High-Quality Syngas Production by Chemical Looping Gasification of Bituminite Based on NiFe2O4 Oxygen Carrier
Abstract
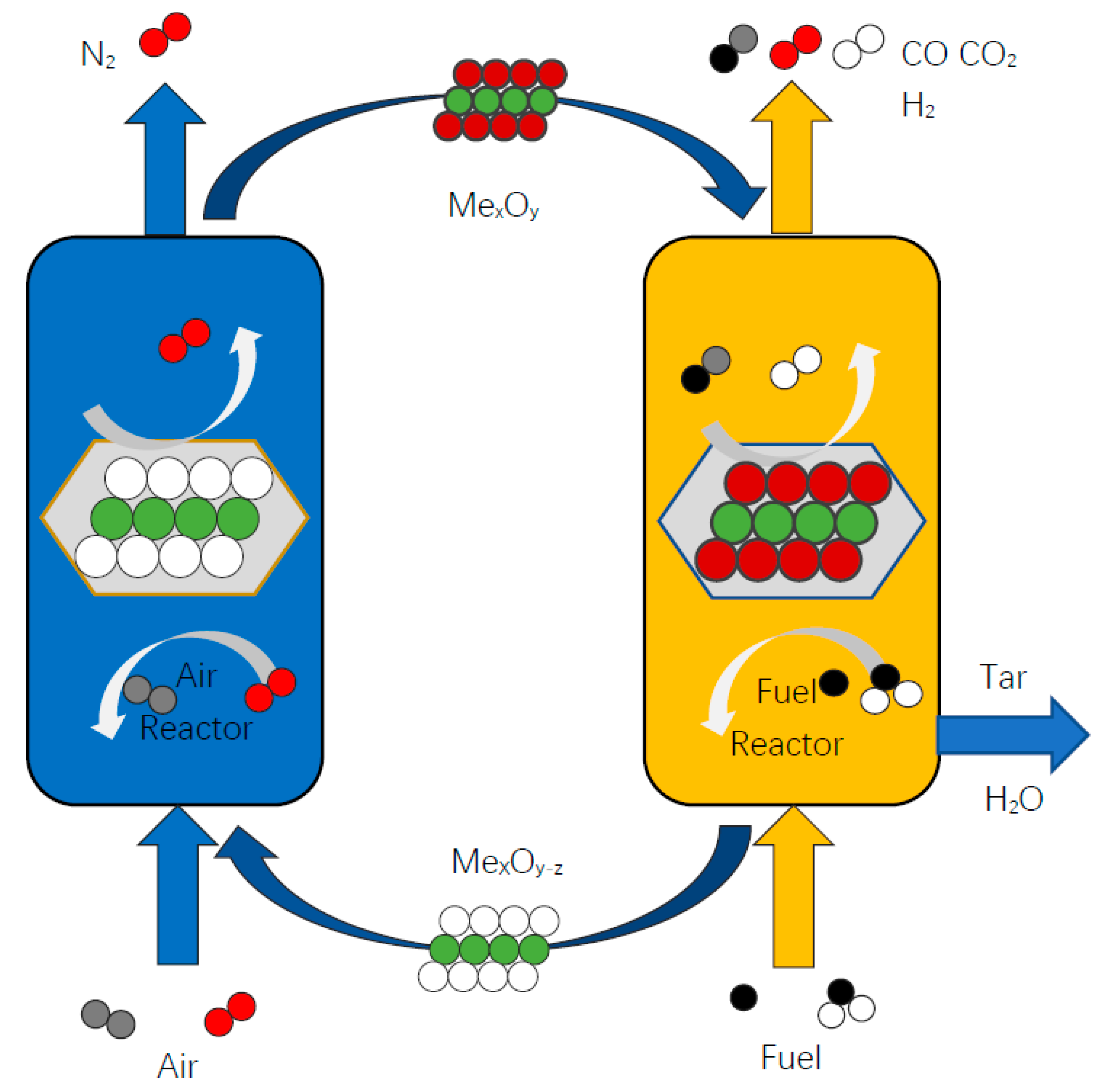
:1. Introduction
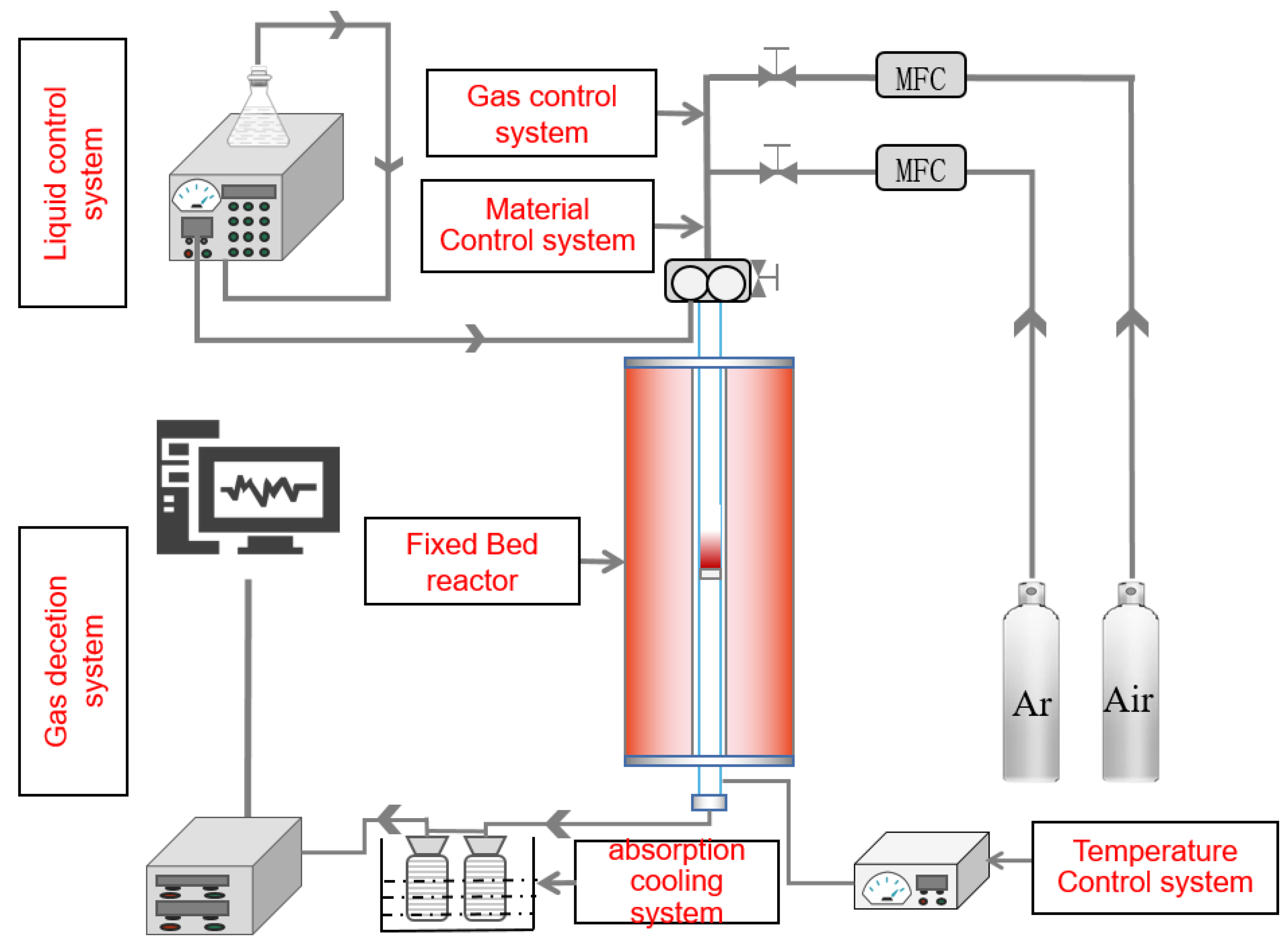
2. Experiment and Apparatus
2.1. Materials
2.2. Experimental Section
2.3. Data Evaluation
3. Results and Discussion
3.1. Effect of Temperature on CLG
3.2. Effect of O/B on CLG
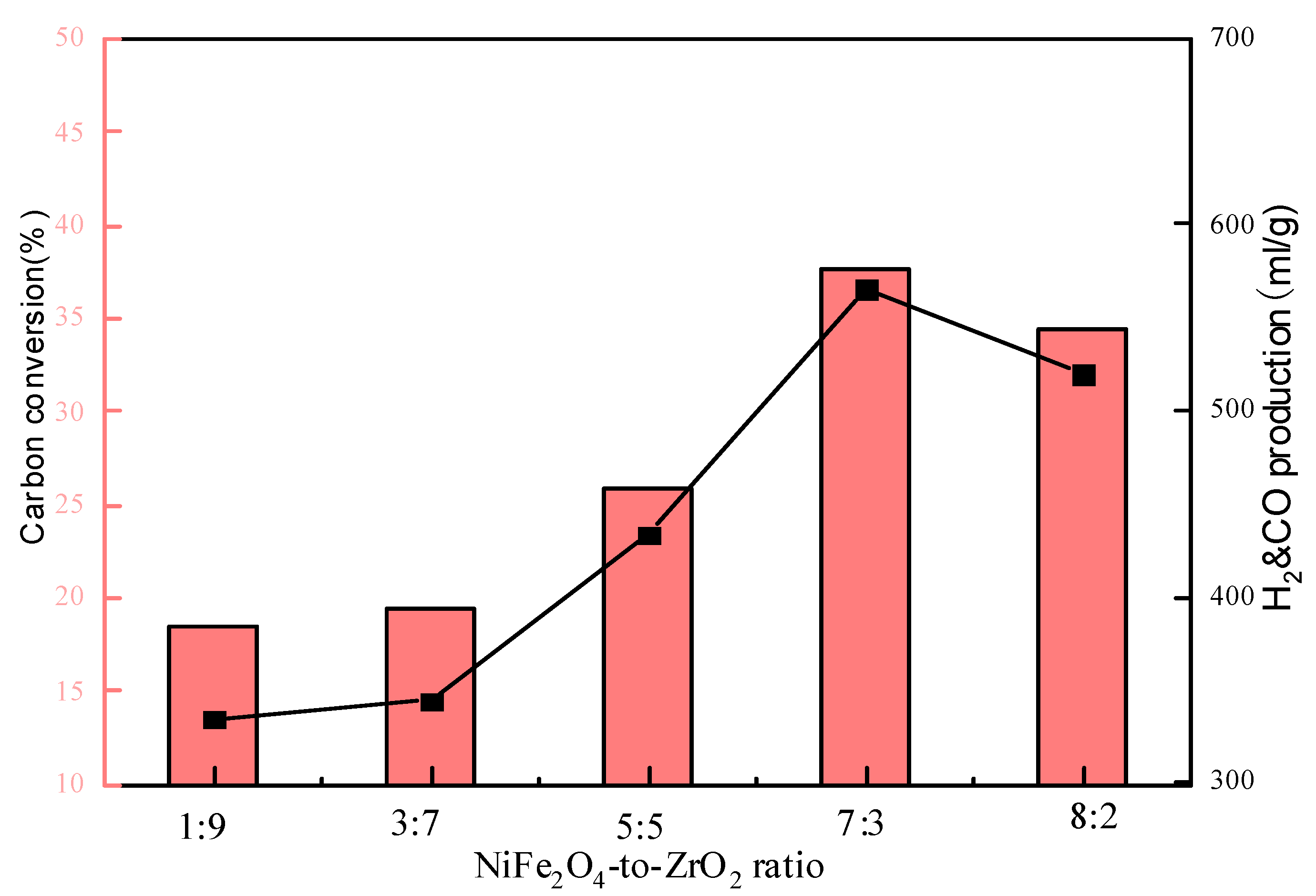
3.3. Effect of NiFe2O4-to-ZrO2 Ratio on CLG
3.4. Effect of Alkali Metals Addition on CLG
3.5. Effect of Steam Addition on CLG
3.6. Long-Term Redox CLG
3.7. XRD Analysis
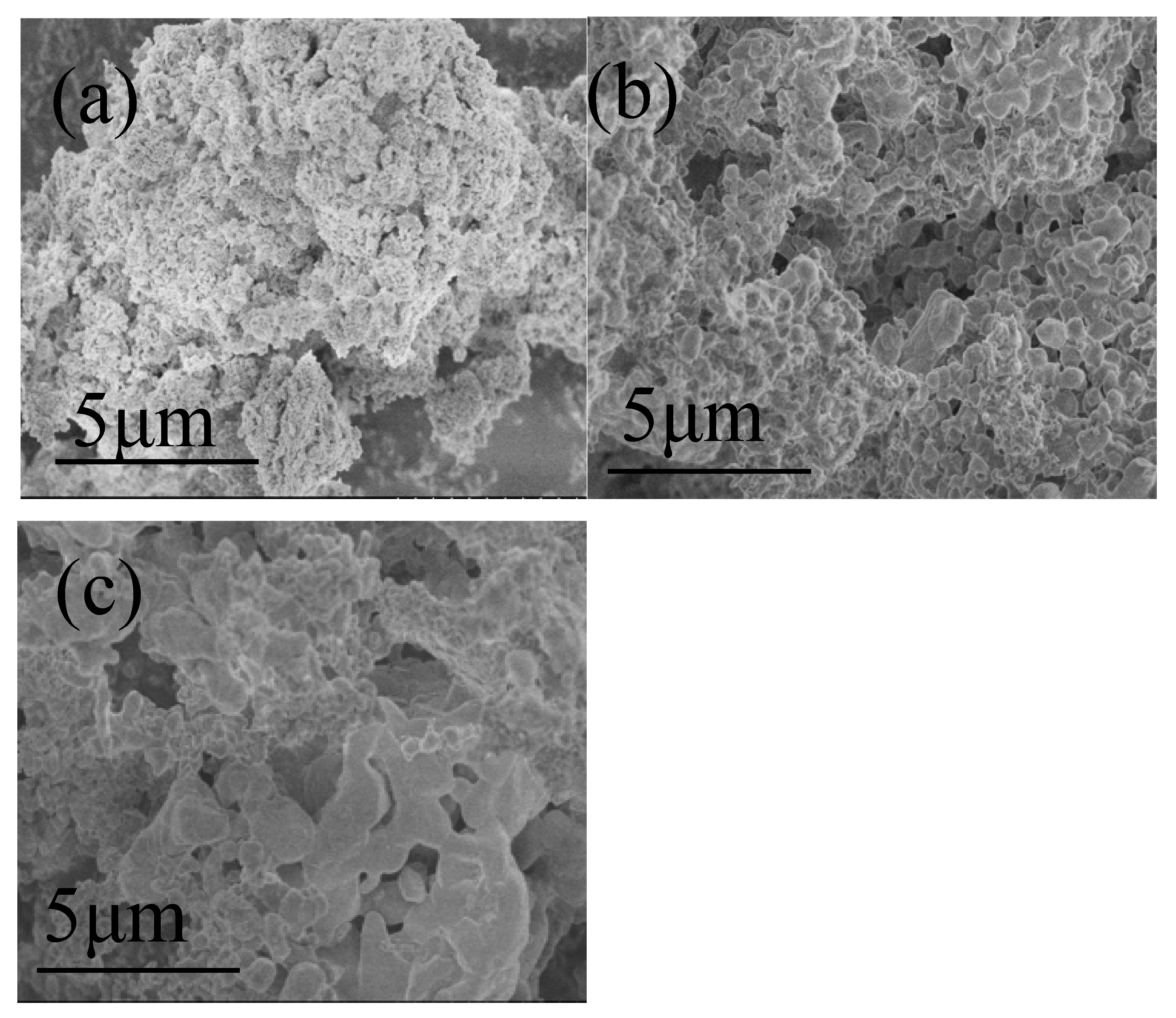
3.8. SEM Analysis
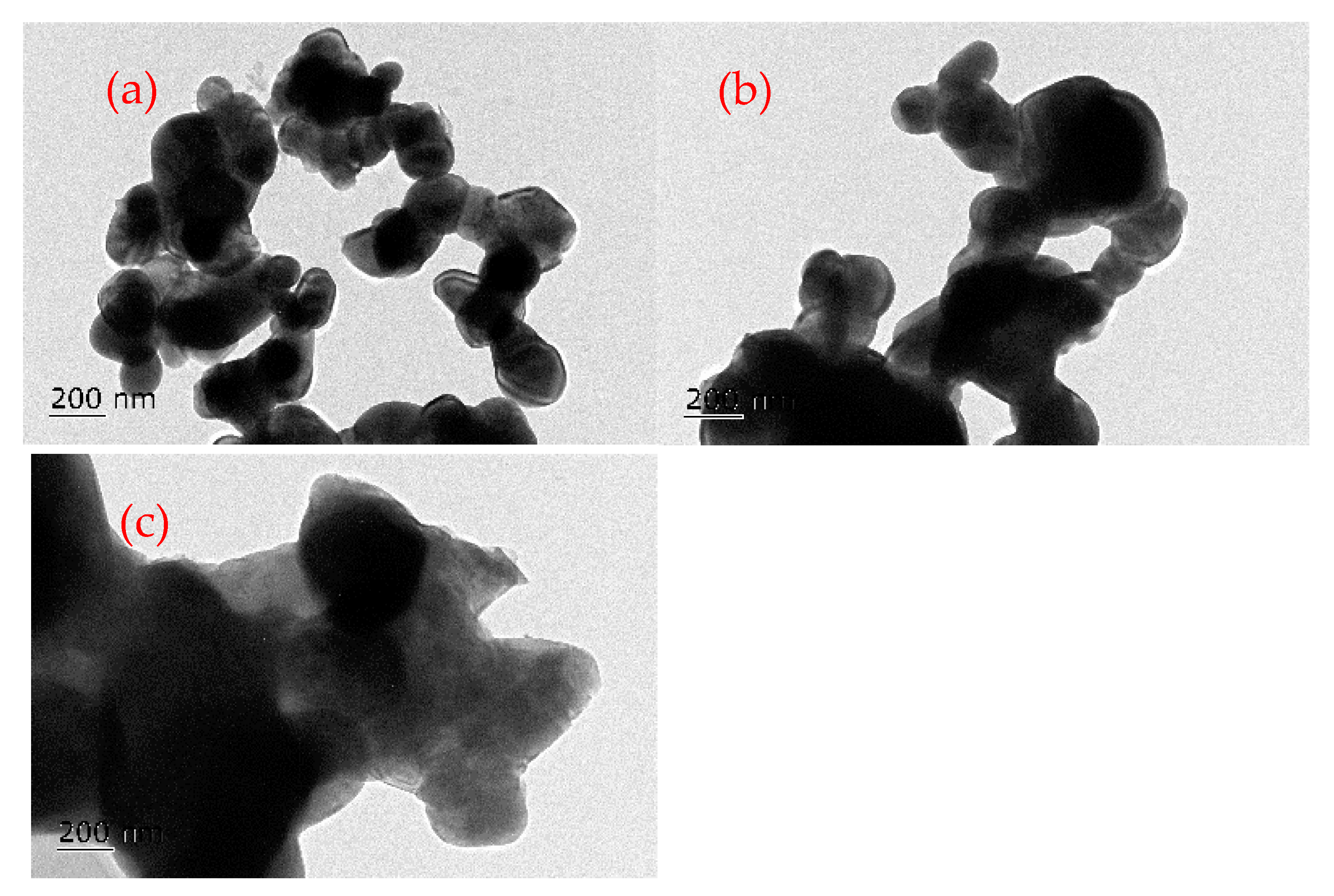
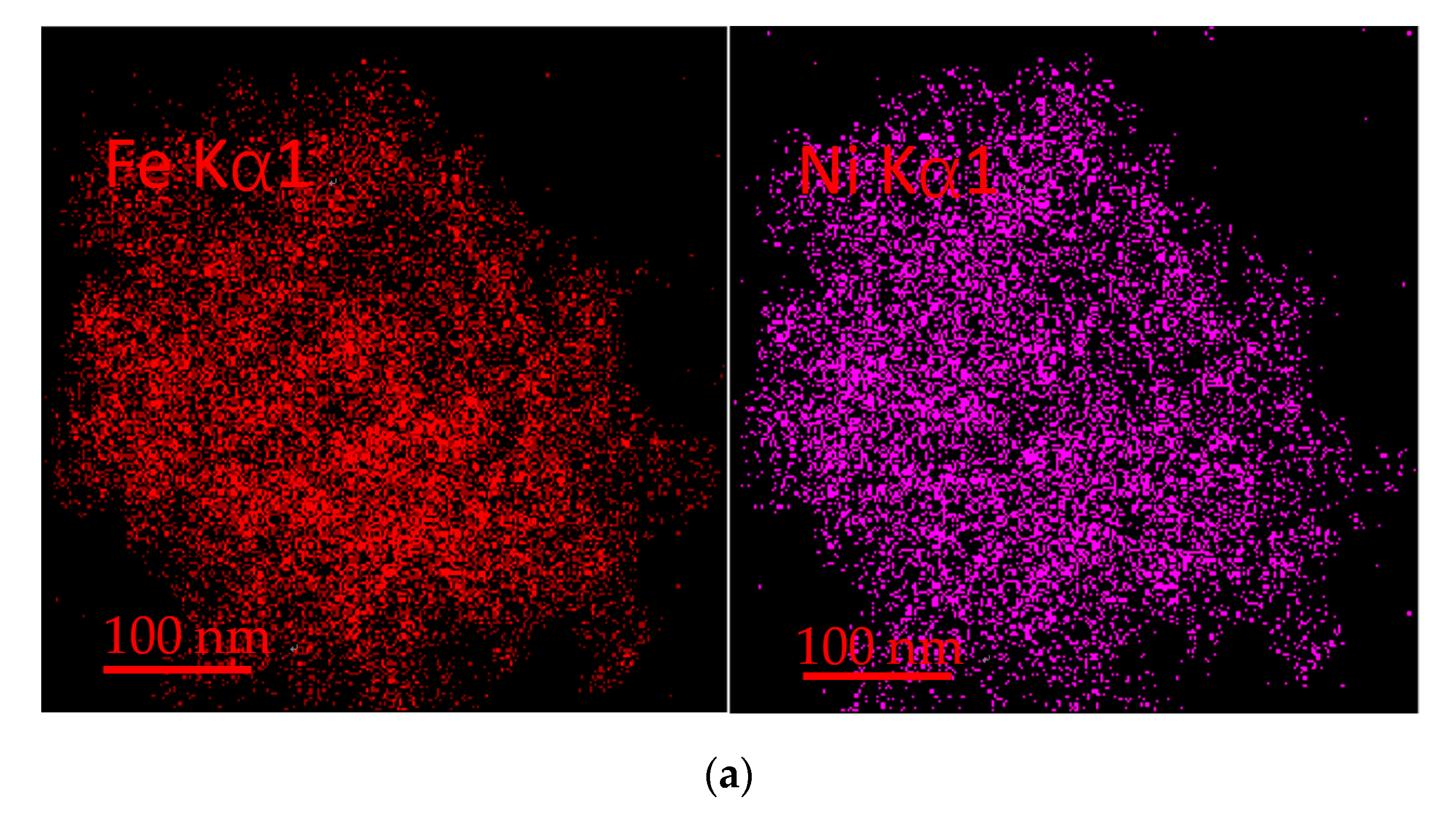
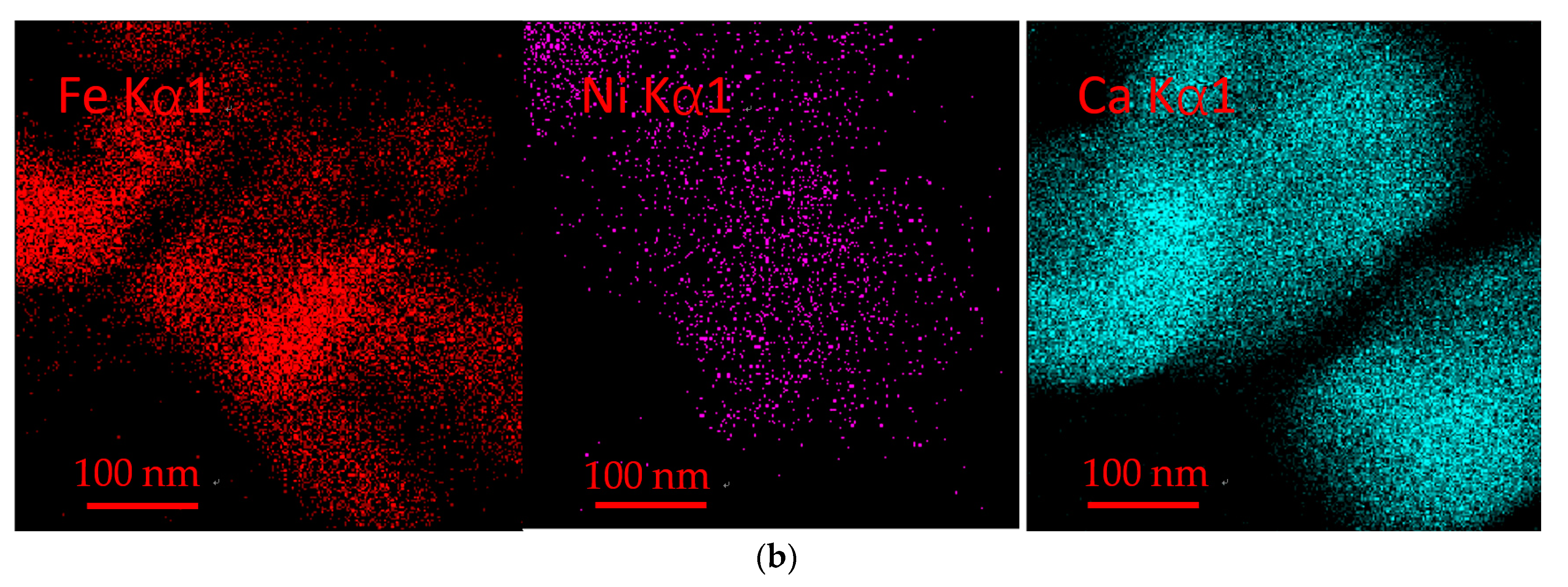
3.9. TEM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.-H.; Li, H.-J.; Wang, R.-Q.; Feng, J.; Li, W.-Y. Acid pretreatment effect on oxygen migration during lignite pyrolysis. Fuel 2020, 262, 116650. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Zhou, A.; Wang, J.; Cai, J.; Dang, Y. A Comparative Study on the Performance of Direct Carbon Solid Oxide Fuel Cells Powered with Different Rank Coals. Energy Fuels 2021, 35, 6835–6844. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y.; Ren, H. Intelligent and ecological coal mining as well as clean utilization technology in China: Review and prospects. Int. J. Min. Sci. Technol. 2019, 29, 161–169. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Z.; Wu, L.; Gao, W. The green behavioral effect of clean coal technology on China’s power generation industry. Sci. Total Environ. 2019, 675, 286–294. [Google Scholar] [CrossRef]
