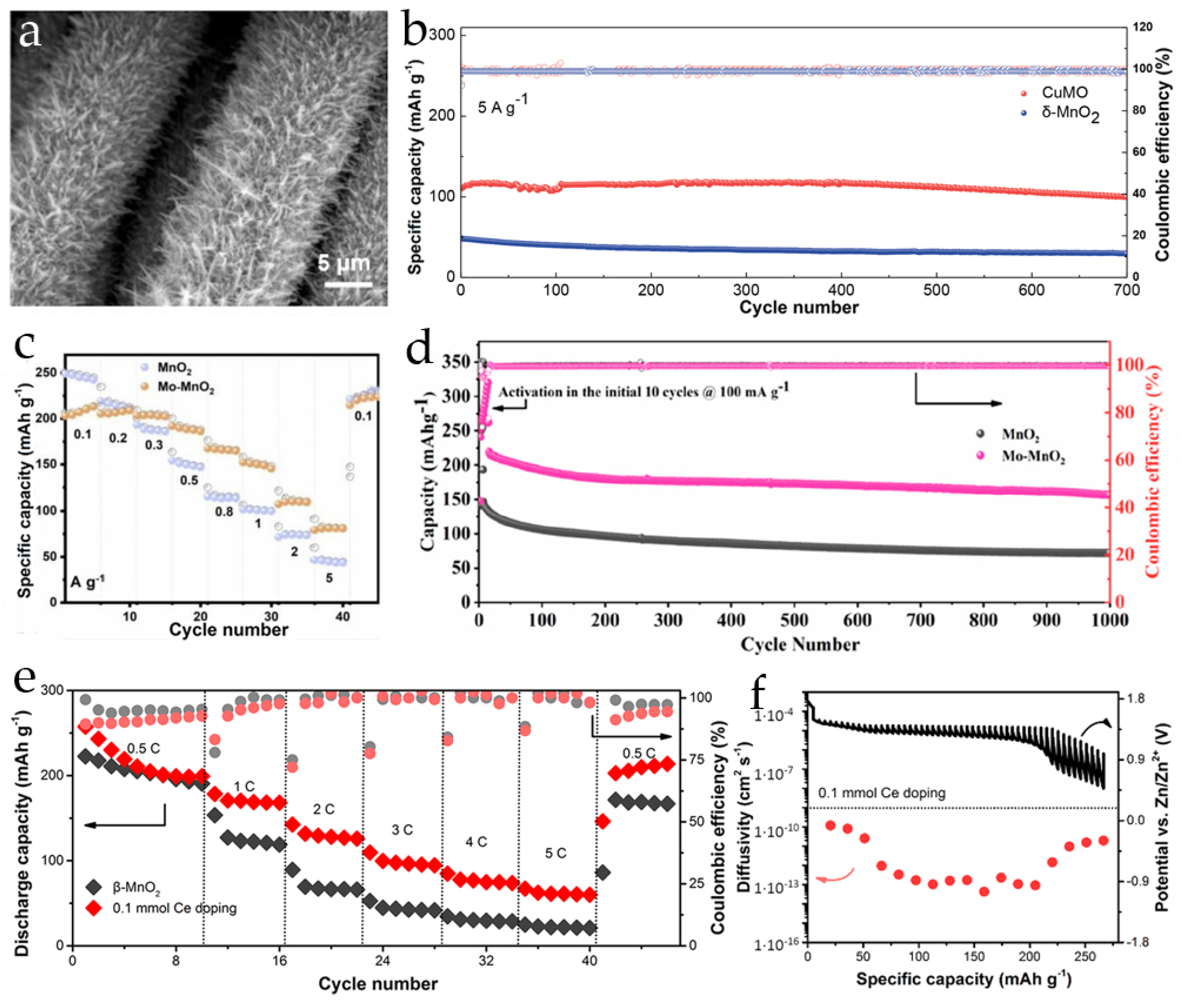
2.3. Post-Transition Metal Ions

3. Manganese-Oxide-Conducting Polymer Composite Cathodes for AZIBs
3.1. Polyaniline-Modified MnO2 Cathodes
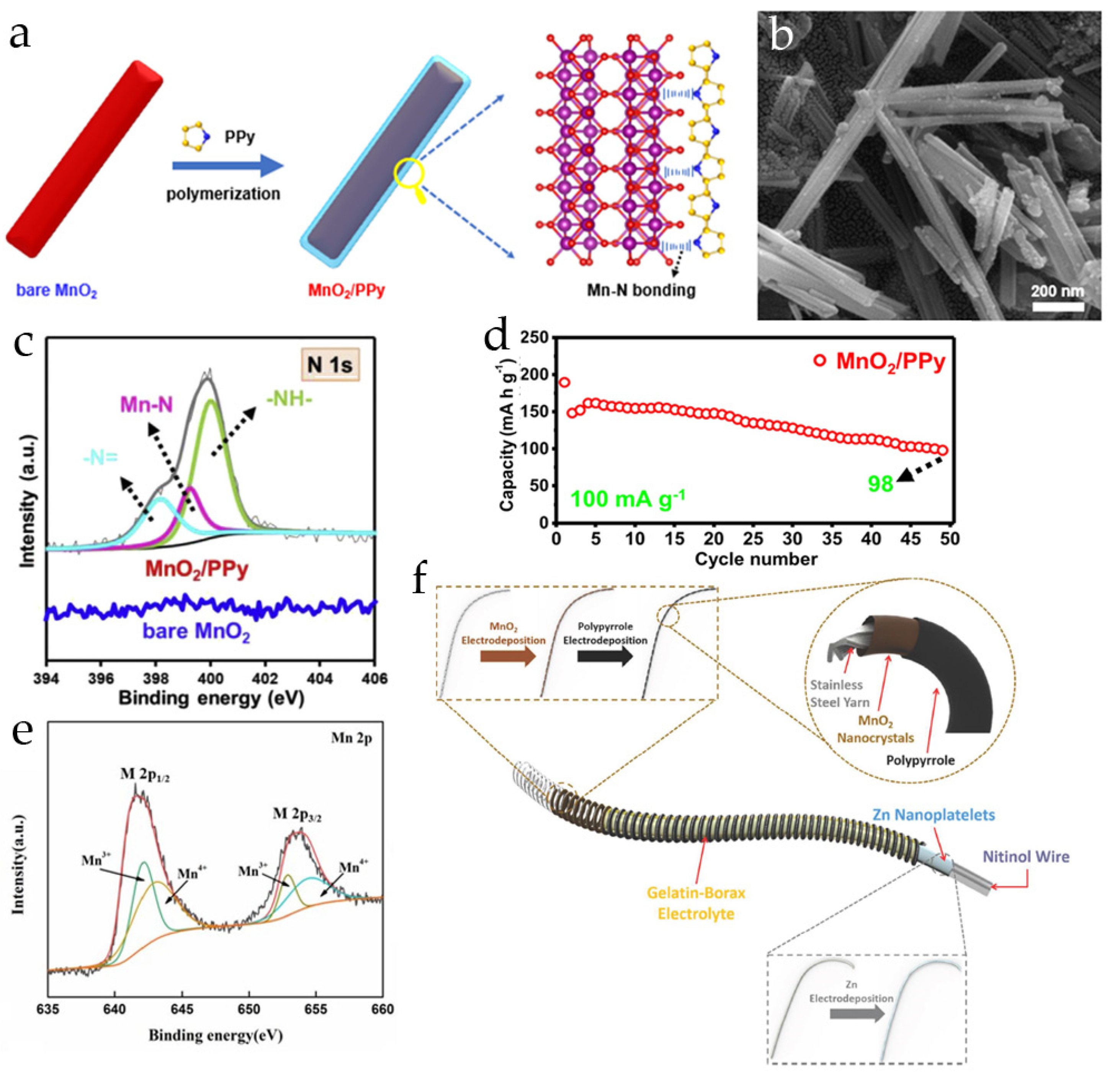
3.2. Polypyrrole-Modified MnO2 Cathodes
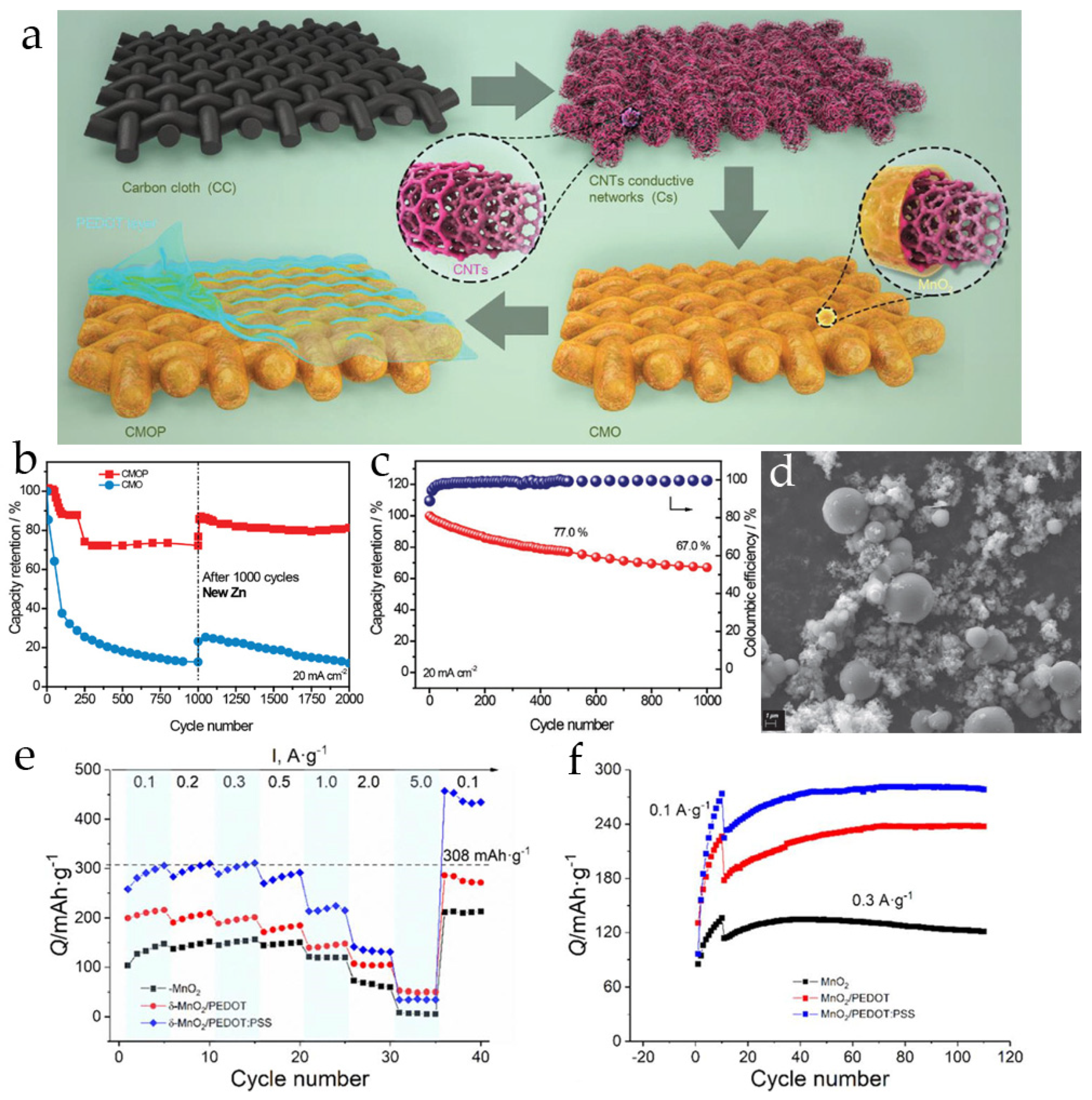
3.3. Poly(3,4-Ethylenedioxythiophene)-Modified MnO2 Cathodes
