Application of Bio-Derived Alternatives for the Assured Flow of Waxy Crude Oil: A Review
Abstract
1. Introduction

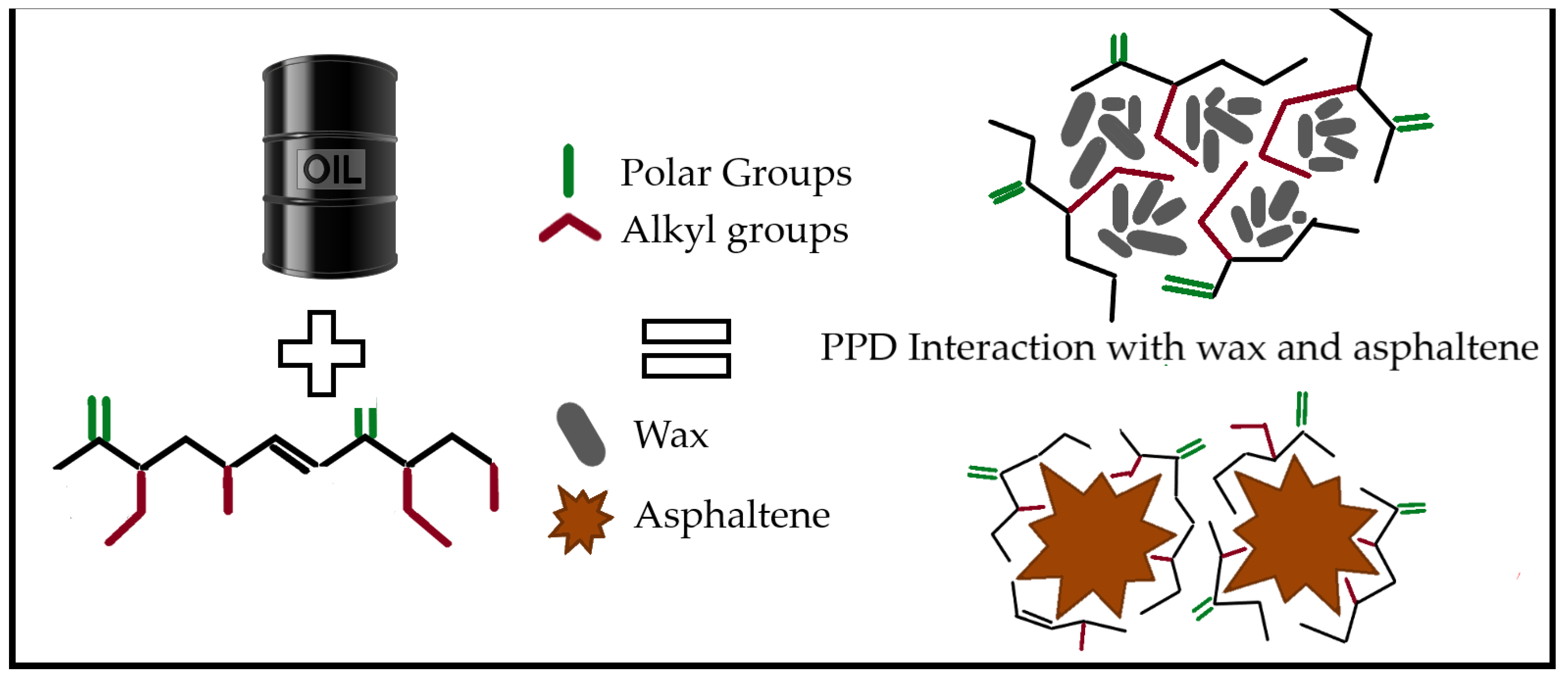
2. Pour Point Depressants
3. Bio-Derived Crude Flow Improvers
4. Transesterification of Bio-Based Oils
5. Nanoparticles as Crude Flow Improvers
| Polymeric PPD | Nanoparticle | Blending Type | %PPR | %VR | %YSR | Highlights | Ref |
|---|---|---|---|---|---|---|---|
| poly(octadecyl acrylate), (POA) | Silica (SiO2) | Solvent blending | 80 |
| [13] | ||
| Poly(methyl methacrylate) PMMA | Graphene Oxide (GO) | Solvent blending | 77.77 | 82.19 | 71.93 |
| [121] |
| POA | Montmorillo-nite (MMT) | Solvent blending | 33.75 | 82.3 |
| [115] | |
| POA | MMT clay | Melt blending | 63.15 | 91.37 |
| [118] | |
| ethylene vinyl acetate (EVA) | MMT | Solvent blending | 94.11 |
| [110] | ||
| EVA | SiO2 | Solvent blending | >100 | 95.46 | 99.95 |
| [122] |
| EVA | SiO2 | Solvent blending | 100 | 41.67 |
| [8] | |
| Poly (Octadecyl Acrylate)-Co-(Maleic Anhydride), PODAMA | MMT | Free-radical polymerization | 100 | 81.74 | 87.70 |
| [73] |
| poly(2-ethylhexyl acrylate), P(2EHA) | GO | Free-radical polymerization | 50 | 99.20 |
| [116] | |
| PMMA | GO | Free-radical polymerization | 60.53 | 99.80 | 99.80 |
| [99] |
| EVA | Iron Oxide (Fe3O4) | Melt blending | 97.3 | 95 |
| [123] | |
| poly(octadecylacrylate-co-1-vinyldodecanoate-co-4-vinylbenzyl trioctylphosphonium), (VTOP-PODA-VL) | Bentonite clay (BT) | Solvent blending | >100 | 85.14 | 92.7 |
| [124] |
| poly (ethylene-butene), (PEB) | Aluminum oxide (Al2O3) | Mixing | 77.9 |
| [125] | ||
| Octadecyl methacrylate, styrene, maleic anhydride, and acrylamide copolymer | 3-propyl trimethoxysi-lane (KH570) modified SiO2 | Graft copoly-merization | 45.71 | 97.6 |
| [111] | |
| Poly (maleic anhydride-alt-1-octadecene), (MA) | sodium cloisite Na+ | Solvent blending | 94 |
| [126] | ||
| PEB | Zinc Oxide (ZnO) | Mixing | 33.33 |
| [127] | ||
| Ethylenevinyl alcohol copolymer (EVAL) | GO | Graft copoly merization | 62.50 | 99.5 | 78.30 |
| [120] |
| Poly(octadecyl acrylate-co-vinyl neodecanoate), (PODA-co-VND) | oleic acid-modified graphene oxide, (OL-GO) | Solvent blending | >100 | 84.14 | 93.53 |
| [128] |
| 2,5,8,11 Tetramethyl 6 dodecyn-5,8 Diol Ethoxylate, (GS) | SiO2, tin oxide (SnO), Nickel oxide (Ni2O3) | Mixing | 92.78 |
| [109] | ||
| poly-a-olefins-acrylate high-carbon ester (PAA-18) | GO, carbon nanospheres (Cna), carbon nanotubes (OCNTs) | Solvothermal | 53 | 92.10 |
| [114] | |
| EVA | SiO2 | Solvent blending | 29.16 | 92.70 | 76.89 |
| [129] |
| 1-octyl 3-methylimidazolium chloride, [(OMIM) Cl] | GO | Free-radical polymerization | 76.92 | 98.78 |
| [130] |
5.1. Carbon-Based Nanohybrid PPD
5.2. Silica-Based Nanohybrid PPD
5.3. Other Nanoparticle-Based PPD Studies
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elkatory, M.R.; Soliman, E.A.; El Nemr, A.; Hassaan, M.A.; Ragab, S.; El-Nemr, M.A.; Pantaleo, A. Mitigation and Remediation Technologies of Waxy Crude Oils’: Deposition within Transportation Pipelines: A Review. Polymers 2022, 14, 3231. [Google Scholar] [CrossRef] [PubMed]
- IEA. World Energy Outlook 2019; IEA: Paris, France, 2019. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 3 January 2023).
