Preparation of an Anodic Alumina-Supported Ni Catalyst and Development of a Catalytic Plate Reformer for the Steam Reforming of Methane
Abstract
:1. Introduction
2. Experimental
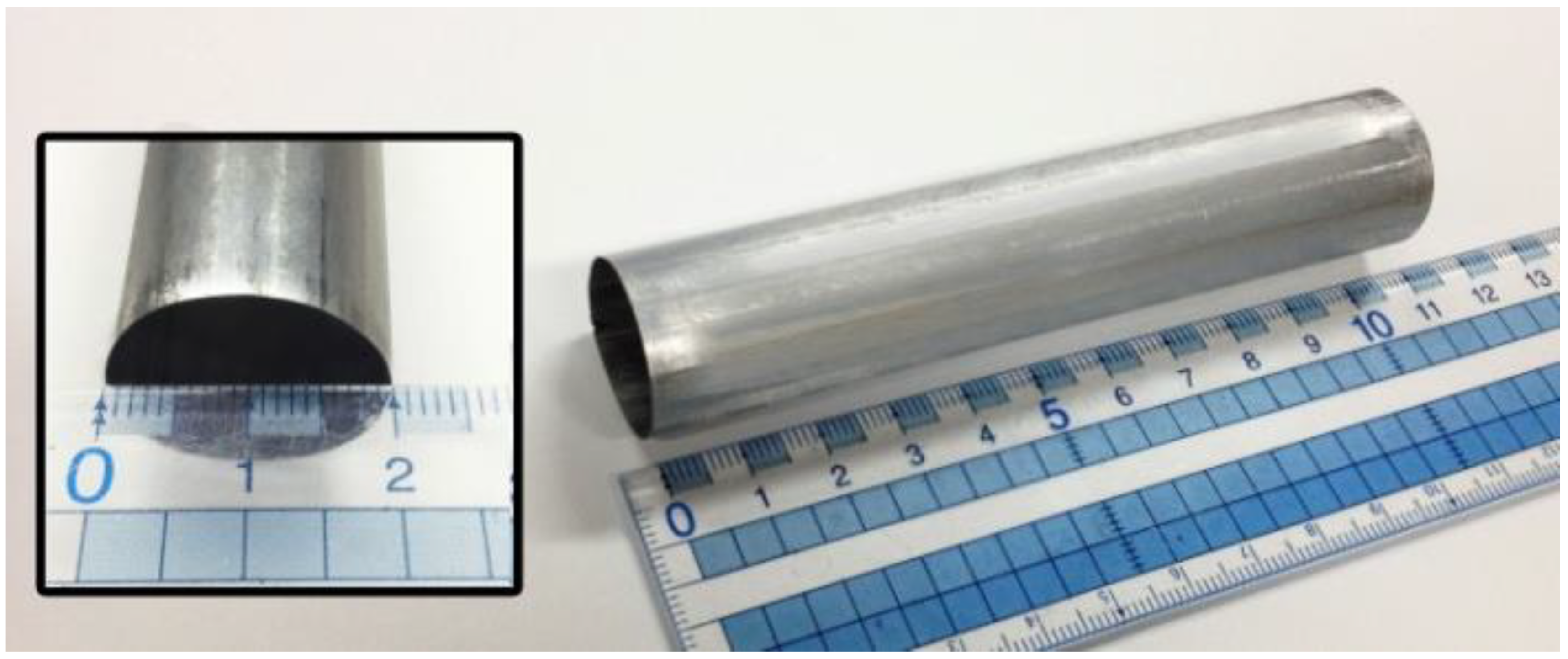
2.1. Preparation of Anodic Alumina Catalyst
2.2. Characterization of Anodic Alumina Catalyst
2.3. Steam Reforming of Methane
3. Mathematical Model for the SMR Reformer
