A Review of Mineral and Rock Wettability Changes Induced by Reaction: Implications for CO2 Storage in Saline Reservoirs
Abstract
1. Introduction
2. Contact Angle Measurements
3. Reaction-Induced Wettability Changes in the CO2-Brine-Mineral Systems
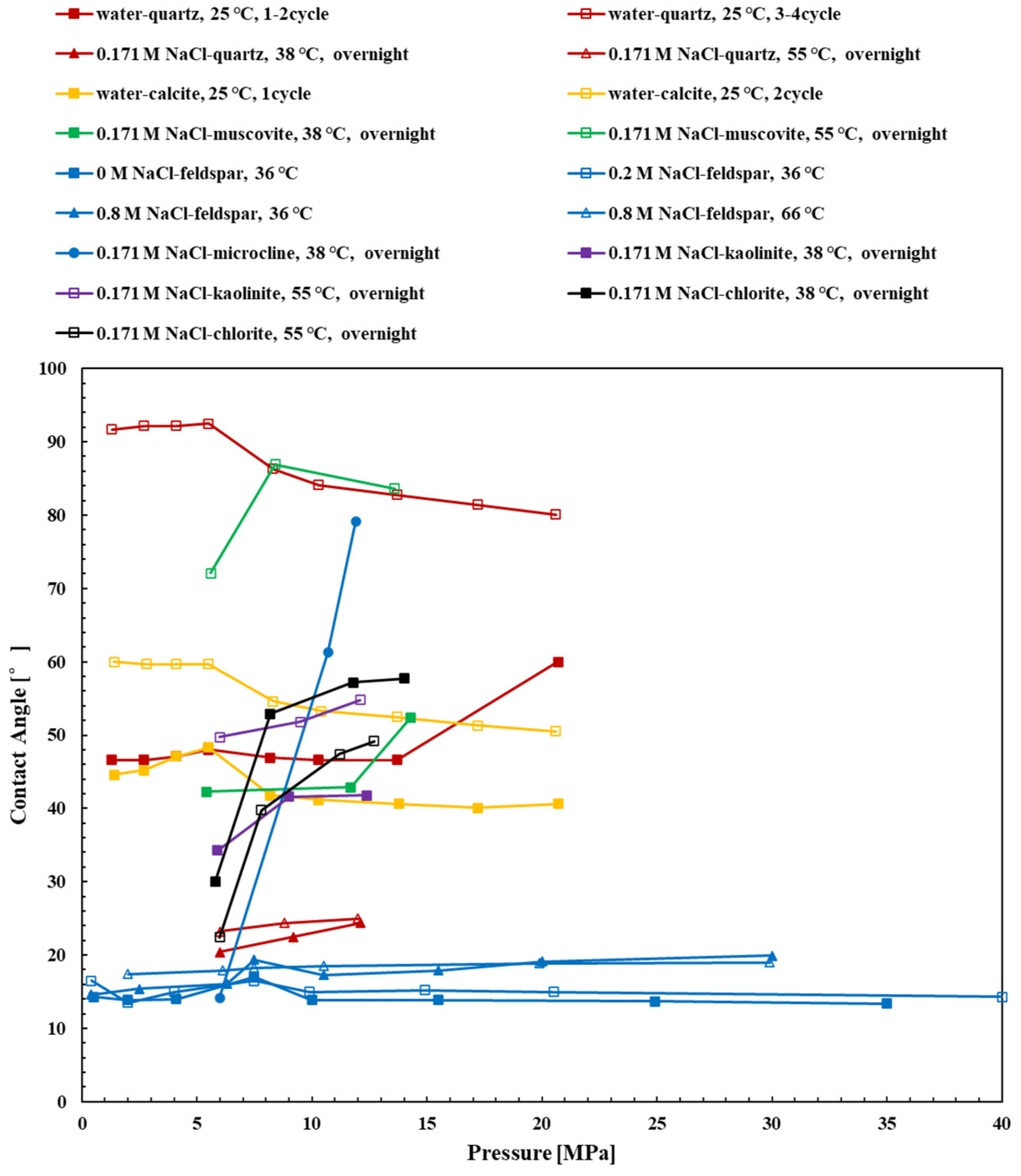
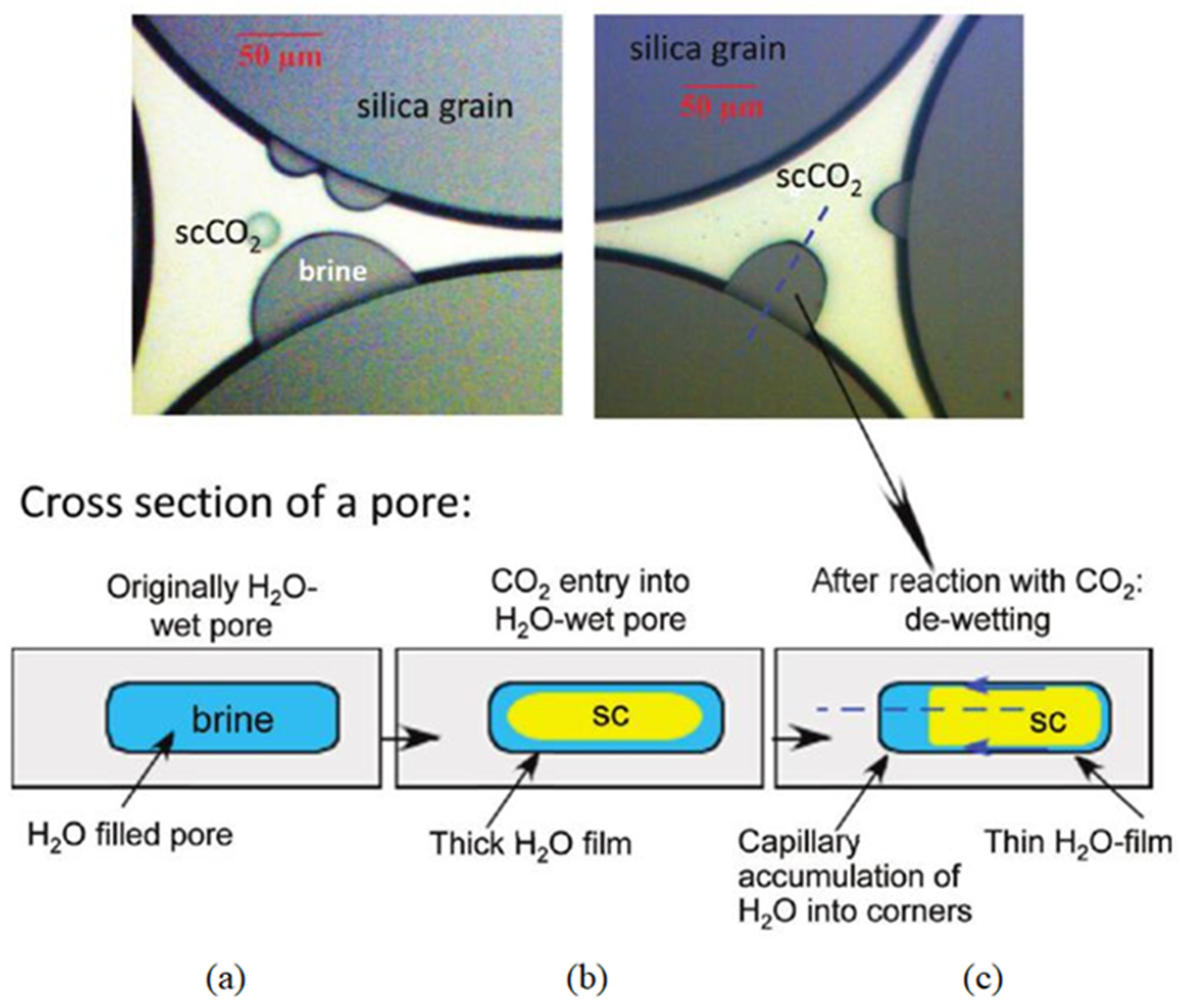
3.1. Quartz
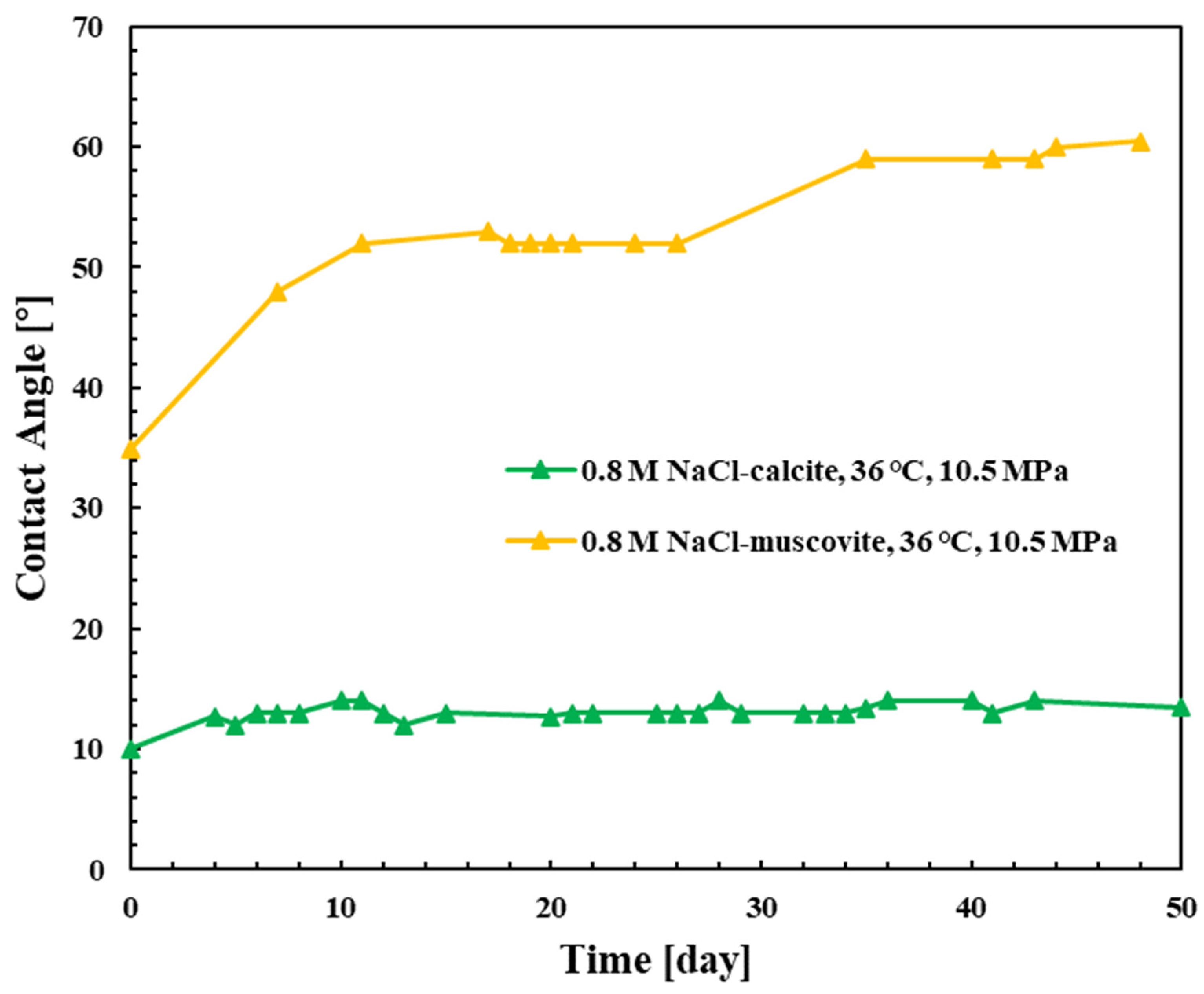
3.2. Calcite
3.3. Mica
3.4. Feldspar and Microcline
3.5. Kaolinite and Illite
3.6. Chlorite
4. Reaction-Induced Wettability Changes in the CO2-Brine-Rock Systems
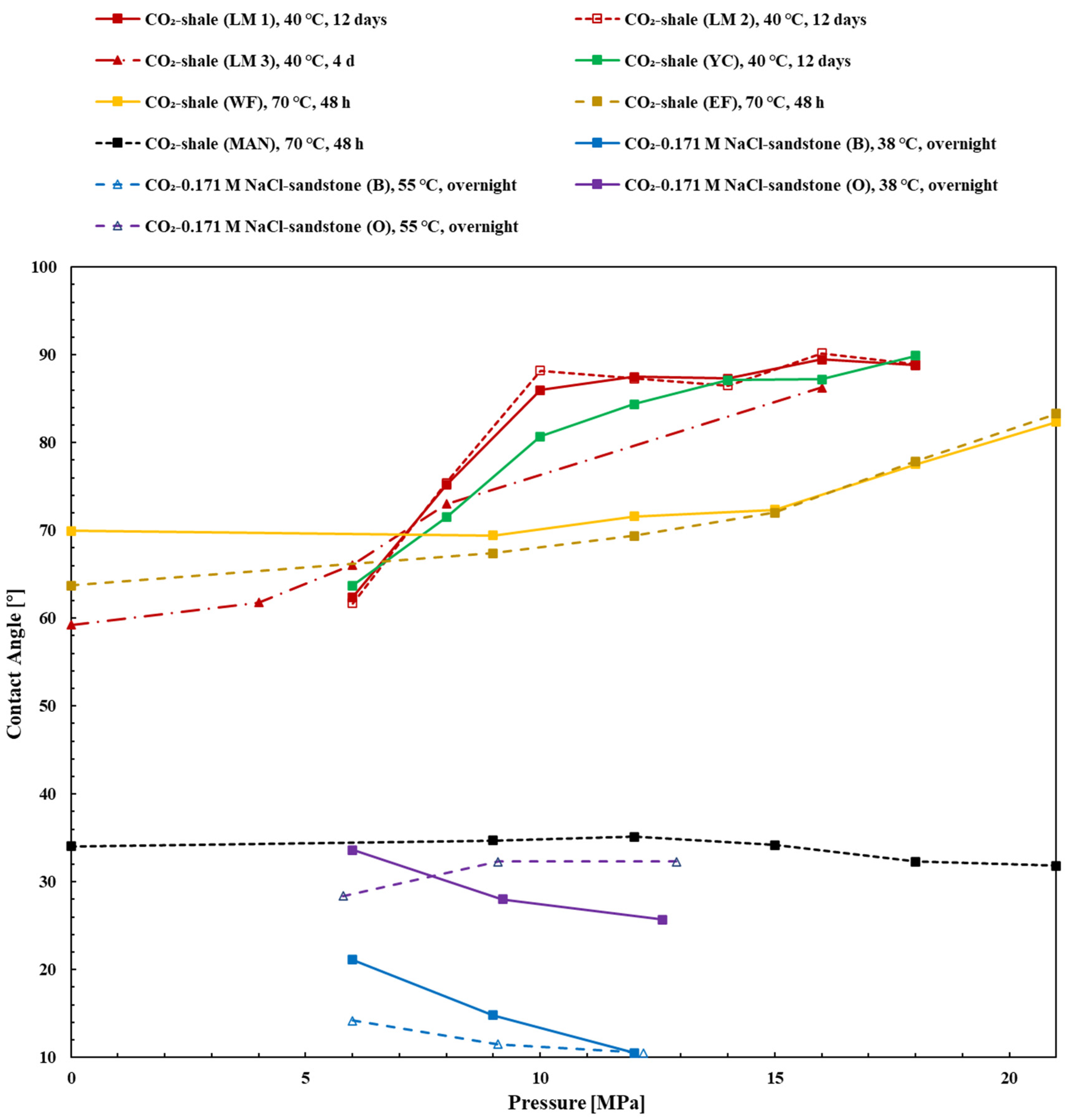
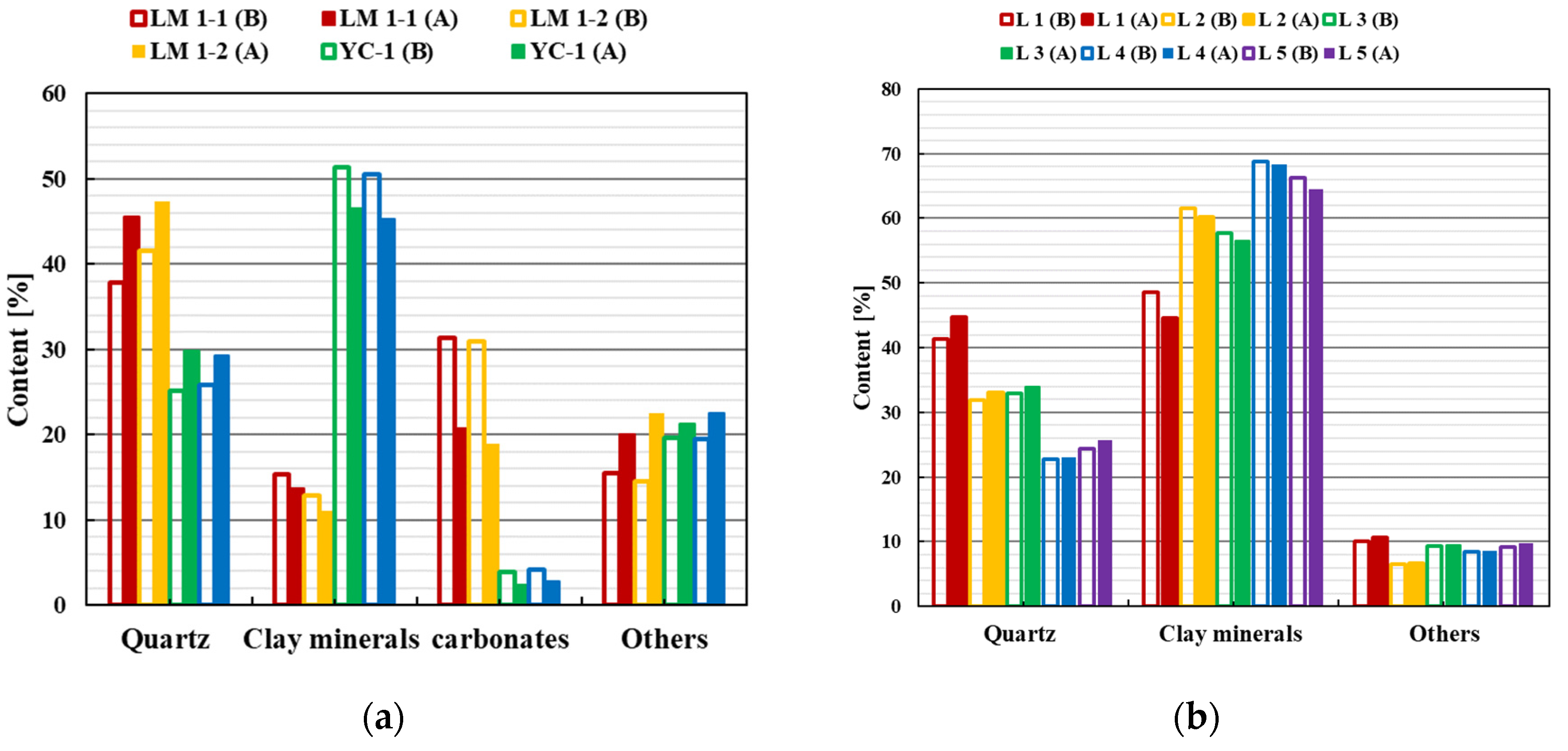
4.1. Shale


4.2. Sandstone
4.3. Limestone
5. Research Gaps and Future Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | Method |
|---|---|
| Chao Qin et al., 2022 [13] | The sessile drop method |
| Raoof Gholami et al., 2021 [28] | The sessile drop method |
| Ahmed Fatah et al., 2021 [29] | The sessile drop method |
| Prem Kumar Bikkina 2011 [36] | The sessile drop method |
| Yongman Kim 2012 [37] | Image processing method |
| Xiaozhou Zhang et al., 2020 [38] | The bubble capture method |
| Raheleh Farokhpoor et al., 2013 [40] | The bubble capture method |
| Lijie Zhang et al., 2016 [41] | The bubble capture method |
| Shibo Wang et al., 2013 [44] | The bubble capture method |
| Chao Qin et al., 2016 [54] | The sessile drop method |
| Ahmed Fatah et al., 2021 [55] | The sessile drop method |
| Yiyu Lu et al., 2021a [56] | The sessile drop method |
| Yiyu Lu et al., 2021b [57] | The sessile drop method |
| Cut Aja Fauziah et al., 2021 [65] | The sessile drop method |
| Shibo Wang et al., 2015 [66] | The sessile drop method |
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control. 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; De Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Iglauer, S.; Pentland, C.H.; Busch, A. CO2 wettability of seal and reservoir rocks and the implications for carbon geo-sequestration. Water Resour. Res. 2015, 51, 729–774. [Google Scholar] [CrossRef]
- Hu, R.; Wan, J.; Kim, Y.; Tokunaga, T.K. Wettability impact on supercritical CO2 capillary trapping: Pore-scale visualization and quantification. Water Resour. Res. 2017, 53, 6377–6394. [Google Scholar] [CrossRef]
- Baban, A.; Keshavarz, A.; Amin, R.; Iglauer, S. Impact of Wettability Alteration on CO2 Residual Trapping in Oil-Wet Sandstone at Reservoir Conditions Using Nuclear Magnetic Resonance. Energy Fuels 2022, 36, 13722–13731. [Google Scholar] [CrossRef]
- Al-Khdheeawi, E.A.; Vialle, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. Influence of CO2-wettability on CO2 migration and trapping capacity in deep saline aquifers. Greenh. Gases Sci. Technol. 2017, 7, 328–338. [Google Scholar] [CrossRef]
