Calorific Value of Zea mays Biomass Derived from Soil Contaminated with Chromium (VI) Disrupting the Soil’s Biochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Procedure of the Experiment
2.3. Calorific Value Determination
- Hv—calorific value of air-dried plant biomass (MJ kg−1);
- Q—heat of combustion of air-dried plant biomass;
- MC—biomass moisture content (%);
- 0.0244—correction coefficient for water vaporization enthalpy (MJ kg−1 per 1% moisture content).
- YEP—energy yield of plant biomass (MJ);
- Hv—calorific value of air-dried plant biomass (MJ kg−1);
- Y—aerial biomass yield of (kg) Zea mays per 1 kg of soil.
2.4. Determination of Enzyme Activity
2.5. Physicochemical and Chemical Analyzes of Soil
2.6. Statistical Analyzes and Calculations
- IF—index of the effect;
- y—chromium, compost, or HumiAgra, respectively;
- B—value of the dependent variable of the tested object;
- C—value of the dependent variable of the control object;
- x—tested parameter, e.g., biomass, enzyme activity, etc.
3. Results
3.1. Yields of Zea mays in Soil Contaminated with Cr(VI) and the Crop’s Energy Efficiency
3.2. Activity of Soil Enzymes in Soil Contaminated with Cr(VI)
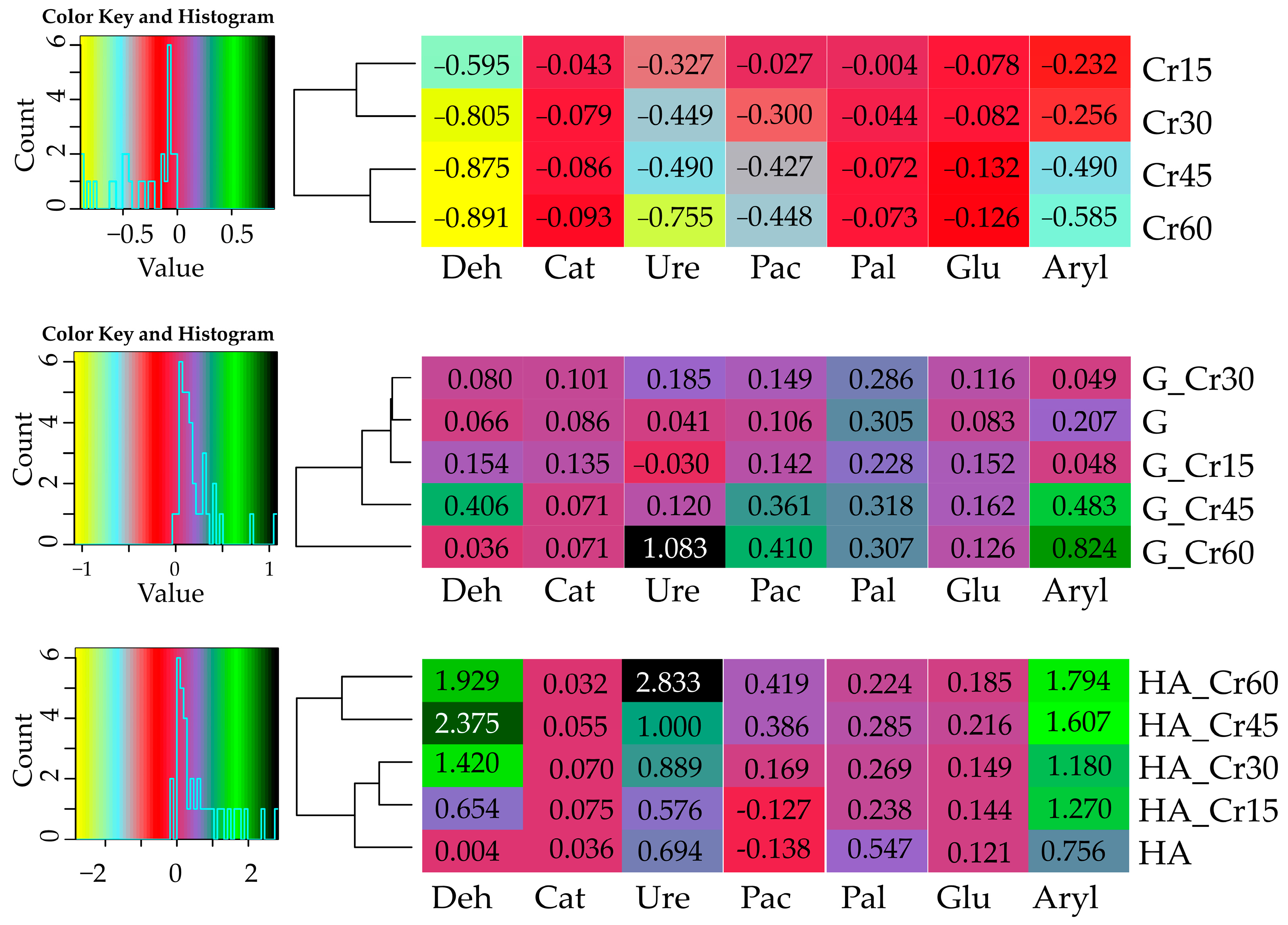
3.3. Interactions between the Yield of Zea mays and its Energy Yield and Soil Enzyme Activity
4. Discussion
4.1. Yield of Zea mays in Soil Contaminated with Cr(VI) and its Energy Efficiency
4.2. Activity of Soil Enzymes in Soil Contaminated with Cr(VI)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Butler, C.D. Climate Change, Health and Existential Risks to Civilization: A Comprehensive Review (1989–2013). Int. J. Environ. Res. Public Health 2018, 15, 2266. [Google Scholar] [CrossRef] [PubMed]
- Huggel, C.; Bouwer, L.M.; Juhola, S.; Mechler, R.; Muccione, V.; Orlove, B.; Wallimann-Helmer, I. The Existential Risk Space of Climate Change. Clim. Change 2022, 174, 8. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Climate & Energy Framework. 2030. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2030-climate-energy-framework_pl (accessed on 2 February 2023).
- Hoang, A.T.; Varbanov, P.S.; Nižetić, S.; Sirohi, R.; Pandey, A.; Luque, R.; Ng, K.H.; Pham, V.V. Perspective Review on Municipal Solid Waste-to-Energy Route: Characteristics, Management Strategy, and Role in Circular Economy. J. Clean. Product 2022, 359, 131897. [Google Scholar] [CrossRef]
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2023, 10, 29. [Google Scholar] [CrossRef]
- Gradziuk, P.; Gradziuk, B.; Trocewicz, A.; Jendrzejewski, B. Potential of Straw for Energy Purposes in Poland—Forecasts Based on Trend and Causal Models. Energies 2020, 13, 5054. [Google Scholar] [CrossRef]
- Gocławski, J.; Korzeniewska, E.; Sekulska-Nalewajko, J.; Kiełbasa, P.; Dróżdż, T. Method of Biomass Discrimination for Fast Assessment of Calorific Value. Energies 2022, 15, 2514. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Peters, B.; Smuła-Ostaszewska, J. Simultaneous Prediction of Potassium Chloride and Sulphur Dioxide Emissions during Combustion of Switchgrass. Fuel 2012, 96, 29–42. [Google Scholar] [CrossRef]
