Theoretical Analysis of InGaN Solar Energy Converters Based on Photon-Enhanced Thermionic Emission
Abstract
1. Introduction
2. Theoretical Model
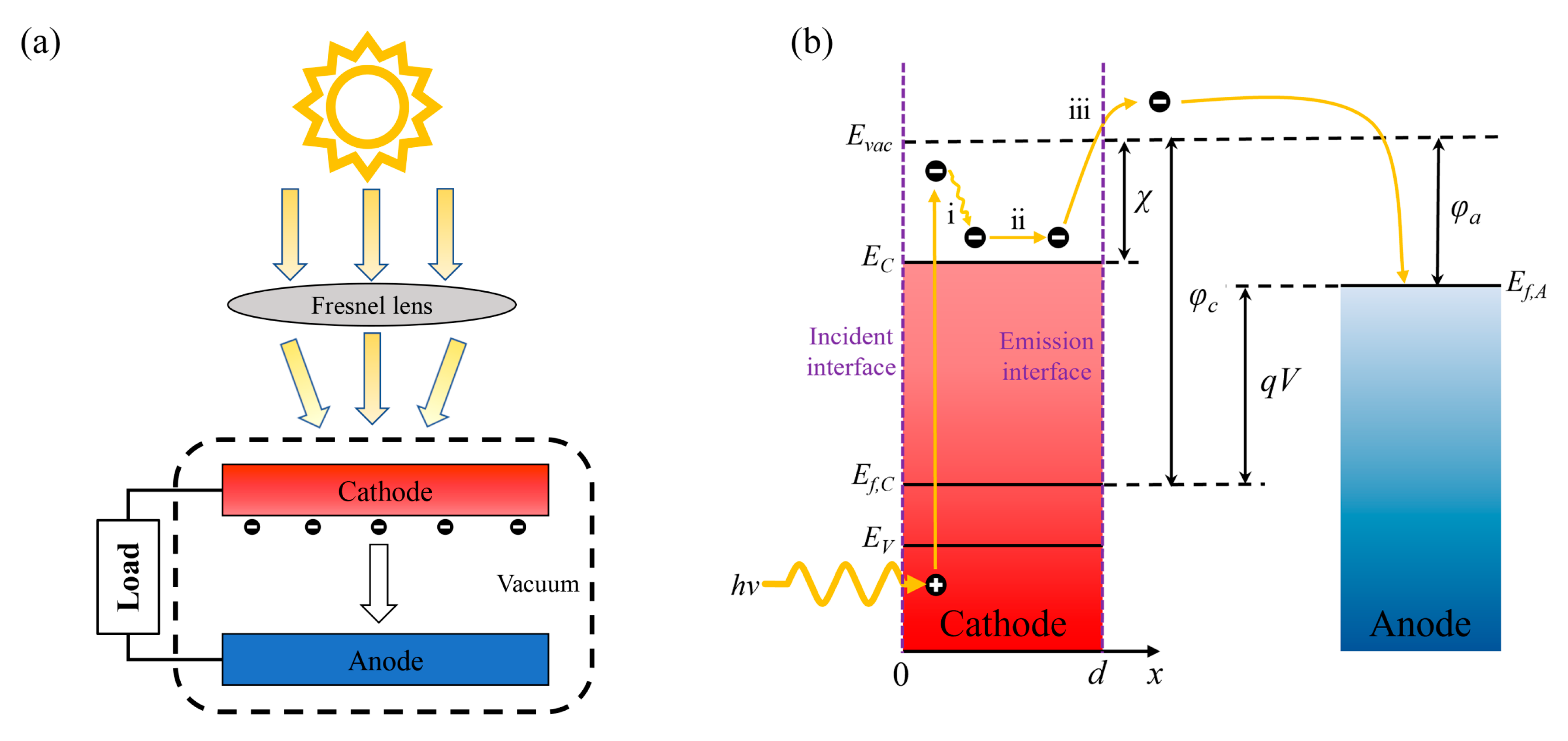
2.1. Structure and Principle
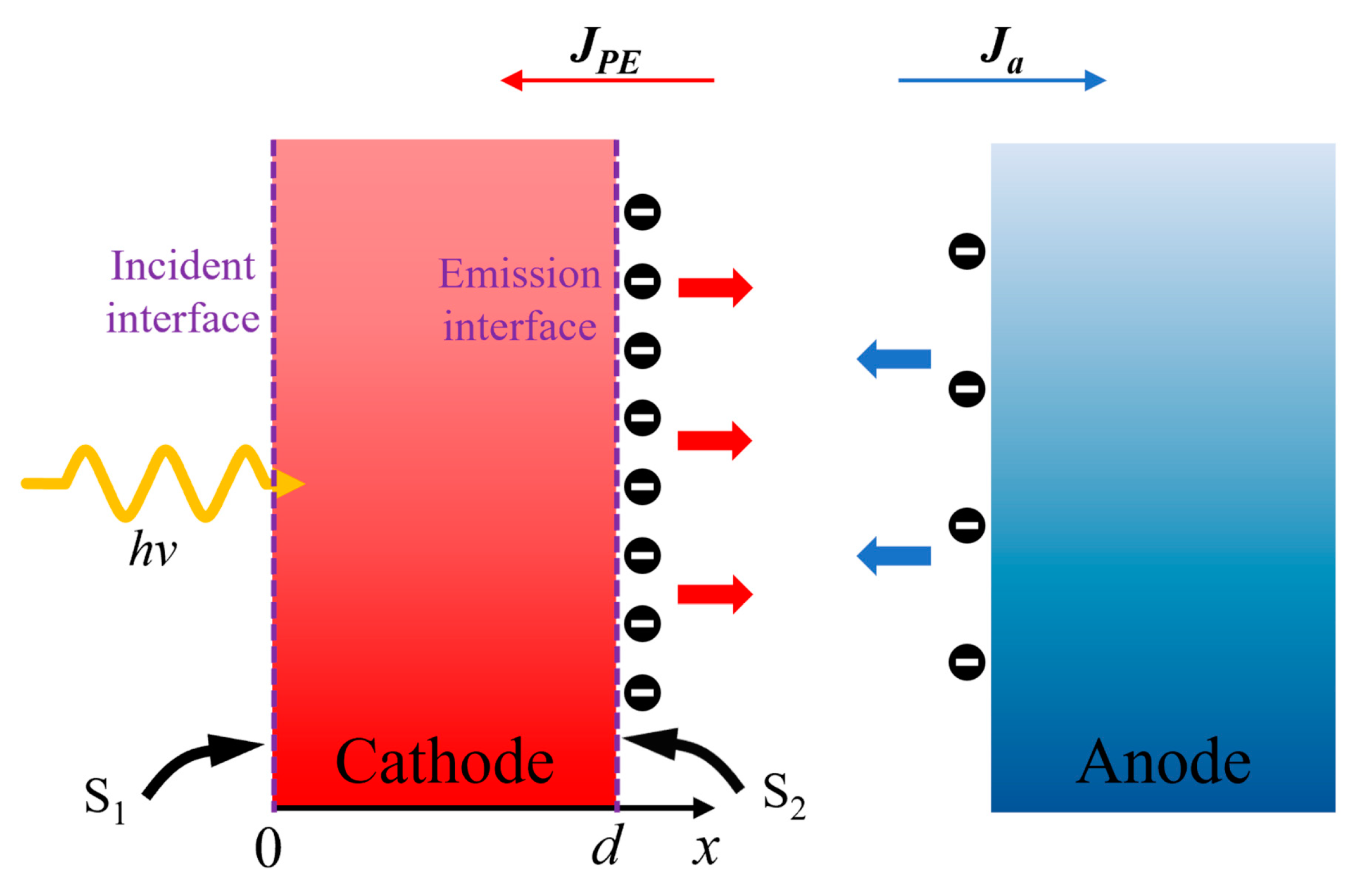
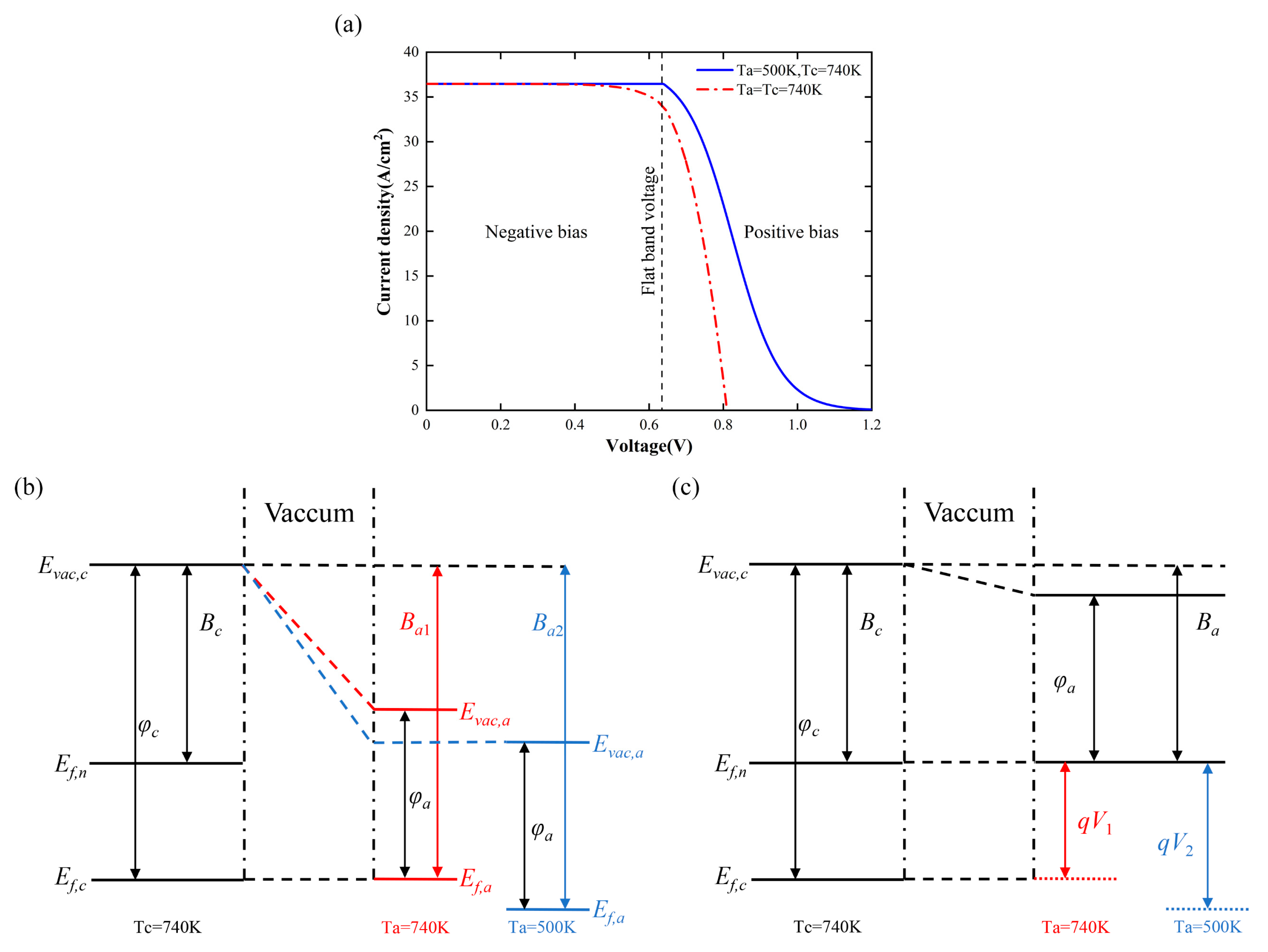
2.2. Calculation Model
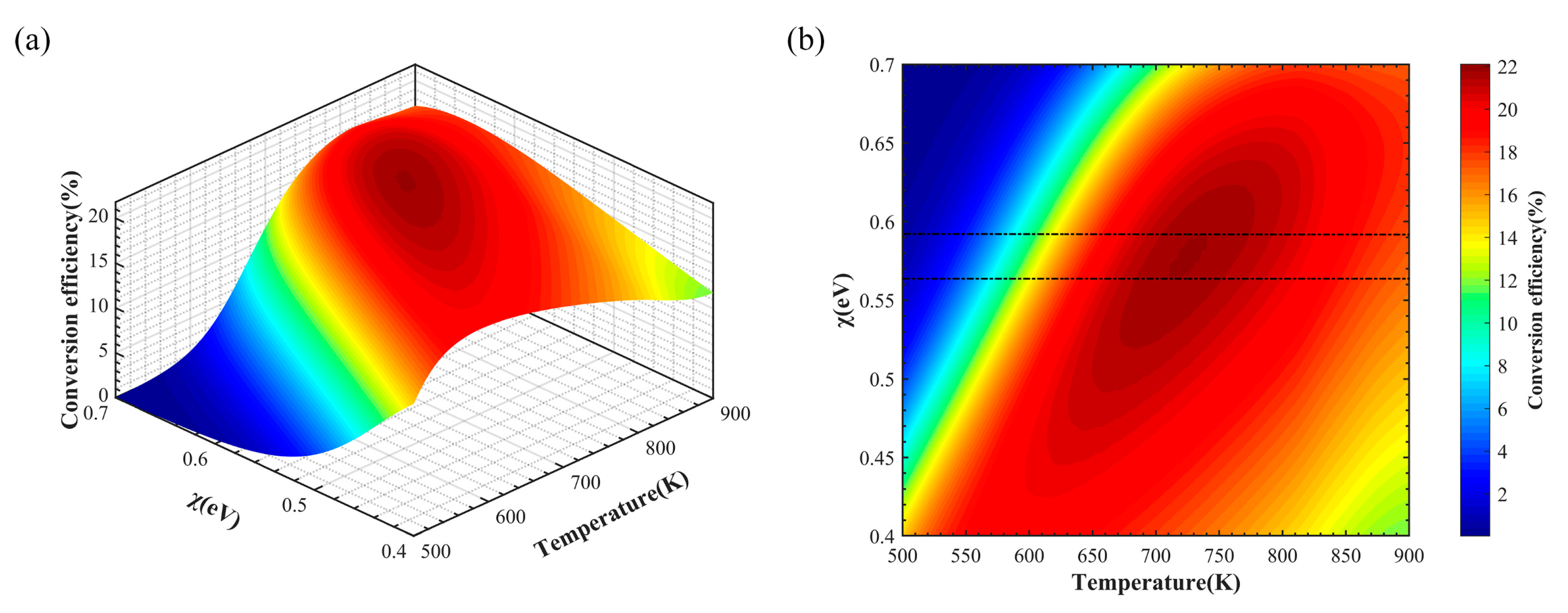
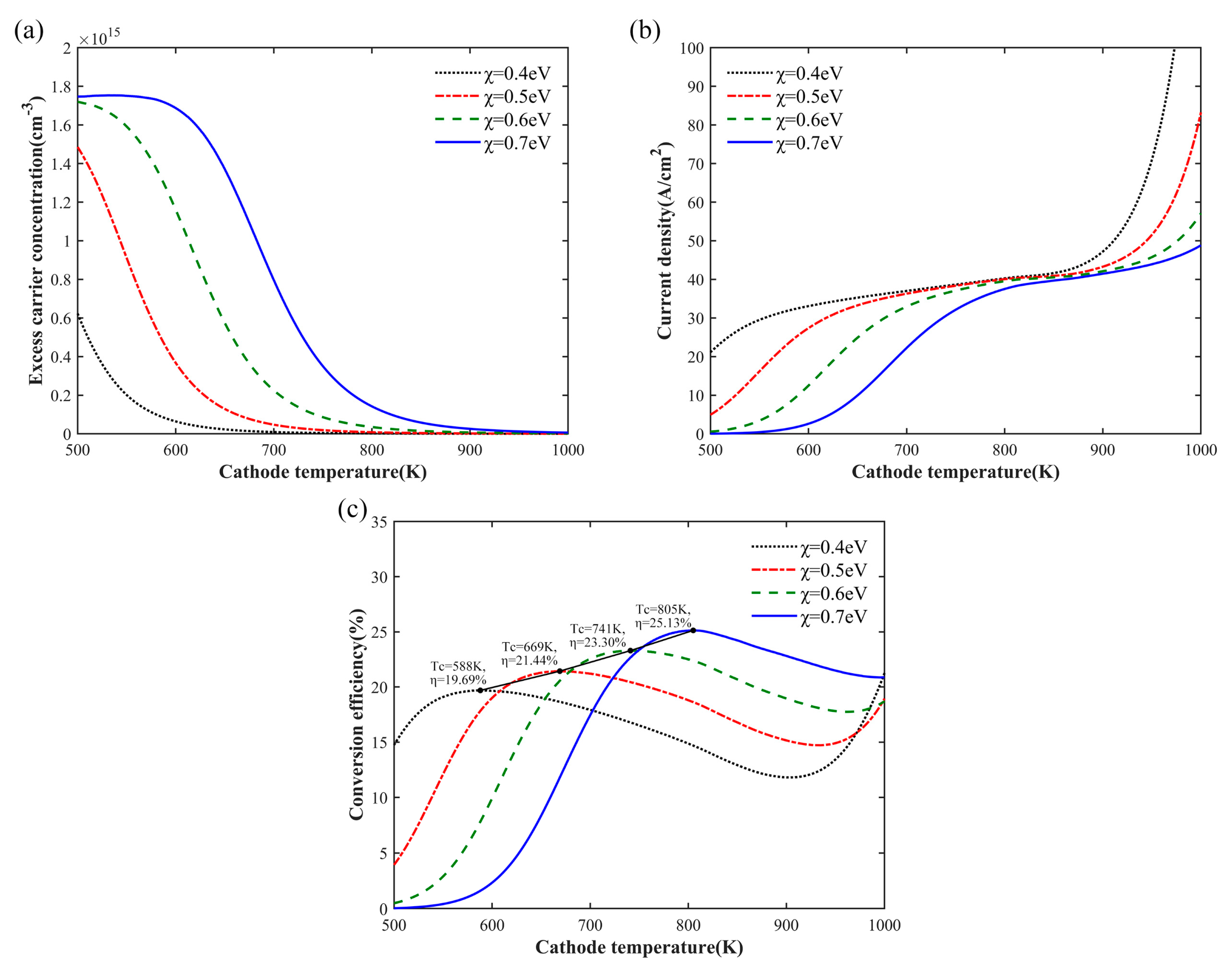
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schwartz, R.J. Photovoltaic power generation. Proc. IEEE 1993, 81, 355–364. [Google Scholar] [CrossRef]
- Sukhatme, S.P. Solar thermal power generation. J. Chem. Sci. 1997, 109, 521–531. [Google Scholar] [CrossRef]
- Luque, A.; Marti, A. Limiting efficiency of coupled thermal and photovoltaic converters. Sol. Energy Mater. Sol. Cells 1999, 58, 147–165. [Google Scholar] [CrossRef]
- Green, M.A. General temperature dependence of solar cell performance and implications for device modelling. Prog. Photovolt. 2003, 11, 333–340. [Google Scholar] [CrossRef]
- Kribus, A.; Mittelman, G. Potential of polygeneration with solar thermal and photovoltaic systems. J. Sol. Energy Eng. 2008, 130, 011001. [Google Scholar] [CrossRef]
- Smestad, G.P. Conversion of heat and light simultaneously using a vacuum photodiode and the thermionic and photoelectric effects. Sol. Energy Mater. Sol. Cells 2004, 82, 227–240. [Google Scholar] [CrossRef]
