Nitrogen-Doped Porous Carbon Nanosheets Based on a Schiff Base Reaction for High-Performance Lithium-Ion Batteries Anode
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of COF, 2D-PNC, and NC
2.2. Electrochemical Measurements
2.3. Material Characterizations
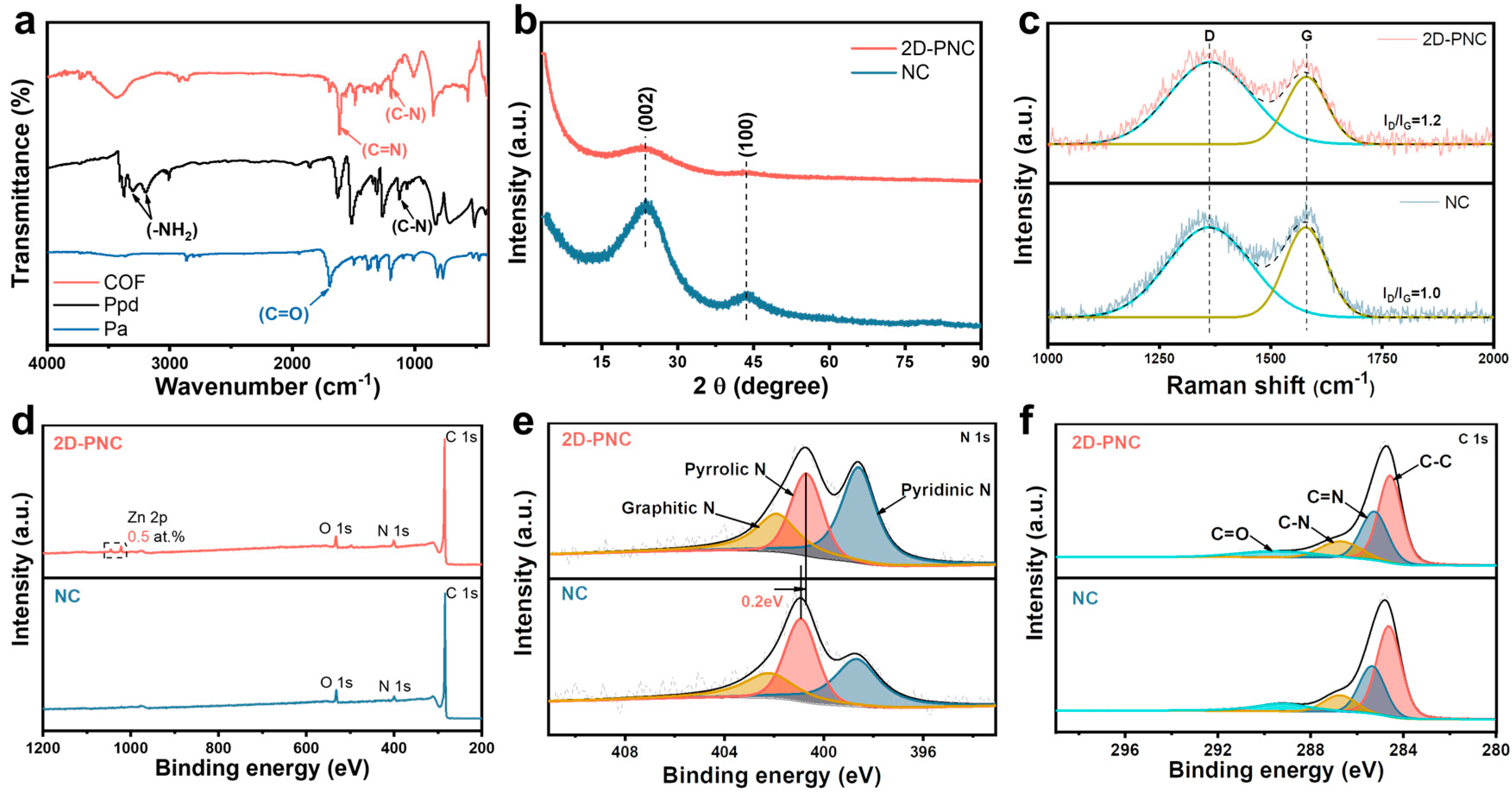
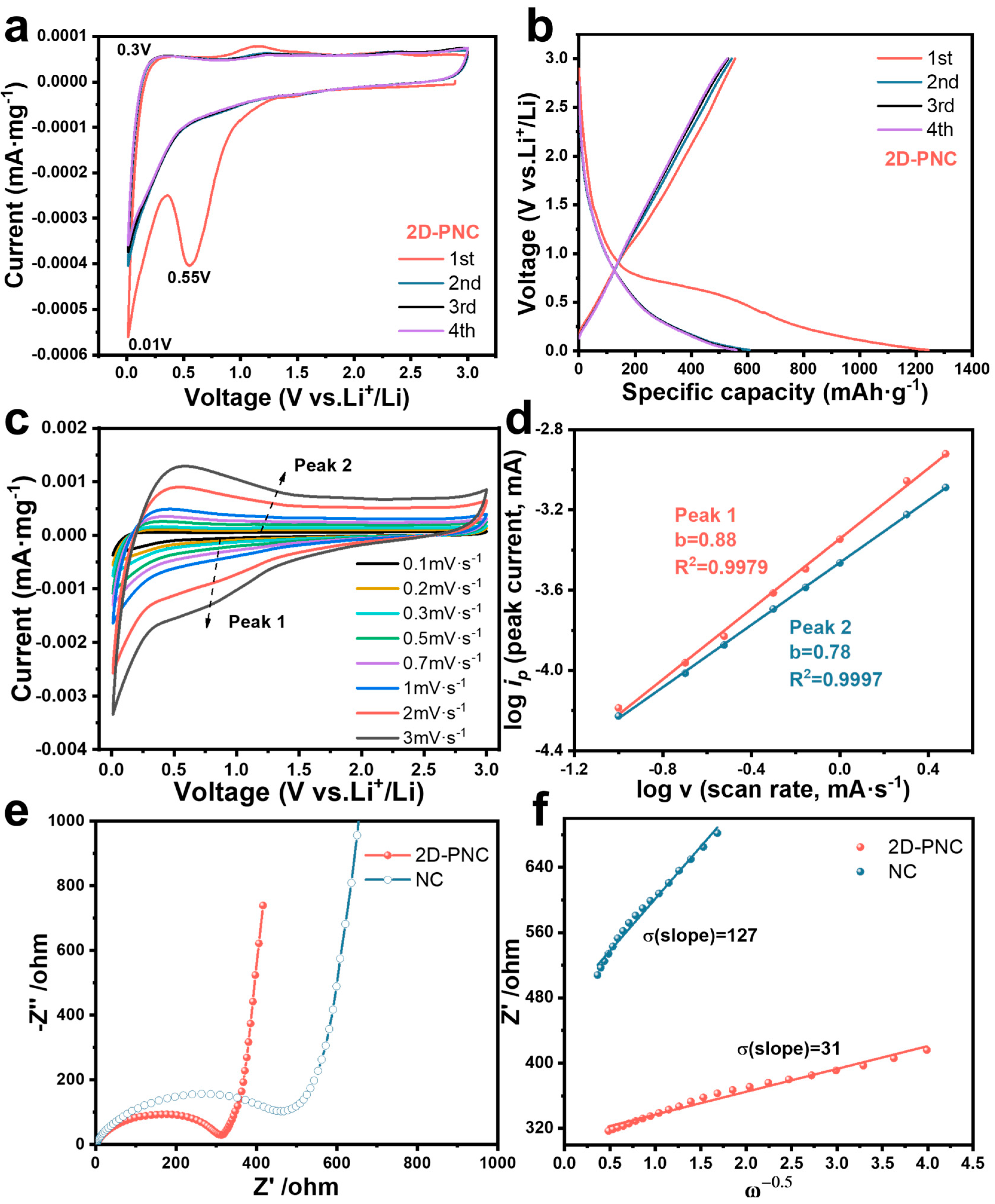
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lai, X.; Halpert, J.E.; Wang, D. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems. Energy Environ. Sci. 2012, 5, 5604–5618. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, J.; Moon, J.; Jung, K.-N.; Kim, J.H.; Park, G.-J.; Choi, J.-H.; Rhee, D.Y.; Kim, J.-S.; Lee, J.-W.; et al. A cooperative biphasic MoOx–MoPx promoter enables a fast-charging lithium-ion battery. Nat. Commun. 2021, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lei, S.; Kheimeh Sari, H.M.; Zhong, L.; Kakimov, A.; Wang, J.; Chen, J.; Liu, D.; Huang, L.; Hu, J.; et al. Controllable S-Vacancies of monolayered Mo–S nanocrystals for highly harvesting lithium storage. Nano Energy 2020, 78, 105235. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Chen, L.-F.; Ma, S.-X.; Lu, S.; Feng, Y.; Zhang, J.; Xin, S.; Yu, S.-H. Biotemplate synthesis of three-dimensional porous MnO/C-N nanocomposites from renewable rapeseed flower pollen: An anode material for lithium-ion batteries. Nano Res. 2016, 10, 1–10. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Ghigna, P.; Airoldi, L.; Fracchia, M.; Callegari, D.; Anselmi-Tamburini, U.; D’Angelo, P.; Pianta, N.; Ruffo, R.; Cibin, G.; de Souza, D.O.; et al. Lithiation Mechanism in High-Entropy Oxides as Anode Materials for Li-Ion Batteries: An Operando XAS Study. ACS Appl. Mater. Interfaces 2020, 12, 50344–50354. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Wang, X.; Liu, W.; Liang, J.; Liang, C. Preparation of porous carbon anode materials for lithium-ion battery from rice husk. Mater. Lett. 2019, 253, 405–408. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Xiong, J.; Ou, X.; Su, W.; Zhong, G.; Yang, C. Activated Amorphous Carbon With High-Porosity Derived From Camellia Pollen Grains as Anode Materials for Lithium/Sodium Ion Batteries. Front. Chem. 2018, 6, 366. [Google Scholar] [CrossRef]