- Narnaware, S.L.; Panwar, N.L. Biomass Gasification for Climate Change Mitigation and Policy Framework in India: A Review. Bioresour Technol. Rep. 2022, 17, 100892. [Google Scholar] [CrossRef]
- Panoutsou, C.; Giarola, S.; Ibrahim, D.; Verzandvoort, S.; Elbersen, B.; Sandford, C.; Malins, C.; Politi, M.; Vourliotakis, G.; Zita, V.E.; et al. Opportunities for Low Indirect Land Use Biomass for Biofuels in Europe. Appl. Sci. 2022, 12, 4623. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Energetic Value of Elymus Elongatus L. and Zea Mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals. Energies 2021, 14, 4903. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. Calorific Value of Festuca Rubra Biomass in the Phytostabilization of Soil Contaminated with Nickel, Cobalt and Cadmium Which Disrupt the Microbiological and Biochemical Properties of Soil. Energies 2022, 15, 3445. [Google Scholar] [CrossRef]
- Vahabisani, A.; An, C. Use of biomass-derived adsorbents for the removal of petroleum pollutants from water: A mini-review. Environ. Syst. Res. 2021, 10, 25. [Google Scholar] [CrossRef]
- Guerin, T.F. The effect of interactions between soil compaction and phenol contamination on plant growth characteristics: Implications for scaling bioremediation at industrial sites. J. Environ. Manag. 2022, 302, 114017. [Google Scholar] [CrossRef]
- Jakl, M.; Kovač, I.; Zeljković, S.Ć.; Dytrtová, J.J. Triazole Fungicides in Soil Affect the Yield of Fruit, Green Biomass, and Phenolics Production of Solanum lycopersicum L. Food Chem. 2021, 351, 129328. [Google Scholar] [CrossRef]
- Serowaniec, M. Sustainable Development Policy and Renewable Energy in Poland. Energies 2021, 14, 2244. [Google Scholar] [CrossRef]
- Ezzahra Yatim, F.; Boumanchar, I.; Srhir, B.; Chhiti, Y.; Jama, C.; Ezzahrae, M.; Alaoui, F. Waste-to-Energy as a Tool of Circular Economy: Prediction of Higher Heating Value of Biomass by Artificial Neural Network (ANN) and Multivariate Linear Regression (MLR). Waste Manag. 2022, 153, 293–303. [Google Scholar] [CrossRef]
- la Rosa, A.D.; Greco, S.; Tosto, C.; Cicala, G. LCA and LCC of a Chemical Recycling Process of Waste CF-thermoset Composites for The Production of Novel CF-thermoplastic Composites. Open Loop and Closed Loop Scenarios. J. Clean. Prod. 2021, 304, 127158. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Santiago-Herrera, M.; Ibáñez, J.; Yousaf, S.; Iqbal, M.; Martel-Martín, S.; Barros, R. Sustainability of Phytoremediation: Post-harvest stratagems and Economic Opportunities for the Produced Metals Contaminated Biomass. J. Environ. Manag. 2023, 326 Pt B, 116700. [Google Scholar] [CrossRef]
- Mata, T.M.; Rodrigues, S.; Caetano, N.S.; Martins, A.A. Life Cycle Assessment of Bioethanol from Corn Stover from Soil Phytoremediation. Energy Rep. 2022, 8, 468–474. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A.; Thimmanagari, M.; Defersha, F. Techno-economic Assessment of Corn Stover for Hybrid Bioenergy Production: A sustainable approach. Case Stud. Therm. Eng. 2019, 13, 100408. [Google Scholar] [CrossRef]
