Improving the Efficiency of Organic Solar Cells via the Molecular Engineering of Simple Fused Non-Fullerene Acceptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Methods
2.3. Synthetic Procedures
3. Results and Discussion
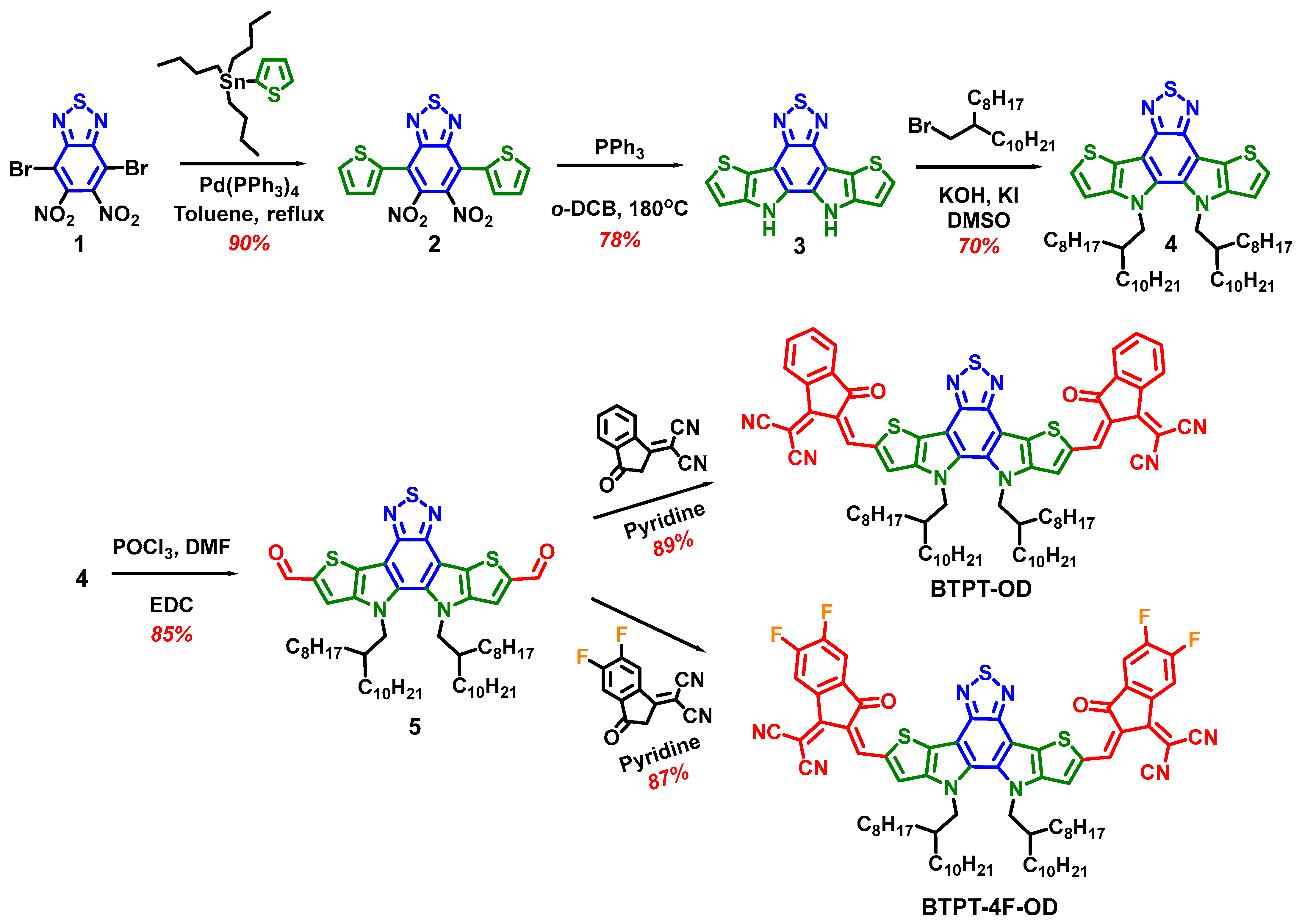
3.1. Synthesis and Characterization
3.2. Optical and Electrochemical Properties
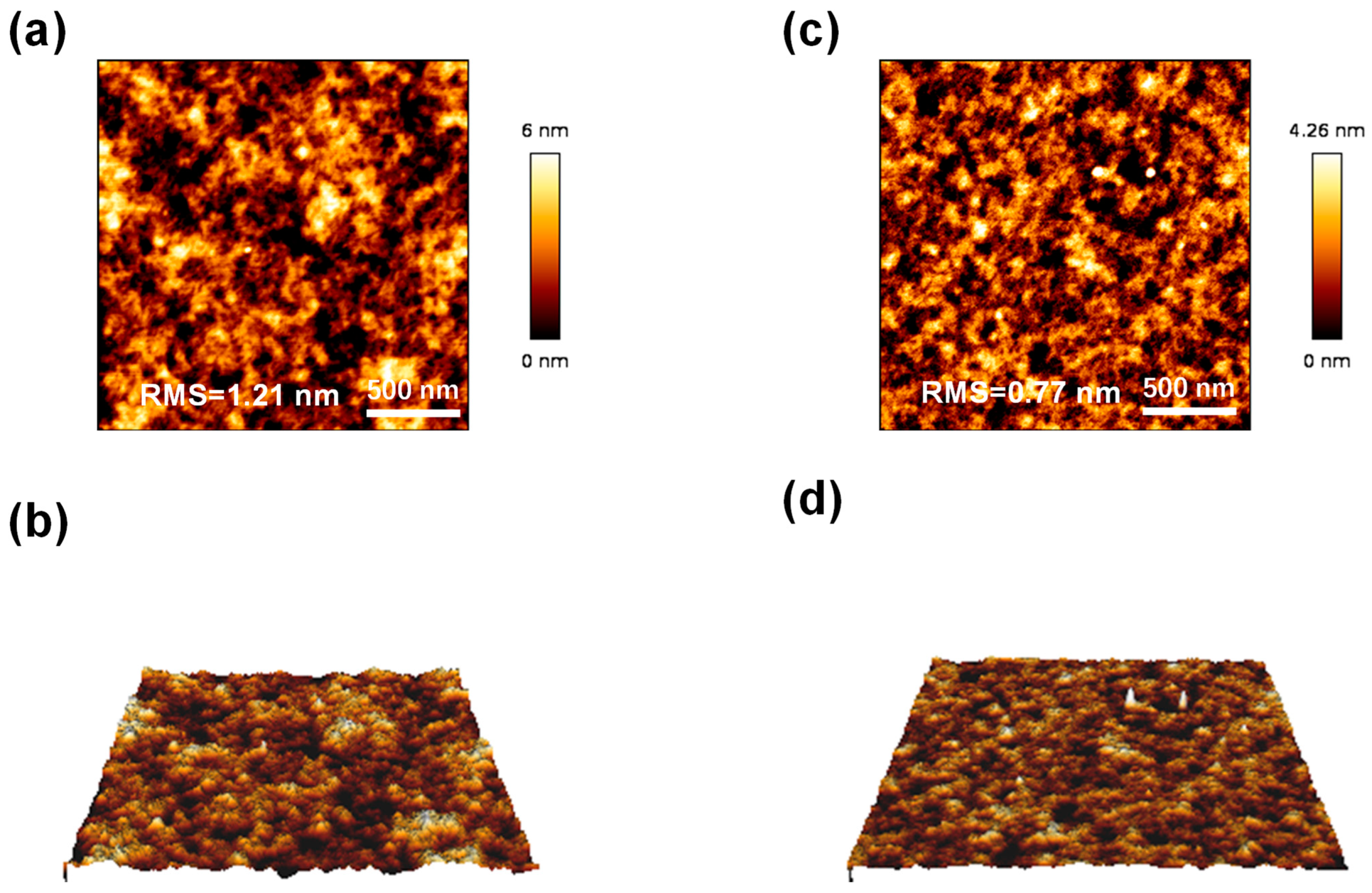
3.3. Thermal and Structural Properties
3.4. Photovoltaic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Yan, H.; Huang, F.; Yan, H. Non-fullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef] [PubMed]
- Riede, M.; Spoltore, D.; Leo, K. Organic Solar Cells—The Path to Commercial Success. Adv. Energy Mater. 2021, 11, 2002653. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, J.L. Machine Learning for High Performance Organic Solar Cells: Current Scenario and Future Prospects. Energy Environ. Sci. 2021, 14, 90–105. [Google Scholar] [CrossRef]
- Moser, M.; Wadsworth, A.; Gasparini, N.; McCulloch, I. Challenges to the Success of Commercial Organic Photovoltaic Products. Adv. Energy Mater. 2021, 11, 2100056. [Google Scholar] [CrossRef]
- Kini, G.P.; Jeon, S.J.; Moon, D.K. Latest Progress on Photoabsorbent Materials for Multifunctional Semitransparent Organic Solar Cells. Adv. Funct. Mater. 2021, 31, 2007931. [Google Scholar] [CrossRef]
- Sun, R.; Wu, Y.; Yang, X.; Gao, Y.; Chen, Z.; Li, K.; Qiao, J.; Wang, T.; Guo, J.; Liu, C.; et al. Single-Junction Organic Solar Cells with 19.17% Efficiency Enabled by Introducing One Asymmetric Guest Acceptor. Adv. Mater. 2022, 34, 2110147. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-Junction Organic Photovoltaic Cell with 19% Efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Zhang, S.; Chen, Z.; Xu, Y.; Cui, Y.; Zhang, T.; Ren, J.; Qin, J.; Hong, L.; Hao, X.; et al. Reduced Non-Radiative Charge Recombination Enables Organic Photovoltaic Cell Approaching 19% Efficiency. Joule 2021, 5, 2408–2419. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Xu, J.; Li, C.; Yan, J.; Zhou, G.; Zhong, W.; Hao, T.; Song, J.; Xue, X.; et al. Single-Junction Organic Solar Cells with over 19% Efficiency Enabled by a Refined Double-Fibril Network Morphology. Nat. Mater. 2022, 21, 656–663. [Google Scholar] [CrossRef]