- Bakhshian, S.; Hosseini, S.A. Pore–scale analysis of supercritical CO2–brine immiscible displacement under fractional–wettability conditions. Adv. Water Resour. 2019, 126, 96–107. [Google Scholar] [CrossRef]
- Liang, Y.; Tsuji, S.; Jia, J.; Tsuji, T.; Matsuoka, T. Modeling CO2-Water-Mineral Wettability and Mineralization for Carbon Geosequestration. Acc. Chem. Res. 2017, 50, 1530–1540. [Google Scholar] [CrossRef]
- Valle, L.M.; Rodríguez, R.; Grima, C.; Martínez, C. Effects of supercritical CO2 injection on sandstone wettability and capillary trapping. Int. J. Greenh. Gas Control. 2018, 78, 341–348. [Google Scholar] [CrossRef]
- Iglauer, S.; Al-Yaseri, A.Z.; Rezaee, R.; Lebedev, M. CO2 wettability of caprocks: Implications for structural storage capacity and containment security. Geophys. Res. Lett. 2015, 42, 9279–9284. [Google Scholar] [CrossRef]
- Iglauer, S. Optimum storage depths for structural CO2 trapping. Int. J. Greenh. Gas Control. 2018, 77, 82–87. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Zuo, S.; Chen, S.; Liu, Z.; Yin, H.; Li, Y. Influence of supercritical CO2 exposure on water wettability of shale: Implications for CO2 sequestration and shale gas recovery. Energy 2022, 242, 122551. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Iglauer, S. CO2-Water-Rock Wettability: Variability, Influencing Factors, and Implications for CO2 Geostorage. Acc. Chem. Res. 2017, 50, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Li, Y.; Zhang, M.; Wang, X.; Iglauer, S. Effect of total organic carbon (TOC) content on shale wettability at high pressure and high temperature conditions. J. Pet. Sci. Eng. 2020, 193, 107374. [Google Scholar] [CrossRef]
- Pan, B.; Yin, X.; Iglauer, S. A review on clay wettability: From experimental investigations to molecular dynamics simulations. Adv. Colloid Interface Sci. 2020, 285, 102266. [Google Scholar] [CrossRef] [PubMed]
- Al-Yaseri, A.Z.; Lebedev, M.; Barifcani, A.; Iglauer, S. Receding and advancing (CO2 + brine + quartz) contact angles as a function of pressure, temperature, surface roughness, salt type and salinity. J. Chem. Thermodyn. 2016, 93, 416–423. [Google Scholar] [CrossRef]
- Pan, B.; Li, Y.; Wang, H.; Jones, F.; Iglauer, S. CO2 and CH4 Wettabilities of Organic-Rich Shale. Energy Fuels 2018, 32, 1914–1922. [Google Scholar] [CrossRef]
- Pan, B.; Li, Y.; Xie, L.; Wang, X.; He, Q.; Li, Y.; Hejazi, S.H.; Iglauer, S. Role of fluid density on quartz wettability. J. Pet. Sci. Eng. 2019, 172, 511–516. [Google Scholar] [CrossRef]
- Arif, M.; Al-Yaseri, A.Z.; Barifcani, A.; Lebedev, M.; Iglauer, S. Impact of pressure and temperature on CO2-brine-mica contact angles and CO2-brine interfacial tension: Implications for carbon geo-sequestration. J. Colloid Interface Sci. 2016, 462, 208–215. [Google Scholar] [CrossRef]
