Thermocatalytic Decomposition of Sesame Waste Biomass over Ni-Co-Doped MCM-41: Kinetics and Physicochemical Properties of the Bio-Oil
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Method
2.2. Synthesis and Characterization of Catalyst
2.3. Preparation of Biomass for Analysis
2.4. Thermogravimetry
2.5. Pyrolysis of Biomass
2.6. Physicochemical Properties
3. Results and Discussion
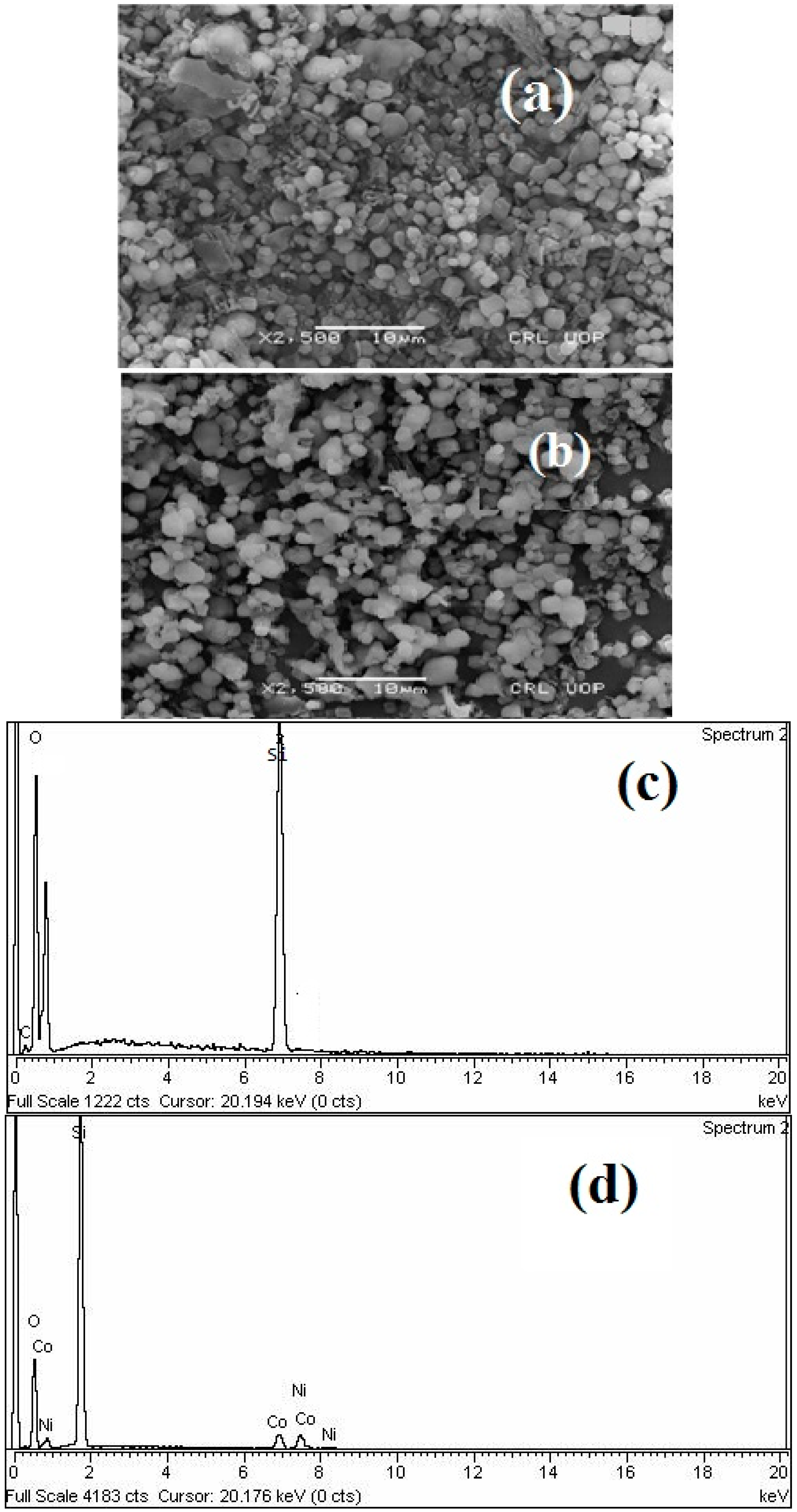
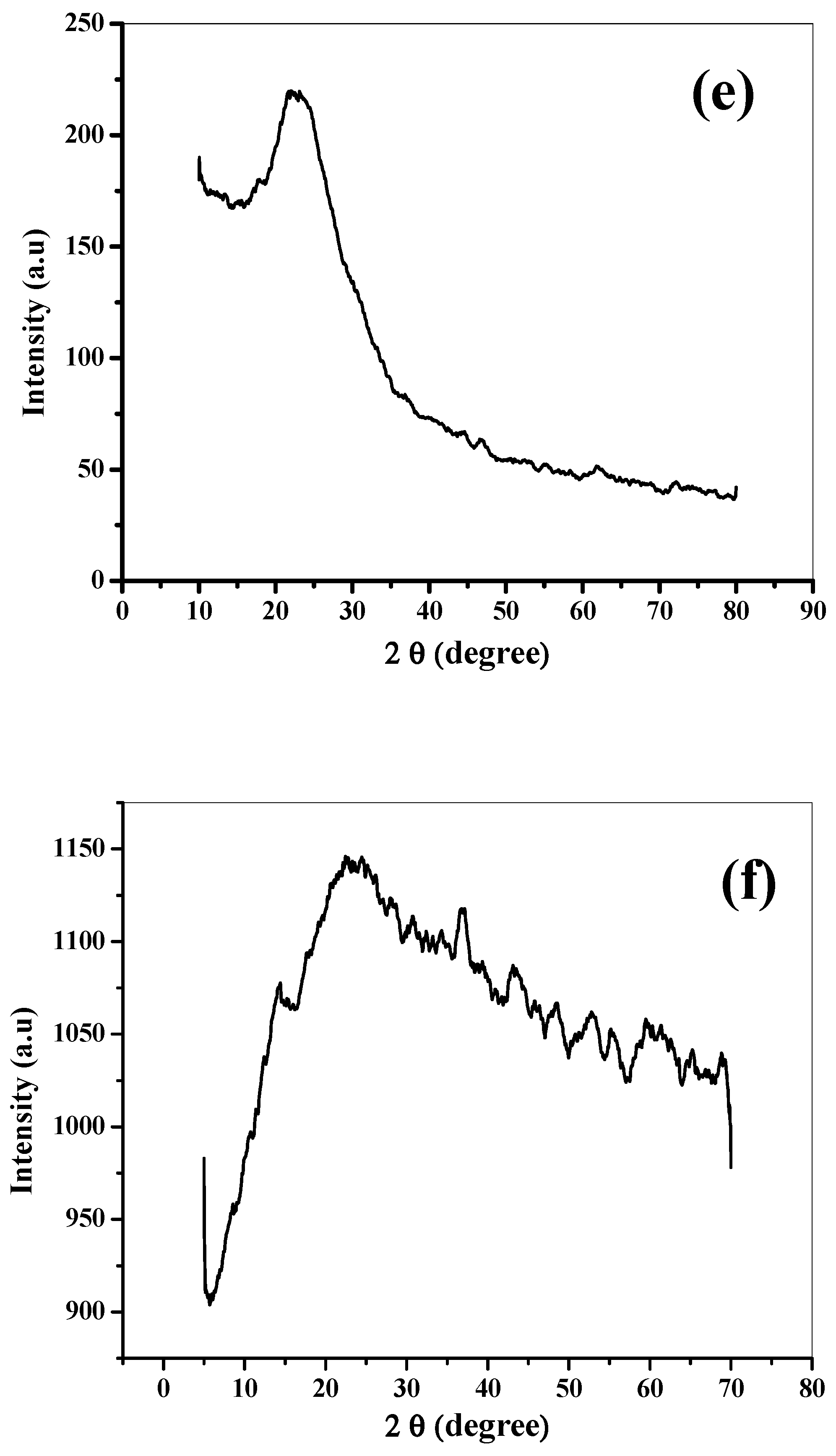
3.1. Characterization of Catalyst
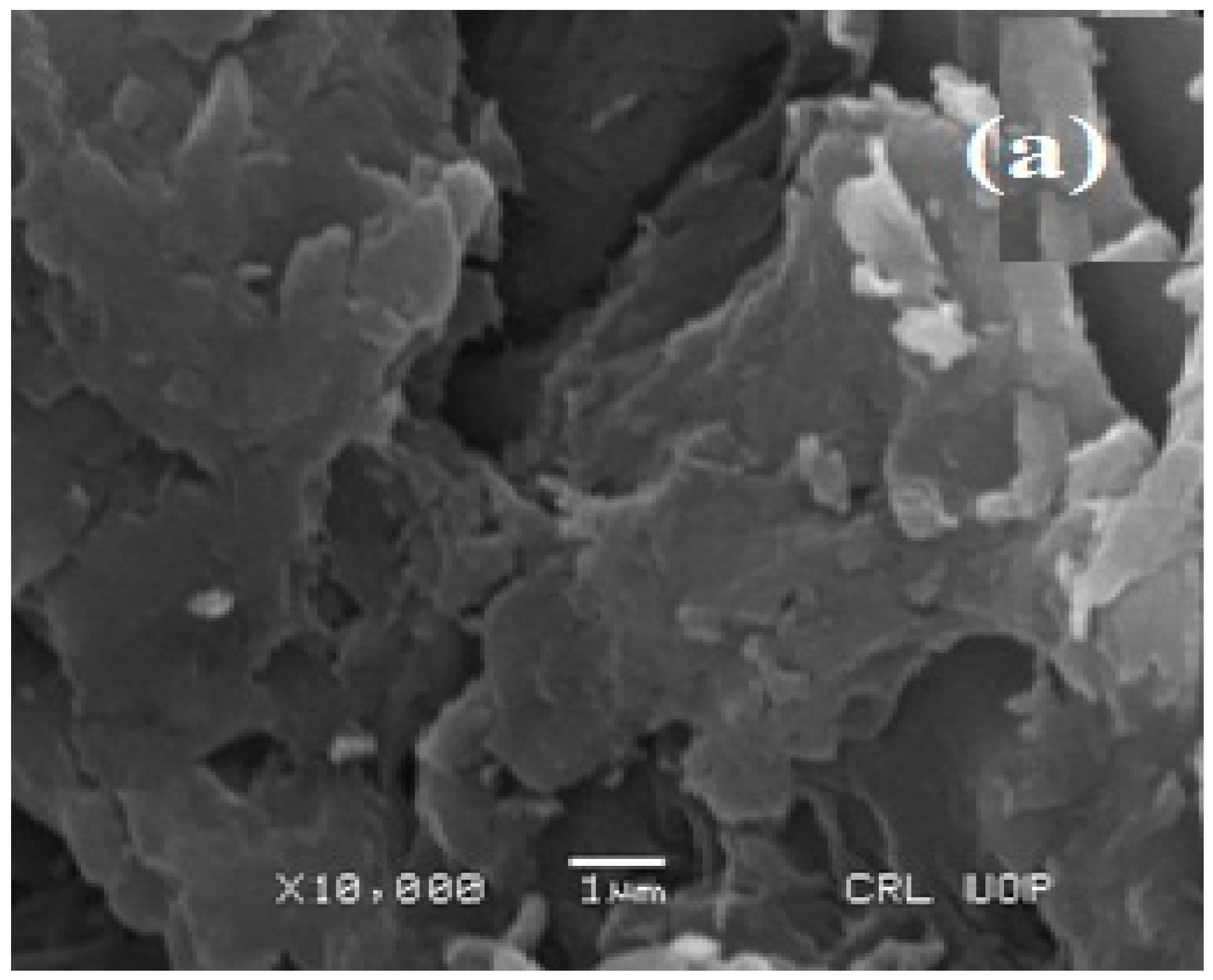
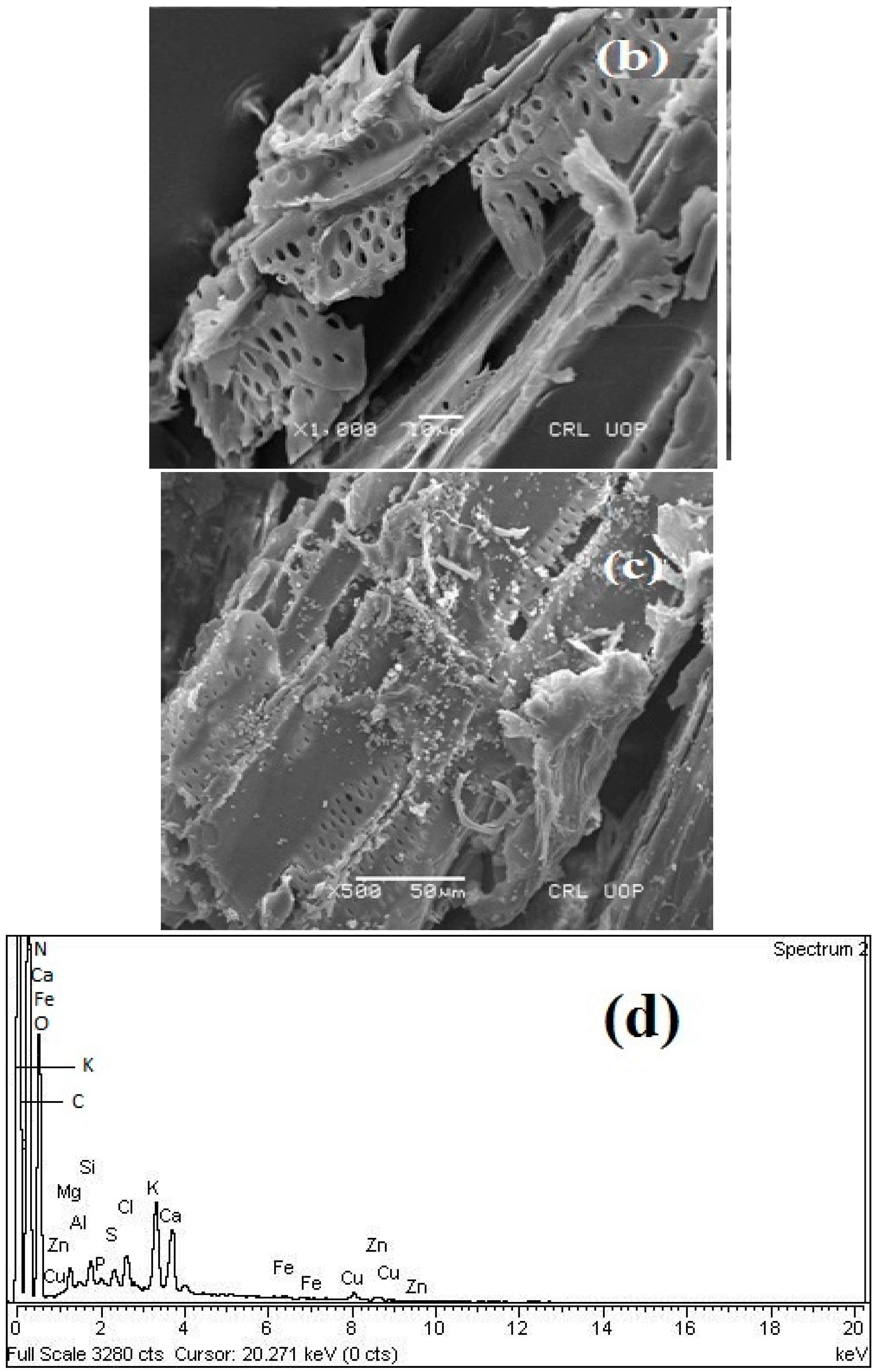
3.2. Surface Morphology and Chemical Composition of Waste Sesame Biomass
3.3. Thermogravimetric Analysis and Kinetic Study
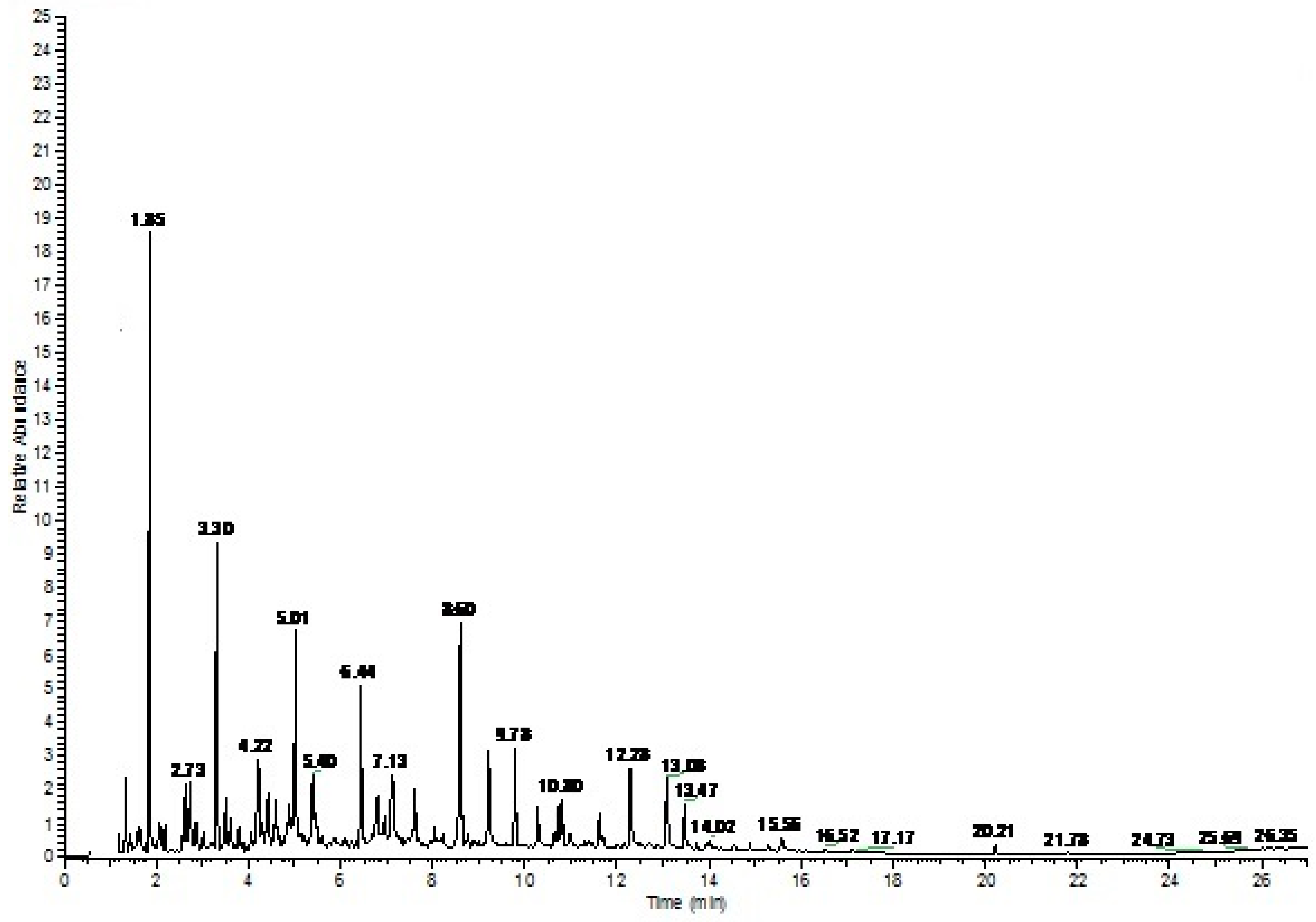
3.4. Pyrolysis of Biomass and Characterization of Bio-Oil
3.5. Physicochemical Characteristics of Bio-Oil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Y.; Wu, T.; Lester, E.; Tang, L.; Meng, Y.; Fang, Y.; Pang, C.H. The kinetics studies and thermal characterisation of biomass. Energy Procedia 2019, 158, 357–363. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis characteristics, fuel properties, and compositional study of Madhuca longifolia seeds over metal oxide catalysts. Biomass Convers. Biorefinery 2019, 10, 621–637. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Slow pyrolysis of chemically treated walnut shell for valuable products: Effect of process parameters and in-depth product analysis. Energy 2019, 181, 665–676. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Nisar, J.; Ullah, N.; Awan, I.A.; Iqbal, M.; Khan, T.A. Pyrolysis–gas chromatography of sugar beet bagasse. Waste Biomass Valorization 2016, 7, 79–85. [Google Scholar] [CrossRef]
- Nisar, J.; Nasir, U.; Ali, G.; Shah, A.; Farooqi, Z.H.; Iqbal, M.; Shah, M.R. Kinetics of pyrolysis of sugarcane bagasse: Effect of catalyst on activation energy and yield of pyrolysis products. Cellulose 2021, 28, 7593–7607. [Google Scholar] [CrossRef]