- Chiristina, E.N.; Rahayu, S.U.; Tubtimtae, A.; Shi, J.-B.; Lee, M.-W. Rare-Earth-Incorporated Ternary Ce x Cd 1−x S Quantum Dot-Sensitized Solar Cells. RSC Adv. 2022, 12, 31093–31101. [Google Scholar] [CrossRef]
- Sun, S.; Xu, M.; Zhang, Y.; Liu, R.; Wang, X.; Zhang, L.; Fang, Y.; Wang, P. Study of Molybdenum Oxide Optimized Hole Carrier Transport in Perovskite Solar Cells. Org. Electron. 2023, 113, 106697. [Google Scholar] [CrossRef]
- Tai, Q.; Yan, F. Emerging Semitransparent Solar Cells: Materials and Device Design. Adv. Mater. 2017, 29, 1700192. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ameri, T.; Forberich, K.; Brabec, C.J. Semitransparent Polymer Solar Cells. Polym. Int. 2013, 62, 1408–1412. [Google Scholar] [CrossRef]
- Dai, S.; Zhan, X. Nonfullerene Acceptors for Semitransparent Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1800002. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and Semitransparent Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Landerer, D.; Bahro, D.; Röhm, H.; Koppitz, M.; Mertens, A.; Manger, F.; Denk, F.; Heidinger, M.; Windmann, T.; Colsmann, A. Solar Glasses: A Case Study on Semitransparent Organic Solar Cells for Self-Powered, Smart, Wearable Devices. Energy Technol. 2017, 5, 1936–1945. [Google Scholar] [CrossRef]
- Hollingsworth, J.A.; Ravishankar, E.; O’Connor, B.; Johnson, J.X.; DeCarolis, J.F. Environmental and Economic Impacts of Solar-Powered Integrated Greenhouses. J. Ind. Ecol. 2020, 24, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Sumaiya, S.; Kardel, K.; El-Shahat, A. Organic Solar Cell by Inkjet Printing—An Overview. Technologies 2017, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Xue, P.; Cheng, P.; Han, R.P.S.; Zhan, X. Printing Fabrication of Large-Area Non-Fullerene Organic Solar Cells. Mater. Horizons 2022, 9, 194–219. [Google Scholar] [CrossRef]
- Burlingame, Q.; Huang, X.; Liu, X.; Jeong, C.; Coburn, C.; Forrest, S.R. Intrinsically Stable Organic Solar Cells under High-Intensity Illumination. Nature 2019, 573, 394–397. [Google Scholar] [CrossRef]
