Pt-Fe-Co Ternary Metal Single Atom Catalyst for toward High Efficiency Alkaline Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
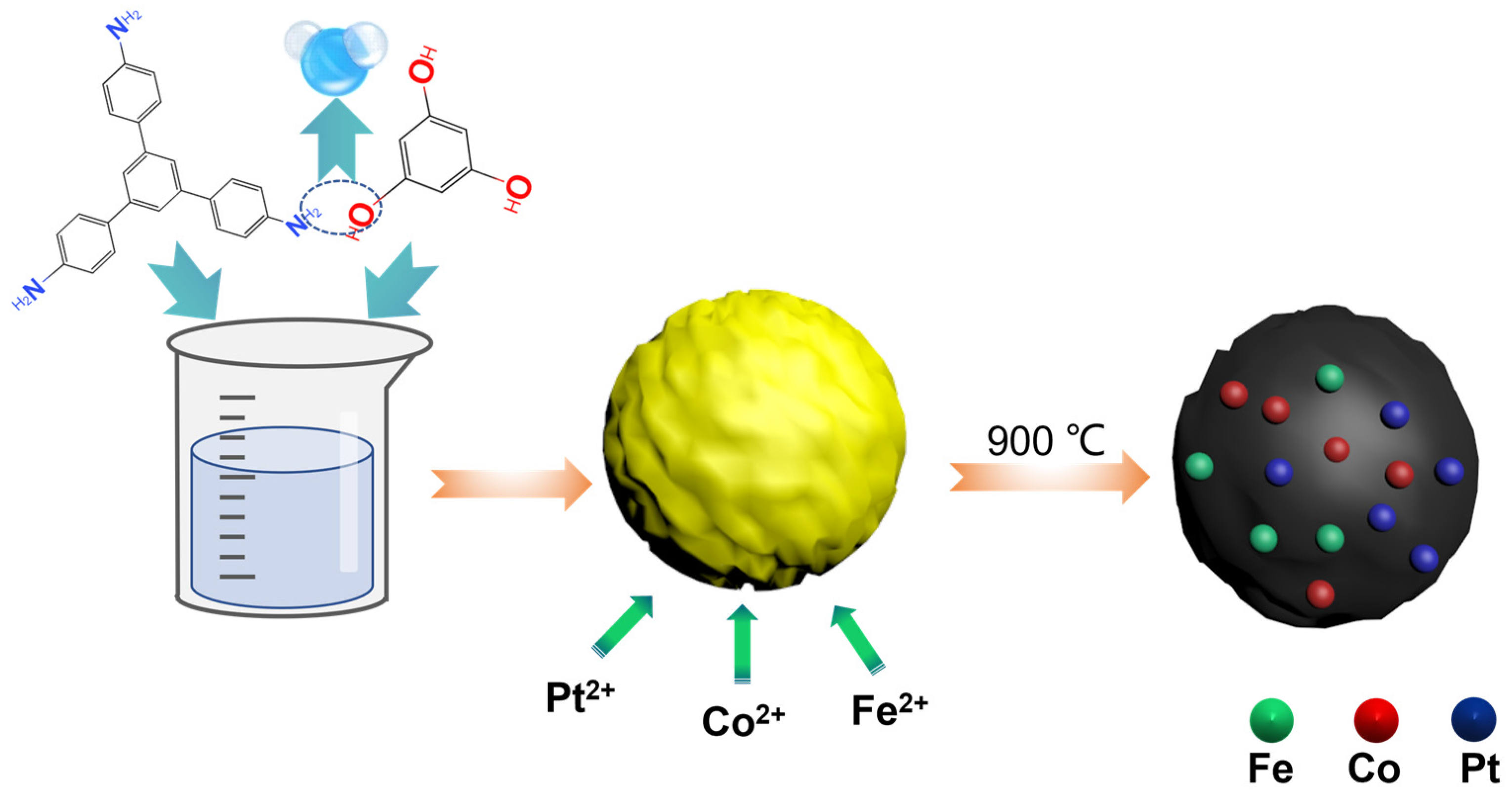
2.2. Preparation of COF [35]
2.3. Preparation of Pt, Fe, Co/N-C
2.4. Preparation of Fe, Co/N-C
2.5. Preparation of Pt/N-C
2.6. Preparation of N-C
2.7. Materials Characterizations
2.8. Electrocatalytic Measurements
3. Results and Discussion
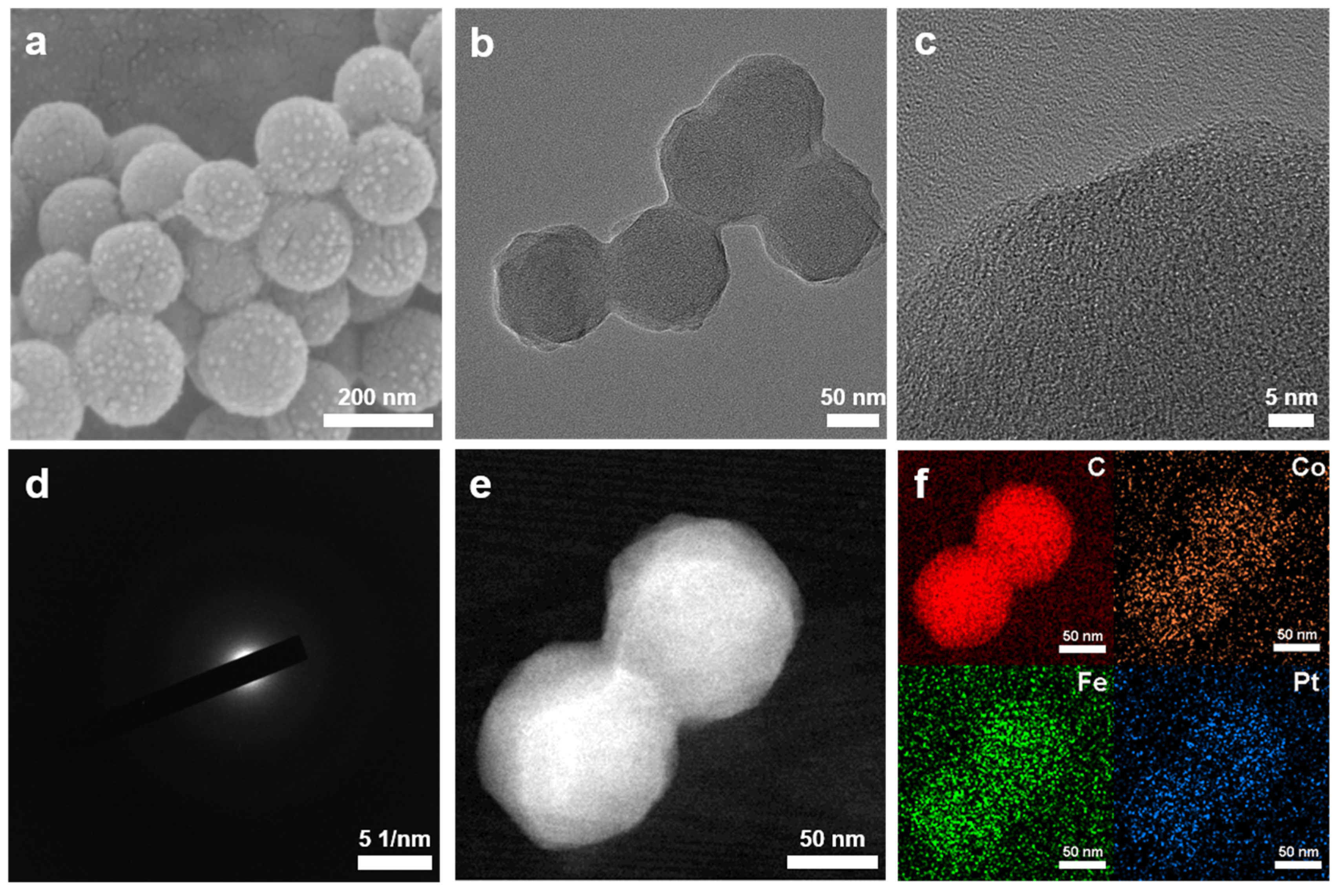
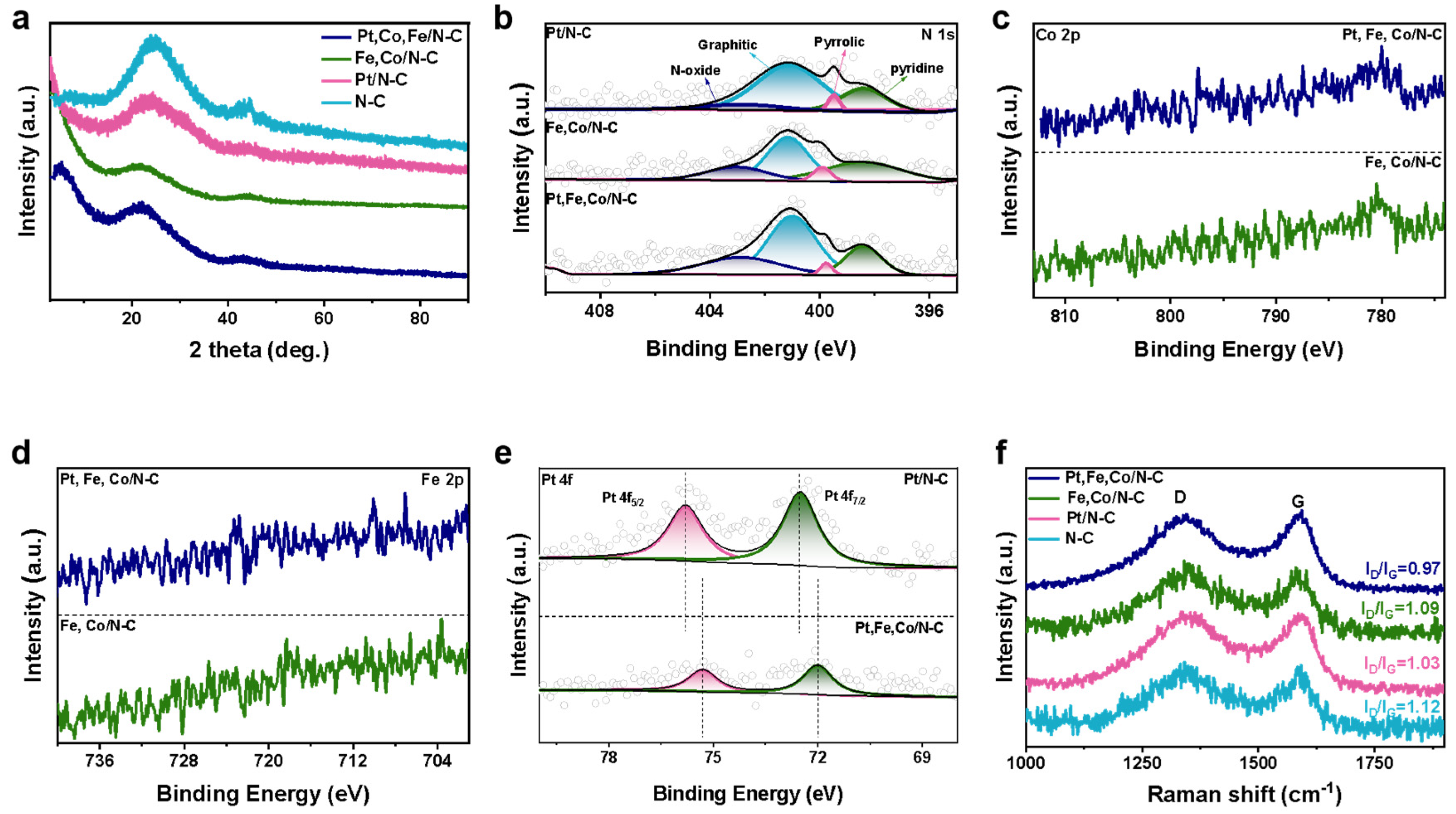
3.1. Design and Characterizations of Catalysts
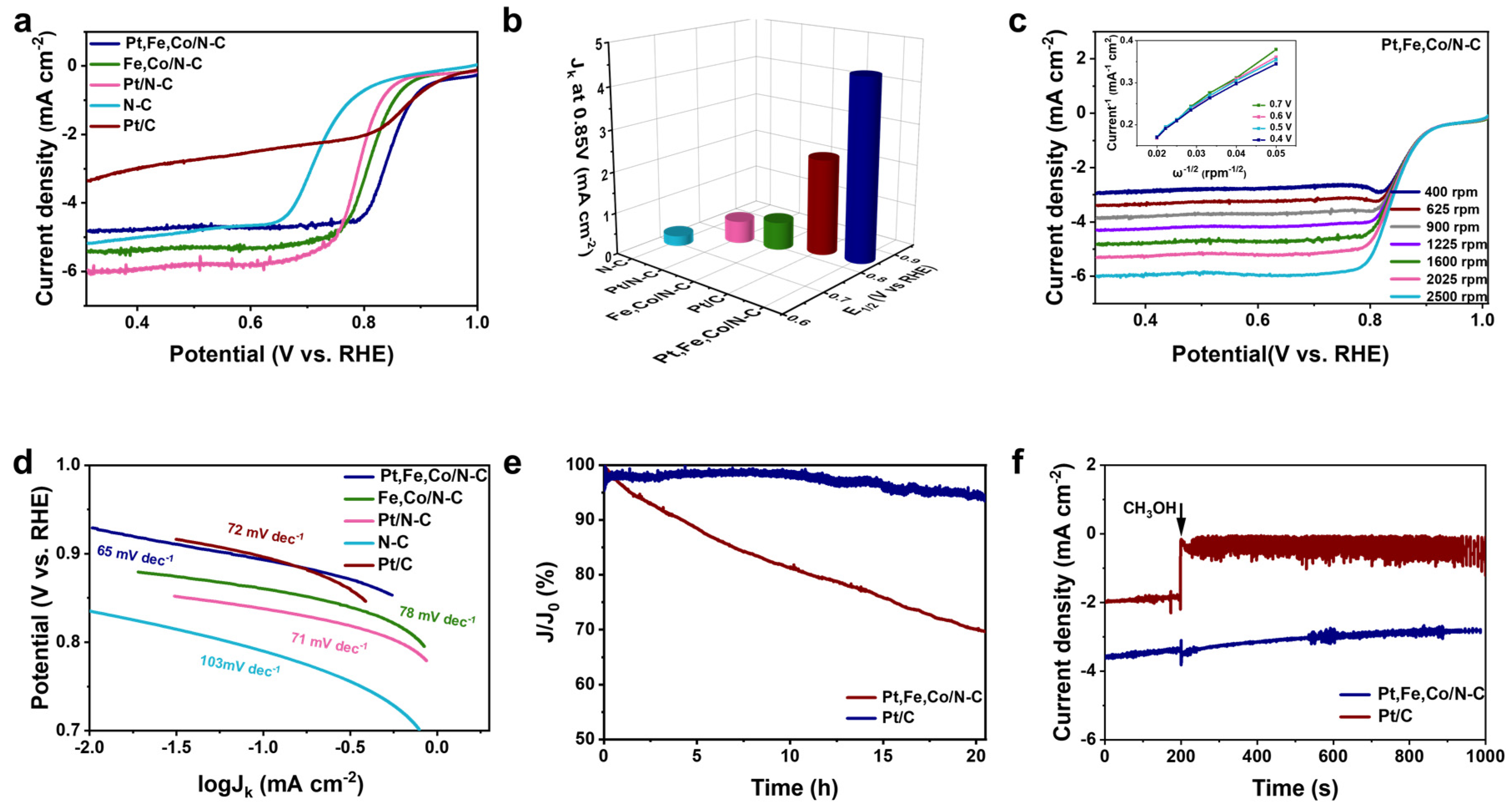
3.2. Electrocatalytic Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Guo, W.; Han, X.; Hong, X. Two-dimensional Noble Metal Nanomaterials for Electrocatalysis. Chem. Res. Chin. Univ. 2020, 36, 597–610. [Google Scholar] [CrossRef]
- Wang, Z.; Lei, Q.; Wang, Z.; Yuan, H.; Cao, L.; Qin, N.; Lu, Z.; Xiao, J.; Liu, J. In-situ synthesis of free-standing FeNi-oxyhydroxide nanosheets as a highly efficient electrocatalyst for water oxidation. Chem. Eng. J. 2020, 395, 125180. [Google Scholar] [CrossRef]
- Xu, D.; Long, X.; Xiao, J.; Zhang, Z.; Liu, G.; Tong, H.; Liu, Z.; Li, N.; Qian, D.; Li, J.; et al. Rationally constructing CoO and CoSe2 hybrid with CNTs-graphene for impressively enhanced oxygen evolution and DFT calculations. Chem. Eng. J. 2021, 422, 129982. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Song, Y.; Zhou, W.; Shao, Z. Designing High-Valence Metal Sites for Electrochemical Water Splitting. Adv. Funct. Mater. 