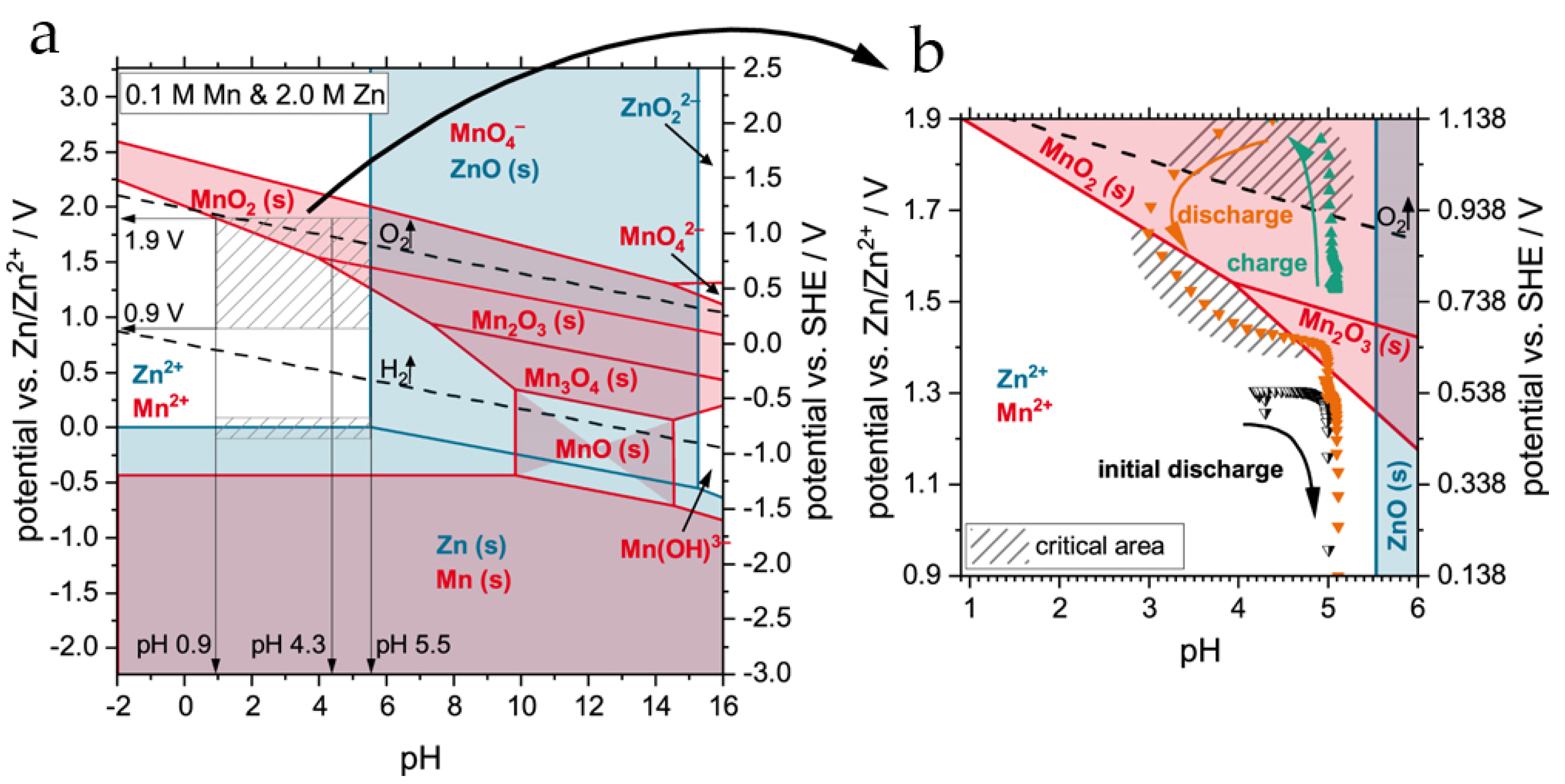
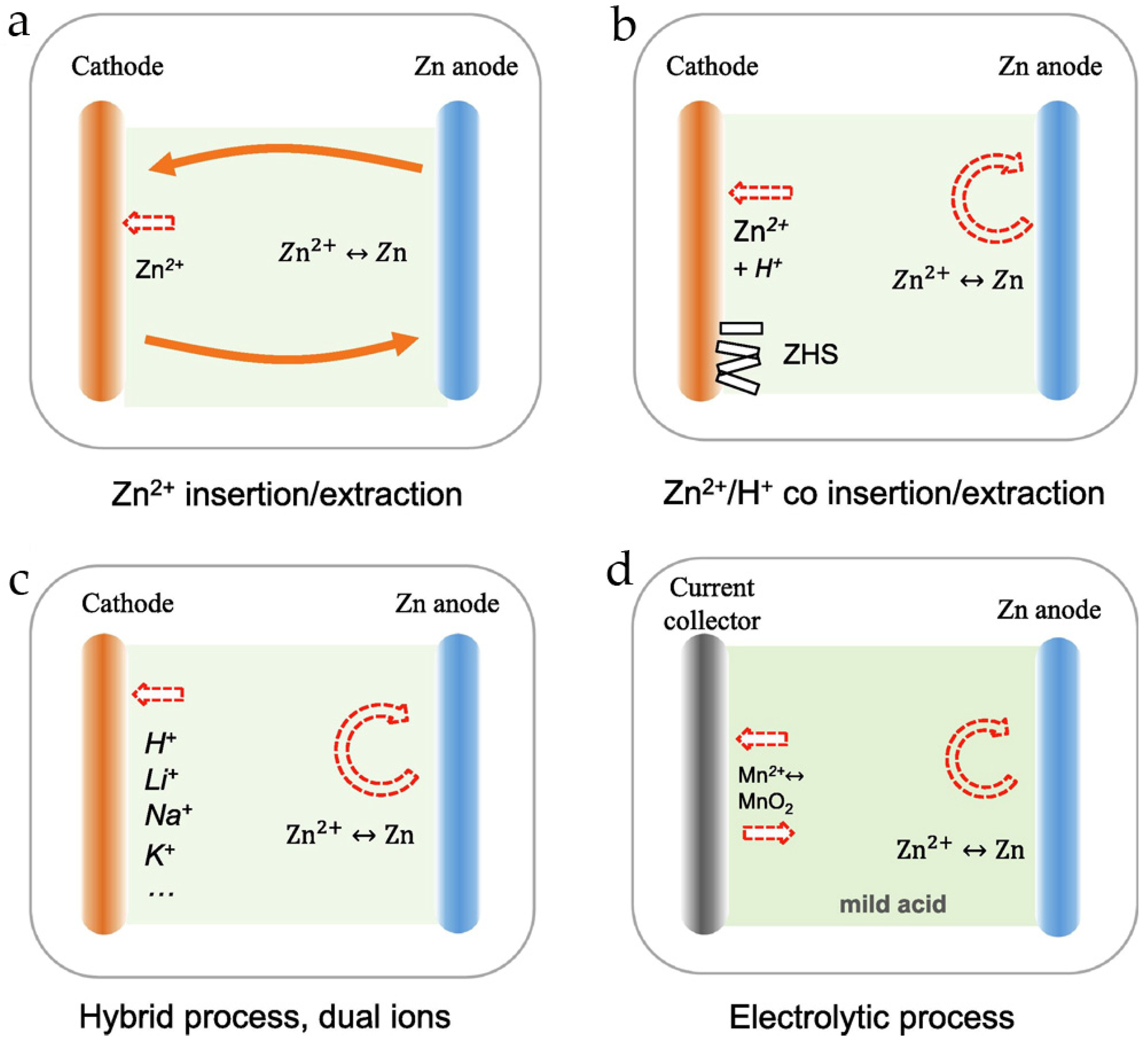
4. On the Charge–Discharge Mechanism in Rechargeable Zn//MnO2 Batteries
- (i)
- Reversible (de)intercalation of Zn2+ ions,
- (ii)
- Reversible (de)intercalation of H+,
- (iii)
- Co-(de)intercalation of Zn2+ and H+,
- (iv)
- Electrolytic deposition/dissolution of MnO2.