- Nisar, J.; Waris, S.; Shah, A.; Anwar, F.; Ali, G.; Ahmad, A.; Muhammad, F. Production of Bio-Oil from De-Oiled Karanja (Pongamia pinnata L.) Seed Press Cake Via Pyrolysis: Kinetics and Evaluation of Anthill as the Catalyst. Sustain. Chem. 2022, 3, 345–357. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Thermocatalytic conversion of non-edible Neem seeds towards clean fuel and chemicals. J. Anal. Appl. Pyrolysis 2018, 134, 83–92. [Google Scholar] [CrossRef]
- Salema, A.A.; Ting, R.M.W.; Shang, Y.K. Pyrolysis of blend (oil palm biomass and sawdust) biomass using TG-MS. Bioresour. Technol. 2019, 274, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhang, H.; Xiao, R.; Li, X.; Cai, Y. Controlled regeneration of ZSM-5 catalysts in the combined oxygen and steam atmosphere used for catalytic pyrolysis of biomass-derivates. Energy Convers. Manag. 2018, 155, 175–181. [Google Scholar] [CrossRef]
- Dabros, T.M.; Stummann, M.Z.; Høj, M.; Jensen, P.A.; Grunwaldt, J.-D.; Gabrielsen, J.; Mortensen, P.M.; Jensen, A.D. Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis. Prog. Energy Combust. Sci. 2018, 68, 268–309. [Google Scholar] [CrossRef]
- Rehman, N.U.; Nisar, J.; Ali, G.; Ahmad, A.; Shah, A.; Farooqi, Z.H.; Muhammad, F. Production of Bio-Oil from Thermo-Catalytic Decomposition of Pomegranate Peels over a Sulfonated Tea Waste Heterogeneous Catalyst: A Kinetic Investigation. Energies 2023, 16, 1908. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Steele, P.; Puettmann, M.E.; Penmetsa, V.K.; Cooper, J.E. Life-cycle assessment of pyrolysis bio-oil production. For. Prod. J. 2012, 62, 326–334. [Google Scholar] [CrossRef]
- Nisar, J.; Sharaf, M.; Ali, G.; Farooqi, Z.; Iqbal, M.; Khan, S. Pyrolysis of juice-squeezed grapefruit waste: Effect of nickel oxide on kinetics and bio-oil yield. Int. J. Environ. Sci. Technol. 2022, 19, 10211–10222. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil–a review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Ali, G.; Afraz, M.; Muhammad, F.; Nisar, J.; Shah, A.; Munir, S.; Hussain, S.T. Production of Fuel Range Hydrocarbons from Pyrolysis of Lignin over Zeolite Y, Hydrogen. Energies 2022, 16, 215. [Google Scholar] [CrossRef]
- Nisar, J.; Ahmad, A.; Ali, G.; Rehman, N.U.; Shah, A.; Shah, I. Enhanced Bio-Oil Yield from Thermal Decomposition of Peanut Shells Using Termite Hill as the Catalyst. Energies 2022, 15, 1891. [Google Scholar] [CrossRef]