- Xu, C.; Wei, Q.; Li, M.; You, J.; Song, W.; Wang, X.; Zhang, G.; Li, H.; He, Y.; Liu, Z. Ultrafast and simple integration engineering of graphene-based flexible films with extensive tunability and simple trial in lithium-ion batteries. J. Alloys Compd. 2022, 922, 166282. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Yan, X. Hierarchically porous and nitrogen, sulfur-codoped graphene-like microspheres as a high capacity anode for lithium ion batteries. Chem. Commun. 2015, 51, 2134–2137. [Google Scholar] [CrossRef]
- Fu, L.; Tang, K.; Song, K.; van Aken, P.A.; Yu, Y.; Maier, J. Nitrogen doped porous carbon fibres as anode materials for sodium ion batteries with excellent rate performance. Nanoscale 2014, 6, 1384–1389. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, D.; Xia, G.; Yang, Y.; Liu, T.; Wu, M.; Chen, Q. Biomass waste inspired nitrogen-doped porous carbon materials as high-performance anode for lithium-ion batteries. J. Alloys Compd. 2017, 693, 1197–1204. [Google Scholar] [CrossRef]
- Alkarmo, W.; Ouhib, F.; Aqil, A.; Thomassin, J.-M.; Vertruyen, B.; Piedboeuf, M.-L.; Job, N.; Detrembleur, C.; Jérôme, C. Continuous-porous N-doped carbon network as high-performance electrode for lithium-ion batteries. J. Mater. Sci. 2018, 53, 6135–6146. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; Ramirez-Rico, J. An electrochemical evaluation of nitrogen-doped carbons as anodes for lithium ion batteries. Carbon 2020, 164, 261–271. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Park, H.-Y.; Kim, M.-S.; Choi, H.-S.; Inamdar, S.N.; Yu, J.-S. Nitrogen-Doped Carbon Nanoparticles by Flame Synthesis as Anode Material for Rechargeable Lithium-Ion Batteries. Langmuir 2014, 30, 318–324. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Wang, H.-G.; Wu, Z.; Zhang, X.-B. In Situ Fabrication of Porous Graphene Electrodes for High-Performance Energy Storage. ACS Nano 2013, 7, 2422–2430. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Q.; Han, L.; Wang, S.; Fang, S.; Zhang, Z.; Sun, S. Synthesis of conjugated covalent organic frameworks/graphene composite for supercapacitor electrodes. RSC Adv. 2015, 5, 27290–27294. [Google Scholar] [CrossRef]