- Leonard, N.G.; Lee, S.W.; Chang, D.W.; Hodgkiss, J.M.; Vak, D. Organic Photovoltaics’ New Renaissance: Advances Toward Roll-to-Roll Manufacturing of Non-Fullerene Acceptor Organic Photovoltaics. Adv. Mater. Technol. 2022, 7, 2101556. [Google Scholar] [CrossRef]
- Peng, C.; Huang, Y.; Wu, Z. Building-integrated photovoltaics (BIPV) in architectural design in China. Energy Build. 2011, 43, 3592–3598. [Google Scholar] [CrossRef]
- Biyik, E.; Araz, M.; Hepbasli, A.; Shahrestani, M.; Yao, R.; Shao, L.; Essah, E.; Oliveira, A.C.; del Caño, T.; Rico, E.; et al. A Key Review of Building Integrated Photovoltaic (BIPV) Systems. Eng. Sci. Technol. Int. J. 2017, 20, 833–858. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, D.; Li, M.; Zhong, W.; Zeng, Z.; Ying, L.; Huang, F.; Cao, Y. Achieving over 16% efficiency for single-junction organic solar cells. Sci. China Chem. 2019, 62, 746–752. [Google Scholar] [CrossRef]
- Zhao, J.J.; Yao, C.; Ali, M.U.; Miao, J.; Meng, H. Recent Advances in High-performance Organic Solar Cells Enabled by Acceptor-Donor-Acceptor-Donor-Acceptor (A-DA’D-A) Type Acceptors. Mater. Chem. Front. 2020, 4, 3487–3504. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Zou, Y.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Luo, D.; Jang, W.; Babu, D.D.; Kim, M.S.; Wang, D.H.; Kyaw, A.K.K. Recent progress in organic solar cells based on non-fullerene acceptors: Materials to devices. J. Mater. Chem. A 2022, 10, 3255–3295. [Google Scholar] [CrossRef]
- He, Q.; Ufimkin, P.; Aniés, F.; Hu, X.; Kafourou, P.; Rimmele, M.; Rapley, C.L.; Ding, B. Molecular engineering of Y-series acceptors for nonfullerene organic solar cells. SusMat 2022, 2, 591–606. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, Y.; Zheng, S. From Y6 to BTPT-4F: A theoretical insight into the influence of the individual change of fused-ring skeleton length or side alkyl chains on molecular arrangements and electron mobility. New J. Chem. 2021, 45, 12247–12259. [Google Scholar] [CrossRef]
- Ming, R.; Gao, J.; Gao, W.; Ning, W.; Luo, Z.; Zhang, F.; Yang, C. Benzo[c][1,2,5]thiadiazole-Fused Pentacyclic Small Molecule Acceptors for Organic Solar Cells. Dyes Pigm. 2020, 185, 108970. [Google Scholar] [CrossRef]