- Adam, J.; Blazso, M.; Meszaros, E.; Stöcker, M.; Nilsen, M.H.; Bouzga, A.; Hustad, J.E.; Grønli, M.; Øye, G. Pyrolysis of biomass in the presence of Al-MCM-41 type catalysts. Fuel 2005, 84, 1494–1502. [Google Scholar] [CrossRef]
- Nilsen, M.H.; Antonakou, E.; Bouzga, A.; Lappas, A.; Mathisen, K.; Stöcker, M. Investigation of the effect of metal sites in Me–Al-MCM-41 (Me = Fe, Cu or Zn) on the catalytic behavior during the pyrolysis of wooden based biomass. Microporous Mesoporous Mater. 2007, 105, 189–203. [Google Scholar] [CrossRef]
- Antonakou, E.; Lappas, A.; Nilsen, M.H.; Bouzga, A.; Stöcker, M. Evaluation of various types of Al-MCM-41 materials as catalysts in biomass pyrolysis for the production of bio-fuels and chemicals. Fuel 2006, 85, 2202–2212. [Google Scholar] [CrossRef]
- Maria do Socorro, B.F.; Melo, D.M.; Fontes, L.A.; Braga, R.M.; Costa, C.C.; Martinelli, A.E. Ex situ catalytic biomass pyrolysis using mesoporous Ti-MCM-41. Environ. Sci. Pollut. Res. 2019, 26, 5983–5989. [Google Scholar]
- Li, X.; Hu, C.; Shao, S.; Cai, Y.; Zhang, X. In situ catalytic upgrading of pyrolysis vapours from vacuum pyrolysis of rape straw over La/MCM-41. J. Anal. Appl. Pyrolysis 2019, 140, 213–218. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; Su, Y.; John, V.T.; Valla, J.A. MCM-41/ZSM-5 composite particles for the catalytic fast pyrolysis of biomass. Appl. Catal. A Gen. 2020, 602, 117727. [Google Scholar] [CrossRef]
- Xue, Z.; Zhong, Z.; Zhang, B. Microwave-Assisted Catalytic Fast Pyrolysis of Biomass for Hydrocarbon Production with Physically Mixed MCM-41 and ZSM-5. Catalysts 2020, 10, 685. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Yang, W.; Jönsson, P.G. Kinetic Study of an H-ZSM-5/Al–MCM-41 Catalyst Mixture and Its Application in Lignocellulose Biomass Pyrolysis. Energy Fuels 2019, 33, 5360–5367. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Bolatibieke, D.-N.; Nan, D.-H.; Zhang, Z.-X.; Cui, M.-S.; Lu, Q. Catalytic fast pyrolysis of biomass with Ni-P-MCM-41 to selectively produce levoglucosenone. J. Anal. Appl. Pyrolysis 2020, 148, 104824. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Grün, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel pathways for the preparation of mesoporous MCM-41 materials: Control of porosity and morphology. Microporous Mesoporous Mater. 1999, 27, 207–216. [Google Scholar] [CrossRef]