- Jia, C.; Huang, Z.; Sepehrnoori, K.; Yao, J. Modification of two-scale continuum model and numerical studies for carbonate matrix acidizing. J. Pet. Sci. Eng. 2021, 197, 107972. [Google Scholar] [CrossRef]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth-Sci. Rev. 2016, 157, 86–110. [Google Scholar] [CrossRef]
- Liu, Q.; Maroto-Valer, M.M. Parameters affecting mineral trapping of CO2 sequestration in brines. Greenh. Gases Sci. Technol. 2011, 1, 211–222. [Google Scholar] [CrossRef]
- Rezaee, R.; Saeedi, A.; Iglauer, S.; Evans, B. Shale alteration after exposure to supercritical CO2. Int. J. Greenh. Gas Control. 2017, 62, 91–99. [Google Scholar] [CrossRef]
- Mito, S.; Xue, Z.; Ohsumi, T. Case study of geochemical reactions at the Nagaoka CO2 injection site, Japan. Int. J. Greenh. Gas Control. 2008, 2, 309–318. [Google Scholar] [CrossRef]
- Jia, C.; Sepehrnoori, K.; Huang, Z.; Yao, J. Modeling and Analysis of Carbonate Matrix Acidizing Using a New Two-Scale Continuum Model. SPE J. 2021, 26, 2570–2599. [Google Scholar] [CrossRef]
- Gholami, R.; Raza, A.; Andersen, P.; Escalona, A.; Cardozo, N.; Marín, D.; Sarmadivaleh, M. Long-term integrity of shaly seals in CO2 geo-sequestration sites: An experimental study. Int. J. Greenh. Gas Control. 2021, 109, 103370. [Google Scholar] [CrossRef]
- Fatah, A.; Bennour, Z.; Mahmud, H.B.; Gholami, R.; Hossain, M. Surface wettability alteration of shales exposed to CO2: Implication for long-term integrity of geological storage sites. Int. J. Greenh. Gas Control. 2021, 110, 103426. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Kong, D.; pan, B.; Yue, M. Analytical model of hydraulic fracturing horizontal well gas production capacity of a water-bearing tight sandstone reservoir considering planar heterogeneity. Phys. Fluids 2022, 34, 126603. [Google Scholar] [CrossRef]
- Kalam, S.; Olayiwola, T.; Al-Rubaii, M.M.; Amaechi, B.I.; Jamal, M.S.; Awotunde, A.A. Carbon dioxide sequestration in underground formations: Review of experimental, modeling, and field studies. J. Pet. Explor. Prod. Technol. 2020, 11, 303–325. [Google Scholar] [CrossRef]
- Ringrose, P.S.; Furre, A.K.; Gilfillan, S.M.V.; Krevor, S.; Landro, M.; Leslie, R.; Meckel, T.; Nazarian, B.; Zahid, A. Storage of Carbon Dioxide in Saline Aquifers: Physicochemical Processes, Key Constraints, and Scale-Up Potential. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 471–494. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.; Bera, A. A review on pore-scale modeling and CT scan technique to characterize the trapped carbon dioxide in impermeable reservoir rocks during sequestration. Renew. Sustain. Energy Rev. 2021, 144, 110986. [Google Scholar] [CrossRef]
