Improving Thermal Stability of Perovskite Solar Cells by Thermoplastic Additive Engineering
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Film and Device Fabrication
2.3. Instrumentation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic–organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.-J.; Fiala, P.; Jeangros, Q.; Ballif, C. High-Bandgap Perovskite Materials for Multijunction Solar Cells. Joule 2018, 2, 1421–1436. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photon. 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Zheng, G.; Huang, Y.; Chen, Y.; Li, L.; et al. A Eu3+-Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Laboratory (NREL). Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 10 March 2023).
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of Metal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Schoonman, J. Organic–inorganic lead halide perovskite solar cell materials: A possible stability problem. Chem. Phys. Lett. 2015, 619, 193–195. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskitesolar cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef]
- Leguy, A.M.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; Van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible hydration of CH3NH3PbI3 in films, single crystals, and solar cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Q.; Huo, J.; Gao, F.; Gan, Z.; Zhao, Q.; Li, H. Mechanisms and Suppression of Photoinduced Degradation in Perovskite Solar Cells. Adv. Energy Mater. 2020, 11, 2002326–2002356. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Anoop, M.K.; Visoly-Fisher, I.; Kolusheva, S.; Galagan, Y.; Di Giacomo, F.; Vukovic, O.; Patil, B.R.; Sherafatipour, G.; Turkovic, V.; et al. Dynamics of Photoinduced Degradation of Perovskite Photovoltaics: From Reversible to Irreversible Processes. ACS Appl. Energy Mater. 2018, 1, 799–806. [Google Scholar] [CrossRef]
- Zhu, W.; Xin, G.; Scott, S.M.; Xu, W.; Yao, T.; Gong, B.; Wang, Y.; Li, M.; Lian, J. Deciphering the degradation mechanism of the lead-free all inorganic perovskite Cs2SnI6. NPJ Mater. Degrad. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 15218–15227. [Google Scholar] [CrossRef]
- Mohammadi, M.; Gholipour, S.; Byranvand, M.M.; Abdi, Y.; Taghavinia, N.; Saliba, M. Encapsulation Strategies for Highly Stable Perovskite Solar Cells under Severe Stress Testing: Damp Heat, Freezing, and Outdoor Illumination Conditions. ACS Appl. Mater. Interfaces 2021, 13, 45455–45464. [Google Scholar] [CrossRef]
- Rolston, N.; Printz, A.D.; Tracy, J.M.; Weerasinghe, H.C.; Vak, D.; Haur, L.J.; Priyadarshi, A.; Mathews, N.; Slotcavage, D.J.; McGehee, M.D.; et al. Effect of Cation Composition on the Mechanical Stability of Perovskite Solar Cells. Adv. Energy Mater. 2017, 8, 1702116–1702122. [Google Scholar] [CrossRef]
- Ha, S.R.; Jeong, W.H.; Liu, Y.; Oh, J.T.; Bae, S.Y.; Lee, S.; Kim, J.W.; Bandyopadhyay, S.; Jeong, H.I.; Kim, J.Y.; et al. Molecular aggregation method for perovskite–fullerene bulk heterostructure solar cells. J. Mater. Chem. A 2019, 8, 1326–1334. [Google Scholar] [CrossRef]