- Schwede, J.W.; Bargatin, I.; Riley, D.C.; Hardin, B.E.; Rosenthal, S.J.; Sun, Y.; Schmitt, F.; Pianetta, P.; Howe, R.T.; Shen, Z.; et al. Photon-enhanced thermionic emission for solar concentrator systems. Nat. Mater. 2010, 9, 762–767. [Google Scholar] [CrossRef]
- Khalid, K.A.A.; Leong, T.J.; Mohamed, K. Review on Thermionic Energy Converters. IEEE Trans. Electron. Devices 2016, 63, 2231–2241. [Google Scholar] [CrossRef]
- Segev, G.; Kribus, A.; Rosenwaks, Y. High performance isothermal photo-thermionic solar converters. Sol. Energy Mater. Sol. Cells 2013, 113, 114–123. [Google Scholar] [CrossRef]
- Segev, G.; Rosenwaks, Y.; Kribus, A. Limit of efficiency for photon-enhanced thermionic emission vs. photovoltaic and thermal conversion. Sol. Energy Mater. Sol. Cells 2015, 140, 464–476. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, T.; Zhang, Y.; Chen, X.; Su, S.; Chen, J. Effects of nanoscale vacuum gap on photon-enhanced thermionic emission devices. J. Appl. Phys. 2016, 119, 45106. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Hao, H.; Chen, J.; Su, S. Optimal design of the interelectrode space in a photon-enhanced thermionic emission solar cell. Appl. Therm. Eng. 2019, 157, 113758. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, H.; Ni, M.; Xiao, G. Photo-thermo-electric modeling of photon-enhanced thermionic emission with concentrated solar power. Sol. Energy Mater. Sol. Cells 2022, 246, 111922. [Google Scholar] [CrossRef]
- Schwede, J.W.; Sarmiento, T.; Narasimhan, V.K.; Rosenthal, S.J.; Riley, D.C.; Schmitt, F.; Bargatin, I.; Sahasrabuddhe, K.; Howe, R.T.; Harris, J.S.; et al. Photon-enhanced thermionic emission from heterostructures with low interface recombination. Nat. Commun. 2013, 4, 1576. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yang, W.; Yang, Y.; Sun, C.; Cai, Z. GaAs film for photon-enhanced thermionic emission solar harvesters. Mater. Sci. Semicond. Process. 2014, 25, 143–147. [Google Scholar] [CrossRef]
- Diao, Y.; Liu, L.; Xia, S. Theoretical analysis and modeling of photoemission ability and photoelectric conversion characteristics of GaAs nanowire cathodes based on photon-enhanced thermionic emission. Sol. Energy 2019, 194, 510–518. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, P.; Cao, W.; Zhu, B.; Wang, B.; Bai, Y.; Qin, J.; Bai, X.; Chen, Z. High-performance solid-state photon-enhanced thermionic emission solar energy converters with graded bandgap window-layer. J. Phys. D Appl. Phys. 2020, 54, 55502. [Google Scholar] [CrossRef]