- Nejat, T.; Jalalinezhad, P.; Hormozi, F.; Bahrami, Z. Hydrogen production from steam reforming of ethanol over Ni-Co bimetallic catalysts and MCM-41 as support. J. Taiwan Inst. Chem. Eng. 2019, 97, 216–226. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Williams, P.T.; Shi, J.; Huang, J. Hydrogen production from biomass gasification with Ni/MCM-41 catalysts: Influence of Ni content. Appl. Catal. B Environ. 2011, 108, 6–13. [Google Scholar] [CrossRef]
- Parangi, T.F.; Patel, R.M.; Chudasama, U.V. Synthesis and characterization of mesoporous Si-MCM-41 materials and their application as solid acid catalysts in some esterification reactions. Bull. Mater. Sci. 2014, 37, 609–615. [Google Scholar] [CrossRef]
- Abrokwah, R.Y.; Deshmane, V.G.; Kuila, D. Comparative performance of M-MCM-41 (M: Cu, Co, Ni, Pd, Zn and Sn) catalysts for steam reforming of methanol. J. Mol. Catal. A Chem. 2016, 425, 10–20. [Google Scholar] [CrossRef]
- Salam, M.S.A.; Betiha, M.A.; Shaban, S.A.; Elsabagh, A.M.; Abd El-Aal, R.M. Synthesis and characterization of MCM-41-supported nano zirconia catalysts. Egypt. J. Pet. 2015, 24, 49–57. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Scope of doped mesoporous (<10 nm) surfactant-modified alumina templated carbons for hydrogen storage applications. Int. J. Energy Res. 2019, 43, 4264–4280. [Google Scholar]
- Parvulescu, V.; Su, B.-L. Iron, cobalt or nickel substituted MCM-41 molecular sieves for oxidation of hydrocarbons. Catal. Today 2001, 69, 315–322. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal–support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Gucbilmez, Y.; Dogu, T.; Balci, S. Vanadium incorporated high surface area MCM-41 catalysts. Catal. Today 2005, 100, 473–477. [Google Scholar] [CrossRef]