- Chae, S.; Yi, A.; Kim, H.J. Molecular engineering of a conjugated polymer as a hole transporting layer for versatile p–i–n perovskite solar cells. Mater. Today Energy 2019, 14, 100341–100348. [Google Scholar] [CrossRef]
- Meng, Z.; Guo, D.; Yu, J.; Fan, K. Investigation of Al2O3 and ZrO2 spacer layers for fully printable and hole-conductor-free mesoscopic perovskite solar cells. Appl. Surf. Sci. 2018, 430, 632–638. [Google Scholar] [CrossRef]
- Lao, Y.; Zhang, Y.; Yang, S.; Zhang, Z.; Yu, W.; Qu, B.; Xiao, L.; Chen, Z. Efficient Perovskite Solar Cells with Enhanced Thermal Stability by Sulfide Treatment. ACS Appl. Mater. Interfaces 2022, 14, 27427–27434. [Google Scholar] [CrossRef]
- Zou, J.; Liu, W.; Deng, W.; Lei, G.; Zeng, S.; Xiong, J.; Gu, H.; Hu, Z.; Wang, X.; Li, J. An efficient guanidinium isothiocyanate additive for improving the photovoltaic performances and thermal stability of perovskite solar cells. Electrochim. Acta 2018, 291, 297–303. [Google Scholar] [CrossRef]
- Tang, X.; Chen, M.; Jiang, L.; Li, M.; Tang, G.; Liu, H. Improvements in Efficiency and Stability of Perovskite Solar Cells Using a Cesium Chloride Additive. ACS Appl. Mater. Interfaces 2022, 14, 26866–26872. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Guo, P.; Hsu, C.; Lin, M.; Wang, B.; Zeng, L.; Huang, W.; Soe, C.M.M.; Su, W.; Bedzyk, M.J.; et al. Enhanced Efficiency of Hot-Cast Large-Area Planar Perovskite Solar Cells/Modules Having Controlled Chloride Incorporation. Adv. Energy Mater. 2016, 7, 1601660–1601668. [Google Scholar] [CrossRef]