- Qiu, W.; Zheng, S. Designing and Screening High-Performance Non-Fullerene Acceptors: A Theoretical Exploration of Modified Y6. Sol. Rrl 2022, 5, 2100023. [Google Scholar] [CrossRef]
- Murugan, P.; Hu, T.; Hu, X.; Chen, Y. Fused ring A–DA′D–A (Y-series) non-fullerene acceptors: Recent developments and design strategies for organic photovoltaics. J. Mater. Chem. A 2022, 10, 17968–17987. [Google Scholar] [CrossRef]
- Wang, J.; Xue, P.; Jiang, Y.; Huo, Y.; Zhan, X. The principles, design and applications of fused-ring electron acceptors. Nat. Rev. Chem. 2022, 6, 614–634. [Google Scholar] [CrossRef]
- Uno, T.; Takagi, K.; Tomoeda, M. Synthesis of Bisfurazanobenzo-2, 1, 3-thiadiazole and Related Compounds. Chem. Pharm. Bull. 1980, 28, 909–1912. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.; Zheng, C.; Sun, Q.; Liua, G.; Fan, C. Enhancement of cyclization quantum yields of perfluorodiarylethenes via weak intramolecular interactions. Chem. Commun. 2013, 48, 8036–8038. [Google Scholar] [CrossRef]
- Uddin, M.A.; Lee, T.H.; Xu, S.; Park, S.Y.; Kim, T.; Song, S.; Nguyen, T.L.; Ko, S.J.; Hwang, S.; Kim, J.Y.; et al. Interplay of Intramolecular Noncovalent Coulomb Interactions for Semicrystalline Photovoltaic Polymers. Chem. Mater. 2015, 27, 5997–6007. [Google Scholar] [CrossRef]
- Yao, H.; Cui, Y.; Yu, R.; Gao, B.; Zhang, H.; Hou, J. Design, Synthesis, and Photovoltaic Characterization of a Small Molecular Acceptor with an Ultra-Narrow Band Gap Angew. Chem. Int. Ed. 2017, 56, 3045–3049. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Rasulova, N.N.; Luponosov, Y.N.; Surin, N.M.; Buzin, M.I.; Leshchiner, I.; Peregudova, S.M.; Muzafarov, A.M. Bithiophenesilane-Based Dendronized Polymers: Facile Synthesis and Properties of Novel Highly Branched Organosilicon Macromolecular Structures. Macromolecules 2012, 45, 2014–2024. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P.; Cravino, A. From one- to three-dimensional organic semiconductors: In search of the organic silicon? Adv. Mater. 2007, 19, 2045–2060. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, O.V.; Luponosov, Y.N.; Ponomarenko, S.A.; Kausch-Busies, N.; Paraschuk, D.Y.; Olivier, Y.; Beljonne, D.; Cornil, J.; Pshenichnikov, M.S. Ultrafast charge generation pathways in photovoltaic blends based on novel star-shaped conjugated molecules. Adv. Energy Mater. 2014, 5, 1401657. [Google Scholar] [CrossRef]