- Ma, X.; Chi, H.; Yue, H.; Zhao, Y.; Xu, Y.; Lv, J.; Wang, S.; Gong, J. Hydrogenation of dimethyl oxalate to ethylene glycol over mesoporous Cu-MCM-41 catalysts. AIChE J. 2013, 59, 2530–2539. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, F.; Malana, M.A.; Ali, G.; Iqbal, M.; Shah, A.; Bhatti, I.A.; Khan, T.A.; Rashid, U. Kinetics of the pyrolysis of cobalt-impregnated sesame stalk biomass. Biomass Convers. Biorefinery 2020, 10, 1179–1187. [Google Scholar] [CrossRef]
- Balonek, C.M.; Lillebø, A.H.; Rane, S.; Rytter, E.; Schmidt, L.D.; Holmen, A. Effect of alkali metal impurities on Co–Re catalysts for Fischer–Tropsch synthesis from biomass-derived syngas. Catal. Lett. 2010, 138, 8–13. [Google Scholar] [CrossRef]
- Zhu, W.; Song, W.; Lin, W. Catalytic gasification of char from co-pyrolysis of coal and biomass. Fuel Process. Technol. 2008, 89, 890–896. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Al Ayed, O.S.; Ye, G.; Luo, H.; Ibrahim, M.; Rashid, U.; Nehdi, I.A.; Qadir, G. Kinetic analyses and pyrolytic behavior of Para grass (Urochloa mutica) for its bioenergy potential. Bioresour. Technol. 2017, 224, 708–713. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Nisar, J.; Rahman, A.; Ali, G.; Shah, A.; Farooqi, Z.H.; Bhatti, I.A.; Iqbal, M.; Rehman, N.U. Pyrolysis of almond shells waste: Effect of zinc oxide on kinetics and product distribution. Biomass Convers. Biorefinery 2020, 12, 2583–2595. [Google Scholar] [CrossRef]
- Torres-García, E.; Ramírez-Verduzco, L.; Aburto, J. Pyrolytic degradation of peanut shell: Activation energy dependence on the conversion. Waste Manag. 2020, 106, 203–212. [Google Scholar] [CrossRef]
- Westerhof, R.J.; Brilman, D.W.; van Swaaij, W.P.; Kersten, S.R. Effect of temperature in fluidized bed fast pyrolysis of biomass: Oil quality assessment in test units. Ind. Eng. Chem. Res. 2010, 49, 1160–1168. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of temperature on pyrolysis products from four nut shells. J. Anal. Appl. Pyrolysis 2006, 76, 285–289. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresour. Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Mubarak, N.M.; Riaz, S.; Siddiqui, M.T.H.; Takkalkar, P.; Tunio, M.; Mazari, S.; Bhutto, A.W. Solvothermal liquefaction of corn stalk: Physico-chemical properties of bio-oil and biochar. Waste Biomass Valorization 2019, 10, 1957–1968. [Google Scholar] [CrossRef]