- Yang, N.; Xie, L.; Wang, P.; Xu, Y.; Li, S.; Shen, X.; Fu, Y.; He, H. Theoretical analysis and experimental research of photon-enhanced thermionic emission solar energy converters with InN photocathode. Sol. Energy Mater. Sol. Cells 2022, 242, 111766. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Meyer, J.R. Band parameters for nitrogen-containing semiconductors. J. Appl. Phys. 2003, 94, 3675–3696. [Google Scholar] [CrossRef]
- Wu, J.; Walukiewicz, W. Band gaps of InN and group III nitride alloys. Superlattices Microstruct. 2003, 34, 63–75. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Fu, H.; Li, D.; Zhang, C.; Chen, H.; Fang, Y.; Fu, K.; DenBaars, S.P.; Nakamura, S.; et al. High-Temperature Polarization-Free III-Nitride Solar Cells with Self-Cooling Effects. ACS Photonics 2019, 6, 2096–2103. [Google Scholar] [CrossRef]
- Moses, G.; Huang, X.; Zhao, Y.; Auf Der Maur, M.; Katz, E.A.; Gordon, J.M. InGaN/GaN multi-quantum-well solar cells under high solar concentration and elevated temperatures for hybrid solar thermal-photovoltaic power plants. Prog. Photovolt. 2020, 28, 1167–1174. [Google Scholar] [CrossRef]
- Bhuiyan, A.G.; Sugita, K.; Hashimoto, A.; Yamamoto, A. InGaN Solar Cells: Present State of the Art and Important Challenges. IEEE J. Photovolt. 2012, 2, 276–293. [Google Scholar] [CrossRef]