- Li, X.; Zhang, F.; Liu, G. Review on new heavy oil viscosity reduction technologies. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, 012059. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Anto, R.; Deshmukh, S.; Sanyal, S.; Bhui, U.K. Nanoparticles as flow improver of petroleum crudes: Study on temperature-dependent steady-state and dynamic rheological behavior of crude oils. Fuel 2020, 275, 117873. [Google Scholar] [CrossRef]
- Souas, F.; Safri, A.; Benmounah, A. A review on the rheology of heavy crude oil for pipeline transportation. Pet. Res. 2021, 6, 116–136. [Google Scholar] [CrossRef]
- Ridzuan, N.; Subramanie, P.; Uyop, M.F. Effect of pour point depressant (PPD) and the nanoparticles on the wax deposition, viscosity and shear stress for Malaysian crude oil. Pet. Sci. Technol. 2020, 38, 929–935. [Google Scholar] [CrossRef]
- Sun, Z.; Jing, G.; Tu, Z. Effect of modified nano-silica/EVA on flow behavior and wax crystallization of model oils with different wax contents. J. Dispers. Sci. Technol. 2018, 39, 71–76. [Google Scholar] [CrossRef]
- Mozaffari, S.; Tchoukov, P.; Mozaffari, A.; Atias, J.; Czarnecki, J.; Nazemifard, N. Capillary driven flow in nanochannels—Application to heavy oil rheology studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 178–187. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Jeon, B.-H.; Salama, E.-S.; Eraky, M.; Kim, W.B.; Wang, J.; Ahn, T. Occurrence and Characterization of Paraffin Wax Formed in Developing Wells and Pipelines. Energies 2019, 12, 967. [Google Scholar] [CrossRef]
- Ganeeva, Y.M.; Yusupova, T.N.; Romanov, G.V. Waxes in asphaltenes of crude oils and wax deposits. Pet. Sci. 2016, 13, 737–745. [Google Scholar] [CrossRef]
- Lim, B.; Said Ak Salim, H.; Ridzuan, N. A review of the mechanism and role of wax inhibitors in the wax deposition and pre-cipitation. Pertanika J. Sci. Technol. 2019, 27, 499–526. [Google Scholar]
- Yang, F.; Paso, K.; Norrman, J.; Li, C.; Oschmann, H.; Sjöblom, J. Hydrophilic Nanoparticles Facilitate Wax Inhibition. Energy Fuels 2015, 29, 1368–1374. [Google Scholar] [CrossRef]
- Ragunathan, T.; Husin, H.; Wood, C.D. Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition. Appl. Sci. 2020, 10, 479. [Google Scholar] [CrossRef]
- Ragunathan, T.; Husin, H.; Wood, C.D. Effects of Crude Palm Oil and Crude Palm Kernel Oil Upon Wax Inhibition. ACS Omega 2020, 5, 19342–19349. [Google Scholar] [CrossRef]
- Vijayakumar, S.D.; Zakaria, J.; Ridzuan, N. Molecular dynamics approach on intermolecular interaction between n-icosane and gemini surfactant assisted nanoparticles. Pet. Res. 2022, 7, 366–371. [Google Scholar] [CrossRef]
- Moschopedis, S.E.; Speight, J.G. Investigation of hydrogen bonding by oxygen functions in Athabasca bitumen. Fuel 1976, 55, 187–192. [Google Scholar] [CrossRef]
- da Costa, L.M.; Stoyanov, S.R.; Gusarov, S.; Tan, X.; Gray, M.R.; Stryker, J.M.; Tykwinski, R.; de M. Carneiro, J.W.; Seidl, P.R.; Kovalenko, A. Density Functional Theory Investigation of the Contributions of π–π Stacking and Hydrogen-Bonding Interactions to the Aggregation of Model Asphaltene Compounds. Energy Fuels 2012, 26, 2727–2735. [Google Scholar] [CrossRef]
- Xin, S.-M.; Liu, Q.-K.; Wang, K.; Chen, Y.; Yuan, P.-Q.; Cheng, Z.-M.; Yuan, W.-K. Solvation of asphaltenes in supercritical water: A molecular dynamics study. Chem. Eng. Sci. 2016, 146, 115–125. [Google Scholar] [CrossRef]
- Joonaki, E.; Hassanpouryouzband, A.; Burgass, R.; Hase, A.; Tohidi, B. Effects of Waxes and the Related Chemicals on Asphaltene Aggregation and Deposition Phenomena: Experimental and Modeling Studies. ACS Omega 2020, 5, 7124–7134. [Google Scholar] [CrossRef]
- Gharbi, K.; Benamara, C.; Benyounes, K.; Kelland, M.A. Toward Separation and Characterization of Asphaltene Acid and Base Fractions. Energy Fuels 2021, 35, 14610–14617. [Google Scholar] [CrossRef]
- Ansari, F.; Shinde, S.B.; Paso, K.G.; Sjöblom, J.; Kumar, L. Chemical Additives as Flow Improvers for Waxy Crude Oil and Model Oil: A Critical Review Analyzing Structure–Efficacy Relationships. Energy Fuels 2022, 36, 3372–3393. [Google Scholar] [CrossRef]
- Murgich, J. Intermolecular forces in aggregates of asphaltenes and resins. Pet. Sci. Technol. 2002, 20, 983–997. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Daraboina, N.; Sarica, C. Wax deposition mechanisms: Is the current description sufficient? Fuel 2020, 275, 117937. [Google Scholar] [CrossRef]
- Alnaimat, F.; Ziauddin, M. Wax deposition and prediction in petroleum pipelines. J. Pet. Sci. Eng. 2020, 184, 106385. [Google Scholar] [CrossRef]
- Rosvold, K. Wax Deposition Models; Norwegian University of Science and Technology: Stavenger, Norway, 2008. [Google Scholar]