2021, 31, 2009779. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Y.; Ren, X.; Ji, X.; Liu, Y.; Guo, X.; Liu, Z.; Asiri, A.M.; Wei, Q.; Sun, X. Co(OH)2 Nanoparticle-Encapsulating Conductive Nanowires Array: Room-Temperature Electrochemical Preparation for High-Performance Water Oxidation Electrocatalysis. Adv. Mater. 2018, 30, 1705366. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, L.; Yue, L.; Deng, B.; Cao, Y.; Liu, Q.; Luo, Y.; Lu, S.; Zheng, B.; Sun, X. A NiCo LDH nanosheet array on graphite felt: An efficient 3D electrocatalyst for the oxygen evolution reaction in alkaline media. Inorg. Chem. Front. 2021, 8, 3162–3166. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, H.; Liang, J.; Wang, Y.; Li, T.; Luo, Y.; Shi, X.; Lu, S.; Feng, Z.; Wu, Q.; et al. Noble-metal-free electrospun nanomaterials as electrocatalysts for oxygen reduction reaction. Mater. Today Phys. 2020, 15, 100280. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, J.; Wang, Z.; Yuan, H.; Lu, Z.; Luo, B.; Tian, E.; Waterhouse, G.I.N. Structural and Electronic Engineering of Ir-Doped Ni-(Oxy)hydroxide Nanosheets for Enhanced Oxygen Evolution Activity. ACS Catal. 2021, 11, 5386–5395. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Waterhouse, G.I.N. Complex alloy nanostructures as advanced catalysts for oxygen electrocatalysis: From materials design to applications. J. Mater. Chem. A 2020, 8, 23142–23161. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Zheng, Y.; Vasileff, A.; Qiao, S.