- Richter, H.J.; Knoche, K.F. Reversibility of Combustion Processes. In Efficiency and Costing; ACS Publications: Washington, DC, USA, 1983; pp. 71–85. [Google Scholar] [CrossRef]
- Cormos, A.-M.; Chisalita, D.-A. Assessment of chemical looping combustion process by dynamic simulation. Comput. Aided Chem. Eng. 2016, 38, 271–276. [Google Scholar] [CrossRef]
- Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Chemical Looping Combustion: A Brief Overview. Energies 2022, 15, 1563. [Google Scholar] [CrossRef]
- Noorman, S.; Annaland, M.V.S.; Kuipers, J. Experimental validation of packed bed chemical-looping combustion. Chem. Eng. Sci. 2010, 65, 92–97. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Capone, S.; Anatone, M.; Gallucci, K. Chemical Looping Combustion and Gasification: A Review and a Focus on European Research Projects. Ind. Eng. Chem. Res. 2022, 61, 14403–14432. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Ramkumar, S. Utilization of chemical looping strategy in coal gasification processes. Particuology 2008, 6, 131–142. [Google Scholar] [CrossRef]
- Zhao, K.; Fang, X.; Huang, Z.; Wei, G.; Zheng, A.; Zhao, Z. Hydrogen-rich syngas production from chemical looping gasification of lignite by using NiFe2O4 and CuFe2O4 as oxygen carriers. Fuel 2021, 303, 121269. [Google Scholar] [CrossRef]
- De Diego, L.F.; Garcı´a-Labiano, F.; Gayán, P.; Celaya, J.; Palacios, J.M.; Adánez, J. Operation of a 10 kWth chemical-looping combustor during 200 h with a CuO–Al2O3 oxygen carrier. Fuel 2007, 86, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Marx, F.; Dieringer, P.; Ströhle, J.; Epple, B. Design of a 1 MWth Pilot Plant for Chemical Looping Gasification of Biogenic Residues. Energies 2021, 14, 2581. [Google Scholar] [CrossRef]