- Fang, Q.; Gu, S.; Zheng, J.; Zhuang, Z.; Qiu, S.; Yan, Y. 3D Microporous Base-Functionalized Covalent Organic Frameworks for Size-Selective Catalysis. Angew. Chem. Int. Ed. 2014, 53, 2878–2882. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Fayed, M.G.; Mohamed, S.G.; Wen, Z.; Sun, X.; Abdelhamid, H.N. High-Performance Lithium-Ion Battery and Supercapacitors Using Covalent Organic Frameworks (COFs)/Graphitic Carbon Nitride (g-C3N4)-Derived Hierarchical N-Doped Carbon. ACS Appl. Energy Mater. 2022, 5, 12828–12836. [Google Scholar] [CrossRef]
- Zhuang, G.-L.; Gao, Y.-F.; Zhou, X.; Tao, X.-Y.; Luo, J.-M.; Gao, Y.-J.; Yan, Y.-L.; Gao, P.-Y.; Zhong, X.; Wang, J.-G. ZIF-67/COF-derived highly dispersed Co3O4/N-doped porous carbon with excellent performance for oxygen evolution reaction and Li-ion batteries. Chem. Eng. J. 2017, 330, 1255–1264. [Google Scholar] [CrossRef]
- Li, Z.; Ji, W.; Wang, T.-X.; Zhang, Y.; Li, Z.; Ding, X.; Han, B.-H.; Feng, W. Guiding Uniformly Distributed Li–Ion Flux by Lithiophilic Covalent Organic Framework Interlayers for High-Performance Lithium Metal Anodes. ACS Appl. Mater. Interfaces 2021, 13, 22586–22596. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Hernán, L.; Morales, J. Limitations of Disordered Carbons Obtained from Biomass as Anodes for Real Lithium-Ion Batteries. ChemSusChem 2011, 4, 658–663. [Google Scholar] [CrossRef]
- Boonprachai, R.; Autthawong, T.; Namsar, O.; Yodbunork, C.; Yodying, W.; Sarakonsri, T. Natural Porous Carbon Derived from Popped Rice as Anode Materials for Lithium-Ion Batteries. Crystals 2022, 12, 223. [Google Scholar] [CrossRef]
- Gou, W.; Kong, X.; Wang, Y.; Ai, Y.; Liang, S.; Pan, A.; Cao, G. Yolk-shell structured V2O3 microspheres wrapped in N, S co-doped carbon as pea-pod nanofibers for high-capacity lithium ion batteries. Chem. Eng. J. 2019, 374, 545–553. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, X.; Wang, J.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Facile Synthesis of High-Performance Nitrogen-Doped Hierarchically Porous Carbon for Catalytic Oxidation. ACS Sustain. Chem. Eng. 2020, 8, 4236–4243. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Yang, N.; Deng, M.; Ibraheem, S.; Deng, J.; Li, J.; Li, L.; Wei, Z. Ultrahigh-Loading Zinc Single-Atom Catalyst for Highly Efficient Oxygen Reduction in Both Acidic and Alkaline Media. Angew. Chem. Int. Ed. 2019, 58, 7035–7039. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Li, H.; Ma, M.; Zhang, Y.; Yan, Z.; Agnoli, S.; Zhang, G.; Sun, X. Single-atom Zn for boosting supercapacitor performance. Nano Res. 2022, 15, 1715–1724. [Google Scholar] [CrossRef]
- Fang, Y.; Zeng, Y.; Jin, Q.; Lu, X.F.; Luan, D.; Zhang, X.; Lou, X.W. Nitrogen-Doped Amorphous Zn–Carbon Multichannel Fibers for Stable Lithium Metal Anodes. Angew. Chem. Int. Ed. 2021, 60, 8515–8520. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, F.; Zeng, Q.; Li, Z.; Yang, B.; Lei, L.; Zhang, Q.; He, F.; Wu, X.; Hou, Y. A Universal Principle to Accurately Synthesize Atomically Dispersed Metal–N4 Sites for CO2 Electroreduction. Nanomicro Lett. 2020, 12, 108. [Google Scholar]
- Bian, Z.; Yuan, T.; Xu, Y.; Pang, Y.; Yao, H.; Li, J.; Yang, J.; Zheng, S. Boosting Li–S battery by rational design of freestanding cathode with enriched anchoring and catalytic N-sites carbonaceous host. Carbon 2019, 150, 216–223. [Google Scholar] [CrossRef]
- Yang, L.X.; Liu, R.J.; Bu, H.P.; Liu, H.J.; Zeng, C.L.; Fu, C. TiC Nanomaterials with Varying Dimensionalities as Anode Materials for Lithium-Ion Batteries. ACS Appl. Nano Mater. 2022, 5, 11787–11796. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Cheng, Z.; Sun, J.; Tian, Y.; He, J.; Chen, Y.; Bai, Y.; Liu, Z. Nitrogen-Doped Porous Carbon Nanosheets Based on a Schiff Base Reaction for High-Performance Lithium-Ion Batteries Anode. Energies 2023, 16, 1733. https://doi.org/10.3390/en16041733
Li M, Cheng Z, Sun J, Tian Y, He J, Chen Y, Bai Y, Liu Z. Nitrogen-Doped Porous Carbon Nanosheets Based on a Schiff Base Reaction for High-Performance Lithium-Ion Batteries Anode. Energies. 2023; 16(4):1733. https://doi.org/10.3390/en16041733
Chicago/Turabian StyleLi, Mai, Zhi Cheng, Jingrui Sun, Yu Tian, Jiawei He, Yutian Chen, Yang Bai, and Zhiming Liu. 2023. "Nitrogen-Doped Porous Carbon Nanosheets Based on a Schiff Base Reaction for High-Performance Lithium-Ion Batteries Anode" Energies 16, no. 4: 1733. https://doi.org/10.3390/en16041733