3.1. Reactor Schemas
- Operation was in steady state;
- Gases were assumed to be ideal gases obeying the ideal gas law;
- Chemical reaction took place only in the catalyst layer.
3.2. Kinetics Data
- where the equilibrium constant Km (m = 1 or 2) was calculated from thermodynamic data. The pre-exponential factor (A) and the activation energy (Ek) were estimated from experimental data using the least squares method.
3.3. Mathematical Model
3.3.1. Gas Phase
3.3.2. Catalyst Phase
3.3.3. Boundary Conditions
3.3.4. Symmetry
4. Results and Discussion
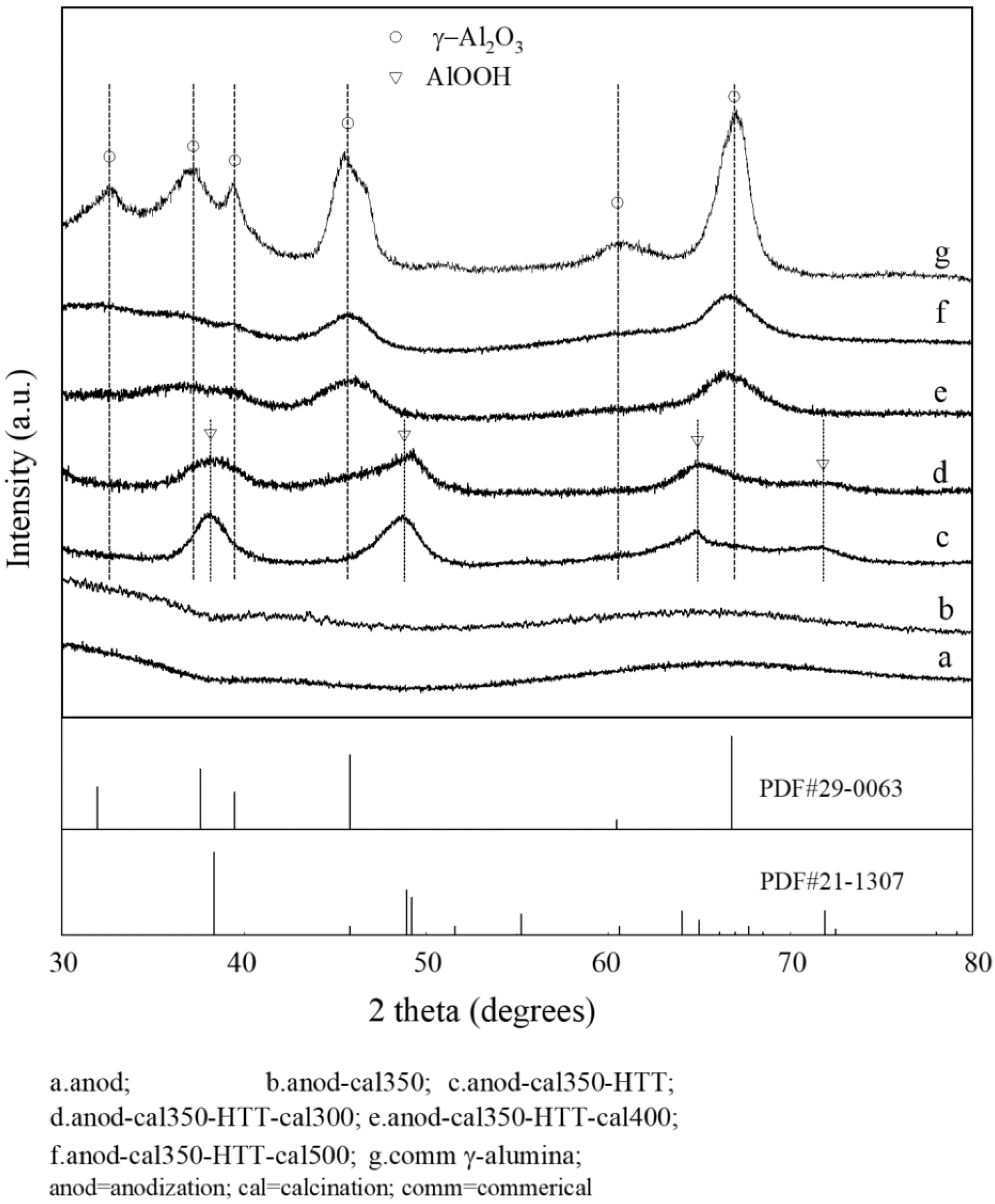
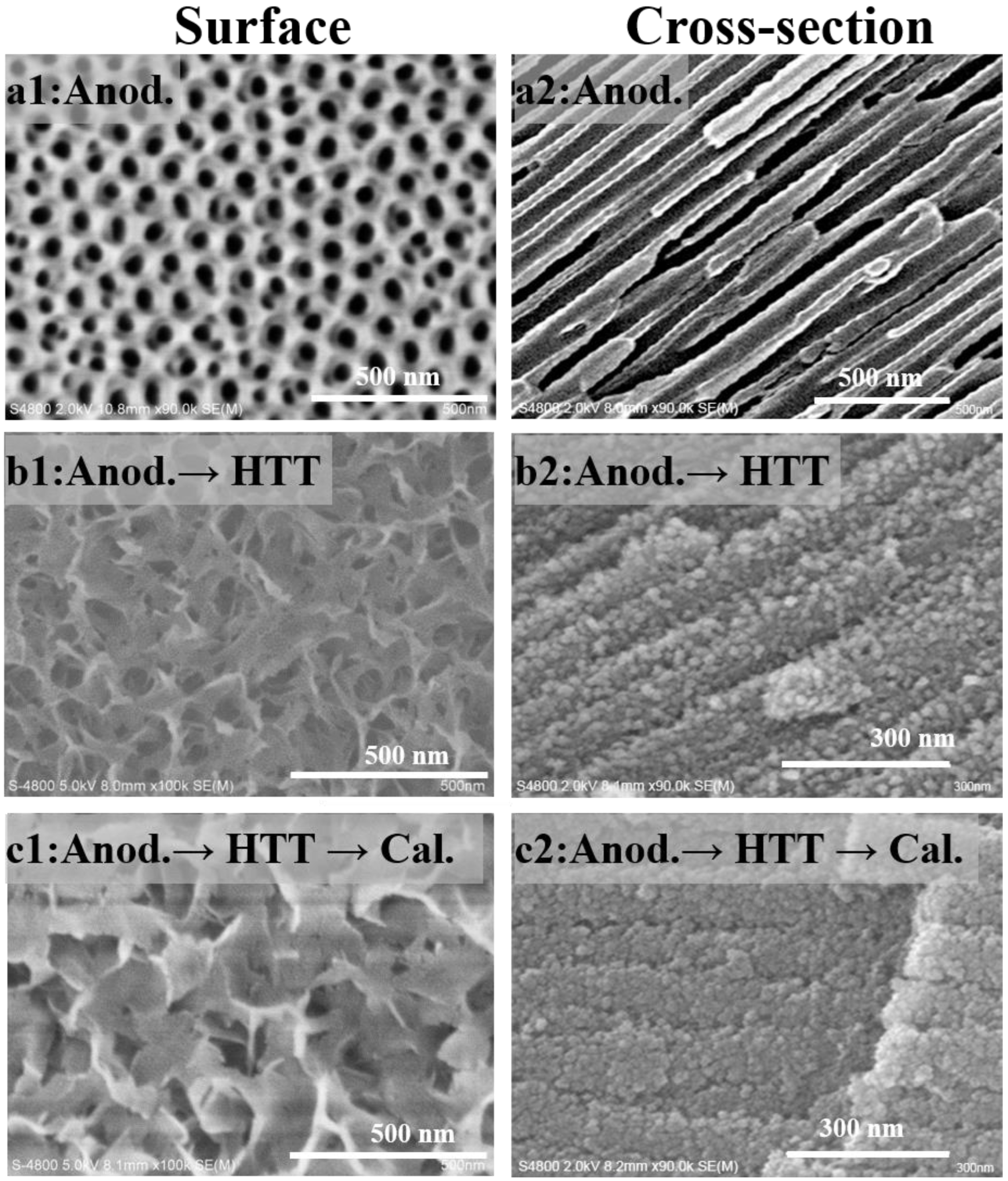
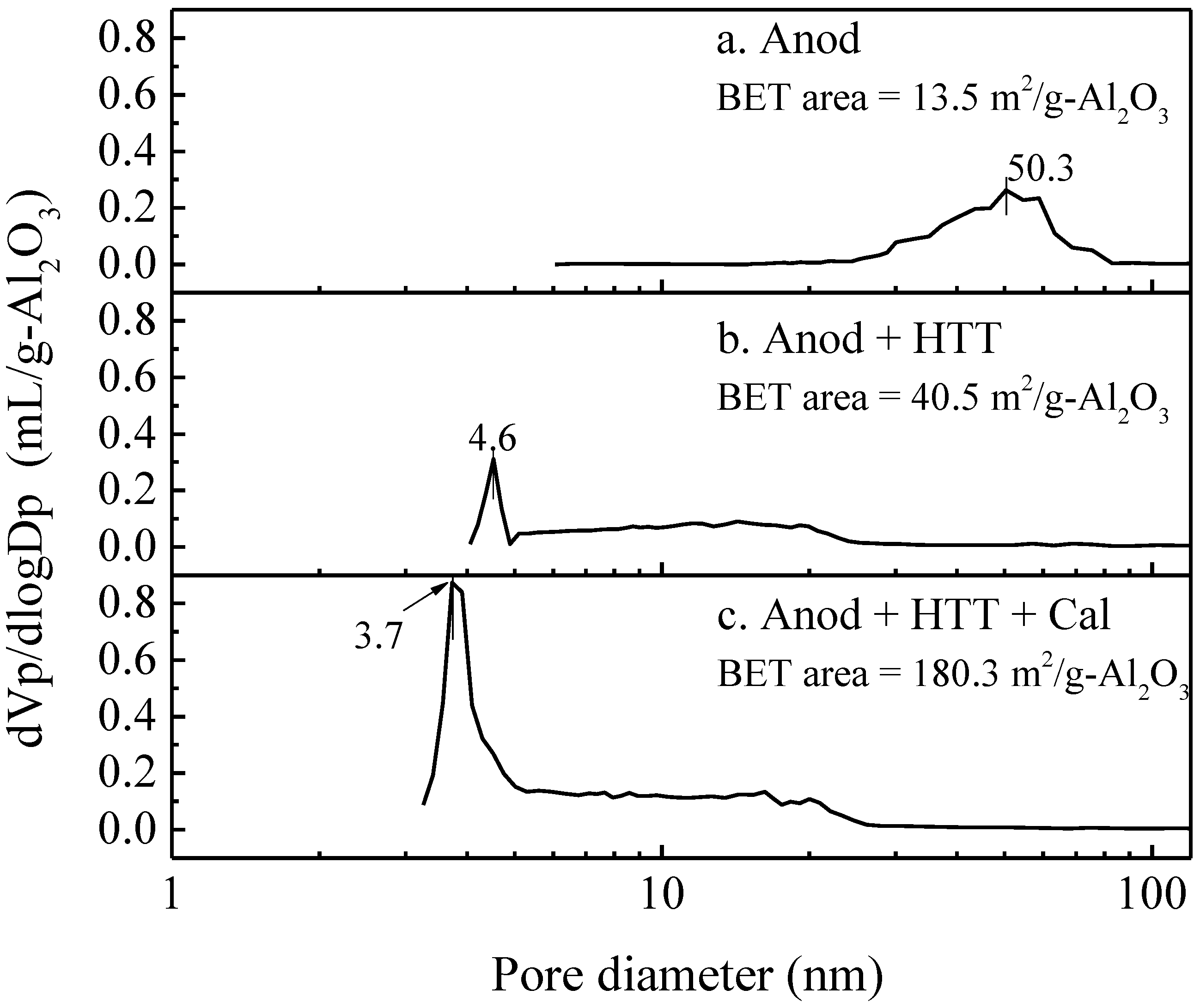
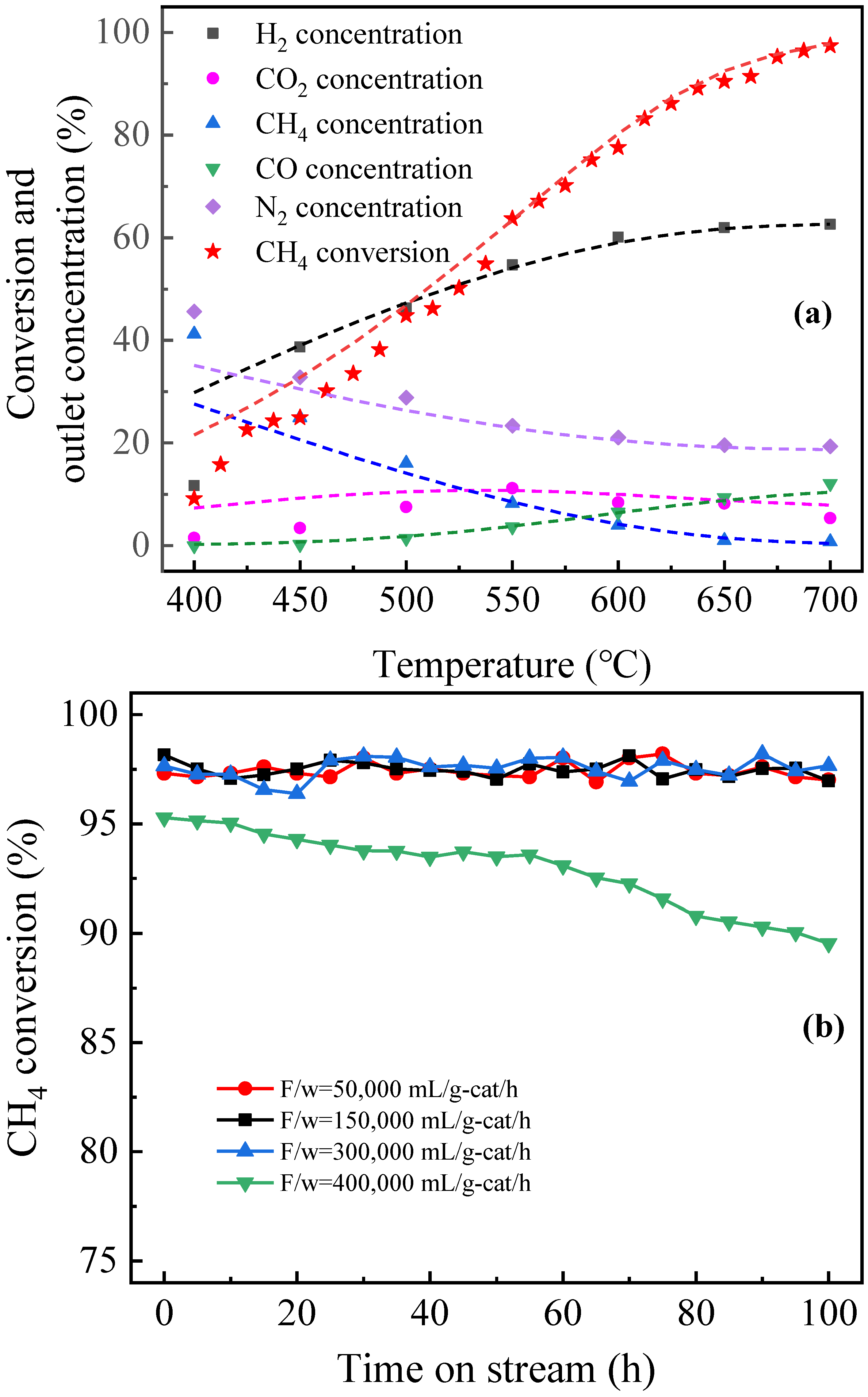