5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and Environmental Aspects in Recycling Lithium-Ion Batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling–Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and Opportunities Facing Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Ali, W.; et al. An Overview and Future Perspectives of Rechargeable Zinc Batteries. Small 2020, 16, 2000730. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Guo, S.; Cao, X.; Pan, A.; Fang, G.; Zhou, J.; Liang, S. Fundamentals and Perspectives in Developing Zinc-Ion Battery Electrolytes: A Comprehensive Review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Fu, H. Recent Advances in Rechargeable Zn-Based Batteries. J. Power Sources 2021, 493, 229677. [Google Scholar] [CrossRef]
- Borchers, N.; Clark, S.; Horstmann, B.; Jayasayee, K.; Juel, M.; Stevens, P. Innovative Zinc-Based Batteries. J. Power Sources 2021, 484, 229309. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chemie-Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Chen, L.; An, Q.; Mai, L. Recent Advances and Prospects of Cathode Materials for Rechargeable Aqueous Zinc-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 1900387. [Google Scholar] [CrossRef]
- Liu, X.; Yi, J.; Wu, K.; Jiang, Y.; Liu, Y.; Zhao, B.; Li, W.; Zhang, J. Rechargeable Zn–MnO2 Batteries: Advances, Challenges and Perspectives. Nanotechnology 2020, 31, 122001. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, N.; De Luna, Y. Recent Progress in Layered Manganese and Vanadium Oxide Cathodes for Zn-Ion Batteries. Energy Technol. 2021, 9, 2100011. [Google Scholar] [CrossRef]
- Liang, R.; Fu, J.; Deng, Y.P.; Pei, Y.; Zhang, M.; Yu, A.; Chen, Z. Parasitic Electrodeposition in Zn-MnO2 Batteries and Its Suppression for Prolonged Cyclability. Energy Storage Mater. 2021, 36, 478–484. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L.; Lu, B.; Wu, X.; Liang, S.; Zhou, J. Issues and Opportunities Facing Aqueous Mn2+/MnO2-based Batteries. ChemSusChem 2022, 15, e202200348. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Kumari, N.; Rai, A.K. A Review on Solutions to Overcome the Structural Transformation of Manganese Dioxide-Based Cathodes for Aqueous Rechargeable Zinc Ion Batteries. J. Power Sources 2023, 555, 232385. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Shen, Y.; Wang, R.; Li, H.; Zhou, M.; Wang, W.; Wang, K.; Jiang, K. Issues and Opportunities of Manganese-Based Materials for Enhanced Zn-Ion Storage Performances. J. Energy Storage 2022, 45, 103729. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Kim, S.S.; Islam, S.; Jo, J.; Kim, S.S.; Mathew, V.; Sun, Y.K.; Kim, J. The Dominant Role of Mn2+ Additive on the Electrochemical Reaction in ZnMn2O4 Cathode for Aqueous Zinc-Ion Batteries. Energy Storage Mater. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, X.; Xue, L.; Ni, M.; Zhao, Y.; Liu, B.; Xia, H. The Function of Mn2+ Additive in Aqueous Electrolyte for Zn/δ-MnO2 Battery. Electrochim. Acta 2020, 351, 136445. [Google Scholar] [CrossRef]
- Perez-Antolin, D.; Sáez-Bernal, I.; Colina, A.; Ventosa, E. Float-Charging Protocol in Rechargeable Zn–MnO2 Batteries: Unraveling the Key Role of Mn2+ Additives in Preventing Spontaneous pH Changes. Electrochem. Commun. 2022, 138, 107271. [Google Scholar] [CrossRef]
- Lv, H.; Song, Y.; Qin, Z.; Zhang, M.; Yang, D.; Pan, Q.; Wang, Z.; Mu, X.; Meng, J.; Sun, X.; et al. Disproportionation Enabling Reversible MnO2/Mn2+ Transformation in a Mild Aqueous Zn-MnO2 Hybrid Battery. Chem. Eng. J. 2022, 430, 133064. [Google Scholar] [CrossRef]
- Bischoff, C.F.; Fitz, O.S.; Burns, J.; Bauer, M.; Gentischer, H.; Birke, K.P.; Henning, H.-M.; Biro, D. Revealing the Local pH Value Changes of Acidic Aqueous Zinc Ion Batteries with a Manganese Dioxide Electrode during Cycling. J. Electrochem. Soc. 2020, 167, 020545. [Google Scholar] [CrossRef]
- Yong, B.; Ma, D.; Wang, Y.; Mi, H.; He, C.; Zhang, P. Understanding the Design Principles of Advanced Aqueous Zinc-Ion Battery Cathodes: From Transport Kinetics to Structural Engineering, and Future Perspectives. Adv. Energy Mater. 2020, 10, 2002354. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Sun, W.; Shao, Y.; Zhang, L.; Zhao, K. Challenges and Perspectives for Doping Strategy for Manganese-Based Zinc-Ion Battery Cathode. Energies 2022, 15, 4698. [Google Scholar] [CrossRef]
- Xie, Q.; Cheng, G.; Xue, T.; Huang, L.; Chen, S.; Sun, Y.; Sun, M.; Wang, H.; Yu, L. Alkali Ions Pre-Intercalation of δ-MnO2 Nanosheets for High-Capacity and Stable Zn-Ion Battery. Mater. Today Energy 2022, 24, 100934. [Google Scholar] [CrossRef]
- Chen, C.; Shi, M.; Zhao, Y.; Yang, C.; Zhao, L.; Yan, C. Al-Intercalated MnO2 Cathode with Reversible Phase Transition for Aqueous Zn-Ion Batteries. Chem. Eng. J. 2021, 422, 130375. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Mathew, V.; Song, J.; Kim, S.; Tung, D.P.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Ambient Redox Synthesis of Vanadium-Doped Manganese Dioxide Nanoparticles and Their Enhanced Zinc Storage Properties. Appl. Surf. Sci. 2017, 404, 435–442. [Google Scholar] [CrossRef]
- Kataoka, F.; Ishida, T.; Nagita, K.; Kumbhar, V.; Yamabuki, K.; Nakayama, M. Cobalt-Doped Layered MnO2 Thin Film Electrochemically Grown on Nitrogen-Doped Carbon Cloth for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4720–4726. [Google Scholar] [CrossRef]
- Ko, W.Y.; Lubis, A.L.; Wang, H.Y.; Wu, T.C.; Lin, S.T.; Lin, K.J. Facile Construction of Zn-Doped Mn3O4 −MnO2 Vertical Nanosheets for Aqueous Zinc-Ion Battery Cathodes. ChemElectroChem 2022, 9, e202200750. [Google Scholar] [CrossRef]
- Li, X.; Qu, J.; Xu, J.; Zhang, S.; Wang, X.; Wang, X.; Dai, S. K-Preintercalated MnO2 Nanosheets as Cathode for High-Performance Zn-Ion Batteries. J. Electroanal. Chem. 2021, 895, 115529. [Google Scholar] [CrossRef]
- Lin, M.; Shao, F.; Tang, Y.; Lin, H.; Xu, Y.; Jiao, Y.; Chen, J. Layered Co Doped MnO2 with Abundant Oxygen Defects to Boost Aqueous Zinc-Ion Storage. J. Colloid Interface Sci. 2022, 611, 662–669. [Google Scholar] [CrossRef]