- Etumuluru, J.S. Comparison of Chemical Composition and Energy Property of Torrefied Switchgrass and Corn Stover. Front. Energy Res. 2015, 3, 46. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Cheng, M.; Su, Y.; Li, Y.; Jiang, W.; Li, H.; Zhao, Y.; Wen, X.; Zhang, L.; Ali, A.; et al. All Polyploidization Facilitates Gene Flow and Speciation Among Corn, Zea perennis and Tripsacum dactyloides. Planta 2019, 249, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the Global Number and Distribution of Maize and Wheat Farms. Glob. Food Sec. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea mays and Soil Microorganisms to the Toxic Effect of Chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Murthy, M.K.; Khandayataray, P.; Padhiary, S.; Samal, D. A Review on Chromium Health Hazards and Molecular Mechanism of Chromium Bioremediation. Rev. Environ. Health 2022. online ahead of print. [Google Scholar] [CrossRef]
- Wu, Q.; Mo, W.; Liu, J.; Peng, S.; Li, Q.; Wan, R. Remediation of High-Concentration Cr(VI)-Contaminated Soils with FeSO4 Combined with Biostimulation: Cr(VI) Transformation and Stabilization. J. Hazard. Mater. Adv. 2022, 8, 100161. [Google Scholar] [CrossRef]
- Laurenti, R.; Redwood, M.; Puig, R.; Frostell, B. Measuring the Environmental Footprint of Leather Processing Technologies. J. Ind. Ecol. 2017, 21, 1180–1187. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in Agricultural Oils and Crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and Biotransformation of Hexavalent Chromium [Cr(VI)]: A Comprehensive Review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef]
- Namieśnik, J.; Rabajczyk, A. Speciation Analysis of Chromium in Environmental Samples. Crit. Rev. Environ. Sci. Technol. 2012, 42, 327–377. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium Speciation, Bioavailability, Uptake, Toxicity and Detoxification in Soil-plant System: A Review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, Toxicity, Microbial Remediation and Phytoremediation of Soil Chromium Contamination. Environ. Chem. Lett. 2021, 19, 1413–1431. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, M.; Fahad, S.; Qayyum, A.; Chen, Y.; Zhu, G. Chromium Induces Toxicity at Different Phenotypic, Physiological, Biochemical, and Ultrastructural Levels in Sweet Potato (Ipomoea batatas L.) Plants. Int. J. Mol. Sci. 2022, 23, 13496. [Google Scholar] [CrossRef]
- Houri, T.; Khairallah, Y.; Zahab, A.A.; Osta, B.; Romanos, D.; Haddad, G. Heavy Metals Accumulation Effects on the Photosynthetic Performance of Geophytes in Mediterranean reserve. J. King Saud. Univ. Sci. 2020, 32, 874–880. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Atresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Terzi, H.; Yıldız, M. Proteomic Analysis Reveals the Role of Exogenous Cysteine in Alleviating Chromium Stress in Maize Seedlings. Ecotoxicol. Environ. Saf. 2021, 209, 111784. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Proline and Betaine Provide Protection to Antioxidant and Methylglyoxal Detoxification Systems During Cold Stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol. Plant 2009, 31, 261–269. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.X.; Peng Tian, P.; Yu-Juan Lin, Y.J.; Yu, X.Z. Proline-mediated regulation on jasmonate signals repressed anthocyanin accumulation through the MYB-bHLH-WDR complex in rice under chromium exposure. Front. Plant Sci. 2022, 13, 953398. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, S.; Sirohi, R.; Kim, S.H.; Ngo, H.H.; Pandey, A. Uptake and Mobilization of Heavy Metals Through Phytoremediation Process from Native Plants Species Growing on Complex Pollutants: Antioxidant Enzymes and Photosynthetic Pigments Response. Environ. Technol. Innov. 2021, 23, 101629. [Google Scholar] [CrossRef]
- Kazan, K. Auxin and the Integration of Environmental Signals into Plant Root Development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Aftab, N.; Saleem, K.; Khan, A.H.A.; Butt, T.A.; Mirza, C.R.; Hussain, J.; Farooq, G.; Tahir, A.; Yousaf, S.; Zafar, M.I.; et al. Cosmos Sulphureous Cav. is More Tolerant to Lead than Copper and Chromium in Hydroponics System. Int. J. Environ. Sci. Technol. 2021, 18, 2325–2334. [Google Scholar] [CrossRef]
- EC. Circular Economy—Implementation of the Circular Economy Action Plan. European Commission. 2018. Available online: http://ec.europa.eu/environment/circular-economy/index (accessed on 12 February 2023).
- EC. Proposal for a Regulation on the Making Available on the Market of CE Marked Products Fertilising and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009. COM (2016) 157 Final 2016/00084 (COD). European Commission. 2016. Available online: https://ec.europa.eu/transparency/regdoc/rep/1/2016/EN/1-2016-157-EN-F1-1.PDF (accessed on 12 February 2023).
- Qian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a New Option for Soil Improvement: Application in Various Problem Soils. Sci. Total. Environ. 2023, 870, 162024. [Google Scholar] [CrossRef] [PubMed]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of Soil Amendments to Contaminated Soils for Heavy Metal Immobilization and Improved Soil Quality—A Critical Review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Response of soil alkaline phosphatase to biochar amendments: Changes in kinetic and thermodynamic Characteristics. Geoderma 2019, 337, 44–54. [Google Scholar] [CrossRef]
- Marx, M.C.; Kandeler, E.; Wood, M.; Wermbter, N.; Jarvis, S.C. Exploring the Enzymatic Landscape: Distribution and Kinetics of Hydrolytic Enzymes in Soil Particle Size Fractions. Soil Biol. Biochem. 2005, 37, 35–48. [Google Scholar] [CrossRef]
- PN-EN ISO 18125:2017-07; Solid Biofuels—Determination of Calorific Value. European Committee for Standardization: Brussels, Belgium, 2017. Available online: https://pkn.pl/pn-en-iso-18125-2017-07 (accessed on 10 December 2022).