- Liu, S.; Guan, Y.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. A Review on Additives for Halide Perovskite Solar Cells. Adv. Energy Mater. 2019, 10, 1902492–1902519. [Google Scholar] [CrossRef]
- Afroz, M.A.; Ghimire, N.; Reza, K.M.; Bahrami, B.; Bobba, R.S.; Gurung, A.; Chowdhury, A.H.; Iyer, P.K.; Qiao, Q. Thermal Stability and Performance Enhancement of Perovskite Solar Cells Through Oxalic Acid-Induced Perovskite Formation. ACS Appl. Energy Mater. 2020, 3, 2432–2439. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Lu, M.; Shi, Z.; Yu, W.W.; Hu, J.; Bai, X.; Zhang, Y. Ionic additive engineering for stable planar perovskite solar cells with efficiency >22%. Chem. Eng. J. 2021, 426, 130841–130847. [Google Scholar] [CrossRef]
- Li, X.; Ke, S.; Feng, X.; Zhao, X.; Zhang, W.; Fang, J. Enhancing the stability of perovskite solar cells through cross-linkable and hydrogen bonding multifunctional additives. J. Mater. Chem. A 2021, 9, 12684–12689. [Google Scholar] [CrossRef]
- Peng, J.; Khan, J.I.; Liu, W.; Ugur, E.; Duong, T.; Wu, Y.; Shen, H.; Wang, K.; Dang, H.; Aydin, E.; et al. A Universal Double-Side Passivation for High Open-Circuit Voltage in Perovskite Solar Cells: Role of Carbonyl Groups in Poly(methyl methacrylate). Adv. Energy Mater. 2018, 8, 1801208–1801216. [Google Scholar] [CrossRef]
- Collavini, S.; Cabrera-Espinoza, A.; Delgado, J.L. Organic Polymers as Additives in Perovskite Solar Cells. Macromolecules 2021, 54, 5451–5463. [Google Scholar] [CrossRef]