- Uçar, S.; Karagöz, S. The slow pyrolysis of pomegranate seeds: The effect of temperature on the product yields and bio-oil properties. J. Anal. Appl. Pyrolysis 2009, 84, 151–156. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of torrefaction on the pyrolysis behavior and bio-oil properties of rice husk by using TG-FTIR and Py-GC/MS. Energy Fuels 2014, 28, 5857–5863. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Min, M.; Ding, K.; Xie, Q.; Ruan, R. Catalytic fast co-pyrolysis of biomass and food waste to produce aromatics: Analytical Py–GC/MS study. Bioresour. Technol. 2015, 189, 30–35. [Google Scholar] [CrossRef]
- Ateş, F.; Pütün, E.; Pütün, A.E. Fast pyrolysis of sesame stalk: Yields and structural analysis of bio-oil. J. Anal. Appl. Pyrolysis 2004, 71, 779–790. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Jeon, J.-K.; Suh, D.J.; Park, S.H.; Sa, Y.J.; Joo, S.H.; Park, Y.-K. Catalytic pyrolysis of biomass components over mesoporous catalysts using Py-GC/MS. Catal. Today 2013, 204, 170–178. [Google Scholar] [CrossRef]
- He, R.; Ye, X.P.; English, B.C.; Satrio, J.A. Influence of pyrolysis condition on switchgrass bio-oil yield and physicochemical properties. Bioresour. Technol. 2009, 100, 5305–5311. [Google Scholar] [CrossRef]
- Guo, X.; Wang, S.; Wang, Q.; Guo, Z.; Luo, Z. Properties of bio-oil from fast pyrolysis of rice husk. Chin. J. Chem. Eng. 2011, 19, 116–121. [Google Scholar] [CrossRef]
- Bakar, M.S.A.; Titiloye, J.O. Catalytic pyrolysis of rice husk for bio-oil production. J. Anal. Appl. Pyrolysis 2013, 103, 362–368. [Google Scholar] [CrossRef]
- Lazzari, E.; Schena, T.; Primaz, C.T.; da Silva Maciel, G.P.; Machado, M.E.; Cardoso, C.A.L.; Jacques, R.A.; Caramão, E.B. Production and chromatographic characterization of bio-oil from the pyrolysis of mango seed waste. Ind. Crops Prod. 2016, 83, 529–536. [Google Scholar] [CrossRef]
- Grioui, N.; Halouani, K.; Agblevor, F.A. Bio-oil from pyrolysis of Tunisian almond shell: Comparative study and investigation of aging effect during long storage. Energy Sustain. Dev. 2014, 21, 100–112. [Google Scholar] [CrossRef]

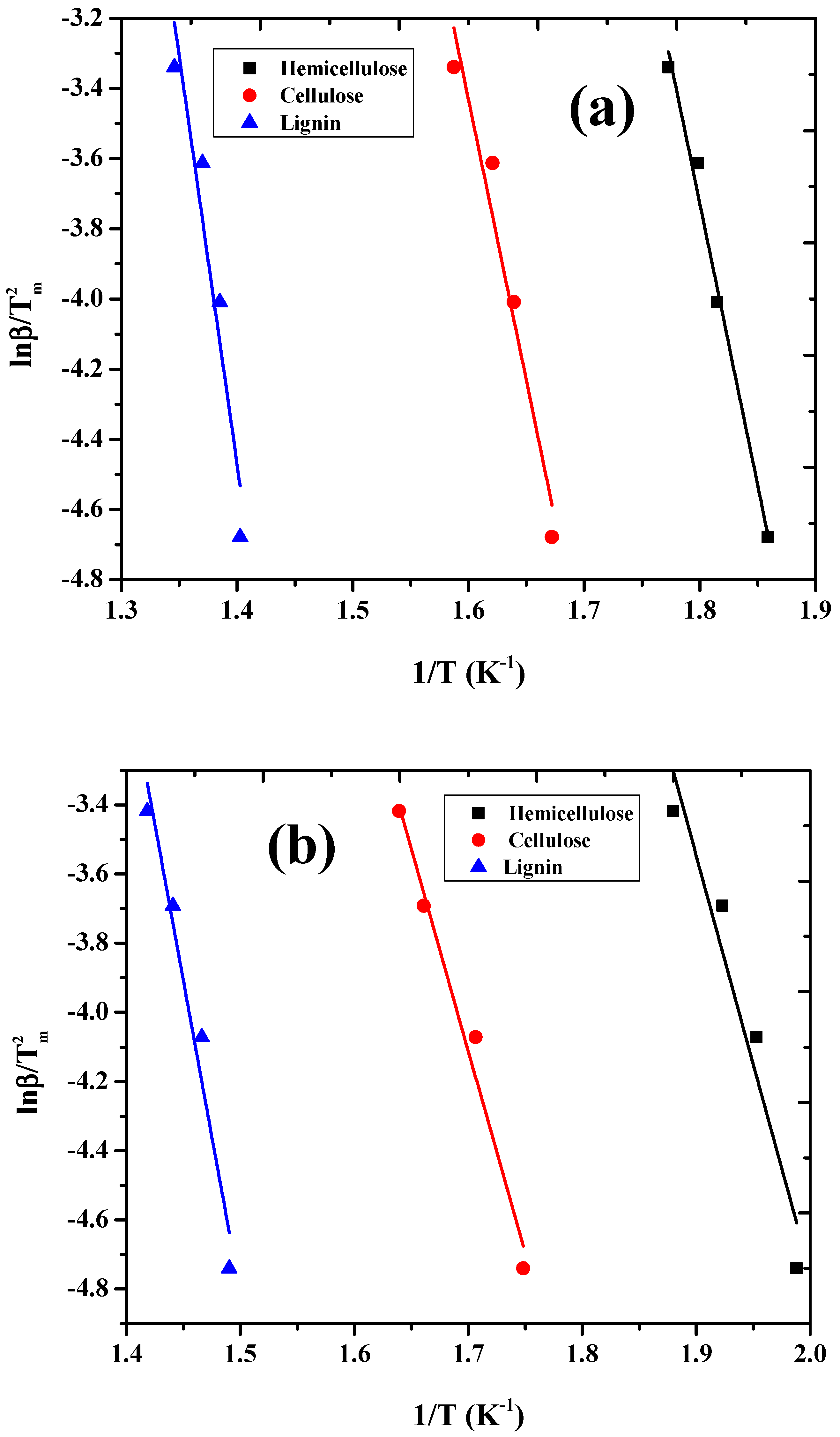