-Z. Self-Supported Earth-Abundant Nanoarrays as Efficient and Robust Electrocatalysts for Energy-Related Reactions. ACS Catal. 2018, 8, 6707–6732. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Guo, C.; Vasileff, A.; Qiao, S. Design Strategies toward Advanced MOF-Derived Electrocatalysts for Energy-Conversion Reactions. Adv. Energy Mater. 2017, 7, 1700518. [Google Scholar] [CrossRef]
- Jiang, R.; Li, L.; Sheng, T.; Hu, G.; Chen, Y.; Wang, L. Edge-Site Engineering of Atomically Dispersed Fe–N4 by Selective C–N Bond Cleavage for Enhanced Oxygen Reduction Reaction Activities. J. Am. Chem. Soc. 2018, 140, 11594–11598. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, J.; Li, Y.; Li, W.; Zhai, J.; Jiang, Q.; Xu, X.; Gao, Y. Enhanced oxygen reduction with carbon-polyhedron-supported discrete cobalt-nitrogen sites for Zn-air batteries. Chem. Eng. J. 2022, 431, 134084. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.; et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Li, W.; Hou, Y. Noble metal-free catalysts for oxygen reduction reaction. Sci. China Chem. 2017, 60, 1494–1507. [Google Scholar] [CrossRef]
- Zong, M.; Ding, Z.; He, W.; Luo, J.; Tang, Z. Peptide Based Noble Metal Nanomaterials for Oxygen Reduction Reaction: A Review. Int. J. Electrochem. Sci. 2020, 15, 2634–2647. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Z.; Kong, D.; Iqbal, R.; Yang, Q.-H.; Zhi, L. N,P co-doped hollow carbon nanofiber membranes with superior mass transfer property for trifunctional metal-free electrocatalysis. Nano Energy 2019, 64, 103879. [Google Scholar] [CrossRef]