- Ni, Z.; Liang, X.; Zhao, L.; Zhao, H.; Ge, B.; Li, W. Tin Doping Manganese Dioxide Cathode Materials with the Improved Stability for Aqueous Zinc-Ion Batteries. Mater. Chem. Phys. 2022, 287, 126238. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, J.; Kou, Z.; Liao, C.; Liu, Z.; Zhou, L.; Wang, J.; Mai, L. Zn2+ Pre-Intercalation Stabilizes the Tunnel Structure of MnO2 Nanowires and Enables Zinc-Ion Hybrid Supercapacitor of Battery-Level Energy Density. Small 2020, 16, 2000091. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Liang, X.; Zhao, L.; Zhao, H.; Ge, B.; Li, W. MnO2 Cathode Materials with High Reversible Stability through Li Intercalating for Aqueous Zinc Ion Battery. Solid State Ionics 2022, 386, 116049. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.; Tao, Y.; Zhang, K.; Cai, M.; Ding, Y.; Liu, X.; Hayat, T.; Alsaedi, A.; Dai, S. Electrochemically Derived Graphene-Like Carbon Film as a Superb Substrate for High-Performance Aqueous Zn-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1907120. [Google Scholar] [CrossRef]
- Lin, M.X.; Shao, F.; Weng, S.; Xiong, S.; Liu, S.; Jiang, S.; Xu, Y.; Jiao, Y.; Chen, J. Boosted Charge Transfer in Oxygen Vacancy-Rich K+ Birnessite MnO2 for Water Oxidation and Zinc-Ion Batteries. Electrochim. Acta 2021, 378, 138147. [Google Scholar] [CrossRef]
- Lv, W.; Meng, J.; Li, Y.; Yang, W.; Tian, Y.; Lyu, X.; Duan, C.; Ma, X.; Wu, Y. Inexpensive and Eco-Friendly Nanostructured Birnessite-Type δ-MnO2: A Design Strategy from Oxygen Defect Engineering and K+ Pre-Intercalation. Nano Energy 2022, 98, 107274. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Li, C.; Chen, X.; Wang, W.; Han, Y.; Lin, H.; Shi, Z.; Feng, S. Engineering the Interplanar Spacing of K-Birnessite for Ultra-Long Cycle Zn-Ion Battery through “Hydrothermal Potassium Insertion” Strategy. Chem. Eng. J. 2022, 435, 134754. [Google Scholar] [CrossRef]
- Liu, G.; Huang, H.; Bi, R.; Xiao, X.; Ma, T.; Zhang, L. K+ Pre-Intercalated Manganese Dioxide with Enhanced Zn2+ Diffusion for High Rate and Durable Aqueous Zinc-Ion Batteries. J. Mater. Chem. A 2019, 7, 20806–20812. [Google Scholar] [CrossRef]
- Tan, J.; Feng, T.; Hu, S.; Liang, Y.; Zhang, S.; Xu, Z.; Zhou, H.; Wu, M. In Situ Synthesis of a Self-Supported MnO2-Based Cathode for High-Performance Zinc-Ion Batteries by K+ Pre-Intercalation. Appl. Surf. Sci. 2022, 604, 154578. [Google Scholar] [CrossRef]
- Xu, T.H.; Liou, S.; Hou, F.L.; Li, Y.Y. Potassium-Doped Hydrated Manganese Dioxide Nanowires-Carbon Nanotubes on Graphene for High-Performance Rechargeable Zinc-Ion Batteries. J. Alloys Compd. 2022, 913, 165278. [Google Scholar] [CrossRef]
- Sun, T.; Nian, Q.; Zheng, S.; Shi, J.; Tao, Z. Layered Ca0.28MnO2·0.5H2O as a High Performance Cathode for Aqueous Zinc-Ion Battery. Small 2020, 16, 2000597. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.-G.; Liu, H.; Wei, C.; Kang, F. Zinc Ion Stabilized MnO2 Nanospheres for High Capacity and Long Lifespan Aqueous Zinc-Ion Batteries. J. Mater. Chem. A 2019, 7, 13727–13735. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, H.; Chen, C.; Jiang, J.; Zhao, L.; Yan, C. Synergistically Coupling of Graphene Quantum Dots with Zn-Intercalated MnO2 Cathode for High-Performance Aqueous Zn-Ion Batteries. Int. J. Miner. Metall. Mater. 2023, 30, 25–32. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Duan, H.; Xie, S.; Dai, R.; Rong, J.; Kang, F.; Dong, L. Aerogel-Structured MnO2 Cathode Assembled by Defect-Rich Ultrathin Nanosheets for Zinc-Ion Batteries. Chem. Eng. J. 2022, 441, 136008. [Google Scholar] [CrossRef]
- Li, H.; Huang, Z.; Chen, B.; Jiang, Y.; Li, C.; Xiao, W.; Yan, X. A High-Performance MnO2 Cathode Doped with Group VIII Metal for Aqueous Zn-Ion Batteries: In-Situ X-Ray Diffraction Study on Zn2+ Storage Mechanism. J. Power Sources 2022, 527, 231198. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, X.; Veder, J.-P.; Shao, Z. Self-Recovery Chemistry and Cobalt-Catalyzed Electrochemical Deposition of Cathode for Boosting Performance of Aqueous Zinc-Ion Batteries. iScience 2020, 23, 100943. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Luo, M.; Cui, Y.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. Synergistic Combination of a Co-Doped σ-MnO2 Cathode with an Electrolyte Additive for a High-Performance Aqueous Zinc-Ion Battery. ChemPhysMater 2022, 2, 77–82. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, A.; Zhao, W.; Qin, R.; Ding, S.; Chen, X.; Song, Y.; Yang, L.; Lin, H.; Li, S.; et al. Boosting the Energy Density of Aqueous Batteries via Facile Grotthuss Proton Transport. Angew. Chem. 2021, 133, 4215–4220. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, P.; Yang, H.; Min, H.; Niu, M.; Jin, S.; Jiang, Y.; Pan, Z.; Yan, J.; Shen, X.; et al. Manipulating Intercalation-Extraction Mechanisms in Structurally Modulated δ-MnO2 Nanowires for High-Performance Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2022, 433, 133687. [Google Scholar] [CrossRef]
- Long, F.; Xiang, Y.; Yang, S.; Li, Y.; Du, H.; Liu, Y.; Wu, X.; Wu, X. Layered Manganese Dioxide Nanoflowers with Cu2+ and Bi3+ Intercalation as High-Performance Cathode for Aqueous Zinc-Ion Battery. J. Colloid Interface Sci. 2022, 616, 101–109. [Google Scholar] [CrossRef]
- Liao, Y.; Yang, C.; Xu, Q.; Zhao, W.; Zhao, J.; Wang, K.; Chen, H.C. Ag-Doping Effect on MnO2 Cathodes for Flexible Quasi-Solid-State Zinc-Ion Batteries. Batteries 2022, 8, 267. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, G.; Yao, J.; Li, J.; Zheng, J.; Wu, Z.; Gan, Y.; Wang, C.; Lv, L.; Wan, H.; et al. High-Valence Molybdenum Promoted Proton Migration and Inhibited Dissolution for Long-Life Aqueous Zn-MnO2 Batteries. Appl. Surf. Sci. 2022, 592, 153335. [Google Scholar] [CrossRef]
- Wang, Z.; Han, K.; Wan, Q.; Fang, Y.; Qu, X.; Li, P. Mo-Pre-Intercalated MnO2 Cathode with Highly Stable Layered Structure and Expanded Interlayer Spacing for Aqueous Zn-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 859–869. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Wang, J.; Chen, K.; Xue, D.; Liu, J.; Lu, X. Boosting the Zn-Ion Storage Capability of Birnessite Manganese Oxide Nanoflorets by La3+ Intercalation. J. Mater. Chem. A 2019, 7, 22079–22083. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhao, H.; Xu, L.; Xia, J.; Luo, M.; Yang, Y.; Du, Y. Superior-Performance Aqueous Zinc Ion Battery Based on Structural Transformation of MnO2 by Rare Earth Doping. J. Phys. Chem. C 2019, 123, 22735–22741. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Qiao, R.; Dai, X.; Jing, W.; Song, J.; Chen, Y.; Guo, S.; Sun, J.; Tan, Q.; et al. Binder-Free Flexible Zinc-Ion Batteries: One-Step Potentiostatic Electrodeposition Strategy Derived Ce Doped-MnO2 Cathode. Chem. Eng. J. 2022, 431, 133387. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Alam, M.A.; Muhammad, G.; Lv, Y.; Wang, M.; Zhu, C.; Xiong, W. Al-Doped α-MnO2 coated by Lignin for High-Performance Rechargeable Aqueous Zinc-Ion Batteries. RSC Adv. 2021, 11, 35280–35286. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, X.; Du, H.; He, Z.; Wu, X.; Wu, X. Dual Metal Ions and Water Molecular Pre-Intercalated δ-MnO2 Spherical Microflowers for Aqueous Zinc Ion Batteries. J. Colloid Interface Sci. 2022, 623, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Tian, T.; Weng, Q.; Zan, L.; Zhao, S.; Liu, T.; Tang, Z.; Tang, H. Boosting High-Rate Zn-Ion Storage Capability of α-MnO2 through Tri-Ion Co-Intercalation. J. Alloys Compd. 2023, 939, 168813. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Liu, R.; Xiao, H.; Liu, Y.; Wang, X.; Huang, Y.; Yuan, G. Molecular Tailoring of MnO2 by Bismuth Doping to Achieve Aqueous Zinc-Ion Battery with Capacitor-Level Durability. Energy Storage Mater. 2022, 48, 212–222. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Liang, G.; Li, H.; Liu, Z.; Tang, Z.; Liang, J.; Zhi, C. A Superior δ-MnO2 Cathode and a Self-Healing Zn-δ-MnO2 Battery. ACS Nano 2019, 13, 10643–10652. [Google Scholar] [CrossRef]