- Bern, P.A.; Withers, V.R.; Cairns, R.J.R. Wax Deposition in Crude Oil Pipelines. In Proceedings of the European Offshore Technology Conference and Exhibition, London, UK, 21–24 October 1980. [Google Scholar]
- Xiao, R.G.; Wei, B.Q.; Yao, P.F.; Yi, D.R. Study on the Pipeline Wax Deposition Mechanism and Influencing Factors. Adv. Mater. Res. 2012, 516–517, 1018–1021. [Google Scholar] [CrossRef]
- Theyab, M.A. Wax deposition process: Mechanisms, affecting factors and mitigation methods. Opan Access J. Sci. 2018, 2, 112–118. [Google Scholar] [CrossRef]
- Azevedo, L.F.A.; Teixeira, A.M. A Critical Review of the Modeling of Wax Deposition Mechanisms. Pet. Sci. Technol. 2003, 21, 393–408. [Google Scholar] [CrossRef]
- Sousa, A.L.; Matos, H.A.; Guerreiro, L.P. Preventing and removing wax deposition inside vertical wells: A review. J. Pet. Explor. Prod. Technol. 2019, 9, 2091–2107. [Google Scholar] [CrossRef]
- Sousa, A.M.; Matos, H.A.; Guerreiro, L. Wax deposition mechanisms and the effect of emulsions and carbon dioxide injection on wax deposition: Critical review. Petroleum 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Hosseinipour, A.; Japper-Jaafar, A.B.; Yusup, S. The Effect of CO2 on Wax Appearance Temperature of Crude Oils. Procedia Eng. 2016, 148, 1022–1029. [Google Scholar] [CrossRef]
- Han, S.; Huang, Z.; Senra, M.; Hoffmann, R.; Fogler, H.S. Method to Determine the Wax Solubility Curve in Crude Oil from Centrifugation and High Temperature Gas Chromatography Measurements. Energy Fuels 2010, 24, 1753–1761. [Google Scholar] [CrossRef]
- Liu, H.; Duan, J.; Li, J.; Yan, H.; Wang, J.; Lin, K.; Guan, L.; Li, C. Experimental Measurements of Wax Precipitation Using a Modified Method of Simultaneous Centrifugation and High-Temperature Gas Chromatography. Energies 2021, 14, 7035. [Google Scholar] [CrossRef]
- Agarwal, J.R.; Dhingra, S.; Shah, N.; Shah, S.N. Validation of Wax Deposition Models Using Field Data of Western Onshore, India. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Bali, Indonesia, 29–31 October 2019. [Google Scholar]
- Ye, Q.; Xian, L.; Zhang, F.; Yu, H.; Li, X. Research on influence of wax deposition on flow state in coiled tubing with cable inside. AIP Conf. Proc. 2018, 1955, 030045. [Google Scholar] [CrossRef]
- Becker, J.R. Oilfield Paraffin Treatments: Hot Oil and Hot Water Compared to Crystal Modifiers. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 1–4 October 2000. [Google Scholar]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Experimental investigation of comparing electromagnetic and conventional heating effects on the unconventional oil (heavy oil) properties: Based on heating time and upgrading. Fuel 2018, 228, 243–253. [Google Scholar] [CrossRef]
- Ramcharan, T.; Hosein, R. Radio Frequency Heating combined with Solvent Extraction- A method for oil recovery from surface oil sands. J. Pet. Sci. Eng. 2019, 179, 328–336. [Google Scholar] [CrossRef]
- Hanyong, L.; Kexin, C.; Ling, J.; Leilei, W.; Bo, Y. Experimental study on the viscosity reduction of heavy oil with nano-catalyst by microwave heating under low reaction temperature. J. Pet. Sci. Eng. 2018, 170, 374–382. [Google Scholar] [CrossRef]
- Al-Yaari, M. Paraffin Wax Deposition: Mitigation and Removal Techniques. In Proceedings of the SPE Saudi Arabia section Young Professionals Technical Symposium, Dhahran, Saudi Arabia, 14–16 March 2011; p. 10. [Google Scholar]
- Aslanov, H.; Novruzov, A.; Harun, A. Managing Wax-Deposition Risks in Oil Subsea Pipelines by Integrating Wax Modeling and Pigging Performance. SPE Prod. Oper. 2019, 34, 625–634. [Google Scholar] [CrossRef]
- White, M.; Pierce, K.; Acharya, T. A Review of Wax-Formation/Mitigation Technologies in the Petroleum Industry. SPE Prod. Oper. 2018, 33, 476–485. [Google Scholar] [CrossRef]
- Banerjee, S.; Kumar, R.; Mandal, A.; Naiya, T.K. Effect of Natural and Synthetic Surfactant on the Rheology of Light Crude Oil. Pet. Sci. Technol. 2015, 33, 1516–1525. [Google Scholar] [CrossRef]
- Sun, M.; Naderi, K.; Firoozabadi, A. Effect of Crystal Modifiers and Dispersants on Paraffin-Wax Particles in Petroleum Fluids. SPE J. 2018, 24, 32–43. [Google Scholar] [CrossRef]
- Adebiyi, F.M. Paraffin wax precipitation/deposition and mitigating measures in oil and gas industry: A review. Pet. Sci. Technol. 2020, 38, 962–971. [Google Scholar] [CrossRef]
- Adlan, N.A.; Sabri, S.; Masomian, M.; Ali, M.S.M.; Rahman, R.N.Z.R.A. Microbial Biodegradation of Paraffin Wax in Malaysian Crude Oil Mediated by Degradative Enzymes. Front. Microbiol. 2020, 11, 565608. [Google Scholar] [CrossRef] [PubMed]
- Sen, R. Biotechnology in petroleum recovery: The microbial EOR. Prog. Energy Combust. Sci. 2008, 34, 714–724. [Google Scholar] [CrossRef]