- Thon, A.; Kramp, M.; Hartge, E.-U.; Heinrich, S.; Werther, J. Operational experience with a system of coupled fluidized beds for chemical looping combustion of solid fuels using ilmenite as oxygen carrier. Appl. Energy 2014, 118, 309–317. [Google Scholar] [CrossRef]
- Li, F.; Zeng, L.; Velazquez-Vargas, L.G.; Yoscovits, Z.; Fan, L.-S. Syngas chemical looping gasification process: Bench-scale studies and reactor simulations. AIChE J. 2010, 56, 2186–2199. [Google Scholar] [CrossRef]
- Sarafraz, M.; Jafarian, M.; Arjomandi, M.; Nathan, G. The thermo-chemical potential liquid chemical looping gasification with bismuth oxide. Int. J. Hydrogen Energy 2019, 44, 8038–8050. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, Y.; Lin, X.; Wang, B.; Lyu, Q. Research Progress and Perspectives of Solid Fuels Chemical Looping Reaction with Fe-Based Oxygen Carriers. Energy Fuels 2022, 36, 13956–13984. [Google Scholar] [CrossRef]
- Mendiara, T.; Pérez, R.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Low-Cost Fe-Based Oxygen Carrier Materials for the iG-CLC Process with Coal. 1. Ind. Eng. Chem. Res. 2012, 51, 16216–16229. [Google Scholar] [CrossRef]
- Yuan, N.; Bai, H.; An, M.; Zhang, J.; Hu, X.; Guo, Q. Modulation of Fe-based oxygen carriers by low concentration doping of Cu in chemical looping process: Reactivity and mechanism based on experiments combined with DFT calculations. Powder Technol. 2021, 388, 474–484. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, X. Study of reaction mechanism of methane conversion over Ni-based oxygen carrier in chemical looping reforming. Fuel 2017, 210, 866–872. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D.; Tang, Q.; Abuelgasim, S.; Xu, C.; Luo, J.; Zhao, Z.; Abdalazeez, A. Hydrogen-rich syngas production from straw char by chemical looping gasification: The synergistic effect of Mn and Fe on Ni-based spinel structure as oxygen carrier. Fuel 2023, 334, 126803. [Google Scholar] [CrossRef]
- Rasi, N.M.; Karcz, A.; Ponnurangam, S.; Mahinpey, N. Insight into MgO-supported NiO reactivity from atomic-scale electronegativity for oxygen carrier design and catalyst production applications. Catalysis Today 2022, 404, 244–252. [Google Scholar] [CrossRef]
- Gayán, P.; Forero, C.R.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Effect of Support on the Behavior of Cu-Based Oxygen Carriers during Long-Term CLC Operation at Temperatures above 1073 K. Energy Fuels 2011, 25, 1316–1326. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Luo, M.; Wang, G.; Wang, L.; Lv, M. Analysis of Reactivity of a CuO-Based Oxygen Carrier for Chemical Looping Combustion of Coal. Energy Fuels 2012, 26, 3275–3283. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.; Zou, X. Chemical-looping auto-thermal reforming of biomass using Cu-based oxygen carrier. Appl. Energy 2015, 157, 408–415. [Google Scholar] [CrossRef]
- Cao, Y.; He, B.; Yan, L. Process simulation of a dual fluidized bed chemical looping air separation with Mn-based oxygen carrier. Energy Convers. Manag. 2019, 196, 286–295. [Google Scholar] [CrossRef]