- Kopetz, H.; Jossart, J.; Ragossnig, H.; Metschina, C. European Biomass Statistics; European Biomass Association: Brussels, Belgium, 2007. [Google Scholar]
- Öhlinger, R. Dehydrogenase Activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin, Germany, 1996; pp. 241–243. [Google Scholar]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic: London, UK, 1998; pp. 316–365. [Google Scholar]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Role of Chlorella sp. and Rhamnolipid 90 in Maintaining Homeostasis in Soil Contaminated with Bisphenol A. J. Soils Sediments 2021, 21, 27–41. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenol A—A Dangerous Pollutant Distorting the Biological Properties of Soil. Int. J. Mol. Sci. 2021, 22, 12753. [Google Scholar] [CrossRef]
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warsaw, Poland, 1998.
- ISO 11464; Soil Quality—Pre-Treatment of Samples for Physico-Chemical Analysis. International Organization for Standardization: Geneva, Switzerland, 2006.
- Klute, A. Methods of Soil Analysis; American Society of Agronomy, Agronomy Monograph 9: Madison, WI, USA, 1996. [Google Scholar]
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. ISO: Geneva, Switzerland, 1995.
- Nelson, D.; Sommers, L. Total carbon, organic carbon, and organic matter. In Method of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA; Available online: https://posit.co/products/open-source/rstudio/ (accessed on 8 December 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 8 December 2022).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. R Package Version 2.17.0.2020. Available online: https://cran.r-project.org/package=gplots (accessed on 8 December 2022).
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: https://www.tibco.com/products/data-science (accessed on 20 December 2022).
- Tangahu, B.V.; Sheikh-Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants Through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Mohammed, B.; Mohammed, T.; M’hammed, E.; Tarik, A. Physiological and Physico-Chemical Study of the Effect of Chromium (VI) on the Nutritional Quality of Maize (Zea Mays L). Procedia Comput. Sci. 2021, 191, 463–468. [Google Scholar] [CrossRef]
- Polti, M.A.; Atjián, M.C.; Amoroso, M.J.; Abate, C.M. Soil Chromium Bioremediation: Synergic Activity of Actinobacteria and Plants. Int. Biodeterior. Biodegrad. 2011, 65, 1175–1181. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 111. [Google Scholar] [CrossRef]
- Shanker, A.K.; Djanaguiraman, D.; Sudhagar, R.; Chandrashekar, C.N.; Pathmanabhan, G. Differential Antioxidative Response of Ascorbate Glutathione Pathway Enzymes and Metabolites to Chromium Speciation Stress in Green Gram (Vigna radiata (L.) R. Wilczek. cv CO4) Roots. Plant Sci. 2004, 166, 1035–1043. [Google Scholar] [CrossRef]
- Jun, R.; Ling, T.; Guanghua, Z. Effects of Chromium on Seed Germination, Root Elongation and Coleoptiles Growth in Six Pulses. Inter. J. Environ. Sci. Technol. 2009, 6, 571–578. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Khalid, S.; Bibi, I.; Naeem, M.A.; Niazi, N.K.; Tack, F.M.G.; Ippolito, J.A.; Rinklebe, J. Influence of Biochar on Trace Element Uptake, Toxicity and Detoxification in Plants and Associated Health Risks: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2021, 52, 2803–2843. [Google Scholar] [CrossRef]