- Iglauer, S.; Salamah, A.; Sarmadivaleh, M.; Liu, K.; Phan, C. Contamination of silica surfaces: Impact on water–CO2–quartz and glass contact angle measurements. Int. J. Greenh. Gas Control. 2014, 22, 325–328. [Google Scholar] [CrossRef]
- Drelich, J.W. Contact angles: From past mistakes to new developments through liquid-solid adhesion measurements. Adv. Colloid Interface Sci. 2019, 267, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bikkina, P.K. Contact angle measurements of CO2–water–quartz/calcite systems in the perspective of carbon sequestration. Int. J. Greenh. Gas Control. 2011, 5, 1259–1271. [Google Scholar] [CrossRef]
- Kim, Y.; Wan, J.; Kneafsey, T.J.; Tokunaga, T.K. Dewetting of silica surfaces upon reactions with supercritical CO2 and brine: Pore-scale studies in micromodels. Environ. Sci. Technol. 2012, 46, 4228–4235. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, J.; Kamali, F.; Othman, F.; Wang, Y.; Le-Hussain, F. Wettability of sandstone rocks and their mineral components during CO2 injection in aquifers: Implications for fines migration. J. Nat. Gas Sci. Eng. 2020, 73, 103050. [Google Scholar] [CrossRef]
- Farokhpoor, R.; Bjørkvik, B.J.A.; Lindeberg, E.; Torsæter, O. CO2 Wettability Behavior during CO2 Sequestration in Saline Aquifer—An Experimental Study on Minerals Representing Sandstone and Carbonate. Energy Procedia 2013, 37, 5339–5351. [Google Scholar] [CrossRef]
- Farokhpoor, R.; Bjørkvik, B.J.A.; Lindeberg, E.; Torsaeter, O. Wettability behaviour of CO2 at storage conditions. Int. J. Greenh. Gas Control. 2013, 12, 18–25. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, Y.; Jung, H.; Wan, J.; Jun, Y.-S. Effects of Salinity-Induced Chemical Reactions on Biotite Wettability Changes under Geologic CO2 Sequestration Conditions. Environ. Sci. Technol. Lett. 2016, 3, 92–97. [Google Scholar] [CrossRef]
- Chiquet, P.; Broseta, D.; Thibeau, S. Wettability alteration of caprock minerals by carbon dioxide. Geofluids 2007, 7, 112–122. [Google Scholar] [CrossRef]
- Arif, M.; Lebedev, M.; Barifcani, A.; Iglauer, S. CO2 storage in carbonates: Wettability of calcite. Int. J. Greenh. Gas Control. 2017, 62, 113–121. [Google Scholar] [CrossRef]
- Wang, S.; Edwards, I.M.; Clarens, A.F. Wettability phenomena at the CO2-brine-mineral interface: Implications for geologic carbon sequestration. Environ. Sci. Technol. 2013, 47, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.; Shrivastava, V.; Kohse, B. Modeling aqueous phase behavior and chemical reactions in compositional simulation. In Proceedings of the SPE Reservoir Simulation Symposium, The Woodlands, TX, USA, 21–23 February 2011. [Google Scholar] [CrossRef]