| Components | Catalytic | Non-Catalytic | ||
|---|---|---|---|---|
| Ea (kJ/mol) | A (min−1) | Ea (kJ/mol) | A (min−1) | |
| Hemicellulose | 133.02 | 2.3 × 108 | 91.45 | 8.0 × 105 |
| Cellulose | 141.33 | 8.0 × 109 | 99.76 | 3.8 × 106 |
| Lignin | 191.22 | 1.1 × 1011 | 149.65 | 1.6 × 109 |
| Peak No. | R/Time (min) | Compound Name | Mol. Formula | Mol. wt. | Percent Area |
|---|---|---|---|---|---|
| 1 | 1.85 | Furfural | C5H4O2 | 96 | 19.35 |
| 2 | 2.73 | Cyclopropane,2-(1,1-dimethyl-2-pentenyl)-1,1-dimethyl | C12H22 | 166 | 5.78 |
| 3 | 3.30 | 2-Furancarboxaldehyde, 5-methyl | C6H6O2 | 110 | 9.94 |
| 4 | 4.22 | 1,2-Cyclopentanedione, 3-methyl | C6H8O2 | 112 | 10.70 |
| 5 | 5.01 | Phenol, 2-methoxy- | C7H8O2 | 124 | 7.91 |
| 6 | 5.40 | Maltol | C6H6O3 | 126 | 3.72 |
| 7 | 5.87 | 2-Myristynoic acid | C14H24O2 | 224 | 0.48 |
| 8 | 6.44 | Phenol, 2-methoxy-4-methyl- | C8H10O2 | 138 | 2.89 |
| 9 | 6.78 | 1,4:3,6-Dianhydro-à-d-glucopyranose | C6H8O4 | 144 | 3.06 |
| 10 | 7.13 | 2-Furancarboxaldehyde, 5-(hydroxymethyl) | C6H6O3 | 126 | 4.92 |
| 11 | 7.60 | Phenol, 4-ethyl-2-methoxy- | C9H12O2 | 152 | 2.28 |
| 12 | 8.05 | 2-Oxabicyclo[3.3.0]oct-7-en-3-one, 7-(1-hydroxypentyl)- | C12H18O3 | 210 | 1.19 |
| 14 | 8.60 | Phenol, 2,6-dimethoxy- | C8H10O3 | 154 | 6.94 |
| 14 | 9.21 | Vanillin | C8H8O3 | 152 | 3.22 |
| 15 | 9.78 | 1,2,4-Trimethoxybenzene | C9H12O3 | 168 | 2.28 |
| 16 | 10.29 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)- | C9H10O3 | 166 | 1.29 |
| 17 | 10.80 | Silane, dimethoxymethylphenyl | C9H14O2Si | 182 | 3.37 |
| 18 | 11.63 | Phenol,2,6-dimethoxy-4-(2propenyl)- | C11H14O3 | 194 | 1.69 |
| 19 | 12.28 | Benzaldehyde,4-hydroxy-3,5-dimethoxy- | C9H10O4 | 182 | 3.75 |
| 20 | 13.08 | Ethanone,1-(4-hydroxy-3,5-dimethoxyphenyl)- | C10H12O4 | 196 | 2.33 |
| 21 | 13.47 | 3,5-Dimethoxy-4 hydroxyphenylaceticAcid | C10H12O5 | 212 | 1.34 |
| 22 | 14.02 | Ethanone,1-(4-hydroxy-3,5-dimethoxyphenyl)- | C10H12O4 | 196 | 0.58 |
| 23 | 14.55 | 3,9;7,8-Diepoxybicyclo[4.3.0]nonane-6á-ol-2-one,9à-acetoxy-4-isopropenyl-5-methoxycarbonyl-1-methyl- | C18H22O8 | 366 | 0.15 |
| 24 | 14.89 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6, 9-diene-2,8-dione | C17H24O3 | 276 | 0.32 |