- Turkan, I.; Uzilday, B.; Dietz, K.J.; Brautigam, A.; Ozgur, R. Reactive Oxygen Species and Redox Regulation in Mesophyll and Bundle Sheath Cells of C4 Plants. J. Exp. Bot. 2018, 69, 3321–3331. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a Key Player in Metal-induced Oxidative Stress Defences. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Iqbal, M.; Bharwana, S.A.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol Alleviates Chromium Toxicity in Wheat Plants in Relation to Growth, Yield, Stimulation of Anti-oxidative Enzymes, Oxidative Stress and Cr Uptake in Sand and Soil Media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.M.; Young, S.D.; Bailey, E.H.; Humphrey, O.S.; Watts, M.J. Assessment of Chromium Species Dynamics in Root Solutions Using Isotope Tracers. J. Trace Elem. Med. Biol. 2020, 61, 126514. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Xu, M. Chromium Morpho-Phytotoxicity. Plants 2020, 9, 564. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zayed, A.; Lytle, C.M.; Qian, J.-H.; Terry, N. Chromium Accumulation, Translocation and Chemical Speciation in Vegetable Crops. Planta 1998, 206, 293–299. [Google Scholar] [CrossRef]
- Galant, A.; Preuss, M.L.; Cameron, J.C.; Jez, J.M. Plant Glutathione Biosynthesis: Diversity in Biochemical Regulation and Reaction Products. Front. Plant Sci. 2011, 2, 45. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of Water, Salinity, and Cold Stress Responses by Salicylic Acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and Functional Alterations in Photosynthetic Apparatus of Plants under Cadmium Stress. Botan. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological Changes Induced by Chromium Stress in Plants: An Overview. Protoplasma 2012, 249, 599–611. [Google Scholar] [CrossRef]
- Angelova, V.R.; Akova, V.I.; Artinova, N.S.; Ivanov, K.I. The Effect of Organic Amendments on Soil Chemical Characteristics. Bulg. J. Agric. Sci. 2013, 19, 958–971. [Google Scholar]
- Beesley, L.; Marmiroli, M.; Pagano, L.; Pigoni, V.; Fellet, G.; Fresno, T.; Vamerali, T.; Bandiera, M.; Marmiroli, N. Biochar Addition to an Arsenic Contaminated Soil Increases Arsenic Concentrations in the Pore Water but Reduces Uptake to Tomato Plants (Solanum lycopersicum L.). Sci. Total. Environ. 2013, 454–455, 598–603. [Google Scholar] [CrossRef]
- Ahmed, N.; Shah, A.R.; Danish, S.; Fahad, S.; Ali, M.A.; Zarei, T.; Vranová, V.; Datta, R. Immobilization of Cd in Soil by Biochar and New Emerging Chemically Produced Carbon. J. King Saud. Univ. Sci. 2021, 33, 101472. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of Biochar for the Management of Contaminated Soil: Preparation, Application and Prospect. Sci. Total. Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Lima, M.; Eloy, N.; Batista de Siqueira, J.A.; Inzé, D.; Hemerly, A.S.; Ferreira, P.C.G. Molecular Mechanisms of Biomass Increase in Plants. Biotech. Res. Innov. 2017, 1, 14–25. [Google Scholar] [CrossRef]
- Mahapatra, S.; Ali, M.H.; Samal, K. Assessment of compost maturity-stability indices and recent development of composting bin. Energy Nexus. 2022, 6, 100062. [Google Scholar] [CrossRef]
- Wanga, G.; Zhang, J.; Lee, J.Y.; Mao, X.; Ye, L.; Xu, W.; Ning, X.; Zhang, N.; Teng, H.; Wang, C. Hydrothermal Carbonization of Maize Straw for Hydrochar Production and Its Injection for Blast Furnace. Appl. Energy 2020, 266, 114818. [Google Scholar] [CrossRef]
- Sunyong Park, S.; Kim, S.J.; Oh, K.C.; Cho, L.; Jeon, Y.K.; Lee, Y.K.; Kim, D.H. Termogravimetric Analysis-based Proximate Analysis of Agro-byproducts and Prediction of Calorific Value. Enegy. Rep. 2022, 8, 12038–12044. [Google Scholar] [CrossRef]
- Herkowiak, M.; Adamski, M.; Dworecki, Z.; Waliszewska, B.; Pilarski, K.; Witaszek, K.; Niedbała, G.; Piekutowska, M. Analysis of the Possibility of Obtaining Thermal Energy from Combustion of Selected Cereal Straw Species. J. Res. Appl. Agric. Eng. 2018, 63, 68–72. [Google Scholar]
- Naik, S.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian biomass for Alternative Renewable Biofuel. Renew. Energy 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.J.; Graban, Ł.; Lajszner, W.; Kuriata, T. Camelina and Crambe Oil Crops for Bioeconomy—Straw Utilisation for Energy. Energies 2020, 13, 1503. [Google Scholar] [CrossRef]