- Jia, C.; Sepehrnoori, K.; Huang, Z.; Zhang, H.; Yao, J. Numerical studies and analysis on reactive flow in carbonate matrix acidizing. J. Pet. Sci. Eng. 2021, 201, 108487. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, D.; Jun, Y.S. Effects of Phosphonate Structures on Brine–Biotite Interactions under Subsurface Relevant Conditions. ACS Earth Space Chem. 2018, 2, 946–954. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, D.; Jun, Y.S. The Effects of Phosphonate-Based Scale Inhibitor on Brine-Biotite Interactions under Subsurface Conditions. Environ. Sci. Technol. 2018, 52, 6042–6049. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, D.; Kim, Y.; Wan, J.; Jun, Y.S. Effects of phosphate on biotite dissolution and secondary precipitation under conditions relevant to engineered subsurface processes. Phys. Chem. Chem. Phys. 2017, 19, 29895–29904. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Wu, X.; Jun, Y.S. Effects of sulfate on biotite interfacial reactions under high temperature and high CO2 pressure. Phys. Chem. Chem. Phys. 2019, 21, 6381–6390. [Google Scholar] [CrossRef]
- Duc, M.; Gaboriaud, F.; Thomas, F. Sensitivity of the acid-base properties of clays to the methods of preparation and measurement. 1. Literature review. J. Colloid Interface Sci. 2005, 289, 139–147. [Google Scholar] [CrossRef]
- Shao, H.; Ray, J.R.; Jun, Y.S. Effects of salinity and the extent of water on supercritical CO2-induced phlogopite dissolution and secondary mineral formation. Environ. Sci. Technol. 2011, 45, 1737–1743. [Google Scholar] [CrossRef]
- Fatah, A.; Bennour, Z.; Ben Mahmud, H.; Gholami, R.; Hossain, M.M. A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales. Energies 2020, 13, 3200. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Luo, Y.; Xian, X.; Liu, H.; Li, Y. Effect of Supercritical Carbon Dioxide Treatment Time, Pressure, and Temperature on Shale Water Wettability. Energy Fuels 2016, 31, 493–503. [Google Scholar] [CrossRef]
- Fatah, A.; Mahmud, H.B.; Bennour, Z.; Hossain, M.; Gholami, R. Effect of supercritical CO2 treatment on physical properties and functional groups of shales. Fuel 2021, 303, 121310. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, R.; Liu, W.; Tang, J.; Li, H.; Chen, X.; Sun, X. Mechanisms of shale water wettability alteration with chemical groups after CO2 injection: Implication for shale gas recovery and CO2 geo-storage. J. Nat. Gas Sci. Eng. 2021, 90, 103922. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, R.; Tang, J.; Jia, Y.; Lu, Z.; Sun, X. Investigating the Mineralogical and Chemical Effects of CO2 Injection on Shale Wettability at Different Reservoir Temperatures and Pressures. Energy Fuels 2021, 35, 14838–14851. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, M.; Lu, Z.; Du, X.; Yin, H.; Yang, L. Effects of Supercritical CO2 Treatment Temperatures on Mineral Composition, Pore Structure and Functional Groups of Shale: Implications for CO2 Sequestration. Sustainability 2020, 12, 3927. [Google Scholar] [CrossRef]