- Song, Z.X.; Zhu, Y.N.; Liu, H.S.; Banis, M.N.; Zhang, L.; Li, J.J.; Doyle-Davis, K.; Li, R.Y.; Sham, T.K.; Yang, L.J.; et al. Engineering the Low Coordinated Pt Single Atom to Achieve the Superior Electrocatalytic Performance toward Oxygen Reduction. Small 2020, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Y.; Zeng, X.; Chen, Z. Exploration of High-Performance Single-Atom Catalysts on Support M-1/FeOx for CO Oxidation via Computational Study. ACS Catal. 2015, 5, 544–552. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.-Z. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Accounts Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef]
- Ito, Y.; Qiu, H.-J.; Fujita, T.; Tanabe, Y.; Tanigaki, K.; Chen, M. Bicontinuous Nanoporous N-doped Graphene for the Oxygen Reduction Reaction. Adv. Mater. 2014, 26, 4145–4150. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Lv, B.; Li, X.; Guo, K.; Ma, J.; Wang, Y.; Lei, H.; Wang, F.; Jin, X.; Zhang, Q.; Zhang, W.; et al. Controlling Oxygen Reduction Selectivity through Steric Effects: Electrocatalytic Two-Electron and Four-Electron Oxygen Reduction with Cobalt Porphyrin Atropisomers. Angew. Chem. Int. Ed. 2021, 60, 12742–12746. [Google Scholar] [CrossRef]