- Kim, J.J.H.; Lee, J.; You, J.; Park, M.S.; Al Hossain, M.S.; Yamauchi, Y.; Kim, J.J.H. Conductive Polymers for Next-Generation Energy Storage Systems: Recent Progress and New Functions. Mater. Horizons 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Pan, J.; Tian, F.; Zeng, S.; Yang, J.; Qian, Y. Polypyrrole-Controlled Plating/Stripping for Advanced Zinc Metal Anodes. Mater. Today Energy 2020, 17, 100443. [Google Scholar] [CrossRef]
- Ciric-Marjanovic, G. Recent Advances in Polyaniline Research: Polymerization Mechanisms, Structural Aspects, Properties and Applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Huang, J.; Tu, J.; Lv, Y.; Liu, Y.; Huang, H.; Li, L.; Yao, J. Achieving Mesoporous MnO2@polyaniline Nanohybrids via a Gas/Liquid Interfacial Reaction between Aniline and KMnO4 Aqueous Solution towards Zn-MnO2 Battery. Synth. Met. 2020, 266, 116438. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-Intercalated Manganese Dioxide Nanolayers as a High-Performance Cathode Material for an Aqueous Zinc-Ion Battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- Mao, J.; Wu, F.-F.; Shi, W.-H.; Liu, W.-X.; Xu, X.-L.; Cai, G.-F.; Li, Y.-W.; Cao, X.-H. Preparation of Polyaniline-Coated Composite Aerogel of MnO2 and Reduced Graphene Oxide for High-Performance Zinc-Ion Battery. Chinese J. Polym. Sci. 2020, 38, 514–521. [Google Scholar] [CrossRef]
- Ruan, P.; Xu, X.; Gao, X.X.; Feng, J.; Yu, L.; Cai, Y.; Gao, X.X.; Shi, W.; Wu, F.; Liu, W.; et al. Achieving Long-Cycle-Life Zn-Ion Batteries through Interfacial Engineering of MnO2-Polyaniline Hybrid Networks. Sustain. Mater. Technol. 2021, 28, e00254. [Google Scholar] [CrossRef]
- Li, N.; Hou, Z.; Liang, S.; Cao, Y.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.G. Highly Flexible MnO2@polyaniline Core-Shell Nanowire Film toward Substantially Expedited Zinc Energy Storage. Chem. Eng. J. 2023, 452, 139408. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, J.; Zheng, Q.; Huo, Y.; Xie, F.; Lin, D. Polyaniline Intercalation Induced Great Enhancement of Electrochemical Properties in Ammonium Vanadate Nanosheets as an Advanced Cathode for High-Performance Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2022, 448, 137681. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Jiang, H.; Liu, Y.; Meng, C. Polyaniline-Expanded the Interlayer Spacing of Hydrated Vanadium Pentoxide by the Interface-Intercalation for Aqueous Rechargeable Zn-Ion Batteries. J. Colloid Interface Sci. 2021, 603, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xing, F.; Li, T.; Zhang, H.; Yan, J.; Zheng, Q.; Li, X. Intercalated Polyaniline in V2O5 as a Unique Vanadium Oxide Bronze Cathode for Highly Stable Aqueous Zinc Ion Battery. Energy Storage Mater. 2021, 38, 590–598. [Google Scholar] [CrossRef]
- Hao, L.; Yu, D. Progress of Conductive Polypyrrole Nanocomposites. Synth. Met. 2022, 290, 117138. [Google Scholar] [CrossRef]
- Kausar, A. Overview on Conducting Polymer in Energy Storage and Energy Conversion System. J. Macromol. Sci. Part A 2017, 54, 640–653. [Google Scholar] [CrossRef]
- Chavan, U.D.; Prajith, P.; Kandasubramanian, B. Polypyrrole Based Cathode Material for Battery Application. Chem. Eng. J. Adv. 2022, 12, 100416. [Google Scholar] [CrossRef]
- Ruan, P.; Xu, X.; Feng, J.; Yu, L.; Gao, X.; Shi, W.; Wu, F.; Liu, W.; Zang, X.; Ma, F.; et al. Boosting Zinc Storage Performance via Conductive Materials. Mater. Res. Bull. 2021, 133, 111077. [Google Scholar] [CrossRef]
- Morávková, Z.; Taboubi, O.; Minisy, I.M.; Bober, P. The Evolution of the Molecular Structure of Polypyrrole during Chemical Polymerization. Synth. Met. 2021, 271, 116608. [Google Scholar] [CrossRef]
- Huang, J.; Tang, X.; Liu, K.; Fang, G.; He, Z.; Li, Z. Interfacial Chemical Binding and Improved Kinetics Assisting Stable Aqueous Zn–MnO2 Batteries. Mater. Today Energy 2020, 17, 100475. [Google Scholar] [CrossRef]
- Liao, X.; Pan, C.; Pan, Y.; Yin, C. Synthesis of Three-Dimensional β-MnO2/PPy Composite for High-Performance Cathode in Zinc-Ion Batteries. J. Alloys Compd. 2021, 888, 161619. [Google Scholar] [CrossRef]
- Shen, X.; Wang, X.; Yu, N.; Yang, W.; Zhou, Y.; Shi, Y.; Wang, Y.; Dong, L.; Di, J.; Li, Q. A Polypyrrole-Coated MnO2/Carbon Nanotube Film Cathode for Rechargeable Aqueous Zn-Ion Batteries. Acta Phys. Chim. Sin. 2020, 38, 2006059. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Liu, X.; Wei, X.; Cao, J.; Yang, L. Scalable In Situ Reactive Assembly of Polypyrrole-Coated MnO2 Nanowire and Carbon Nanotube Composite as Freestanding Cathodes for High Performance Aqueous Zn-Ion Batteries. ChemElectroChem 2020, 7, 2762–2770. [Google Scholar] [CrossRef]
- Niu, T.; Li, J.; Qi, Y.; Huang, X.; Ren, Y. Preparation and Electrochemical Properties of α-MnO2/RGO-PPy Composite as Cathode Material for Zinc-Ion Battery. J. Mater. Sci. 2021, 56, 16582–16590. [Google Scholar] [CrossRef]
- Huang, A.; Zhou, W.; Wang, A.; Chen, M.; Chen, J.; Tian, Q.; Xu, J. Self-Initiated Coating of Polypyrrole on MnO2/Mn2O3 Nanocomposite for High-Performance Aqueous Zinc-Ion Batteries. Appl. Surf. Sci. 2021, 545, 149041. [Google Scholar] [CrossRef]
- Xu, J.W.; Gao, Q.L.; Xia, Y.M.; Lin, X.S.; Liu, W.L.; Ren, M.M.; Kong, F.G.; Wang, S.J.; Lin, C. High-Performance Reversible Aqueous Zinc-Ion Battery Based on Iron-Doped Alpha-Manganese Dioxide Coated by Polypyrrole. J. Colloid Interface Sci. 2021, 598, 419–429. [Google Scholar] [CrossRef]