- Su, S.; Wang, Y.; Wang, J.; Xu, Z.; Chen, J. Material optimum choices and parametric design strategies of a photon-enhanced solar cell hybrid system. Sol. Energy Mater. Sol. Cells 2014, 128, 112–118. [Google Scholar] [CrossRef]
- Varpula, A.; Tappura, K.; Prunnila, M. Si, GaAs, and InP as cathode materials for photon-enhanced thermionic emission solar cells. Sol. Energy Mater. Sol. Cells 2015, 134, 351–358. [Google Scholar] [CrossRef]
- Varpula, A.; Prunnila, M. Diffusion-emission theory of photon enhanced thermionic emission solar energy harvesters. J. Appl. Phys. 2012, 112, 044506. [Google Scholar] [CrossRef]
- Brown, G.F.; Ager, J.W.; Walukiewicz, W.; Wu, J. Finite element simulations of compositionally graded InGaN solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 478–483. [Google Scholar] [CrossRef]
- Wu, J.; Walukiewicz, W.; Yu, K.M.; Ager, J.W.; Haller, E.E.; Lu, H.; Schaff, W.J. Small band gap bowing in In1−xGaxN alloys. Appl. Phys. Lett. 2002, 80, 4741–4743. [Google Scholar] [CrossRef]
- Muth, J.F.; Lee, J.H.; Shmagin, I.K.; Kolbas, R.M.; Casey, H.C.; Keller, B.P.; Mishra, U.K.; DenBaars, S.P. Absorption coefficient, energy gap, exciton binding energy, and recombination lifetime of GaN obtained from transmission measurements. Appl. Phys. Lett. 1997, 71, 2572–2574. [Google Scholar] [CrossRef]