4.1. Preparation and Activity Test of an Anodic Alumina-Supported Ni Catalyst
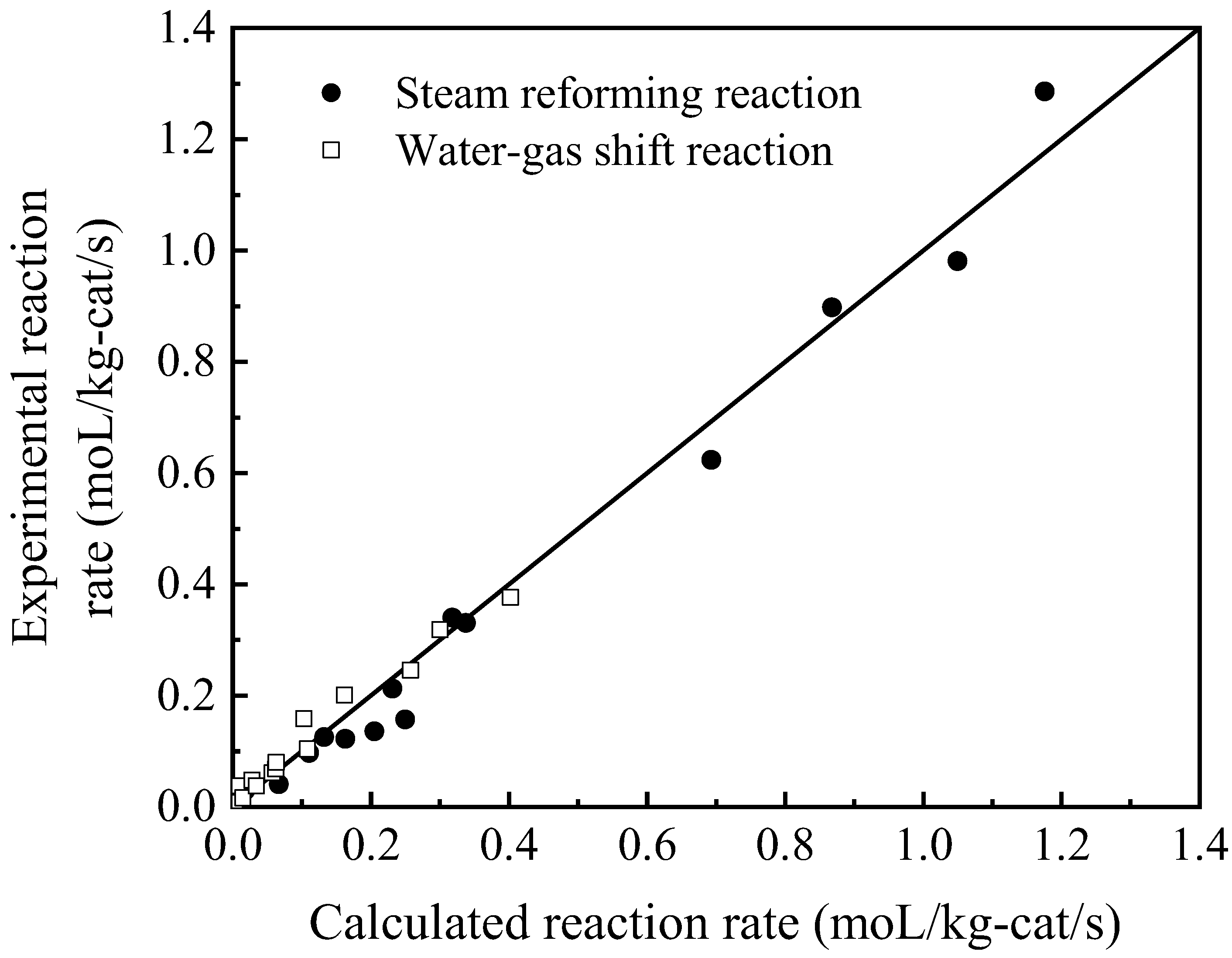
4.2. Reaction Kinetics Data
4.3. Development of Catalytic Plate Reformer
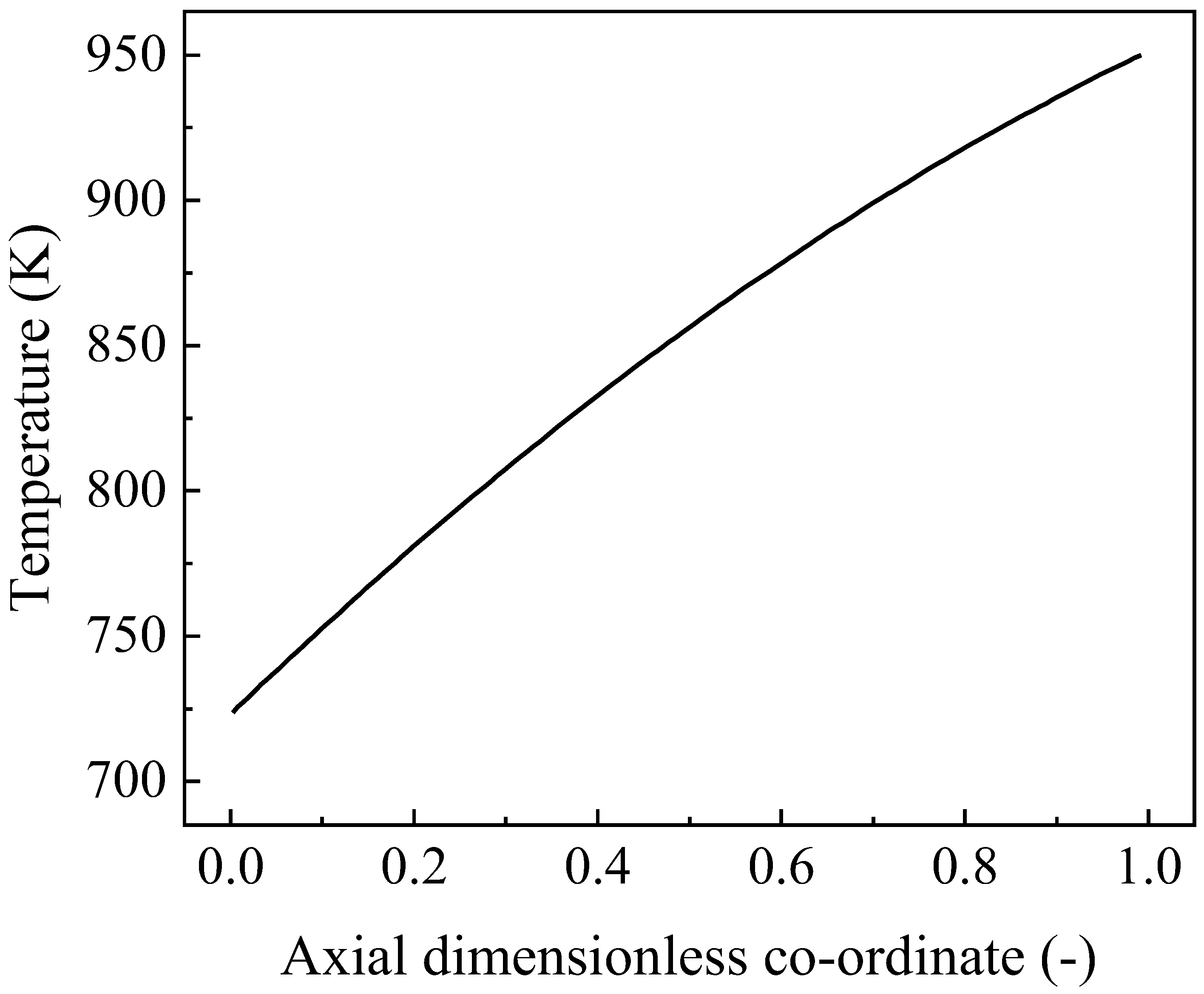
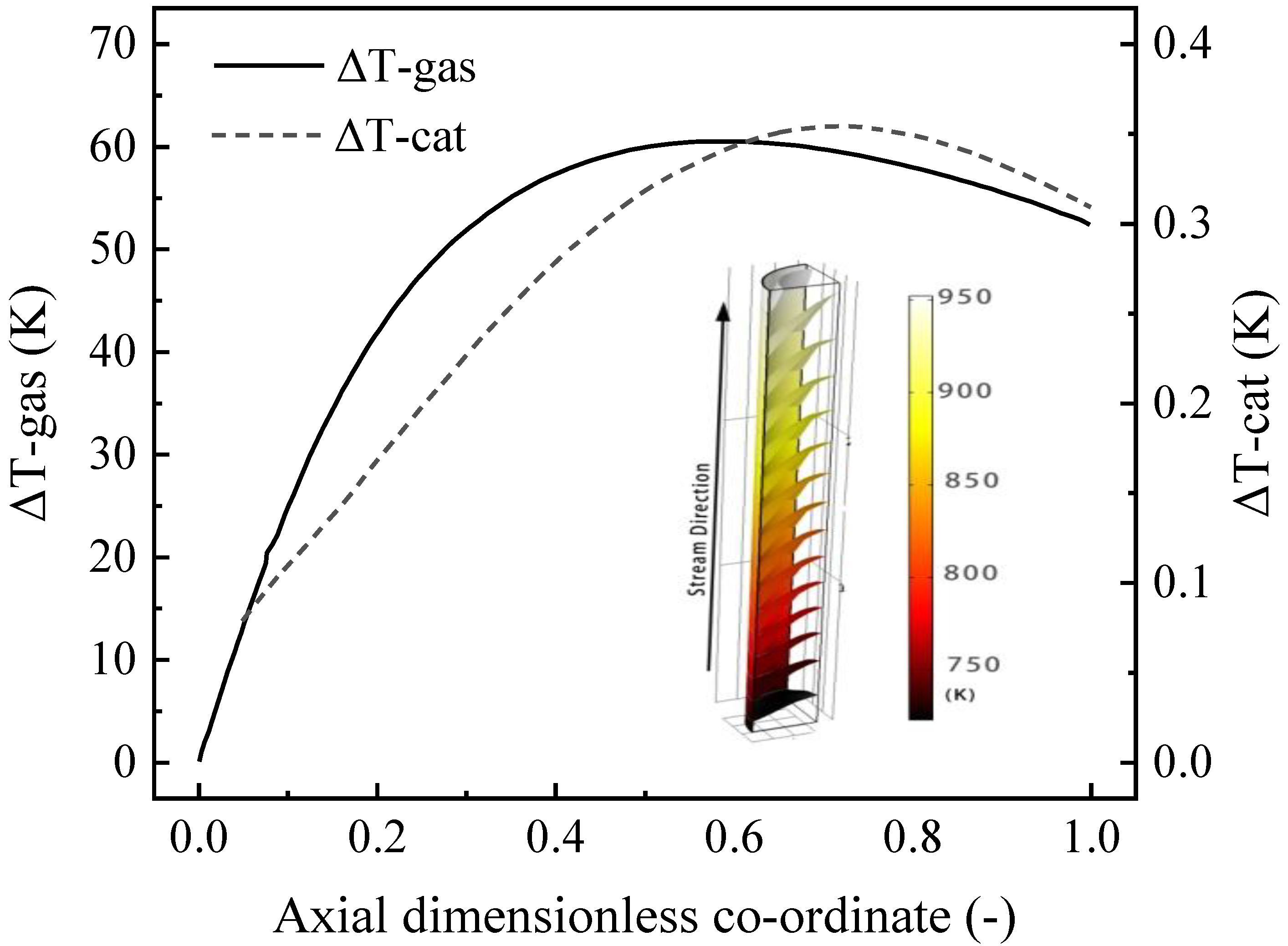
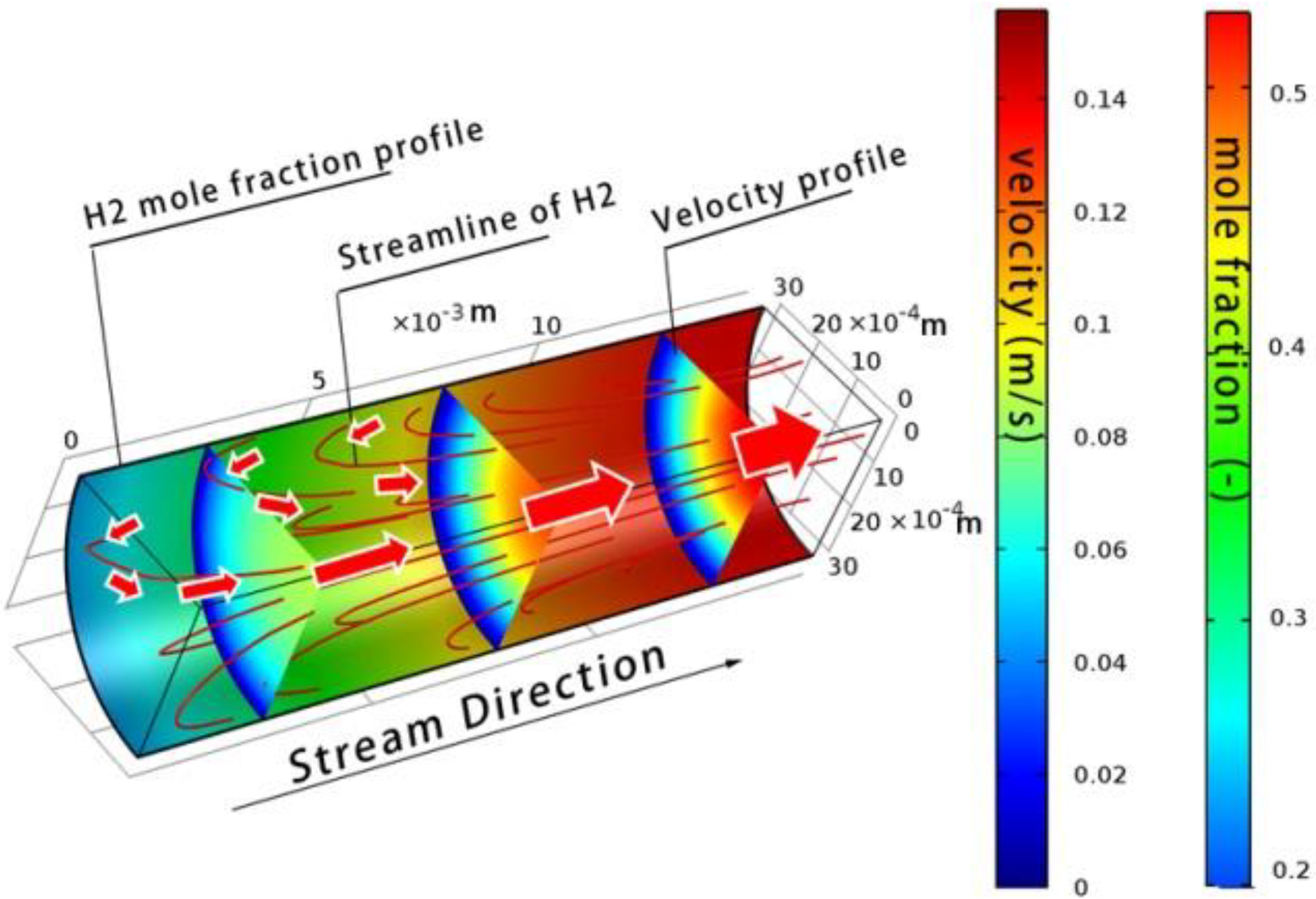
4.3.1. Distribution of Velocity and Temperature
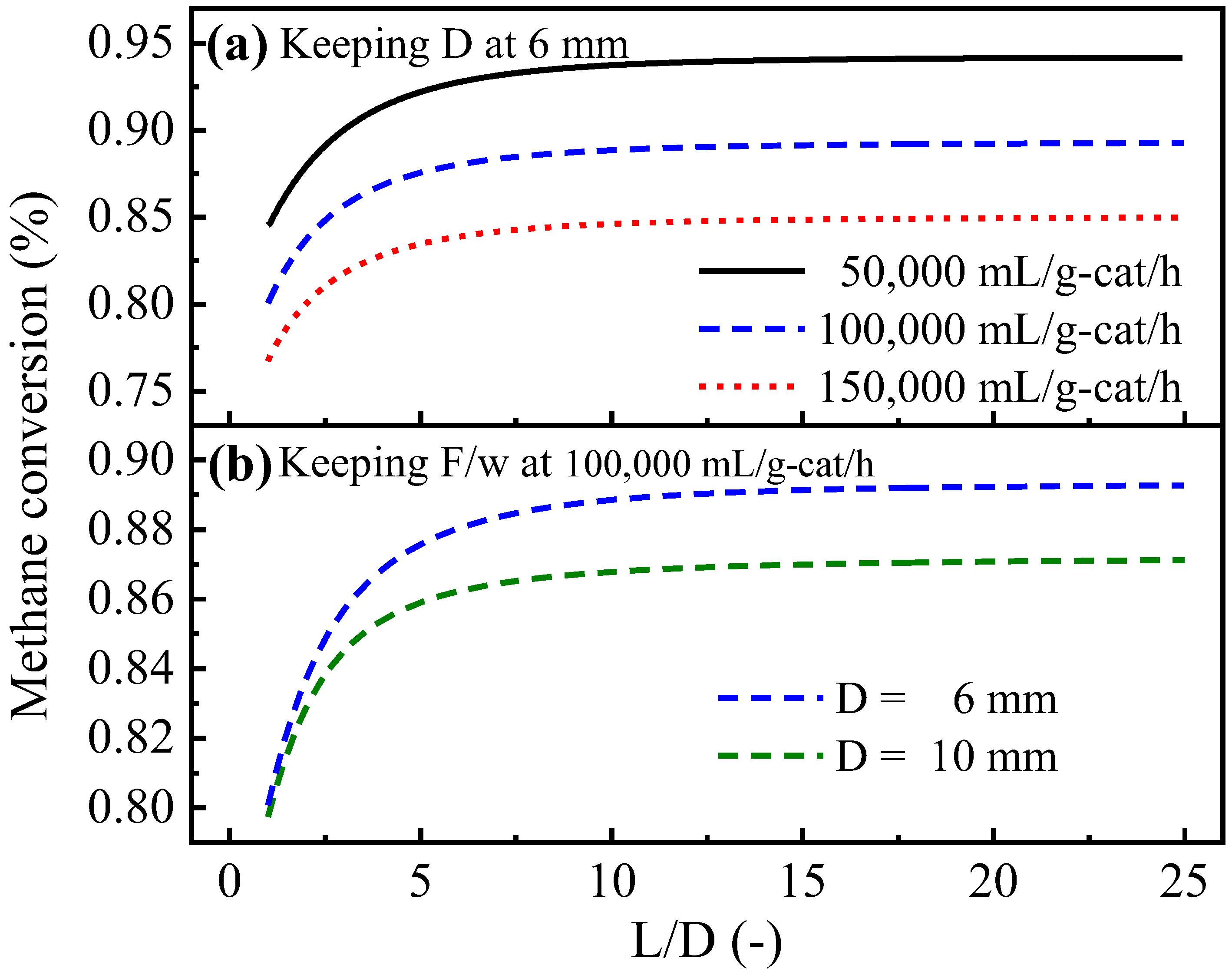