- Wang, Z.; Ruan, Z.; Liu, Z.; Wang, Y.; Tang, Z.; Li, H.; Zhu, M.; Hung, T.F.; Liu, J.; Shi, Z.; et al. A Flexible Rechargeable Zinc-Ion Wire-Shaped Battery with Shape Memory Function. J. Mater. Chem. A 2018, 6, 8549–8557. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on Application of PEDOTs and PEDOT:PSS in Energy Conversion and Storage Devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Chen, H.W.; Li, C. PEDOT: Fundamentals and Its Nanocomposites for Energy Storage. Chin. J. Polym. Sci. (Engl. Ed.) 2020, 38, 435–448. [Google Scholar] [CrossRef]
- Heywang, G.; Jonas, F. Poly(Alkylenedioxythiophene)s—New, Very Stable Conducting Polymers. Adv. Mater. 1992, 4, 116–118. [Google Scholar] [CrossRef]
- Petsagkourakis, I.; Kim, N.; Tybrandt, K.; Zozoulenko, I.; Crispin, X. Poly(3,4-ethylenedioxythiophene): Chemical Synthesis, Transport Properties, and Thermoelectric Devices. Adv. Electron. Mater. 2019, 5, 1800918. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Meng, Y.; Yu, M.; Yi, J.; Wu, Y.; Lu, X.; Tong, Y. Achieving Ultrahigh Energy Density and Long Durability in a Flexible Rechargeable Quasi-Solid-State Zn-MnO2 Battery. Adv. Mater. 2017, 29, 1700274. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, S.; Deng, S.; Wu, W.; Zeng, Y.; Xia, X.; Pan, G.; Tong, Y.; Lu, X. 3D CNTs Networks Enable MnO2 Cathodes with High Capacity and Superior Rate Capability for Flexible Rechargeable Zn–MnO2 Batteries. Small Methods 2019, 3, 1900525. [Google Scholar] [CrossRef]
- Shi, X.; Liu, X.; Wang, E.; Cao, X.; Yu, Y.; Cheng, X.; Lu, X. Boosting the Zn Ion Storage Ability of Amorphous MnO2 via Surface Engineering and Valence Modulation. Carbon Neutraliz. 2023, 2, 28–36. [Google Scholar] [CrossRef]
- Fu, N.; Zhao, Q.; Xu, Y.; Wang, H.; Hu, J.; Wu, Y.; Yang, L.; Wu, X.; Zeng, X. Exploiting the Synergistic Effect of Multiphase MnO2 Stabilized by an Integrated Conducting Network for Aqueous Zinc-Ion Batteries. Mater. Chem. Front. 2022, 6, 1956–1963. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, Q.; He, W.; Lai, Z.; Zhang, X.; Yu, M.; Tong, Y.; Lu, X. Extracting Oxygen Anions from ZnMn2O4: Robust Cathode for Flexible All-Solid-State Zn-Ion Batteries. Energy Storage Mater. 2019, 21, 154–161. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Song, B.; Wang, Z.; Zhang, L.; Lu, Q. Facile in Situ Synthesis of PEDOT Conductor Interface at the Surface of MnO2 Cathodes for Enhanced Aqueous Zinc-Ion Batteries. Surfaces and Interfaces 2022, 33, 102222. [Google Scholar] [CrossRef]
- Chen, H.; Ma, W.; Guo, J.; Xiong, J.; Hou, F.; Si, W.; Sang, Z.; Yang, D. PEDOT-Intercalated MnO2 Layers as a High-Performance Cathode Material for Aqueous Zn-Ion Batteries. J. Alloys Compd. 2023, 932, 167688. [Google Scholar] [CrossRef]
- Kamenskii, M.A.; Volkov, F.S.; Eliseeva, S.N.; Holze, R.; Kondratiev, V.V. Comparative Study of PEDOT- and PEDOT:PSS Modified δ-MnO2 Cathodes for Aqueous Zinc Batteries with Enhanced Properties. J. Electrochem. Soc. 2023, 170, 010505. [Google Scholar] [CrossRef]
- Chen, J.; Liang, J.; Zhou, Y.; Sha, Z.; Lim, S.; Huang, F.; Han, Z.; Brown, S.A.; Cao, L.; Wang, D.; et al. A Vertical Graphene Enhanced Zn–MnO2 Flexible Battery towards Wearable Electronic Devices. J. Mater. Chem. A 2021, 9, 575–584. [Google Scholar] [CrossRef]
- Kamenskii, M.A.; Eliseeva, S.N.; Kondratiev, V.V. The Electrochemical Performance of δ-MnO2 Cathode Material for Aqueous Zinc-Ion Batteries: The Role of Current Collector. ECS Trans. 2021, 105, 135–142. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shoji, T. Rechargeable Zn∣ZnSO4∣MnO2-Type Cells. Inorg. Chim. Acta 1986, 117, L27–L28. [Google Scholar] [CrossRef]
- Xue, T.; Fan, H.J. From Aqueous Zn-Ion Battery to Zn-MnO2 Flow Battery: A Brief Story. J. Energy Chem. 2021, 54, 194–201. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and Perspectives for Manganese-Based Oxides for Advanced Aqueous Zinc-Ion Batteries. InfoMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Fitz, O.; Bischoff, C.; Bauer, M.; Gentischer, H.; Birke, K.P.; Henning, H.M.; Biro, D. Electrolyte Study with in Operando pH Tracking Providing Insight into the Reaction Mechanism of Aqueous Acidic Zn//MnO2 Batteries. ChemElectroChem 2021, 8, 3553–3566. [Google Scholar] [CrossRef]
- Li, L.; Hoang, T.K.A.; Zhi, J.; Han, M.; Li, S.; Chen, P. Functioning Mechanism of the Secondary Aqueous Zn-β-MnO2 Battery. ACS Appl. Mater. Interfaces 2020, 12, 12834–12846. [Google Scholar] [CrossRef]
- Aguilar, I.; Lemaire, P.; Ayouni, N.; Bendadesse, E.; Morozov, A.V.; Sel, O.; Balland, V.; Limoges, B.; Abakumov, A.M.; Raymundo-Piñero, E.; et al. Identifying Interfacial Mechanisms Limitations within Aqueous Zn-MnO2 Batteries and Means to Cure Them with Additives. Energy Storage Mater. 2022, 53, 238–253. [Google Scholar] [CrossRef]
- Wu, Y.; Zhi, J.; Han, M.; Liu, Z.; Shi, Q.; Liu, Y.; Chen, P. Regulating Proton Distribution by Ion Exchange Resin to Achieve Long Lifespan Aqueous Zn-MnO2 Battery. Energy Storage Mater. 2022, 51, 599–609. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Bai, C.; Li, X.; Fang, G.; Liang, S. Zn/MnO2 Battery Chemistry with Dissolution-Deposition Mechanism. Mater. Today Energy 2020, 16, 100396. [Google Scholar] [CrossRef]
- Jin, Y.; Zou, L.; Liu, L.; Engelhard, M.H.; Patel, R.L.; Nie, Z.; Han, K.S.; Shao, Y.; Wang, C.; Zhu, J.; et al. Joint Charge Storage for High-Rate Aqueous Zinc–Manganese Dioxide Batteries. Adv. Mater. 2019, 31, 1900567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Salvador, J.R.; Wu, J.; Liu, B.; Yang, W.; Yang, J.; Zhang, W.; Liu, J.; Yang, J. Reaction Mechanisms for Long-Life Rechargeable Zn/MnO2 Batteries. Chem. Mater. 2019, 31, 2036–2047. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, L.; Xie, L.; Han, Q.; Yang, X.; Chen, L.; Wang, G.; Cao, X. Cathode Materials for Aqueous Zinc-Ion Batteries: A Mini Review. J. Colloid Interface Sci. 2022, 605, 828–850. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mou, J.; Liu, W.; Wang, X.; Dong, L.; Kang, F.; Xu, C. Novel Insights into Energy Storage Mechanism of Aqueous Rechargeable Zn/MnO2 Batteries with Participation of Mn2+. Nano-Micro Lett. 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, H.; Li, J.; Hou, Z.; Liang, J.; Zhou, J.; Zhu, Y.; Qian, Y. Ultrathin δ-MnO2 Nanosheets as Cathode for Aqueous Rechargeable Zinc Ion Battery. Electrochim. Acta 2019, 304, 370–377. [Google Scholar] [CrossRef]