- Mohamed, I.M.; He, J.; Nasr-El-Din, H.A. Carbon dioxide sequestration in sandstone aquifers: How does it affect the permeability? In Proceedings of the Carbon Management Technology Conference, Orlando, FL, USA, 7–9 February 2012. [Google Scholar] [CrossRef]
- Pauwels, H.; Gaus, I.; le Nindre, Y.M.; Pearce, J.; Czernichowski-Lauriol, I. Chemistry of fluids from a natural analogue for a geological CO2 storage site (Montmiral, France): Lessons for CO2–water–rock interaction assessment and monitoring. Appl. Geochem. 2007, 22, 2817–2833. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Song, X.; Liu, Z.; Li, D.; Zhou, F.; Xie, Y.; Xie, C. Effect of supercritical CO2 extraction on CO2/CH4 competitive adsorption in Yanchang shale. Chem. Eng. J. 2021, 412, 128701. [Google Scholar] [CrossRef]
- Zhuravlev, L. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, N.; Li, W.; Song, Y. Water contact angle dependence with hydroxyl functional groups on silica surfaces under CO2 sequestration conditions. Environ. Sci. Technol. 2015, 49, 14680–14687. [Google Scholar] [CrossRef]
- Gholami, R.; Raza, A. CO2 sequestration in sandstone reservoirs: How does reactive flow alter trapping mechanisms? Fuel 2022, 324, 124781. [Google Scholar] [CrossRef]
- Fauziah, C.A.; Al-Yaseri, A.; Al-Khdheeawi, E.; Jha, N.K.; Abid, H.R.; Iglauer, S.; Lagat, C.; Barifcani, A. Effect of CO2 Flooding on the Wettability Evolution of Sand-Stone. Energies 2021, 14, 5542. [Google Scholar] [CrossRef]
- Wang, S.; Tokunaga, T.K. Capillary pressure-saturation relations for supercritical CO2 and brine in limestone/dolomite sands: Implications for geologic carbon sequestration in carbonate reservoirs. Environ. Sci. Technol. 2015, 49, 7208–7217. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.K.; Wan, J. Capillary Pressure and Mineral Wettability Influences on Reservoir CO2 Capacity. Rev. Mineral. Geochem. 2013, 77, 481–503. [Google Scholar] [CrossRef]
- Wang, S.; Tao, Z.; Persily, S.M.; Clarens, A.F. CO2 adhesion on hydrated mineral surfaces. Environ. Sci. Technol. 2013, 47, 11858–11865. [Google Scholar] [CrossRef]
- Mahadevan, J. Comments on the paper titled “Contact angle measurements of CO2–water-quartz/calcite systems in the perspective of carbon sequestration”: A case of contamination? Int. J. Greenh. Gas Control. 2012, 7, 261–262. [Google Scholar] [CrossRef]