- Tong, M.; Sun, F.; Xie, Y.; Wang, Y.; Yang, Y.; Tian, C.; Wang, L.; Fu, H. Operando Cooperated Catalytic Mechanism of Atomically Dispersed Cu-N4 and Zn-N4 for Promoting Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 14005–14012. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Montoro, C.; Rodríguez-San-Miguel, D.; Polo, E.; Escudero-Cid, R.; Ruiz-González, M.L.; Navarro, J.A.R.; Ocón, P.; Zamora, F. Ionic Conductivity and Potential Application for Fuel Cell of a Modified Imine-Based Covalent Organic Framework. J. Am. Chem. Soc. 2017, 139, 10079–10086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pachfule, P.; Li, S.; Langenhahn, T.; Ye, M.; Schlesiger, C.; Praetz, S.; Schmidt, J.; Thomas, A. Macro/Microporous Covalent Organic Frameworks for Efficient Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Bulk COFs and COF nanosheets for electrochemical energy storage and conversion. Chem. Soc. Rev. 2020, 49, 3565–3604. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, Y.; Chen, W.; Li, Z.; Cheong, W.-C.; Zhang, Q.; Gong, Y.; Gu, L.; Chen, C.; Wang, D.; et al. Atomically dispersed Fe atoms anchored on COF-derived N-doped carbon nanospheres as efficient multi-functional catalysts. Chem. Sci. 2020, 11, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; An, Y.; Liu, S.; Sun, F.; Qi, H.; Wu, H.; He, Y.; Liu, P.; Shi, R.; Zhang, J.; et al. Highly accessible and dense surface single metal FeN4 active sites for promoting the oxygen reduction reaction. Energy Environ. Sci. 2022, 15, 2619–2628. [Google Scholar] [CrossRef]