- Luo, C.; Wang, W.; Zhang, H.; Yu, X.; Wang, G. Microbial treatment of waxy crude oil for mitigating wax precipitation and improving liquidity. Pet. Sci. Technol. 2019, 37, 471–478. [Google Scholar] [CrossRef]
- Liu, J.H.; Jia, Y.P.; Chen, Y.T.; Xu, R.D. Microbial treatment for prevention and removal of paraffin deposition on the walls of crude pipelines. Indian J. Microbiol. 2013, 53, 482–484. [Google Scholar] [CrossRef]
- Etoumi, A. Microbial treatment of waxy crude oils for mitigation of wax precipitation. J. Pet. Sci. Eng. 2007, 55, 111–121. [Google Scholar] [CrossRef]
- Chi, Y.; Daraboina, N.; Sarica, C. Effect of the Flow Field on the Wax Deposition and Performance of Wax Inhibitors: Cold Finger and Flow Loop Testing. Energy Fuels 2017, 31, 4915–4924. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sircar, A.; Deka, B.; Silviya Anumegalai, A.; Suresh Moorthi, P.; Yasvanthrajan, N. Flow improvers for assured flow of crude oil in midstream pipeline—A review. J. Pet. Sci. Eng. 2018, 164, 24–30. [Google Scholar] [CrossRef]
- Soni, H.; Bharambe, D. Performance-Based Designing of Wax Crystal Growth Inhibitors. Energy Fuels 2008, 22, 3930–3938. [Google Scholar] [CrossRef]
- Li, R.; Wang, C.; Wang, P.; Pei, J. Preparation of a novel flow improver and its viscosity-reducing effect on bitumen. Fuel 2016, 181, 935–941. [Google Scholar] [CrossRef]
- Induchoodan, G.; Jansson, H.; Mohammadi, A.S.; Swenson, J. The critical role of asphaltene nanoaggregates in stabilizing functionalized graphene in crude oil derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130865. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostina, Y.V.; Antonov, S.V.; Ilyin, S.O. Oxidative Functionalization of Asphaltenes from Heavy Crude Oil. Russ. J. Appl. Chem. 2018, 91, 1835–1840. [Google Scholar] [CrossRef]
- Petrova, L.M.; Abbakumova, N.A.; Zaidullin, I.M.; Borisov, D.N. Polar-solvent fractionation of asphaltenes from heavy oil and their characterization. Pet. Chem. 2013, 53, 81–86. [Google Scholar] [CrossRef]
- Ilyin, S.; Arinina, M.; Polyakova, M.; Bondarenko, G.; Konstantinov, I.; Kulichikhin, V.; Malkin, A. Asphaltenes in heavy crude oil: Designation, precipitation, solutions, and effects on viscosity. J. Pet. Sci. Eng. 2016, 147, 211–217. [Google Scholar] [CrossRef]
- Mostowfi, F.; Indo, K.; Mullins, O.C.; McFarlane, R. Asphaltene Nanoaggregates Studied by Centrifugation. Energy Fuels 2009, 23, 1194–1200. [Google Scholar] [CrossRef]
- Quan, H.; Xing, L. The effect of hydrogen bonds between flow improvers with asphaltene for heavy crude oil. Fuel 2019, 237, 276–282. [Google Scholar] [CrossRef]
- Ragunathan, T.; Zaqwan, J.; Wood, C.D.; Husin, H. The rheological behavior of crude oil in the presence of palm oil additives. J. Pet. Explor. Prod. Technol. 2021, 11, 2833–2843. [Google Scholar] [CrossRef]
- Pandian, S.; Dahyalal, P.C.; Krishna, S.; Hari, S.; Subramanian, D. A study on cashew nut shell liquid as a bio-based flow improver for heavy crude oil. J. Pet. Explor. Prod. Technol. 2021, 11, 2287–2297. [Google Scholar] [CrossRef]
- Oladepo, A.; Ogunkunle, T.; Fadairo, A.; Adesina, A. Evaluating the Potential of Bio-Derived Flow Improver and Its Effect on Nigeria Waxy Crude. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2019. [Google Scholar]
- Alade, O.S.; Hassan, A.; Mahmoud, M.; Al-Shehri, D.; Al-Majed, A. Novel Approach for Improving the Flow of Waxy Crude Oil Using Thermochemical Fluids: Experimental and Simulation Study. ACS Omega 2020, 5, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Olusegun, S.; Omoladun, R.; Alade, O.; Taiwo, E. Experimental and Simulation Studies on the Use of Plant Oils as Flow Improver for Waxy Crude Oil. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Virtual, 11–13 August 2020. [Google Scholar]
- Soni, H.; Kiranbala; Agrawal, K.S.; Nagar, A.; Bharambe, D.P. Designing maleic anhydride-alpha-olifin copolymeric combs as wax crystal growth nucleators. Fuel Process. Technol. 2010, 91, 997–1004. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Hamouly, S.H.; Khidr, T.T.; El-Ghazawy, R.A.; Higazy, S.A. Preparation the Esters of Oleic Acid-Maleic Anhydride Copolymer and Their Evaluation as Flow Improvers for Waxy Crude Oil. J. Dispers. Sci. Technol. 2013, 34, 1585–1596. [Google Scholar] [CrossRef]
- Patel, M.R.; Chitte, P.S.; Bharambe, D.P. Oleic acid based polymeric flow improvers for Langhnaj (North Gujarat, India) crude oil. Egypt. J. Pet. 2017, 26, 895–903. [Google Scholar] [CrossRef]
- Deka, B.; Sharma, R.; Mandal, A.; Mahto, V. Synthesis and evaluation of oleic acid based polymeric additive as pour point depressant to improve flow properties of Indian waxy crude oil. J. Pet. Sci. Eng. 2018, 170, 105–111. [Google Scholar] [CrossRef]