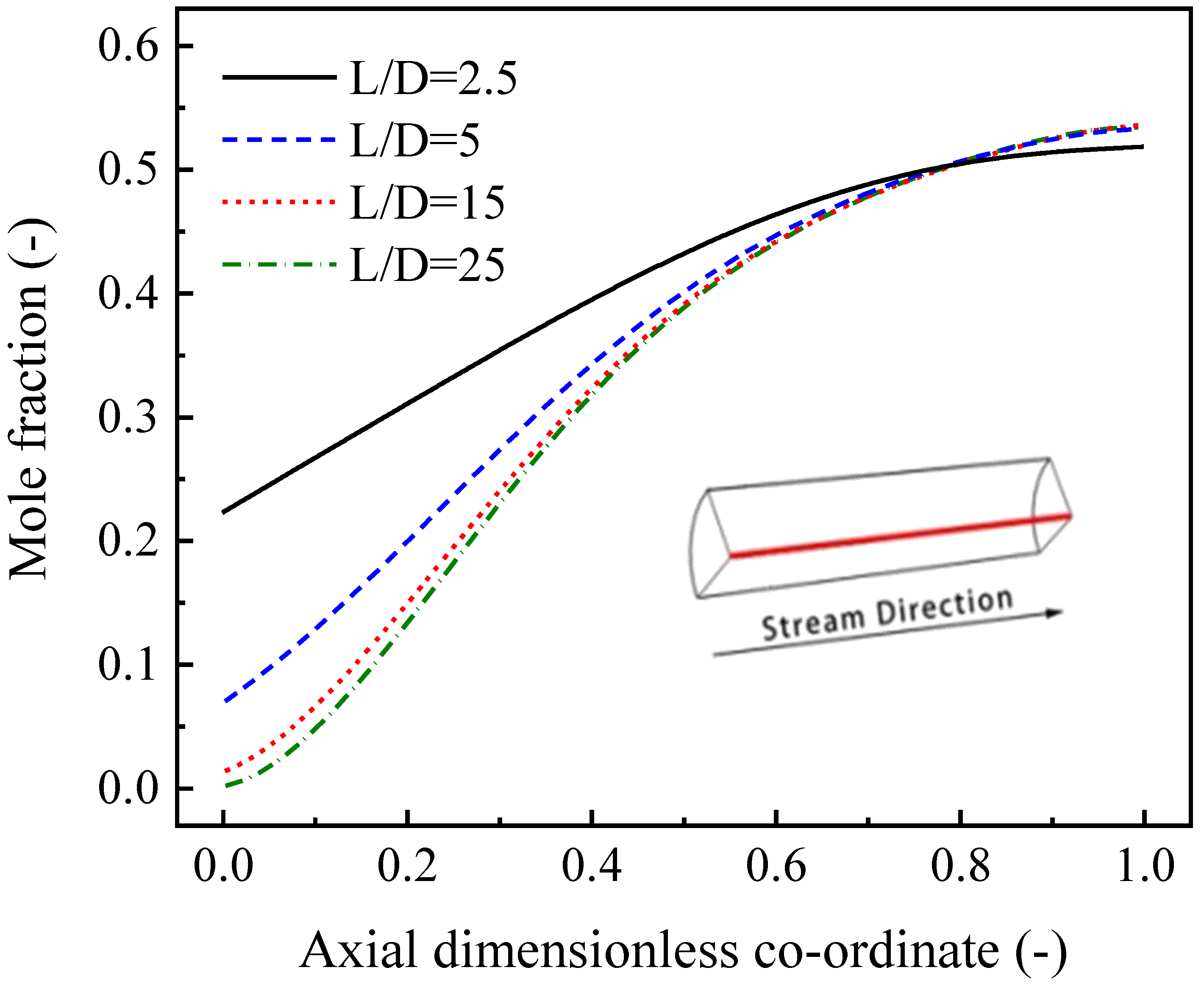
4.3.2. Effects of the L/D of Tube-Type Channels and the L/W of Box-Type Channels
4.3.3. Analysis of Mass Transfer
4.3.4. Analysis of Backmixing
4.3.5. Effect of Height of Box-Type Channel
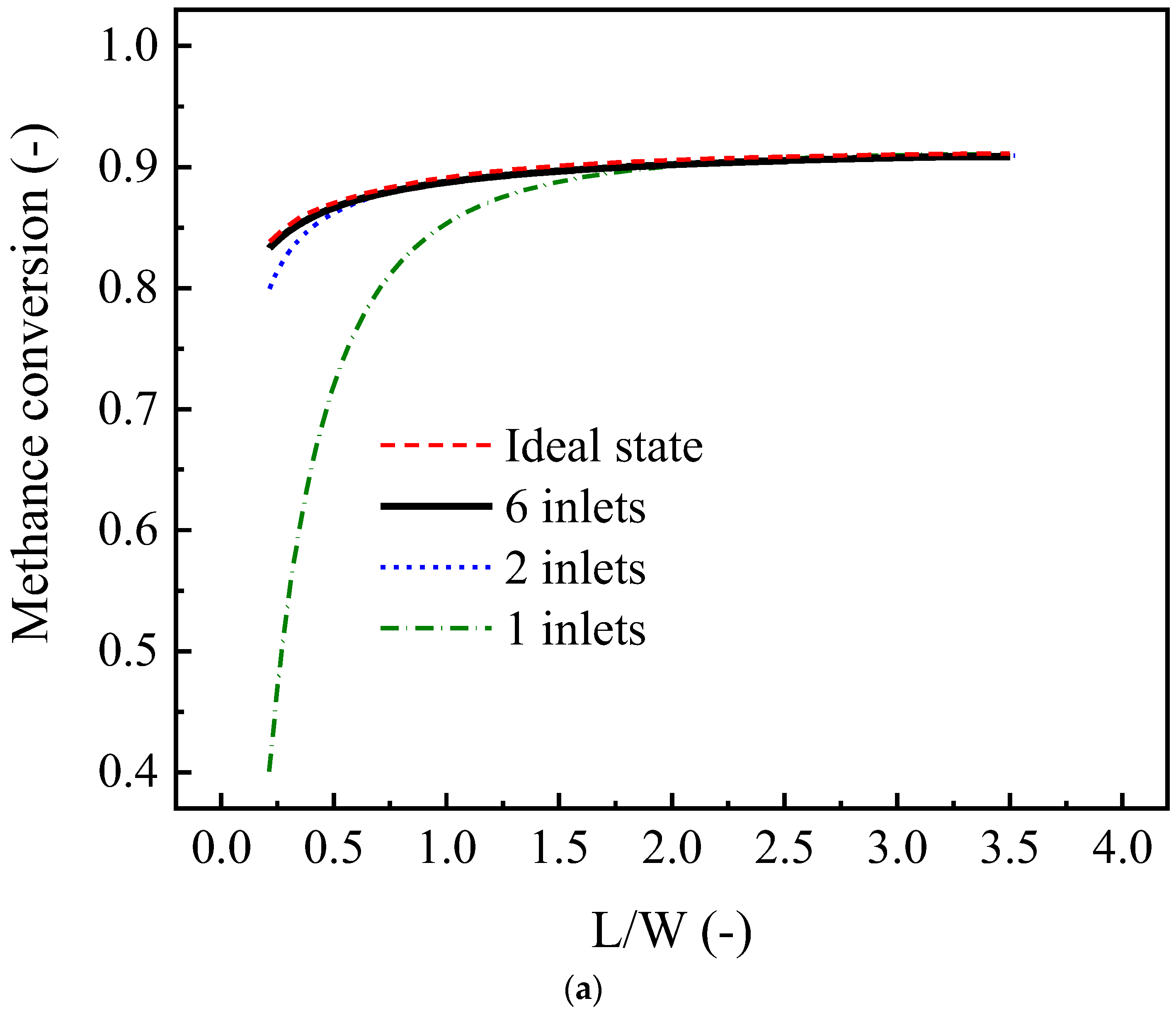
4.3.6. Effect of Number of Inlet Ports in a Box-Type Channel
4.3.7. Comparison of Tube-Type and Box-Type Channels
5. Conclusions
- (1)
- Hydrothermal treatment and subsequent high-temperature calcination can transform amorphous skeletal alumina in the conventional PAA layer into r-alumina, and significantly increase its specific surface area, without destroying the macroscopically integrated structure and microscopically ordered pore structure of the PAA material.