- Jacobs, M.; van der Kolk, T.; Albertsen, K.; Mattisson, T.; Lyngfelt, A.; Snijkers, F. Synthesis and upscaling of perovskite Mn-based oxygen carrier by industrial spray drying route. Int. J. Greenh. Gas Control. 2018, 70, 68–75. [Google Scholar] [CrossRef]
- Bi, W.; Chen, T.; Zhao, R.; Wang, Z.; Wu, J.; Wu, J. Characteristics of a CaSO4 oxygen carrier for chemical-looping combustion: Reaction with polyvinylchloride pyrolysis gases in a two-stage reactor. RSC Adv. 2015, 5, 34913–34920. [Google Scholar] [CrossRef]
- Liu, S.; Lee, D.; Liu, M.; Li, L.; Yan, R. Selection and Application of Binders for CaSO4Oxygen Carrier in Chemical-Looping Combustion. Energy Fuels 2010, 24, 6675–6681. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, M.; Zhang, X.; Hu, X.; Guo, Q. Characteristics of a CaSO4 composite oxygen carrier supported with an active material for in situ gasification chemical looping combustion of coal. RSC Adv. 2018, 8, 23372–23381. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Wang, H.; Zhao, W.; Huang, Z.; Yi, Q.; He, F.; Zhao, K.; Zheng, A.; Meng, J.; Deng, Z.; et al. Synthesis gas production from chemical looping gasification of lignite by using hematite as oxygen carrier. Energy Convers. Manag. 2019, 185, 774–782. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, Y.; Liu, G.; Mo, F.; Ma, X. Application of Mn–Fe Composite Oxides Loaded on Alumina as Oxygen Carrier for Chemical Looping Gasification. Waste Biomass-Valorization 2019, 11, 6395–6409. [Google Scholar] [CrossRef]
- Maya, J.C.; Chejne, F.; Bhatia, S.K. Effect of sintering on the reactivity of copper-based oxygen carriers synthesized by impregnation. Chem. Eng. Sci. 2017, 162, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Sikarwar, S.S.; Vooradi, R.; Patnaikuni, V.S.; Kakunuri, M.; Surywanshi, G.D. A novel thermally stable Fe2O3/Al2O3 nanofiber-based oxygen carrier for chemical-looping combustion. Chem. Papers 2022, 76, 3987–3993. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q.; Wang, Q.; Hua, J.; Peng, R. Oxygen Uncoupling Property and Kinetics of a Copper Manganese Composite Oxygen Carrier in a Packed-Bed Reactor. Energy Fuels 2020, 34, 6158–6167. [Google Scholar] [CrossRef]
- Song, T.; Shen, T.; Shen, L.; Xiao, J.; Gu, H.; Zhang, S. Evaluation of hematite oxygen carrier in chemical-looping combustion of coal. Fuel 2013, 104, 244–252. [Google Scholar] [CrossRef]
- Zheng, A.; Fan, Y.; Wei, G.; Zhao, K.; Huang, Z.; Zhao, Z.; Li, H. Chemical Looping Gasification of Torrefied Biomass Using NiFe2O4 as an Oxygen Carrier for Syngas Production and Tar Removal. Energy Fuels 2020, 34, 6008–6019. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; He, F.; Liu, S.; Zhao, K.; Wei, G.; Zheng, A.; Zhao, Z.; Li, H. Thermodynamic analysis and kinetic investigations on biomass char chemical looping gasification using Fe-Ni bimetallic oxygen carrier. Energy 2017, 141, 1836–1844. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fuidized-bed combustion process with inherent CO2 separation;application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar] [CrossRef]
- Wei, G.; He, F.; Huang, Z.; Zheng, A.; Zhao, K.; Li, H. Continuous Operation of a 10 kW(th) Chemical Looping Integrated Fluidized Bed Reactor for Gasifying Biomass Using an Iron-Based Oxygen Carrier. Energy Fuels 2015, 29, 233–241. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Direct conversion of methane to synthesis gas using lattice oxygen of CeO2–Fe2O3 complex oxides. Chem. Eng. J. 2010, 156, 512–518. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Catalytic effects of inherent alkali and alkaline earth metallic species on steam gasification of biomass. Int. J. Hydrogen Energy 2015, 40, 15460–15469. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Ge, H.; Shen, L.; Gu, H.; Jiang, S. Effect of co-precipitation and impregnation on K-decorated Fe2O3/Al2O3 oxygen carrier in Chemical Looping Combustion of bituminous coal. Chem. Eng. J. 2015, 262, 1065–1076. [Google Scholar] [CrossRef]
- Zhao, H.; Mei, D.; Ma, J.; Zheng, C. Comparison of preparation methods for iron-alumina oxygen carrier and its reduction kinetics with hydrogen in chemical looping combustion. Asia-Pacific J. Chem. Eng. 2014, 9, 610–622. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, S.; Xiao, R. Insights into the relationship between microstructural evolution and deactivation of Al2O3 supported Fe2O3 oxygen carrier in chemical looping combustion. Energy Convers. Manag. 2019, 188, 429–437. [Google Scholar] [CrossRef]
- Shen, L.; Wu, J.; Gao, Z.; Xiao, J. Reactivity deterioration of NiO/Al2O3 oxygen carrier for chemical looping combustion of coal in a 10 kWth reactor. Combust. Flame 2009, 156, 1377–1385. [Google Scholar] [CrossRef]
- Yu, Z.; Li, C.; Fang, Y.; Huang, J.; Wang, Z. Reduction Rate Enhancements for Coal Direct Chemical Looping Combustion with an Iron Oxide Oxygen Carrier. Energy Fuels 2012, 26, 2505–2511. [Google Scholar] [CrossRef]