- Elkatory, M.; Soliman, E.; Hassaan, M.; Ali, R.; Hafez, E.; Ibrahim, H.S.; Hashem, A. Chemical mitigation technology for wax deposition in submarine oil pipeline systems. Egypt. J. Chem. 2021, 64, 5989–5997. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Betiha, M.A.; Osman, D.I.; Mahmoud, T. Synthesis and characterization of nanohybrid of poly(octadecylacrylates derivatives)/montmorillonite as pour point depressants and flow improver for waxy crude oil. J. Appl. Polym. Sci. 2019, 136, 47333. [Google Scholar] [CrossRef]
- Elkatory, M.R.; Hassaan, M.A.; Soliman, E.A.; Niculescu, V.-C.; Raboaca, M.S.; El Nemr, A. Influence of Poly (benzyl oleate-co-maleic anhydride) Pour Point Depressant with Di-Stearyl Amine on Waxy Crude Oil. Polymers 2023, 15, 306. [Google Scholar] [CrossRef]
- Pal, B.; Naiya, T.K. Application of Synthesized Novel Biodegradable Pour-Point Depressant from Natural Source on Flow Assurance of Indian Waxy Crude Oil and Comparative Studies with Commercial Pour-Point Depressant. SPE J. 2022, 27, 864–876. [Google Scholar] [CrossRef]
- Alpandi, A.H.; Husin, H.; Jeffri, S.I.; Sidek, A.; Mingyuan, L. Investigation on Wax Deposition Reduction Using Natural Plant-Based Additives for Sustainable Energy Production from Penara Oilfield Malaysia Basin. ACS Omega 2022, 7, 30730–30745. [Google Scholar] [CrossRef]
- Kumar, R.; Bora, G.S.; Banerjee, S.; Mandal, A.; Naiya, T.K. Application of naturally extracted surfactant from Madhuca longifolia to improve the flow properties of heavy crude oil through horizontal pipeline. J. Pet. Sci. Eng. 2018, 168, 178–189. [Google Scholar] [CrossRef]
- Negi, H.; Faujdar, E.; Saleheen, R.; Singh, R.K. Viscosity Modification of Heavy Crude Oil by Using a Chitosan-Based Cationic Surfactant. Energy Fuels 2020, 34, 4474–4483. [Google Scholar] [CrossRef]
- Jie, Z.; Guo, Z.; Du, W.; Gu, X.; Wang, M.; Zhang, Z.; Ma, Y.; Chen, G. Preparation and Performance of Vegetable Oils Fatty Acids Hydroxylmethyl Triamides as Crude Oil Flow Improvers. Pet. Chem. 2018, 58, 1070–1075. [Google Scholar] [CrossRef]
- Hafiz, A.A.; Khidr, T.T. Hexa-triethanolamine oleate esters as pour point depressant for waxy crude oils. J. Pet. Sci. Eng. 2007, 56, 296–302. [Google Scholar] [CrossRef]
- Soni, H.P.; Bharambe, D.P. Synthesis and evaluation of polymeric additives as flow improvers for indian crude oil. Iran. Polym. J. 2006, 15, 943–954. [Google Scholar]
- Akinyemi, O.P.; Udonne, J.D.; Efeovbokhan, V.E.; Ayoola, A.A. A study on the use of plant seed oils, triethanolamine and xylene as flow improvers of Nigerian waxy crude oil. J. Appl. Res. Technol. 2016, 14, 195–205. [Google Scholar] [CrossRef]
- Taiwo, E.; Otolorin, J.; Afolabi, T. Crude Oil Transportation: Nigerian Niger Delta Waxy Crude. In Crude Oil Exploration in the World; IntechOpen: London, UK, 2012. [Google Scholar]
- Gateau, P.; Hénaut, I.; Barré, L.; Argillier, J.F. Heavy Oil Dilution. Oil Gas Sci. Technol.-Rev. D’ifp Energ. Nouv. 2004, 59, 503–509. [Google Scholar] [CrossRef]
- Eke, W.; Ozioma, A.; Ofordile, S.; Ajienka, J.; Akaranta, O. Performance Evaluation of Cashew Nut Shell Liquid CNSL as Flow Improver for Waxy Crude Oils. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2019. [Google Scholar]
- Eke, W.I.; Achugasim, O.; Ajienka, J.; Akaranta, O. Glycerol-modified cashew nut shell liquid as eco-friendly flow improvers for waxy crude oil. Pet. Sci. Technol. 2021, 39, 101–114. [Google Scholar] [CrossRef]
- Yao, B.; Chen, W.; Li, C.; Yang, F.; Sun, G.; Wang, G.; Xu, H. Polar asphaltenes facilitate the flow improving performance of polyethylene-vinyl acetate. Fuel Process. Technol. 2020, 207, 106481. [Google Scholar] [CrossRef]
- Huyen, Q.; Nguyen, V. Synthesis of crude oil pour-point depressants via polycondensation of cashew nut shell liquids. PetroVietnam J. 2014, 6, 48–52. [Google Scholar]
- Chen, G.; Bai, Y.; Zhang, J.; Yuan, W.; Song, H.; Jeje, A. Synthesis of new flow improvers from canola oil and application to waxy crude oil. Pet. Sci. Technol. 2016, 34, 1285–1290. [Google Scholar] [CrossRef]
- Eke, W.I.; Kyei, S.K.; Achugasim, O.; Ajienka, J.A.; Akaranta, O. Pour point depression and flow improvement of waxy crude oil using polyethylene glycol esters of cashew nut shell liquid. Appl. Petrochem. Res. 2021, 11, 199–208. [Google Scholar] [CrossRef]
- Kumar, R.; Banerjee, S.; Mandal, A.; Kumar Naiya, T. Flow improvement of heavy crude oil through pipelines using surfactant extracted from soapnuts. J. Pet. Sci. Eng. 2017, 152, 353–360. [Google Scholar] [CrossRef]