- Du, Z.Z.; Chen, X.J.; Hu, W.; Chuang, C.H.; Xie, S.; Hu, A.J.; Yan, W.S.; Kong, X.H.; Wu, X.J.; Ji, H.X.; et al. Cobalt in Nitrogen-Doped Graphene as Single-Atom Catalyst for High-Sulfur Content Lithium-Sulfur Batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Aguey-Zinsou, K.-F.; Amal, R. Single Atom and Nanoclustered Pt Catalysts for Selective CO2 Reduction. ACS Appl. Energ. Mater. 2018, 1, 6781–6789. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, Q.; Yin, K.; Li, Y.; Tao, L.; Tan, H.; Yang, Y.; Guo, S. Engineering eg Orbital Occupancy of Pt with Au Alloying Enables Reversible Li-O2 Batteries. Angew. Chem. Int. Ed. 2022, 61, e202201416. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, K.; Wang, P.; He, Y.; Liu, Z. Pt-Fe-Co Ternary Metal Single Atom Catalyst for toward High Efficiency Alkaline Oxygen Reduction Reaction. Energies 2023, 16, 3684. https://doi.org/10.3390/en16093684
Zhang R, Wang K, Wang P, He Y, Liu Z. Pt-Fe-Co Ternary Metal Single Atom Catalyst for toward High Efficiency Alkaline Oxygen Reduction Reaction. Energies. 2023; 16(9):3684. https://doi.org/10.3390/en16093684
Chicago/Turabian StyleZhang, Ruimin, Ke Wang, Peng Wang, Yan He, and Zhiming Liu. 2023. "Pt-Fe-Co Ternary Metal Single Atom Catalyst for toward High Efficiency Alkaline Oxygen Reduction Reaction" Energies 16, no. 9: 3684. https://doi.org/10.3390/en16093684
APA StyleZhang, R., Wang, K., Wang, P., He, Y., & Liu, Z. (2023). Pt-Fe-Co Ternary Metal Single Atom Catalyst for toward High Efficiency Alkaline Oxygen Reduction Reaction. Energies, 16(9), 3684. https://doi.org/10.3390/en16093684