- Liu, D.-S.; Mai, Y.; Chen, S.; Liu, S.; Ang, E.H.; Ye, M.; Yang, Y.; Zhang, Y.; Geng, H.; Li, C.C. A 1D–3D Interconnected δ-MnO2 Nanowires Network as High-Performance and High Energy Efficiency Cathode Material for Aqueous Zinc-Ion Batteries. Electrochim. Acta 2021, 370, 137740. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, A.; Huang, A.; Chen, M.; Tian, Q.; Chen, J.; Xu, X. Hybridizing δ-Type MnO2 With Lignin-Derived Porous Carbon as a Stable Cathode Material for Aqueous Zn–MnO2 Batteries. Front. Energy Res. 2020, 8, 182. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, H.C.; Yang, C.; Liu, R.; Peng, Z.; Cao, H.; Wang, K. Unveiling Performance Evolution Mechanisms of MnO2 Polymorphs for Durable Aqueous Zinc-Ion Batteries. Energy Storage Mater. 2022, 44, 508–516. [Google Scholar] [CrossRef]
- Siamionau, U.; Aniskevich, Y.; Mazanik, A.; Kokits, O.; Ragoisha, G.; Jo, J.H.; Myung, S.T.; Streltsov, E. Rechargeable Zinc-Ion Batteries with Manganese Dioxide Cathode: How Critical Is Choice of Manganese Dioxide Polymorphs in Aqueous Solutions? J. Power Sources 2022, 523, 231023. [Google Scholar] [CrossRef]
- Stoševski, I.; Bonakdarpour, A.; Fang, B.; Lo, P.; Wilkinson, D.P. Formation of MnxZny(OH)ZSO4⋅5H2O–Not Intercalation of Zn–Is the Basis of the Neutral MnO2/Zn Battery First Discharge Reaction. Electrochim. Acta 2021, 390, 138852. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, S.; Lu, B.; Zhou, J. pH-Buffer Contained Electrolyte for Self-Adjusted Cathode-Free Zn–MnO2 Batteries with Coexistence of Dual Mechanisms. Small Struct. 2021, 2, 2100119. [Google Scholar] [CrossRef]
- Efremova, A.O.; Volkov, A.I.; Tolstopyatova, E.G.; Kondratiev, V.V. EQCM Study of Intercalation Processes into Electrodeposited MnO2 Electrode in Aqueous Zinc-Ion Battery Electrolyte. J. Alloys Compd. 2022, 892, 162142. [Google Scholar] [CrossRef]
- Kim, S.J.; Wu, D.; Sadique, N.; Quilty, C.D.; Wu, L.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Zhu, Y. Unraveling the Dissolution-Mediated Reaction Mechanism of α-MnO2 Cathodes for Aqueous Zn-Ion Batteries. Small 2020, 16, 2005406. [Google Scholar] [CrossRef]
- Han, S.D.; Kim, S.; Li, D.; Petkov, V.; Yoo, H.D.; Phillips, P.J.; Wang, H.; Kim, J.J.; More, K.L.; Key, B.; et al. Mechanism of Zn Insertion into Nanostructured δ-MnO2: A Nonaqueous Rechargeable Zn Metal Battery. Chem. Mater. 2017, 29, 4874–4884. [Google Scholar] [CrossRef]

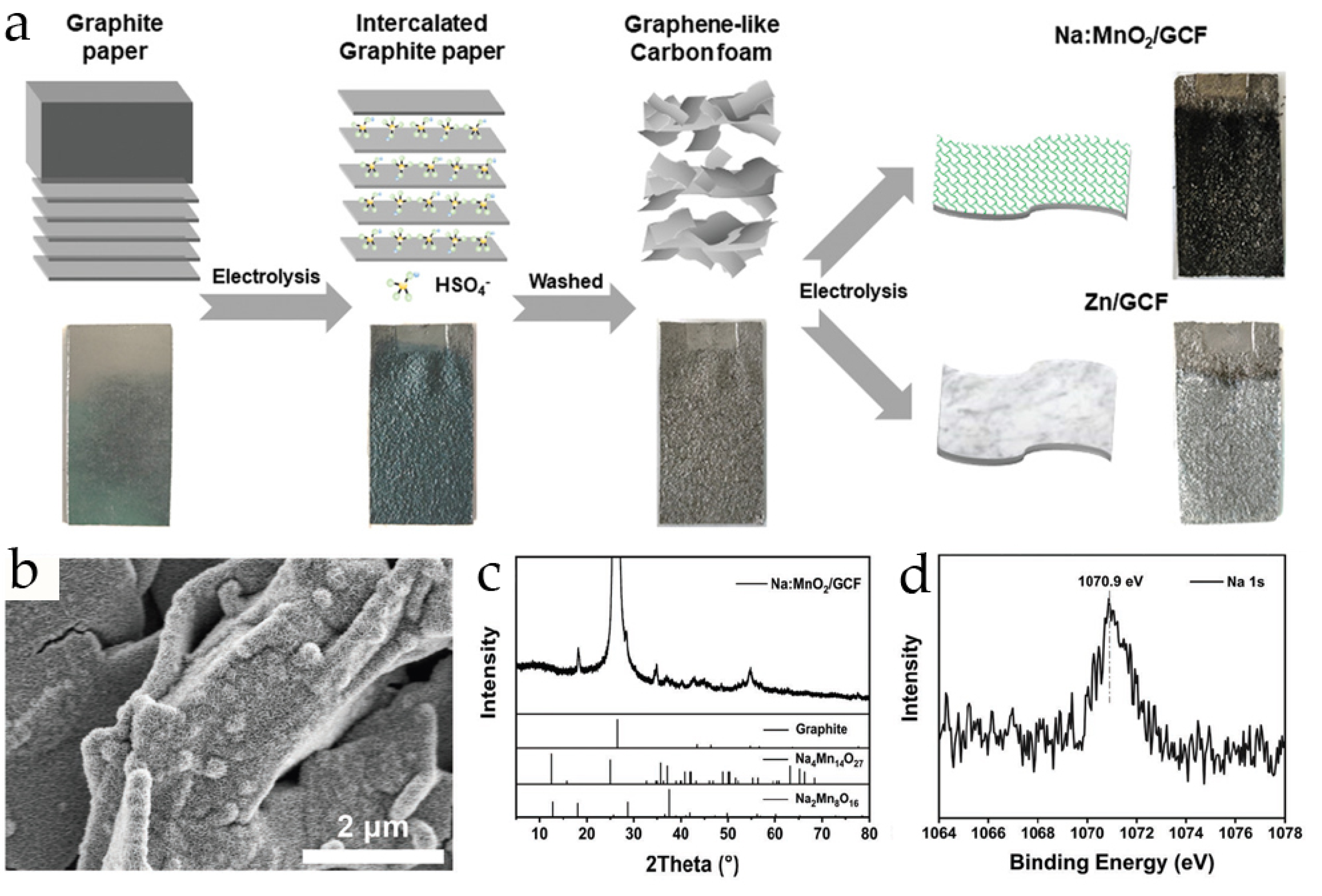




| Material | Synthesis Method | Morphology | Electrolyte | Specific Capacity, mAh g−1 (Current Density, A·g−1) | Capacity Retention, (Number of Cycles and Current, A·g−1) | Ref. |
|---|---|---|---|---|---|---|
| K-δ-MnO2 | precipitation | two-dimensional nanosheets | 2 M ZnSO4 + 0.1 M MnSO4 | 270.5 (0.1); 220.1 (1); 95.1 (3) | 104.6% (100, 0.3) | [23] |
| Li0.023Mn0.87O2 | two-stage hydrothermal method (140/110 °C) | nanorods | 2 M ZnSO4 + 0.1 M MnSO4 | 184 (0.1); 0.54 (2) | 89% (1000, 1) 100% (100, 0.1) | [32] |
| Na-δ-MnO2 | redox reaction | nanoplates | 2 M ZnSO4 + 0.2 M MnSO4 | 278 (0.308) 161 (1.85) 103 (6.16) | ~100% (2000, 2.46/3.08) | [60] |
| Na:MnO2/GCF | electrochemical deposition on graphene-like carbon foam | nanosheets | 2 M ZnSO4 + 0.1 M MnSO4 | 381.8 (0.1) 258.5 (1) 94.8 (3) | ~80% (100, 0.1) ~75% (1000, 1) | [33] |
| K0.19MnO2·0.56H2O | one-pot hydrothermal method (180 °C) | nanosheets | 3 M Zn(CF3SO3)2 | 107 (1) | 87.5% (2000, 10) | [28] |
| K-δ-MnO2-V | one-pot hydrothermal method (160 °C) | layered structure | 2 M ZnSO4 + 0.1 M MnSO4 + 0.1 M K2SO4 | 288.8 (0.1) 85.7 (1) | 91.9% (1500, 1) 89.4% (500, 0.6) | [35] |
| K0.29MnO2 0.67H2O | one-pot hydrothermal method (180 °C) | nanosheets | 2.5 M ZnSO4 + 0.2 M MnSO4 | 300 (0.2) 219 (1) 136 (3) | 92 % (500, 0.2) | [36] |
| α-K0.19MnO2 | self-sacrificial template method | nanotubes | 3 M Zn(CF3SO3)2 + 0.2 M Mn(CF3SO3)2 + 3 M K(CF3SO3) | 270 (0.308) 222.8 (0.616) 200 (1.54) | 98.5% (50, 0.308) 92% (200, 0.616) 90% (400, 1.54) | [37] |
| LGP@K0.15MnO2 | in-situ hydrothermal synthesis (150 °C) | nanosheets | 2 M ZnSO4 + 0.1 M MnSO4 | 402.6 (0.05) 196.1 (0.8) 116.1 (2) | 92.5% (100, 0.2) 83.3% (1000, 1) | [38] |
| KMO-CNT/graphene | polyol reduction method | nanowires | 2 M ZnSO4 + 0.4 M MnSO4 | 373.1 (0.1) 213.6 (1) 108.8 (3) | 82.5% (350, 0.5) 77% (1000, 3) | [39] |
| Ca0.28MnO2·0.5H2O | one-step hydrothermal method | interconnected nanoflakes | 1 M ZnSO4 + 0.1 M MnSO4 | 298 (0.175) 277.7 (0.35) 124.5 (3.5) | 85% (1000, 4) 92% (5000, 3.5) | [40] |
| Zn-δ-MnO2 | redox reaction | flower-like nanospheres | 2 M ZnSO4 + 0.1 M MnSO4 | 275 (0.3) 121 (3) | 100% (100, 0.3/0.6) 100% (500, 1) | [41] |
| GQDs·ZnxMnO2 | redox reaction | nanoflowers | 1 M ZnSO4 | 403.6 (0.3) 211.5 (4) | 88.1% (500, 1) | [42] |
| Zn-doped Mn3O4-MnO2-NSs | electrochemical deposition | vertical nanosheets | 2 M ZnSO4 + 0.1 M MnSO4 | 562.1 (0.3) 272.7 (6) | 69.4% (200, 3) | [27] |
| V-doped δ-MnO2 | redox reaction | nanoparticles | 1 M ZnSO4 | 266 (0.066) 150 (0.266) 67 (1.064) | 52.4% (100, 0.066) | [25] |
| V-doped δ-MnO2 | modified coprecipitation | nanosheets with aerogel-like morphology | 2 M ZnSO4 | 194 (0.2) 74 (2) | 71% (100, 0.3) 52% (600, 3) | [43] |
| Fe-doped δ-MnO2 | one-step hydrothermal process (120 °C) | nanoflowers | 2 M ZnSO4 + 0.1 M MnSO4 | 390 (0.1) 320 (1) 160 (3) | 86.3% (200, 1) | [44] |
| Co/Zn-doped δ-MnO2 on N-doped CC | electrochemical deposition | film on the carbon nanowires | 2 M ZnSO4 + 0.07 M MnSO4 | 280 (1.2) 30 (10.5) | ~100% (600, 1.2) | [26] |
| Co-doped δ-MnO2 | molten-salt synthesis process | nanosheets | 2 M ZnSO4 + 0.2 M MnSO4 | 500 (0.1) 125 (5) | 63% (5000, 2) 100% (0.3, 100) | [45] |
| Co-doped α-MnO2 on CC | one-step hydrothermal process (120 °C) + plasma treatment | nanowires | 2 M ZnSO4 + 0.1 M MnSO4 | 511 (0.5) 337 (1) 100 (5) | 98% (1000, 3) | [29] |
| Co-doped σ-MnO2 | one-step electrodeposition | nanosheets | 2 M ZnSO4 + 0.2 M MnSO4 + 0.02 CoAc | 313.8 (0.5) | 91.8% (1000, 1) | [46] |
| Ni-doped α-MnO2 (Ni0.052K0.119Mn0.948O2 0.208H2O) | one-step hydrothermal process (120 °C) | nanorods | 3 M ZnSO4 + 0.2 M MnSO4 | 303 (0.015) | 71.4% (2000, 1.232) | [47] |
| Cu-doped δ–MnO2 on CC | one-step hydrothermal process (160 °C) | nanowires | 2 M ZnSO4 + 0.2 M MnSO4 | 398.2 (0.1) 224.9 (1) 124.9 (5) | 90.1% (700, 5) | [48] |
| Cu0.06MnO2·1.7H2O (δ–MnO2) | one-step hydrothermal process (180 °C) | nanoflowers | 2 M ZnSO4 + 0.1 M MnSO4 | 493.3 (0.1) 350 (0.5) 125.8 (5) | 80% (150, 0.5) | [49] |
| Bi0.09MnO2·1.5H2O (δ–MnO2) | one-step hydrothermal process (180 °C) | nanoflowers | 2 M ZnSO4 + 0.1 M MnSO4 | 175.5 (0.1) 65 (2) | 96% (1100, 1) 72.3 % (500, 0.5) | [49] |
| Ag-doped α-MnO2 | one-step hydrothermal process (120 °C) | nanowires | 2 M ZnSO4 + 0.1 M MnSO4 | 315 (0.05) 177 (0.5) 85 (2) | 94.4% (500, 0.5) | [50] |
| Mo-doped α-MnO2 | one-step hydrothermal process (120 °C) | nanorods | 2 M ZnSO4 + 0.2 M MnSO4 | 222.8 (0.1); 65.8 (5) | 82.6% (1000, 2) | [51] |