- Siriwardane, R.; Riley, J.; Tian, H.; Richards, G. Chemical looping coal gasification with calcium ferrite and barium ferrite via solid–solid reactions. Appl. Energy 2016, 165, 952–966. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, Y.; Liu, G.; Ma, X. Syngas production by chemical looping gasification of biomass with steam and CaO additive. Int. J. Hydrogen Energy 2018, 43, 19375–19383. [Google Scholar] [CrossRef]







| Ultimate analysis (wt%, daf) | C | H | N | S | O* | Qnet, v, d 26.72 KJ/g |
| 69.77 | 3.91 | 0.61 | 1.06 | 12.97 | ||
| Proximate analysis (wt%) | M | V | A | FC | ||
| 10.22 | 40.30 | 11.68 | 37.8 | |||
| Element | O | Ca | Na | Fe | Si | Al | Mg | S | Others |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 50.12 | 17.07 | 13.14 | 7.05 | 4.62 | 2.90 | 1.70 | 1.55 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Song, D.; Li, Y.; Cao, J.; Wei, G.; He, F. High-Quality Syngas Production by Chemical Looping Gasification of Bituminite Based on NiFe2O4 Oxygen Carrier. Energies 2023, 16, 3385. https://doi.org/10.3390/en16083385
Yang M, Song D, Li Y, Cao J, Wei G, He F. High-Quality Syngas Production by Chemical Looping Gasification of Bituminite Based on NiFe2O4 Oxygen Carrier. Energies. 2023; 16(8):3385. https://doi.org/10.3390/en16083385
Chicago/Turabian StyleYang, Ming, Da Song, Yang Li, Jinzeng Cao, Guoqiang Wei, and Fang He. 2023. "High-Quality Syngas Production by Chemical Looping Gasification of Bituminite Based on NiFe2O4 Oxygen Carrier" Energies 16, no. 8: 3385. https://doi.org/10.3390/en16083385
APA StyleYang, M., Song, D., Li, Y., Cao, J., Wei, G., & He, F. (2023). High-Quality Syngas Production by Chemical Looping Gasification of Bituminite Based on NiFe2O4 Oxygen Carrier. Energies, 16(8), 3385. https://doi.org/10.3390/en16083385