- Ogunkunle, T.; Lana, O.; Oladepo, A.; Babajide, L.; Fadairo, A. The use of bio-diesel based additive as rheology improver and pour point depressant of Nigerian waxy crude. Pet. Sci. Technol. 2019, 37, 1747–1754. [Google Scholar] [CrossRef]
- Fred, O.T.; Damilola, A.V.; Ashonibare, A.A.; Adenike, R.; Sylvia, T.-O.E. Study of linseed oil, its biodiesel and xylene as flow improver for Nigerian waxy crude oils. Pet. Res. 2022, 7, 138–143. [Google Scholar] [CrossRef]
- Ragunathan, T.; Husin, H.; Wood, C.D. Inhibiting Wax Deposition using Palm Oil Additives. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 2–6 November 2020. [Google Scholar]
- Sivaprakasam, S.; Saravanan, C.G. Optimization of the Transesterification Process for Biodiesel Production and Use of Biodiesel in a Compression Ignition Engine. Energy Fuels 2007, 21, 2998–3003. [Google Scholar] [CrossRef]
- Widayat, W.; Wibowo, A.; Hadiyanto, H. Study on Production Process of Biodiesel from Rubber Seed (Hevea Brasiliensis) by in Situ (Trans)Esterification Method with Acid Catalyst. Energy Procedia 2013, 32, 64–73. [Google Scholar] [CrossRef]
- Huang, H.; Wang, W.; Peng, Z.; Ding, Y.; Li, K.; Li, Q.; Gong, J. The influence of nanocomposite pour point depressant on the crystallization of waxy oil. Fuel 2018, 221, 257–268. [Google Scholar] [CrossRef]
- Wen, H.; Zhang, S.; Lian, Y.; Zhao, Z.; Wang, W.; Wei, Y.; Duan, Y.; Dong, S. Effect of nano pour point depressant on the flow properties of the waxy crude oil from Changqing Oilfield. E3S Web Conf. 2021, 329, 01050. [Google Scholar] [CrossRef]
- Sharma, R.; Mahto, V.; Vuthaluru, H. Synthesis of PMMA/modified graphene oxide nanocomposite pour point depressant and its effect on the flow properties of Indian waxy crude oil. Fuel 2019, 235, 1245–1259. [Google Scholar] [CrossRef]
- Al-saba, M.T.; Al Fadhli, A.; Marafi, A.; Hussain, A.; Bander, F.; Al Dushaishi, M.F. Application of Nanoparticles in Improving Rheological Properties of Water Based Drilling Fluids. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar]
- Mahmoud, O.; Nasr-El-Din, H.A.; Vryzas, Z.; Kelessidis, V.C. Effect of Ferric Oxide Nanoparticles on the Properties of Filter Cake Formed by Calcium Bentonite-Based Drilling Muds. SPE Drill. Complet. 2018, 33, 363–376. [Google Scholar] [CrossRef]
- Minakov, A.V.; Zhigarev, V.A.; Mikhienkova, E.I.; Neverov, A.L.; Buryukin, F.A.; Guzei, D.V. The effect of nanoparticles additives in the drilling fluid on pressure loss and cutting transport efficiency in the vertical boreholes. J. Pet. Sci. Eng. 2018, 171, 1149–1158. [Google Scholar] [CrossRef]
- Thakkar, A.; Raval, A.; Chandra, S.; Shah, M.; Sircar, A. A comprehensive review of the application of nano-silica in oil well cementing. Petroleum 2020, 6, 123–129. [Google Scholar] [CrossRef]
- Bashir Abdullahi, M.; Rajaei, K.; Junin, R.; Bayat, A.E. Appraising the impact of metal-oxide nanoparticles on rheological properties of HPAM in different electrolyte solutions for enhanced oil recovery. J. Pet. Sci. Eng. 2019, 172, 1057–1068. [Google Scholar] [CrossRef]
- Pandey, A.; Telmadarreie, A.; Trifkovic, M.; Bryant, S. Cellulose Nanocrystal Stabilized Emulsions for Conformance Control and Fluid Diversion in Porous Media. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018. [Google Scholar]
- Ajulibe, D.; Ogolo, N.; Ikiensikimama, S. Viability of SiO2 Nanoparticles for Enhanced Oil Recovery in the Niger Delta: A Comparative Analysis. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 6–8 August 2018. [Google Scholar]
- Farid Ibrahim, A.; Nasr-El-Din, H. An Experimental Study for the Using of Nanoparticle/VES Stabilized CO2 Foam to Improve the Sweep Efficiency in EOR Applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018. [Google Scholar]
- Sircar, A.; Rayavarapu, K.; Bist, N.; Yadav, K.; Singh, S. Applications of nanoparticles in enhanced oil recovery. Pet. Res. 2022, 7, 77–90. [Google Scholar] [CrossRef]
- VijayaKumar, S.D.; Zakaria, J.; Ridzuan, N. The role of Gemini surfactant and SiO2/SnO/Ni2O3 nanoparticles as flow improver of Malaysian crude oil. J. King Saud Univ.-Eng. Sci. 2021, 34, 384–390. [Google Scholar] [CrossRef]
- Li, N.; Mao, G.; Wu, W.; Liu, Y. Effect evaluation of ethylene vinyl acetate/nano-montmorillonite pour-point depressant on improving the flow properties of model oil. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 296–303. [Google Scholar] [CrossRef]
- Mao, J.; Kang, Z.; Yang, X.; Lin, C.; Zheng, L.; Zuo, M.; Mao, J.; Dai, S.; Xue, J.; Ouyang, D. Synthesis and Performance Evaluation of a Nanocomposite Pour-Point Depressant and Viscosity Reducer for High-Pour-Point Heavy Oil. Energy Fuels 2020, 34, 7965–7973. [Google Scholar] [CrossRef]