- Butt, H.-J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Vafaei, S.; Podowski, M.Z. Analysis of the relationship between liquid droplet size and contact angle. Adv. Colloid Interface Sci. 2005, 113, 133–146. [Google Scholar] [CrossRef]
- Pan, B.; Clarkson, C.R.; Atwa, M.; Debuhr, C.; Ghanizadeh, A.; Birss, V.I. Wetting dynamics of nanoliter water droplets in nanoporous media. J. Colloid Interface Sci. 2021, 589, 411–423. [Google Scholar] [CrossRef]
- Pan, B.; Clarkson, C.R.; Debuhr, C.; Younis, A.; Song, C.; Ghanizadeh, A.; Birss, V.I. Low-permeability reservoir sample wettability characterization at multiple scales: Pore-, micro- and macro-contact angles. J. Nat. Gas Sci. Eng. 2021, 95, 104229. [Google Scholar] [CrossRef]
- Arif, M.; Abu-Khamsin, S.A.; Iglauer, S. Wettability of rock/CO2/brine and rock/oil/CO2-enriched-brine systems:Critical parametric analysis and future outlook. Adv. Colloid Interface Sci. 2019, 268, 91–113. [Google Scholar] [CrossRef]
- Pan, B.; Yin, X.; Zhu, W.; Yang, Y.; Ju, Y.; Yuan, Y.; zhang, L.; Iglauer, S. Theoretical study of brine secondary imbibition in sandstone reservoirs: Implications for H2, CH4, and CO2 geo-storage. Int. J. Hydrogen Energy 2022, 47, 18058–18066. [Google Scholar] [CrossRef]
- Pan, B.; Yin, X.; Ju, Y.; Iglauer, S. Underground hydrogen storage: Influencing parameters and future outlook. Adv. Colloid Interface Sci. 2021, 294, 102473. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Yin, X.; Iglauer, S. Rock-fluid interfacial tension at subsurface conditions: Implications for H2, CO2 and natural gas geo-storage. Int. J. Hydrogen Energy 2021, 46, 25578–25585. [Google Scholar] [CrossRef]
- Pan, B.; Liu, K.; Ren, B.; Zhang, M.; Ju, Y.; Gu, J.; Zhang, X.; Clarkson, C.R.; Edlmann, K.; Zhu, W. Impacts of relative permeability hysteresis, wettability, and injection/withdrawal schemes on underground hydrogen storage in saline aquifers. Fuel 2023, 333, 126516. [Google Scholar] [CrossRef]
- Pan, B.; Jones, F.; Huang, Z.; Yang, Y.; Li, Y.; Hejazi, S.H.; Iglauer, S. Methane (CH4) Wettability of Clay-Coated Quartz at Reservoir Conditions. Energy Fuels 2019, 33, 788–795. [Google Scholar] [CrossRef]



| Salinity | Static Contact Angle [°] | Surface Roughness [Rq, nm] | Zeta Potential [mV] | Surface Hydroxyl Content [%] |
|---|---|---|---|---|
| DI water | −19.1 | 82.9 | ||
| 0.1 M NaCl | ||||
| 0.5M NaCl | ||||
| 1.0M NaCl | −47.6 | 92.9 |
| Pressure [MPa] | Temperature [°C] | Static Contact Angle [°] | Mineral Content [%] | Chemical Groups Content | |||
|---|---|---|---|---|---|---|---|
| Quartz | Carbonate (Calcite and Dolomite) | Clay | Oxygen-Containing Groups | Hydroxyl Group | |||