- (2)
- The ΔT-gas (temperature difference between the channel wall and the center of the gas phase) of the thin-walled catalyst is only 30% of that of the particulate catalyst, indicating the potential advantages of the CPR reactor in terms of heat transfer and energy savings.
- (3)
- Insufficient transverse mass transfer and backmixing are two major factors affecting reformer performance, especially when the L/D (or L/W) is small and the channel height is large.
- (4)
- For tube-type channels, an L/D of 10~35 and a diameter of 5~20 mm are favorable choices. Moreover, for box-type type channels, the L/W and the height should be set to 2~4 and 2~5 mm, respectively.
- (5)
- For box-type channels, the number of gas inlet ports has a significant effect on the reformer’s performance. The distribution state provided by two inlet ports is close to the ideal distribution state.
- (6)
- The box-type channel has the potential for reactor downsizing, but it is not as good as the tube-type channel in terms of gas distribution homogeneity and ease of manufacturing.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Cp | heat capacity at constant pressure [J/(kg K)] |
| D | diameter of reformer tube [mm] |
| Deff | effective diffusion coefficient [m2/s] |
| Dr | gas diffusion coefficient [m2/s] |
| Ek | ctivation energy [kJ/mol] |
| F/w | space velocity [mL/(g-cat h)] |
| F0 | Fourier number [-] |
| H | channel height [m] |
| kj | Arrhenius kinetic parameter of reaction j [mol bar-2/(kg-cat s)] |
| Kj | adsorption constant of species component j [bar2] |
| L | channel length [m] |
| MW | molecular weight of species [kg/mol] |
| p | pressure [Pa] |
| Q | heat source [W/m3] |
| R | gas constant [J/(mol K)] |
| Ri | reaction rate [kg/(m3-cat s)] |
| Re | Reynolds number [-] |
| S/C | steam to carbon ratio [-] |
| T | temperature [°C] |
| W | channel width [cm] |
| W1 | reaction rate [mol/(kg-cat s)] |
| W2 | reaction rate [mol/(kg-cat s)] |
| R | radius [m] |
| u | velocity [m/s] |
| t | time [s] |
| λ | thermal conductivity [W/(m K)] |
| k | thermal conductivity [W/(m K)] |
| ρ | density [kg/m3] |
| ω | mass fraction [-] |
| μ | viscosity [kg/(m s)] |
| Subscript and superscript | |
| 1D | one-dimensional |
| 2D | two-dimensional |
| 3D | three-dimensional |
| AlOOH | boehmite |
| CPR | catalytic plate reactor |
| HTT | hydrothermal treatment |
| PAA | porous anodic alumina |
| PDE | partial differential equations |
| WGS | water–gas shift reaction |
| SRM | steam reforming of methane |
| cat | catalyst |
| i | component of the reactants |
| j | reformer layers (j = 1, catalyst layer; j = 2, gas phase layer) |
Appendix A
References
- Yong, Z.; Shirong, H.; Xiaohui, J.; Yuntao, Y.; Mu, X.; Xi, Y. Characteristics of proton exchange membrane fuel cell considering “dot matrix” gas distribution zones and waveform staggered flow field with cooling channels. Energy Convers. Manag. 2022, 267, 115881–115902. [Google Scholar] [CrossRef]
- Wang, Z.X.; Mao, J.K.; He, Z.Z.; Liang, F.L. Fuzzy control based on iQPSO in proton-exchange membrane fuel-cell temperature system. J. Energy Eng. 2020, 146, 04020044–04020057. [Google Scholar] [CrossRef]
- Zhao, J.J.; Li, S.; Tu, Z.K. Development of practical empirically and statistically-based equations for predicting the temperature characteristics of PEMFC applied in the CCHP system. Int. J. Hydrogen Energy 2023, 158, 17285–17295. [Google Scholar] [CrossRef]
- Jannelli, E.; Minutillo, M.; Perna, A. Analyzing microcogeneration systems based on LT-PEMFC and HT-PEMFC by energy balances. Appl. Energy 2013, 108, 82–91. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Xue, H.Q.; Jiang, L.Y.; Jin, X.; Fu, H.H.; Xie, X.F. Proposal and assessment of a solid oxide fuel cell cogeneration system in order to produce high-temperature steam aiming at industrial applications. Appl. Therm. Eng. 2023, 221, 119882–119895. [Google Scholar] [CrossRef]
- De Falco, M.; Santoro, G.; Capocelli, M.; Caputo, G.; Giaconia, A. Hydrogen production by solar steam methane reforming with molten salts as energy carriers: Experimental and modelling analysis. Int. J. Hydrogen Energy 2021, 46, 10682–10696. [Google Scholar] [CrossRef]
- Wang, Z.X.; Mao, J.K.; Xu, L.G.; Yang, M.L.; Cheng, M.; Chen, Z.Y.; Liang, F.L. Thermodynamic evaluation of a 5 kW kerosene-fueled PEMFC-based cogeneration system: Component-level and system-level analysis. Energy. Convers. Manag. 2023, 277, 116600–116615. [Google Scholar] [CrossRef]
- Fazil, Q.; Mohammad, Y.; Mohd, K.; Hussameldin, I.; Bernard, E.; Hesam, K.; Mohammed, M.R.; Ashok, K.N.; Shreeshivadasan, C. A state-of-the-art review on the latest trends in hydrogen production, storage, and transportation techniques. Fuel 2023, 340, 127574–127591. [Google Scholar]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteyaz, B.; Irshad, K. Sustainable and energy efficient hydrogen production via glycerol reforming techniques: A review. Int. J. Hydrogen Energy 2022, 47, 41397–41420. [Google Scholar] [CrossRef]
- Rahimipetroudi, I.; Shin, J.S.; Rashid, K.; Yang, J.B.; Dong, S.K. Development and CFD analysis for determining the optimal operating conditions of 250 kg/day hydrogen generation for an on-site hydrogen refueling station (HRS) using steam methane reforming. Int. J. Hydrogen Energy 2021, 46, 35057–35076. [Google Scholar] [CrossRef]
- Cherif, A.; Lee, J.S.; Nebbali, R.; Lee, C.J. Novel design and multi-objective optimization of autothermal steam methane reformer to enhance hydrogen production and thermal matching. Appl. Therm. Eng. 2022, 217, 119140–119154. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.V.N.; Chelliapan, S.; Joo, S.-W.; Vasseghian, Y. Latest eco-friendly avenues on hydrogen production towards a circular bioeconomy: Currents challenges, innovative insights, and future perspectives. Renew. Sust. Energy Rev. 2022, 168, 112916–112931. [Google Scholar] [CrossRef]
- Upadhyay, M.; Lee, H.; Kim, A.; Lee, S.H.; Lim, H. CFD simulation of methane steam reforming in a membrane reactor: Performance characteristics over range of operating window. Int. J. Hydrogen Energy 2021, 40, 30402–30411. [Google Scholar] [CrossRef]
- Ngo, S.I.; Lim, Y.; Kim, W.; Seo, D.J.; Yoon, W.L. Computational fluid dynamics and experimental validation of a compact steam methane reformer for hydrogen production from natural gas. Appl. Energy 2019, 236, 340–353. [Google Scholar] [CrossRef]