- Vakili, S.; Mohammadi, S.; Mirzaei Derazi, A.; Mahmoudi Alemi, F.; Hayatizadeh, N.; Ghanbarpour, O.; Rashidi, F. Effect of metal oxide nanoparticles on wax formation, morphology, and rheological behavior in crude oil: An experimental study. J. Mol. Liq. 2021, 343, 117566. [Google Scholar] [CrossRef]
- Taborda, E.A.; Franco, C.A.; Ruiz, M.A.; Alvarado, V.; Cortés, F.B. Experimental and Theoretical Study of Viscosity Reduction in Heavy Crude Oils by Addition of Nanoparticles. Energy Fuels 2017, 31, 1329–1338. [Google Scholar] [CrossRef]
- Jia, X.; Fu, M.; Xing, X.; Wei, L.; Song, Y.; Zhang, L.; Geng, X.; Guo, H. Submicron carbon-based hybrid nano-pour-point depressant with outstanding pour point depressant and excellent viscosity depressant. Arab. J. Chem. 2022, 15, 104157. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Yang, F.; Sjöblom, J.; Zhang, Y.; Norrman, J.; Paso, K.; Xiao, Z. Organically modified nano-clay facilitates pour point depressing activity of polyoctadecylacrylate. Fuel 2016, 166, 96–105. [Google Scholar] [CrossRef]
- Sharma, R.; Deka, B.; Mahto, V.; Vuthaluru, H.; Li, C.-Z. Investigation into the Flow Assurance of Waxy Crude Oil by Application of Graphene-Based Novel Nanocomposite Pour Point Depressants. Energy Fuels 2019, 33, 12330–12345. [Google Scholar] [CrossRef]
- Huang, H.-R.; Wang, W.; Peng, Z.-H.; Li, K.; Ding, Y.-F.; Yu, W.-J.; Gan, D.-Y.; Wang, C.-S.; Xue, Y.-H.; Gong, J. Synergistic effect of magnetic field and nanocomposite pour point depressant on the yield stress of waxy model oil. Pet. Sci. 2020, 17, 838–848. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Yang, F.; Zhang, Y.; Xiao, Z.; Sun, G. Structural properties of gelled Changqing waxy crude oil benefitted with nanocomposite pour point depressant. Fuel 2016, 184, 544–554. [Google Scholar] [CrossRef]
- Norrman, J.; Solberg, A.; Sjöblom, J.; Paso, K. Nanoparticles for Waxy Crudes: Effect of Polymer Coverage and the Effect on Wax Crystallization. Energy Fuels 2016, 30, 5108–5114. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Jing, G.; Liu, S.; Yang, Y.; Xu, J. Synthesis of chemical grafting pour point depressant EVAL-GO and its effect on the rheological properties of Daqing crude oil. Fuel Process. Technol. 2021, 223, 107000. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Betiha, M.A.; Osman, D.I.; Hashim, A.I.; El-Sukkary, M.M.; Mahmoud, T. Preparation and Evaluation of Poly(methyl methacrylate)-Graphene Oxide Nanohybrid Polymers as Pour Point Depressants and Flow Improvers for Waxy Crude Oil. Energy Fuels 2016, 30, 7610–7621. [Google Scholar] [CrossRef]
- Mansourpoor, M.; Azin, R.; Osfouri, S.; Izadpanah, A.A. Effect of DSO, EVA, and SiO2 and clay nanohybrids on rheological properties of waxy oil mixtures. Mater. Res. Express 2018, 5, 095027. [Google Scholar] [CrossRef]
- Yu, H.; Sun, Z.; Jing, G.; Zhen, Z.; Liu, Y.; Guo, K. Effect of a Magnetic Nanocomposite Pour Point Depressant on the Structural Properties of Daqing Waxy Crude Oil. Energy Fuels 2019, 33, 6069–6075. [Google Scholar] [CrossRef]
- Betiha, M.A.; Mahmoud, T.; Al-Sabagh, A.M. Effects of 4-vinylbenzyl trioctylphosphonium- bentonite containing poly(octadecylacrylate-co-1-vinyldodecanoate) pour point depressants on the cold flow characteristics of waxy crude oil. Fuel 2020, 282, 118817. [Google Scholar] [CrossRef]
- Odutola, T.O.; Idemili, C.A. Effect of poly (ethylene-butene) and nano-aluminium oxide blend on the viscosity of Nigerian crude oil. J. Pet. Explor. Prod. Technol. 2020, 10, 2531–2539. [Google Scholar] [CrossRef]
- Subramanie, P.A.P.; Padhi, A.; Ridzuan, N.; Adam, F. Experimental study on the effect of wax inhibitor and nanoparticles on rheology of Malaysian crude oil. J. King Saud Univ.-Eng. Sci. 2020, 32, 479–483. [Google Scholar] [CrossRef]
- Balogun, A.; Odutola, T.; Balogun, Y. Preventing Wax Deposition in Crude Oil Using Polyethylene Butene and Nano Zinc Oxide. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 6–7 December 2021. [Google Scholar]
- Mahmoud, T.; Betiha, M.A. Poly(octadecyl acrylate-co-vinyl neodecanoate)/Oleic Acid-Modified Nano-graphene Oxide as a Pour Point Depressant and an Enhancer of Waxy Oil Transportation. Energy Fuels 2021, 35, 6101–6112. [Google Scholar] [CrossRef]
- Ning, X.; Song, X.; Zhang, S.; Wang, Y.; Feng, Y. Insights into Flow Improving for Waxy Crude Oil Doped with EVA/SiO2 Nanohybrids. ACS Omega 2022, 7, 5853–5863. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Deka, B.; Mahto, V.; Barifcani, A.; Vuthaluru, H. Experimental investigation into the development and evaluation of ionic liquid and its graphene oxide nanocomposite as novel pour point depressants for waxy crude oil. J. Pet. Sci. Eng. 2022, 208, 109691. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Sharma, R.; Deka, B.; Mandal, A.; Mahto, V. Study the influence of sodium dodecyl sulfate on emulsification of heavy and waxy crude oils to improve their flow ability in low temperature conditions. Asia-Pac. J. Chem. Eng. 2019, 14, e2279. [Google Scholar] [CrossRef]