- Lee, K.-M.; Chan, S.-H.; Ting, C.-C.; Chen, S.-H.; Chiu, W.-H.; Suryanarayanan, V.; Hsu, J.-F.; Liu, C.-Y.; Wu, M.-C. Surfactant Tween 20 Controlled Perovskite Film Fabricated by Thermal Blade Coating for Efficient Perovskite Solar Cells. Nanomaterials 2022, 12, 2651. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Z.; Zhou, Y.; Liu, H.; Qin, Q.; Lu, X.; Gao, X.; Shui, L.; Wu, S.; Liu, J. Enhancing the efficiency of low-temperature planar perovskite solar cells by modifying the interface between perovskite and hole transport layer with polymers. Electrochim. Acta 2018, 261, 445–453. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Zhang, Z.; Li, J.; Uddin, Z.; Zheng, Y.; Shao, Y.; Yuan, Y.; Yang, B. Defect Passivation via Additive Engineering to Improve Photodetection Performance in CsPbI2Br Perovskite Photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 56358–56365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, J.; Li, H.; Yan, Y.; Zhou, W.; Yu, D.; Zhao, Q. A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 2016, 7, 10228. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, X.; Zang, J.; Liu, Q.; Fei, Y.; Yu, Z. Polymer-modified CsPbI2Br films for all-inorganic planar perovskite solar cells with improved performance. Surfaces Interfaces 2020, 22, 100809–100815. [Google Scholar] [CrossRef]
- Jiang, L.L.; Wang, Z.K.; Li, M.; Zhang, C.C.; Ye, Q.Q.; Hu, K.H.; Lu, D.Z.; Fang, P.F.; Liao, L.S. Passivated Perovskite Crystallization via g-C3N4 for High-Performance Solar Cells. Adv. Funct. Mater. 2018, 28, 1705875–1705882. [Google Scholar] [CrossRef]