- Mesrane, A.; Rahmoune, F.; Mahrane, A.; Oulebsir, A. Design and Simulation of InGaN 𝑝-𝑛 Junction Solar Cell. Int. J. Photoenergy 2015, 2015, 594858. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, W.; Tang, W.; Sun, C. High-temperature solar cell for concentrated solar-power hybrid systems. Appl. Phys. Lett. 2013, 103, 083902. [Google Scholar] [CrossRef]
- Chang, C.; Tang, T.; Chang, P.; Chen, N.; Liang, C. Magnesium Doping of In-rich InGaN. Jpn. J. Appl. Phys. 2007, 46, 2840–2843. [Google Scholar] [CrossRef]
- Jeng, M. Simulation of Nonpolar p-GaN/i-N/n-GaN Solar Cells. Int. J. Photoenergy 2012, 2012, 910256. [Google Scholar] [CrossRef]
- Feng, S.; Lai, C.; Tsai, C.; Su, Y.; Tu, L. Modeling of InGaN p-n junction solar cells. Opt. Mater. Express 2013, 3, 1777–1788. [Google Scholar] [CrossRef]
- Chen, K.; Hung, C. Numerical study of InGaN tandem solar cells with intermediate bands. Phys. Status Solidi RRL 2017, 11, 1600429. [Google Scholar] [CrossRef]
- Cuscó, R.; Ibáñez, J.; Alarcón-Lladó, E.; Artús, L.; Yamaguchi, T.; Nanishi, Y. Photoexcited carriers and surface recombination velocity in InN epilayers: A Raman scattering study. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 80, 155204. [Google Scholar] [CrossRef]