- Sharma, R.; Mahto, V.; Vuthaluru, H.; Li, C.-Z. Effect of Thermal/Shear Conditioning and Aging on the Effectiveness of Synthesized Nanocomposite PPD on Waxy Crude Oil. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 2–6 November 2020. [Google Scholar]
- Peng, Z.; Wang, W.; Huang, H.; Yang, S.; Gong, J. Influence of Specific Surface Area and Morphology of Nanocomposite Pour Point Depressant on the Modification of Waxy Oil. In Proceedings of the The 29th International Ocean and Polar Engineering Conference, Honolulu, HI, USA, 16–21 June 2019. [Google Scholar]
- Qing, Y.; Yang, M.; Li, L.; Jiang, W.; Zhao, Y. Effect of Organically Modified Nanosilica on the Viscosity and Rheological Behavior of Karamay Heavy Crude Oil. Energy Fuels 2020, 34, 65–73. [Google Scholar] [CrossRef]
- Lim, Z.H.; Al Salim, H.S.; Ridzuan, N.; Nguele, R.; Sasaki, K. Effect of surfactants and their blend with silica nanoparticles on wax deposition in a Malaysian crude oil. Pet. Sci. 2018, 15, 577–590. [Google Scholar] [CrossRef]
- Singh, R.; Talukdar, P. The effect of silica based nanocomposite pour point depressant on the waxy crude oil of north-east india. Int. J. Eng. Appl. Sci. Technol. 2021, 5, 147–151. [Google Scholar] [CrossRef]
- Song, X.; Yin, H.; Feng, Y.; Zhang, S.; Wang, Y. Effect of SiO2 Nanoparticles on Wax Crystallization and Flow Behavior of Model Crude Oil. Ind. Eng. Chem. Res. 2016, 55, 6563–6568. [Google Scholar] [CrossRef]
- Kim, H.C.; Fthenakis, V. Life Cycle Energy and Climate Change Implications of Nanotechnologies. J. Ind. Ecol. 2013, 17, 528–541. [Google Scholar] [CrossRef]
- Agista, M.N.; Guo, K.; Yu, Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef]
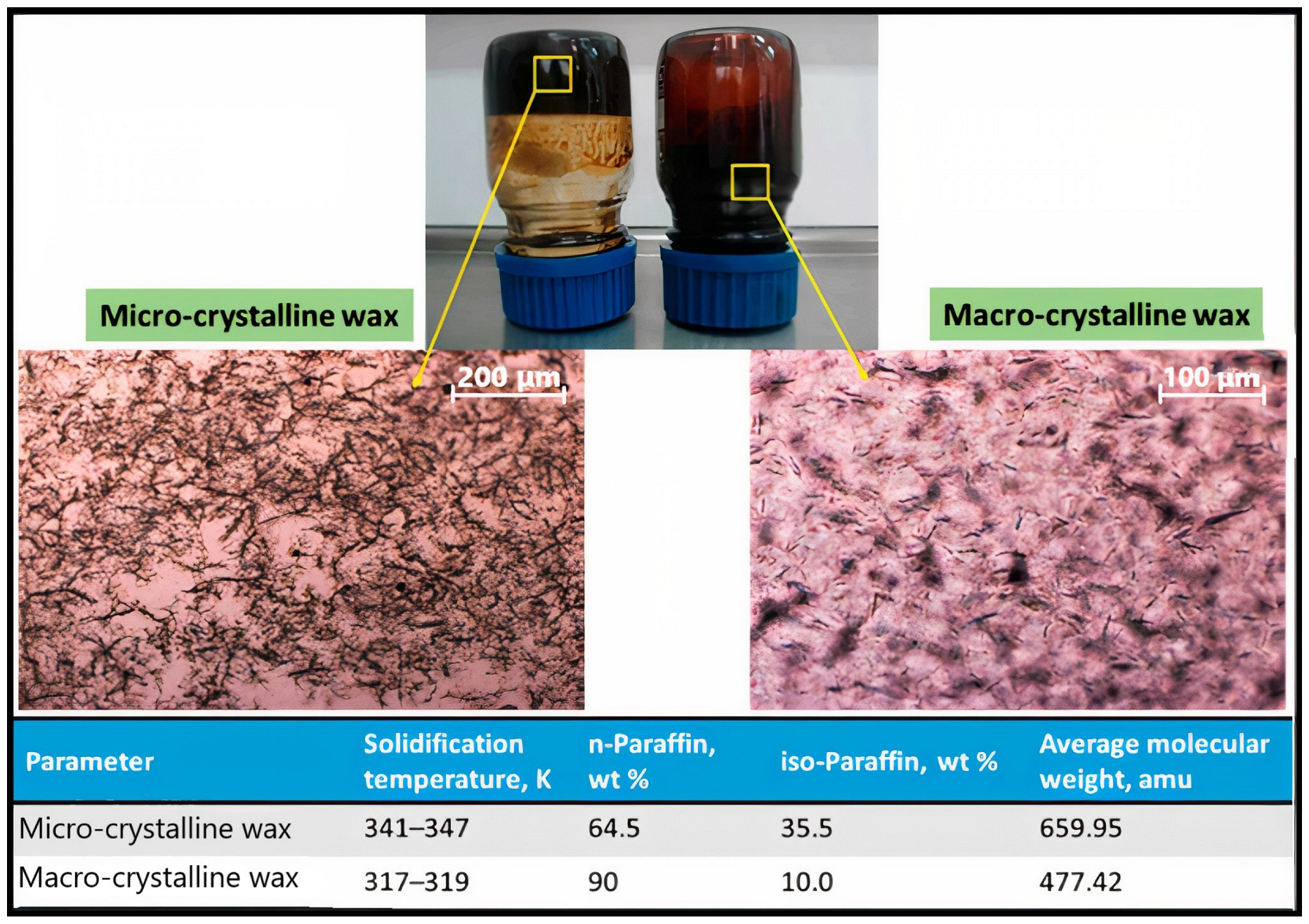






| Synthesized Additive | %PPR | %VR | Findings | Ref |
|---|---|---|---|---|
| poly(n-behenylOleate-co-maleicanhydride) di-methyl ricinolate (22-OMR) | 62.5 | 38 |
| [68] |
| Oleic acid-maleic anhydride copolymer (POMA) | >100 | 73 |
| [69] |
| Hexyl oleate-co-hexadecyl maleimide-co-alkyl oleate (MPO) | 27 | 86.5 |
| [70] |
| Tri-triethanolamine di oleate (TDO) | 54 | 90 |
| [71] |
| Comb-like poly fatty esters (PFES) | 87.5 |
| [72] | |
| 6,6′-(((phenylmethylene,o-olieat)bis(oxy))bis(ethane-2,1-diyl))bis(2-phenyl-o-olieat-1,3,6-dioxazocane)) (SB2) | 55.5 | 56 |
| [73] |
| Poly (benzyl oleate-co-succinic anhydride) Copolymer (PBOCOSA) | 75 | 56.3 |
| [74] |
| Poly (benzyl oleate-co-distearyl amine) (PBOCODSA) | 100 | 62.5 |
| Bio-Derived Additives | Synthetic Additives | %PPR | %VR | PIE | Highlights | Ref |
|---|---|---|---|---|---|---|
| Rubber seed oil (RSO) | 82.5 | 60.5 | 63.2 |
| [82] | |
| Jatropha seed oil (JSO) | 86.3 | 64.6 | 73.5 | |||
| Castor seed oil (CSO) | 83.8 | 64.2 | 77.7 | |||
| Triethanolamine (TEA) | 75 | 45.9 | 66.1 | |||
| Xylene | 62.5 | 56.6 | ||||
| Soapnut | 87.7 |
| [91] | |||
| Brij-30 | 72.4 | |||||
| RSO | 31.8 | 15.6 |
| [92] | ||
| CSO | 33.8 | 38.1 | ||||
| RSO biodiesel | 40 | 51.5 | ||||
| CSO Biodiesel | 53.8 | 59.1 | ||||
| TEA | 22.7 | 8 | ||||
| CSO | 13 |
| [67] | |||
| Moringa seed (MSO) | 13 | |||||
| TEA | 21 | |||||
| Crude palm oil (CPO) | 80.9 |
| [15] | |||
| Crude palm kernel oil (CPKO) | 80.3 | |||||
| Ethylene-co-vinyl acetate (EVA) | 52.4 | |||||
| TEA | 76.6 | |||||
| Linseed Oil (LSO) | 66 |
| [93] | |||
| LSO biodiesel | 67.4 | |||||
| Xylene | 56.5 | |||||
| Coconut oil biodiesel | 26.6 | 62.4 |
| [75] | ||
| PPD-A | 20 | 36.9 | ||||
| JSO | 8.2 | 42.5 |
| [76] | ||
| CPO | 24.1 | 58.8 | ||||
| CPKO | 60.4 | 54.8 | ||||
| EVA | 0 | 75.5 | ||||
| TEA | 79.2 | 89.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabayan, R.C.M.; Sulaimon, A.A.; Jufar, S.R. Application of Bio-Derived Alternatives for the Assured Flow of Waxy Crude Oil: A Review. Energies 2023, 16, 3652. https://doi.org/10.3390/en16093652
Gabayan RCM, Sulaimon AA, Jufar SR. Application of Bio-Derived Alternatives for the Assured Flow of Waxy Crude Oil: A Review. Energies. 2023; 16(9):3652. https://doi.org/10.3390/en16093652
Chicago/Turabian StyleGabayan, Ron Chuck Macola, Aliyu Adebayo Sulaimon, and Shiferaw Regassa Jufar. 2023. "Application of Bio-Derived Alternatives for the Assured Flow of Waxy Crude Oil: A Review" Energies 16, no. 9: 3652. https://doi.org/10.3390/en16093652
APA StyleGabayan, R. C. M., Sulaimon, A. A., & Jufar, S. R. (2023). Application of Bio-Derived Alternatives for the Assured Flow of Waxy Crude Oil: A Review. Energies, 16(9), 3652. https://doi.org/10.3390/en16093652