- Roose, B.; Dey, K.; Chiang, Y.H.; Friend, R.H.; Stranks, S.D. Critical Assessment of the Use of Excess Lead Iodide in Lead Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2020, 11, 6505–6512. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]

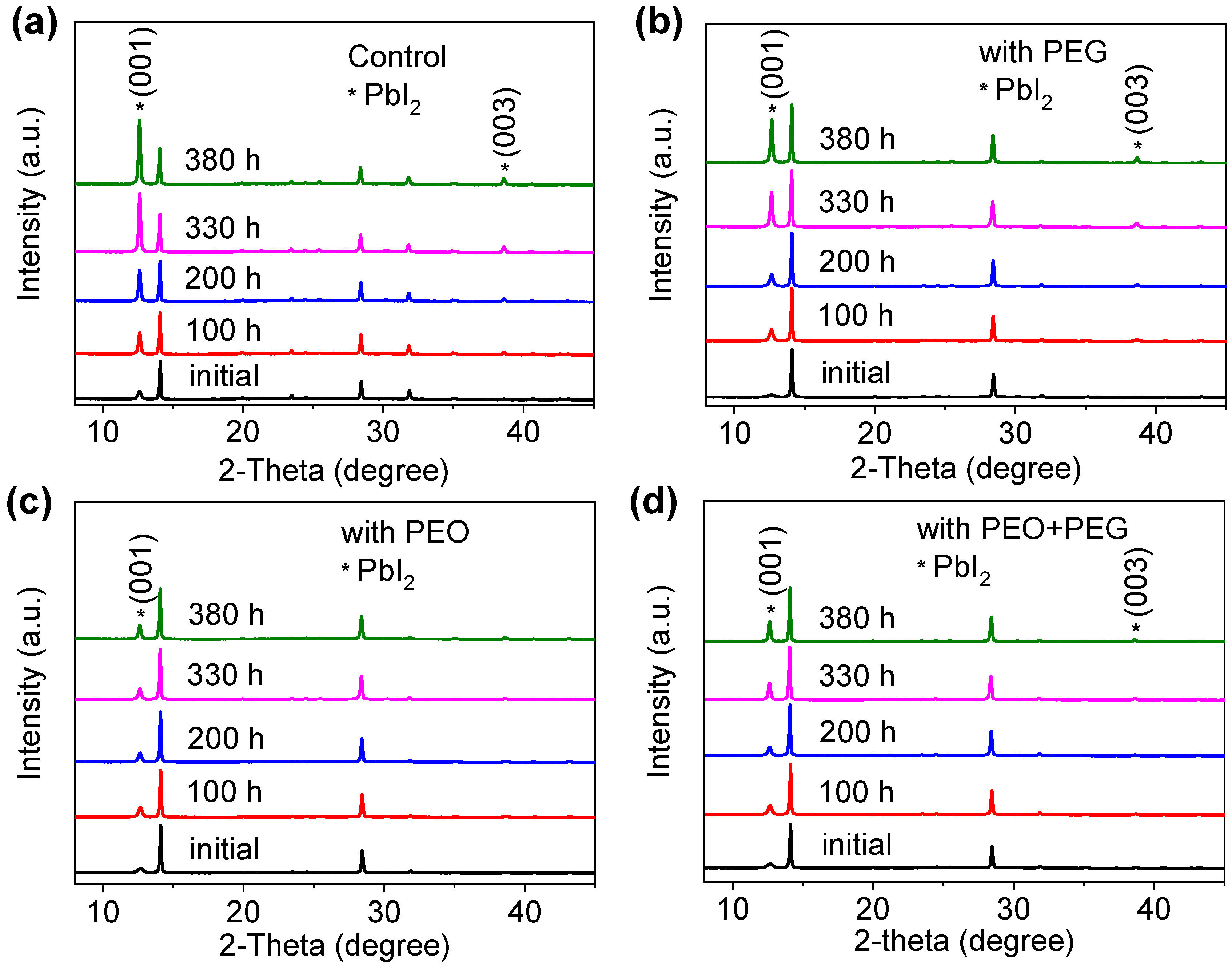
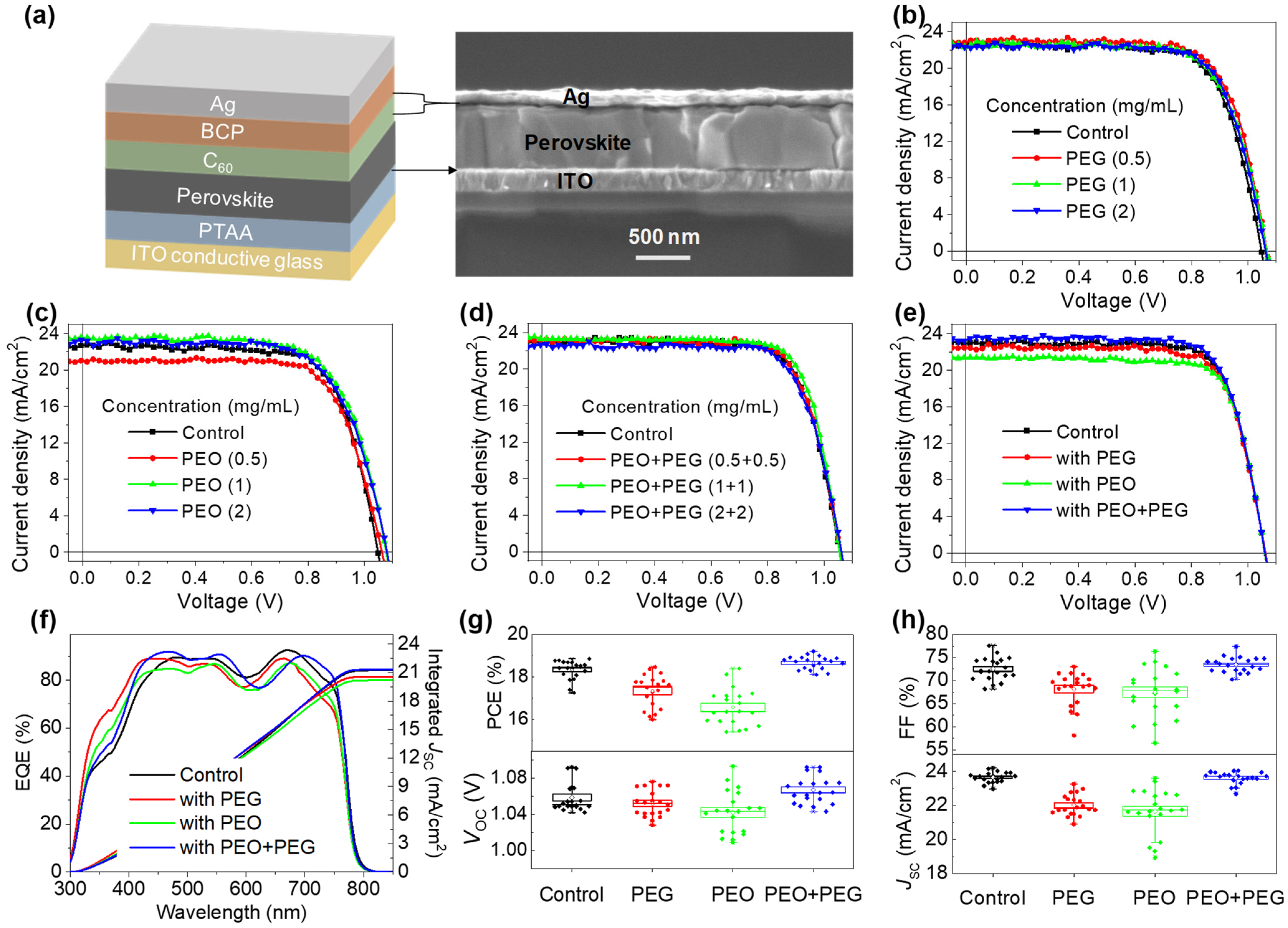
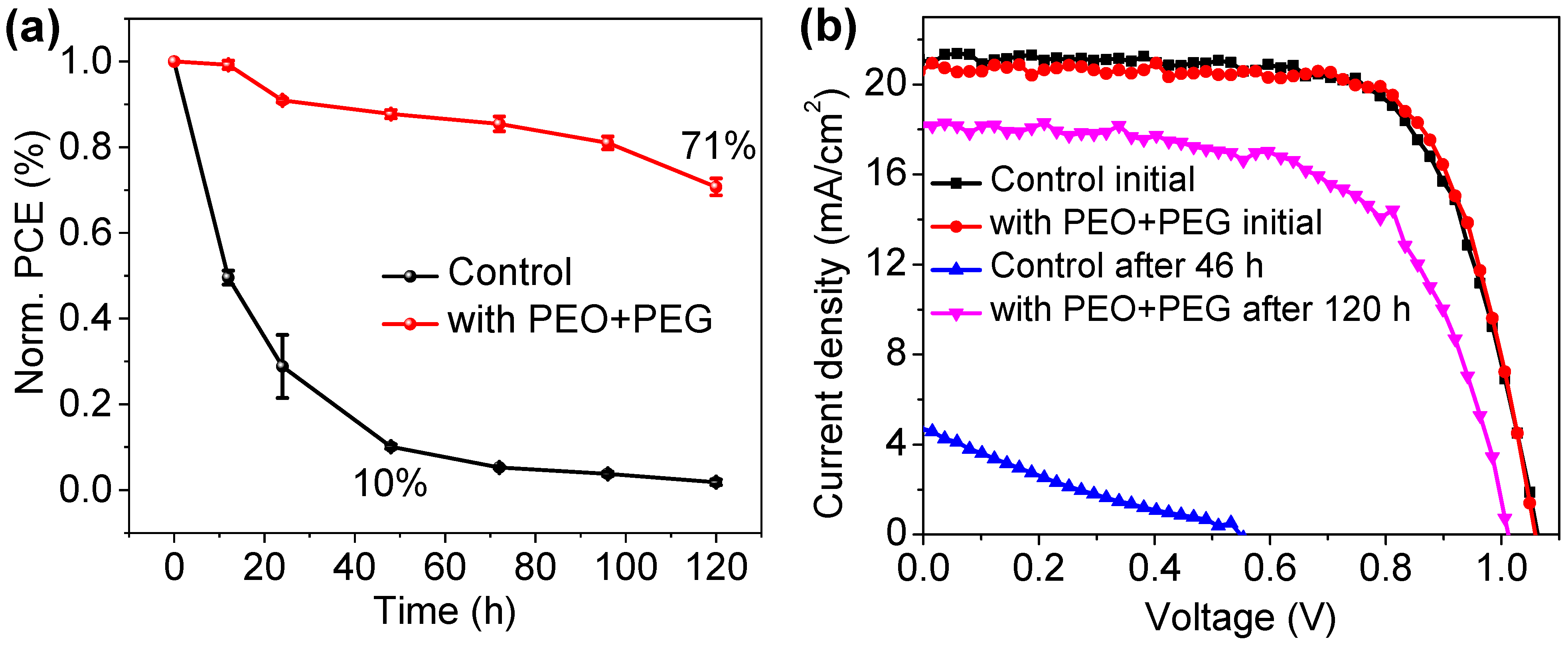
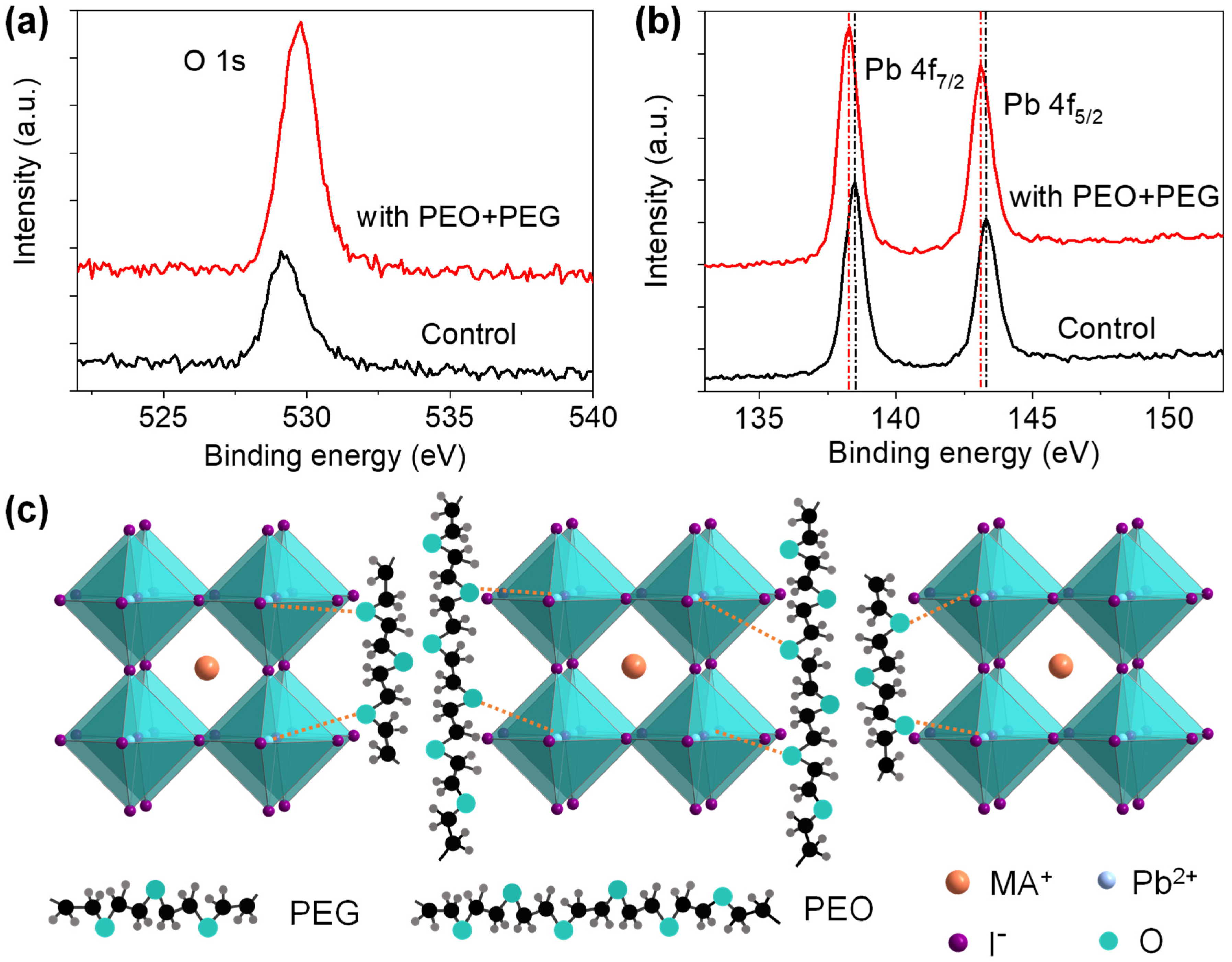
| Devices | VOC (V) | JSC (mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|
| Control | 1.058 | 23.65 | 0.72 | 18.36 |
| With PEG | 1.052 | 22.02 | 0.68 | 17.33 |
| With PEO | 1.042 | 21.66 | 0.67 | 16.56 |
| With PEO + PEG | 1.066 | 23.62 | 0.73 | 18.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, Z.; Ran, J.; Stathatos, E.; Yang, B. Improving Thermal Stability of Perovskite Solar Cells by Thermoplastic Additive Engineering. Energies 2023, 16, 3621. https://doi.org/10.3390/en16093621
Uddin Z, Ran J, Stathatos E, Yang B. Improving Thermal Stability of Perovskite Solar Cells by Thermoplastic Additive Engineering. Energies. 2023; 16(9):3621. https://doi.org/10.3390/en16093621
Chicago/Turabian StyleUddin, Zaheen, Junhui Ran, Elias Stathatos, and Bin Yang. 2023. "Improving Thermal Stability of Perovskite Solar Cells by Thermoplastic Additive Engineering" Energies 16, no. 9: 3621. https://doi.org/10.3390/en16093621
APA StyleUddin, Z., Ran, J., Stathatos, E., & Yang, B. (2023). Improving Thermal Stability of Perovskite Solar Cells by Thermoplastic Additive Engineering. Energies, 16(9), 3621. https://doi.org/10.3390/en16093621