- Katebah, M.; Abousrafa, A.; Linke, P. Hydrogen production using piston reactor technology: Process design and integration for CO2 emission reduction. Energy 2022, 259, 124999–125014. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Ying, Y.X.; Keong, L.K.; Alam, M.A.; Hellgardt, K. Syngas production employing nickel on alumina-magnesia supported catalyst via dry methane reforming. Materialwiss. Werkstofftech. 2021, 52, 1090–1100. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yang, J.; Sunden, B.; Wang, Q.W. Hydraulic and heat transfer characteristics in structured packed beds with methane steam reforming reaction for energy storage. Int. Commun. Heat Mass Transf. 2021, 121, 105109–105122. [Google Scholar] [CrossRef]
- Ding, H.R.; Tong, S.R.; Qi, Z.F.; Liu, F.; Sun, S.E.; Han, L. Syngas production from chemical-looping steam methane reforming: The effect of channel geometry on BaCoO3/CeO2 monolithic oxygen carriers. Energy 2022, 185, 126000–126009. [Google Scholar] [CrossRef]
- Oh, Y.S.; Nam, J.H. A numerical study on the active reaction thickness of nickel catalyst layers used in a low-pressure steam methane reforming process. Int. J. Hydrogen Energy 2021, 46, 7712–7721. [Google Scholar] [CrossRef]
- Kevin, M.; Van, G.; Vladimir, V. Making chemicals with electricity. Science 2019, 364, 734–735. [Google Scholar]
- Zhang, C.Q.; Pu, Y.J.; Wang, F. Hydrothermal treatment of metallic-monolith catalyst support with self-growing porous anodic-alumina film. Chin. J. Chem. Energy 2020, 28, 1311–1319. [Google Scholar] [CrossRef]
- Stefanidis, G.D.; Vlachos, D.G. Methane steam reforming at microcales: Operation strategies for variable power output at millisecond contact times. AIChE J. 2009, 51, 180–191. [Google Scholar] [CrossRef]
- Farsi, A.; Shadravan, V.; Mansouri, S.S.; Zahedi, G.; Manan, Z.A. A new reactor concept for combining oxidative coupling and steam re-forming of methane: Modeling and analysis. Int. J. Hydrogen Energy 2013, 37, 129–152. [Google Scholar] [CrossRef]
- Fukuda, T.; Harada, M.R.; Ookawara, S. Double-layered catalytic wall-plate microreactor for process intensification of dry reforming of methane: Reaction activity improvement and coking suppression. Chem. Eng. Process 2021, 164, 108406–108414. [Google Scholar] [CrossRef]
- Hamzah, A.B.; Fukuda, T.; Ookawara, S. Process intensification of dry reforming of methane by structured catalytic wall-plate microreactor. Chem. Eng. J. 2021, 412, 128636–128646. [Google Scholar] [CrossRef]
- Pashchenko, D.; Eremin, A. Heat flow inside a catalyst particle for steam methane reforming: CFD-modeling and analytical solution. Int. J. Heat Mass Transf. 2021, 165, 120617–120624. [Google Scholar] [CrossRef]
- Wang, Z.X.; Mao, J.K.; He, Z.Z.; Liang, F.L. Energy-exergy analysis of an integrated small-scale LT-PEMFC based on steam methane reforming process. Energy. Convers. Manag. 2021, 246, 114685–114699. [Google Scholar] [CrossRef]
- Oliveira, E.; Grandem, C.A.; Rodriguesm, A.E. Steam methane reforming in a Ni/Al2O3 catalyst: Kinetics and diffusional limitations in extrudates. Can. J. Chem. Eng. 2009, 87, 945–956. [Google Scholar] [CrossRef]
- Xu, J.G.; Froment, G. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Sprung, C.; Arstad, B.; Olsbye, U. Methane steam reforming over a Ni/NiAl2O4 model catalyst-kinetics. Chemcatchem 2014, 6, 1969–1982. [Google Scholar] [CrossRef]
- Numaguchi, T.; Kikuchi, K. Intrinsic kinetics and design simulation in a complex reaction network: Steam-methane reforming. Chem. Eng. Sci. 1988, 43, 2295–2301. [Google Scholar] [CrossRef]
- Liu, M.H.; Shi, Y.X.; Cai, N.S. Modeling of packed bed methanol steam reformer integrated with tubular high temperature proton exchange membrane fuel cell. J. Therm. Sci. 2023, 32, 81–92. [Google Scholar] [CrossRef]
- Seiiedhoseiny, M.; Ghasemzadeh, K.; Jalilnejad, E.; Iulianelli, A. Computational fluid dynamics study on concentration polarization phenomena in silica membrane reactor during methanol steam reforming. Chem. Eng. Process.-Process Intensif. 2023, 183, 109249–109262. [Google Scholar] [CrossRef]
- Chen, W.H.; Lu, C.Y.; Tran, K.; Lin, Y.L.; Naqvi, S.R. A new design of catalytic tube reactor for hydrogen production from ethanol steam reforming. Fuel 2020, 281, 118746–118760. [Google Scholar] [CrossRef]
- Hania, G.; Muhammad, Y.A.; Muhammad, W.T. Production of H2 via sorption enhanced auto-thermal reforming for small scale Applications-A process modeling and machine learning study. Int. J. Hydrogen Energy 2023, 2023 6, 12622–12635. [Google Scholar]
- Ma, J.; Jiang, B.; Tang, D.W. Deciphering high-efficiency solar-thermochemical energy conversion process of heat pipe reactor for steam methane reforming. Fuel 2022, 326, 124972–124981. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, L.; Kameyama, H. Thermal and hydrothermal stability of a metal monolithic anodic alumina support for steam reforming of methane. Chem. Eng. J. 2011, 168, 341–350. [Google Scholar] [CrossRef]
- Hong, R.R.; Feng, J.T.; He, Y.F.; Li, D.Q. Controllable preparation and catalytic performance of Pd/anodic alumina oxide@Al catalyst for hydrogenation of ethylanthraquinone. Chem. Eng. Sci. 2015, 135, 274–284. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; pp. 100–102. [Google Scholar]







| Gas Phase | S/C | 3 | |
| Pressure | 101,325 Pa | ||
| Inlet temperature | 723 K | ||
| Catalyst | Catalyst | 18 wt.% Ni/γ-Al2O3/alloy | |
| Density | Alumina layer | 1900 kg/m3 | |
| Alloy layer | 3900 kg/m3 | ||
| Thickness | Alumina layer | 40 um | |
| Alloy layer | 50 um | ||
| Thermal-conductivity | Alumina layer | 1.039 W/m/K | |
| Alloy layer | 11.3 W/m/K | ||
| Constant | A | E (kJ/mol) |
|---|---|---|
| k1 (mol bar−2/(kg-cat s)) | 1.54 × 1014 | 191 |
| k2 (mol bar−4/(kg-cat s)) | 3.97 × 108 | 59.8 |
| K1 (bar2) | exp (−26,830/T + 30.114) | |
| K2 (−) | exp (4400/T − 4.036) | |
| Tube-Type Reformer Channel | Box-Type Reformer Channel | |
|---|---|---|
| Flow rate | 2.4 L/min | |
| Cat. Size | 77.7~111.6 cm2 | 54.4~84 cm2 |
| Height (D, H) | 5~15 mm | 2~5 mm |
| Width | – | 4 cm |
| volume | 9.71~41.8 cm3 | 6.4~21 cm3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Lin, Y.; Hu, J.; Gu, C.; Ding, L.; Zhang, X.; Jiang, S.; Guo, Y. Preparation of an Anodic Alumina-Supported Ni Catalyst and Development of a Catalytic Plate Reformer for the Steam Reforming of Methane. Energies 2023, 16, 3426. https://doi.org/10.3390/en16083426
Dong S, Lin Y, Hu J, Gu C, Ding L, Zhang X, Jiang S, Guo Y. Preparation of an Anodic Alumina-Supported Ni Catalyst and Development of a Catalytic Plate Reformer for the Steam Reforming of Methane. Energies. 2023; 16(8):3426. https://doi.org/10.3390/en16083426
Chicago/Turabian StyleDong, Shan, Yi Lin, Jiajun Hu, Chenglin Gu, Leilin Ding, Xinjian Zhang, Shi Jiang, and Yu Guo. 2023. "Preparation of an Anodic Alumina-Supported Ni Catalyst and Development of a Catalytic Plate Reformer for the Steam Reforming of Methane" Energies 16, no. 8: 3426. https://doi.org/10.3390/en16083426