| initial | 58 | 8.5 | 48.0 | 12.1 | 131.9 | 21.4 | |
| 16 | 40 | 58 | 9.3 | 47.0 | 11.4 | 76.5 | 21.9 |
| 16 | 50 | 68 | 102.3 | 19.5 | |||
| 16 | 60 | 69 | 113.2 | 18.0 | |||
| 16 | 70 | 75 | 192 | 13.0 | |||
| 16 | 80 | 73 | 9.5 | 48.9 | 11.8 | 132.7 | 15.8 |
| 14 | 80 | 75 | 167.9 | 14.4 | |||
| 12 | 80 | 79 | 9.7 | 46.7 | 11.2 | 190.3 | 10.4 |
| 10 | 80 | 69 | 100 | 17.4 | |||
| Sample | Clay Content [%] | Quartz Content [%] | Surface Hydroxyl Content | |
|---|---|---|---|---|
| WF | Before | 60.9 | 30.2 | |
| After | 46.3 | 46.7 | ||
| EF | Before | 62.5 | 16.6 | 25.779 |
| After | 56.0 | 23.5 | 21.373 | |
| MAN | Before | 37.7 | 49.6 | 27.757 |
| After | 34.3 | 56.5 | 29.814 |
| Pressure [MPa] | Initial | 4 MPa | 6 MPa | 8 MPa | 16 MPa |
|---|---|---|---|---|---|
| Hydrophilic minerals components (%) | 37 | 35.9 | 29.3 | 25.7 | 25.5 |
| Ratio of the Si-OH to the Si-O-Si components | 11.24 | 7 | 6.57 | 6.39 |
| Sandstone | Pressure [MPa] | Contact Angle [°] | Mineral Content [wt%] | ||
|---|---|---|---|---|---|
| Advancing | Receding | ||||
| Berea | Before flooding | 10 | 65 | 58 | Quartz: 84.3 Kaolinite: 4.1 Illite: 1.9 Albite: 4.2 Microcline: 4.1 Chorite: 1.4 |
| 15 | 81 | 75 | |||
| After flooding | 10 | 74 | 67 | Quartz: 58.2 Kaolinite: 3.2 Illite: 3.6 Albite: 12.4 Muscovite: 1.6 Chorite: 5.7 Ankerite: 15.3 | |
| 15 | 89 | 83 | |||
| Bandera Gray | Before flooding | 10 | 86 | 79 | Quartz: 84.9 Kaolinite: 3.9 Illite: 1.8 Albite: 4.2 Microcline: 4.1 Chorite: 1.1 |
| 15 | 105 | 97 | |||
| After flooding | 10 | 96 | 90 | Quartz: 58.4 Kaolinite: 3.1 Illite: 3.2 Albite: 12.2 Muscovite: 3.1 Chorite: 5.2 Ankerite: 14.8 | |
| 15 | 108 | 103 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Song, L.; Zhang, X.; Yang, Y.; Fan, H.; Pan, B. A Review of Mineral and Rock Wettability Changes Induced by Reaction: Implications for CO2 Storage in Saline Reservoirs. Energies 2023, 16, 3484. https://doi.org/10.3390/en16083484
Chen T, Song L, Zhang X, Yang Y, Fan H, Pan B. A Review of Mineral and Rock Wettability Changes Induced by Reaction: Implications for CO2 Storage in Saline Reservoirs. Energies. 2023; 16(8):3484. https://doi.org/10.3390/en16083484
Chicago/Turabian StyleChen, Ting, Laiming Song, Xueying Zhang, Yawen Yang, Huifang Fan, and Bin Pan. 2023. "A Review of Mineral and Rock Wettability Changes Induced by Reaction: Implications for CO2 Storage in Saline Reservoirs" Energies 16, no. 8: 3484. https://doi.org/10.3390/en16083484
APA StyleChen, T., Song, L., Zhang, X., Yang, Y., Fan, H., & Pan, B. (2023). A Review of Mineral and Rock Wettability Changes Induced by Reaction: Implications for CO2 Storage in Saline Reservoirs. Energies, 16(8), 3484. https://doi.org/10.3390/en16083484