| Mo-doped δ-MnO2 | one-step hydrothermal method (120 °C) | flower-like nanospheres | 2 M ZnSO4 + 0.1 M MnSO4 | 327 (0.2) 207 (1) 107 (3) | 76.8% (1000, 1) | [52] |
| La3+-inserted δ -MnO2 | redox reaction | nanoflorets | 1 M ZnSO4 + 0.4 M MnSO4 | 278.5 (0.1) | 71% (200, 0.2) | [53] |
| Ce-doped α- MnO2 | one-step hydrothermal method (140 °C) | nanorod-like structure | 2 M ZnSO4 + 0.1 M MnSO4 | 134 (1.54) | 74% (100, 1.54) | [54] |
| Ce-MnO2@CC | one-step electrodeposition | porous lamellar structures | PAM/2 M ZnSO4 + 0.1 M MnSO4 | 292 (0.1) 212 (0.5) 106 (2) | 64% (450, 0.1) | [55] |
| Al-intercalated α-MnO2 | one-step hydrothermal method (140 °C) | nanorods | PVA: 1 M ZnSO4 (1:4) | 333.6 (1) 198.6 (4) | 94.5% (2000, 2) | [24] |
| Al-Doped α-MnO2 coated by Lignin | one-step hydrothermal process (200 °C) | 1D nanorod structures | 2 M ZnSO4 + 0.2 M MnSO4 | ~420 (0.1) 180 (1.5) | 66.7% (3000, 1.5) | [56] |
| Al3+ pre-intercalated K0.27MnO2·0.54H2O (δ-MnO2) | modified hydrothermal method (160 °C) | spherical microflowers | 2 M ZnSO4 + 0.1 M MnSO4 | 323.7 (0.1) 250 (0.5) 191.7 (2) | 99% (300, 0.5) | [57] |
| α-MnO2@KCoAl | co-precipitation method | irregular lumpy particles with agglomeration | 2 M ZnSO4 + 0.05 M MnSO4 | 524 (0.5) 431 (1) 221 (5) | ~66.4% (100, 0.5) | [58] |
| Bi-doped α-MnO2 | redox process followed by annealing | nanoparticles | 2 M ZnSO4 + 0.2 M MnSO4 | 363 (0.1) 286 (0.6) 197 (1) | 93% (10,000, 1) | [59] |
| Sn-doped α-MnO2 | hydrothermal process (180 °C) with further calcination | nanorods | 2 M ZnSO4 + 0.1 M MnSO4 | 210 (0.1) 106 (1) | 80 % (500, 1) | [30] |
| Material | Synthesis Method | Morphology | Electrolyte | Specific Capacity, mAh g−1 (Current Density, A·g−1) | Capacity Retention, (Number of Cycles and Current, A·g−1) | Ref. |
|---|---|---|---|---|---|---|
| δ-MnO2@polyaniline | gas/liquid interface reaction | mesoporous nanohybrids | 2 M ZnSO4 + 0.2 M MnSO4 | 313 (0.1) 145 (1) 88 (3) | ~100% (500, 0.5) | [65] |
| Polyaniline- intercalated δ-MnO2 | one-step inorganic/ organic interface reaction | nanolayers with spongiform structure | 2 M ZnSO4 + 0.1 M MnSO4 | 298 (0.05) 280 (0.2) 110 (3) | 90% (200, 0.2) 40% (5000, 2) | [66] |
| Polyaniline-coated β-MnO2/rGO | MnO2 ball-milling + hydrothermal process with rGO (160 °C) + in situ polymerization | aerogel-supported | 2 M ZnSO4 | 241.1 (0.1) 111.7 (1) | 82.7% (600, 1) | [67] |
| PANI-δ-MnO2/CC | hydrothermal method (150 °C) + in situ polymerization | nanosheets | 2 M ZnSO4 + 0.1 M MnSO4 | 286 (0.5) 233 (2) 177 (4) | 96.9% (9000, 4) | [68] |
| α-MnO2@PANI | hydrothermal process (160 °C) + in situ interfacial polymerization | core-shell | 2 M ZnSO4 + 0.1 M MnSO4 | 342 (0.2) 100 (3) | 82% (2000, 2) | [69] |
| α-MnO2/PPy | hydrothermal process (160 °C) + in situ polymerization | nanorods | 2 M ZnSO4 + 0.1 M MnSO4 | 256 (0.1) 104 (1) | 100% (500, 1) 100% (50, 0.1) | [78] |
| β-MnO2/PPy | one-step hydrothermal process (120 °C) | micro-spherical structure of nanowires and clusters of nanorods | 2 M ZnSO4 + 0.1 M MnSO4 | 215.4 (0.1) 214.1 (0.2) 171.5 (0.5) 69.9 (1.5) | 100% (160, 0.2) | [79] |
| CNT/α-MnO2-PPy | in situ reactive self- assembly and following vacuum filtration | core-shell structure and rod-shaped morphology | 2 M ZnSO4 + 0.1 M MnSO4 | 253.9 (0.3) 83.3 (2) | 87.4% (1000, 1) 75.5% (200, 0.3) | [81] |
| α-MnO2/rGO-PPy | hydrothermal process (140 °C) + in situ polymerization | nanowires wrapped by PPy | 3 M Zn(CF3SO3)2 | 438.3 (0.1) 248.8 (0.5) | ~85.9% (100, 0.5) | [82] |
| Mn2O3/α-MnO2@PPy | molten salt method + self-initiated polymerization | nanobelts and nanoparticles | 2 M ZnSO4 + 0.2 M MnSO4 | 289.9 (0.2) 252.6 (1) 199.8 (3) | ~100% (1000, 3) 96.7% (1000, 1) | [83] |
| Fe-doped α-MnO2 coated by PPy | chemical precipitation method + in situ polymerization | nanoparticles | 2 M ZnSO4 + 0.1 M MnSO4 | 270 (0.1) 164 (0.4) 73 (1) | 99.6% (100, 0.1) | [84] |
| α-MnO2/PPy@SS | electrodeposition | nanocrystallites | 1 M ZnSO4 + 0.1 M MnSO4 | 143.2 (0.308) 102.2 (0.924) 86.8 (1.54) | 74.2% (850, 1.54) | [85] |
| MnO2@PEDOT | electrodeposition | nanosheets | 2 M ZnCl2 + 0.4 M MnSO4 | 366.6 (0.74) 143 (7.43) | 83.7% (300, 1.11) | [90] |
| PEDOT@Co-MnO2 | low-temperature hydrothermal process + electrochemical polymerization | nanoflakes | 2 M ZnSO4 | 298.9 (1) | 92.3% (1000, 5.0) | [92] |
| δ-MnO2/α-MnO2 /PEDOT | decomposition (δ -MnO2) + hydrothermal process (150 °C, α-MnO2) + electrodeposition (PEDOT) | nanowires of δ-MnO2 and nanoflakes of α-MnO2 | 2 M ZnSO4 + 0.1 M MnSO4 | 360.5 (0.031) 174.5 (0.308) 94 (1.54) | 78% (860, 0.308) | [93] |
| δ-MnO2@PEDOT | redox reaction | nanowires | 2 M ZnSO4 + 0.2 M MnSO4 | 242 (0.2) 133 (1) 120.7 (2) | 85.1% (1000, 2) | [95] |
| VG-α-MnO2 coated with PEDOT:PSS | hydrothermal process (150 °C) | MnO2 nano- particles on VG nanosheets with 3D porous structure | 1 M ZnSO4 + 0.1 M MnSO4 | 367.4 (0.5) 280.5 (1) 148.2 (6) | 73.7% (1000, 5) | [98] |
| K0.46Mn2O4·1.55 H2O (δ-MnO2)/PEDOT:PSS | hydrothermal method (160 °C) +mechanical mixing with PEDOT:PSS | nanoflowers | 2 M ZnSO4 + 0.1 M MnSO4 | 380 (0.3) 243 (1) 40 (5) | 100% (120, 0.3) | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamenskii, M.A.; Volkov, F.S.; Eliseeva, S.N.; Tolstopyatova, E.G.; Kondratiev, V.V. Enhancement of Electrochemical Performance of Aqueous Zinc Ion Batteries by Structural and Interfacial Design of MnO2 Cathodes: The Metal Ion Doping and Introduction of Conducting Polymers. Energies 2023, 16, 3221. https://doi.org/10.3390/en16073221
Kamenskii MA, Volkov FS, Eliseeva SN, Tolstopyatova EG, Kondratiev VV. Enhancement of Electrochemical Performance of Aqueous Zinc Ion Batteries by Structural and Interfacial Design of MnO2 Cathodes: The Metal Ion Doping and Introduction of Conducting Polymers. Energies. 2023; 16(7):3221. https://doi.org/10.3390/en16073221
Chicago/Turabian StyleKamenskii, Mikhail A., Filipp S. Volkov, Svetlana N. Eliseeva, Elena G. Tolstopyatova, and Veniamin V. Kondratiev. 2023. "Enhancement of Electrochemical Performance of Aqueous Zinc Ion Batteries by Structural and Interfacial Design of MnO2 Cathodes: The Metal Ion Doping and Introduction of Conducting Polymers" Energies 16, no. 7: 3221. https://doi.org/10.3390/en16073221
APA StyleKamenskii, M. A., Volkov, F. S., Eliseeva, S. N., Tolstopyatova, E. G., & Kondratiev, V. V. (2023). Enhancement of Electrochemical Performance of Aqueous Zinc Ion Batteries by Structural and Interfacial Design of MnO2 Cathodes: The Metal Ion Doping and Introduction of Conducting Polymers. Energies, 16(7), 3221. https://doi.org/10.3390/en16073221