- Aleksiejūnas, R.; Sūdžius, M.; Malinauskas, T.; Vaitkus, J.; Jarašiūnas, K.; Sakai, S. Determination of free carrier bipolar diffusion coefficient and surface recombination velocity of undoped GaN epilayers. Appl. Phys. Lett. 2003, 83, 1157–1159. [Google Scholar] [CrossRef]
- Sahasrabuddhe, K.; Schwede, J.W.; Bargatin, I.; Jean, J.; Howe, R.T.; Shen, Z.; Melosh, N.A. A model for emission yield from planar photocathodes based on photon-enhanced thermionic emission or negative-electron-affinity photoemission. J. Appl. Phys. 2012, 112, 094907. [Google Scholar] [CrossRef]
- Koeck, F.A.M.; Nemanich, R.J.; Lazea, A.; Haenen, K. Thermionic electron emission from low work-function phosphorus doped diamond films. Diamond Relat. Mater. 2009, 18, 789–791. [Google Scholar] [CrossRef]
- Kazazis, S.A.; Papadomanolaki, E.; Iliopoulos, E. Polarization-Engineered InGaN/GaN Solar Cells: Realistic Expectations for Single Heterojunctions. IEEE J. Photovolt. 2018, 8, 118–124. [Google Scholar] [CrossRef]
- Marouf, Y.; Dehimi, L.; Bouzid, F.; Pezzimenti, F.; Corte, F.G.D. Theoretical design and performance of InxGa1-xN single junction solar cell. Optik 2018, 163, 22–32. [Google Scholar] [CrossRef]