| 25 | 15.28 | Estra-1,3,5(10)-trien-17á-ol | C18H24O | 256 | 0.32 |
| 26 | 15.56 | 3,5-Dimethoxy-4-hydroxycinnamaldehy | C11H12O4 | 208 | 0.88 |
| 27 | 16.09 | Curan-19,20-diol, 16,17-didehydro-, (19S)- | C19H24N2O2 | 312 | 0.07 |
| 28 | 16.52 | 8-Octadecenoic acid, methyl ester | C19H36O2 | 296 | 0.07 |
| 29 | 17.17 | Octadecanoic acid | C18H36O2 | 284 | 0.38 |
| 30 | 18.58 | 2-Nonadecanone 2,4-dinitrophenylhydrazine | C25H42N4O4 | 462 | 0.02 |
| 31 | 18.93 | 9,12,15-Octadecatrienoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester,(Z,Z,Z)- | C27H52O4Si2 | 496 | 0.05 |
| 32 | 20.21 | 1,2-Benzenedicarboxylicacid,diisooctyl ester | C24H38O4 | 390 | 0.32 |
| 33 | 21.78 | 2,5-Dimethoxy-4-ethylamphetamine | C13H21NO2 | 223 | 0.12 |
| 34 | 26.35 | Sertindole | C24H26ClFN4O | 440 | 0.43 |
| Biomass | Viscosity (cP) | Density gm−3 | Fluidity (cP)−1 | Specific Gravity | API Gravity | Reference |
|---|---|---|---|---|---|---|
| Rice husk | 82.43 | 1.190 | - | - | - | [58] |
| Rice husk/Al-MCM-41 | 1.65 | - | - | - | - | [59] |
| Mango seed | 149 | - | - | - | - | [60] |
| Almond shell | <100 | - | - | - | - | [61] |
| Cobalt-impregnated sesame stalk | 176 | 1.047 | [40] | |||
| Sesame waste biomass/Ni/Co/MCM-41 | 1.13 | 1.01 | 0.975 | 1.231 | −1.687 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisar, J.; Ullah, R.; Ali, G.; Shah, A.; Din, M.I.; Hussain, Z.; Amin, R. Thermocatalytic Decomposition of Sesame Waste Biomass over Ni-Co-Doped MCM-41: Kinetics and Physicochemical Properties of the Bio-Oil. Energies 2023, 16, 3731. https://doi.org/10.3390/en16093731
Nisar J, Ullah R, Ali G, Shah A, Din MI, Hussain Z, Amin R. Thermocatalytic Decomposition of Sesame Waste Biomass over Ni-Co-Doped MCM-41: Kinetics and Physicochemical Properties of the Bio-Oil. Energies. 2023; 16(9):3731. https://doi.org/10.3390/en16093731
Chicago/Turabian StyleNisar, Jan, Raqeeb Ullah, Ghulam Ali, Afzal Shah, Muhammad Imran Din, Zaib Hussain, and Roohul Amin. 2023. "Thermocatalytic Decomposition of Sesame Waste Biomass over Ni-Co-Doped MCM-41: Kinetics and Physicochemical Properties of the Bio-Oil" Energies 16, no. 9: 3731. https://doi.org/10.3390/en16093731