- Roncali, J.; Grosu, I. The dawn of single material organic solar cells. Adv. Sci. 2019, 6, 1801026. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| λmax a, nm | ε b 105 (L × mol−1 × cm−1) | λmax c, nm | φox/HOMO d, eV | φred/LUMO d, eV | Eg d, eV | |
|---|---|---|---|---|---|---|
| BTPT-OD | 688 | 2.10 | 734 | 1.42/−5.82 | −0.60/−3.80 | 2.02 |
| BTPT-4F-OD | 711 | 2.05 | 762 | 1.45/−5.85 | −0.46/−3.94 | 1.91 |
| Active Layer | VOC (V) | JSC (Jcalc) a (mA/cm2) | FF (%) | PCE (%, PCEavg b) |
|---|---|---|---|---|
| PM6:BTPT-4F-OD | 0.824 | 8.16 (7.84) | 36.9 | 2.48 (2.32 ± 0.13) |
| PM6:BTPT-OD | 0.952 | 9.86 (9.70) | 43.3 | 4.30 (4.19 ± 0.12) |
| Device | μe (×10−4 cm2 V−1 s−1) | μh (×10−4 cm2 V−1 s−1) | μe/μh |
|---|---|---|---|
| PM6:BTPT-4F-OD | 3.52 ± 0.25 | 1.94 ± 0.31 | 1.81 |
| PM6:BTPT-OD | 4.39 ± 0.27 | 2.86 ± 0.28 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papkovskaya, E.D.; Wan, J.; Balakirev, D.O.; Dyadishchev, I.V.; Bakirov, A.V.; Luponosov, Y.N.; Min, J.; Ponomarenko, S.A. Improving the Efficiency of Organic Solar Cells via the Molecular Engineering of Simple Fused Non-Fullerene Acceptors. Energies 2023, 16, 3443. https://doi.org/10.3390/en16083443
Papkovskaya ED, Wan J, Balakirev DO, Dyadishchev IV, Bakirov AV, Luponosov YN, Min J, Ponomarenko SA. Improving the Efficiency of Organic Solar Cells via the Molecular Engineering of Simple Fused Non-Fullerene Acceptors. Energies. 2023; 16(8):3443. https://doi.org/10.3390/en16083443
Chicago/Turabian StylePapkovskaya, Elizaveta D., Ji Wan, Dmitry O. Balakirev, Ivan V. Dyadishchev, Artem V. Bakirov, Yuriy N. Luponosov, Jie Min, and Sergey A. Ponomarenko. 2023. "Improving the Efficiency of Organic Solar Cells via the Molecular Engineering of Simple Fused Non-Fullerene Acceptors" Energies 16, no. 8: 3443. https://doi.org/10.3390/en16083443