- Parajuli, D.; Shah, D.K.; KC, D.; Kumar, S.; Park, M.; Pant, B. Influence of Doping Concentration and Thickness of Regions on the Performance of InGaN Single Junction-Based Solar Cells: A Simulation Approach. Electrochem 2022, 3, 407–415. [Google Scholar] [CrossRef]


| Tc (K) | Vfb (V) | Vm (V) |
|---|---|---|
| 600 | 0.794 | 0.794 |
| 700 | 0.684 | 0.684 |
| 800 | 0.569 | 0.699 |
| 900 | 0.450 | 0.730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Yang, N.; Xie, L.; Xu, Y.; He, H.; Fu, Y.; Shen, X. Theoretical Analysis of InGaN Solar Energy Converters Based on Photon-Enhanced Thermionic Emission. Energies 2023, 16, 3483. https://doi.org/10.3390/en16083483
Wang P, Yang N, Xie L, Xu Y, He H, Fu Y, Shen X. Theoretical Analysis of InGaN Solar Energy Converters Based on Photon-Enhanced Thermionic Emission. Energies. 2023; 16(8):3483. https://doi.org/10.3390/en16083483
Chicago/Turabian StyleWang, Pingan, Ning Yang, Liubing Xie, Yanpeng Xu, Huan He, Yuechun Fu, and Xiaoming Shen. 2023. "Theoretical Analysis of InGaN Solar Energy Converters Based on Photon-Enhanced Thermionic Emission" Energies 16, no. 8: 3483. https://doi.org/10.3390/en16083483
APA StyleWang, P., Yang, N., Xie, L., Xu, Y., He, H., Fu, Y., & Shen, X. (2023). Theoretical Analysis of InGaN Solar Energy Converters Based on Photon-Enhanced Thermionic Emission. Energies, 16(8), 3483. https://doi.org/10.3390/en16083483