- Kalita, D.; Saikia, C.N. Chemical constituents and energy content of some latex bearing plants. Biores. Technol. 2004, 92, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Jasinskas, A.; Kleiza, V.; Streikus, D.; Domeika, R.; Vaiciukevičius, E.; Gramauskas, G.; Valentin, M.T. Assessment of Quality Indicators of Pressed Biofuel Produced from Coarse Herbaceous Plants and Determination of the Influence of Moisture on the Properties of Pellets. Sustainability 2022, 14, 1068. [Google Scholar] [CrossRef]
- Monedero, E.; Hernández, J.J.; Collado, R. Combustion-Related Properties of Poplar, Willow and Black Locust to Be Used as Fuels in Power Plants. Energies 2017, 10, 997. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Szczukowski, S.; Krzyżaniak, M.; Tworkowski, J. Energy Value of Yield and Biomass Quality in a 7-Year Rotation of Willow Cultivated on Marginal Soil. Energies 2020, 13, 2144. [Google Scholar] [CrossRef]
- Bury, M.; Możdżer, E.; Kitczak, T.; Siwek, H.; Włodarczyk, M. Yields, Calorific Value and Chemical Properties of Cup Plant Silphium Perfoliatum L. Biomass, Depending on the Method of Establishing the Plantation. Agronomy 2020, 10, 851. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Przybył, J.; Czajkowski, Ł.; Majka, J.; Pawłowski, A. Effects of Harvest Maturity on the Chemical and Energetic Properties of Corn Stover Biomass Combustion. Materials 2022, 15, 2831. [Google Scholar] [CrossRef]
- Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Engineering Abiotic Stress Response in Plants for Biomass Production. J. Biol. Chem. 2018, 293, 5035–5043. [Google Scholar] [CrossRef]
- Simonnot, M.O.; Vaughan, J.; Laubie, B. Processing of Bio-ore to Products. In Agromining: Farming for Metals; Springer: Berlin, Germany, 2018; pp. 39–51. [Google Scholar] [CrossRef]
- Burns, R.G.; De Forest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J.; Lajszner, W. The Effects of Copper on Soil Biochemical Properties and Its Interaction with Other Heavy Metals. Pol. J. Environ. Stud. 2006, 15, 927–934. [Google Scholar]
- D’Ascoli, R.; Rao, M.A.; Adamo, P.; Renella, G.; Landi, L.; Rutigliano, F.A.; Terribile, F.; Gianfreda, L. Impact of River Overflowing on Trace Element Contamination of Volcanic Soils in South Italy: Part II. Soil Biological and Biochemical Properties in Relation to Trace Element Speciation. Environ. Pollut. 2006, 144, 317–32686. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J.; Jastrzębska, E.; Hłasko, A. The Biological Properties of Soil as Influenced by Chromium Contamination. Pol. J. Environ. Stud. 2001, 10, 37–42. [Google Scholar]
- Wyszkowska, J. Soil Contamination by Chromium and Its Enzymatic Activity and Yielding. Pol. J. Environ. Stud. 2002, 11, 79–84. [Google Scholar]
- Abdu, N.; Abdullahi, A.A.; Abdulkadir, A. Heavy Metals and Soil Microbes. Environ. Chem. Lett. 2017, 15, 65–84. [Google Scholar] [CrossRef]
- Liu, S.; Pu, S.; Deng, D.; Huang, H.; Yan, C.; Ma, H.; Razavi, B.S. Comparable Effects of Manure and Its Biochar on Reducing Soil Cr Bioavailability and Narrowing the Rhizosphere Extent of Enzyme Activities. Environ. Inter. 2020, 134, 105277. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Burns, R.G. Ecology of Extracellular Enzyme Activities and Organic Matter Degradation in Soil: A Complex Community-driven Process. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 35–55. [Google Scholar]
- Stursova, M.; Sinsabaugh, R.L. Stabilization of Oxidative Enzymes in Desert Soil May Limit Organic Matter Accumulation. Soil Biol. Biochem. 2008, 40, 550–553. [Google Scholar] [CrossRef]
- Yuan, B.; Yue, D. Soil Microbial and Enzymatic Activities Across a Chrono sequence of Chinese Pine Plantation Development on the Loess Plateau of China. Pedosphere 2012, 22, 1–12. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Abdelly, C. Nickel, Cadmium and Lead Phytotoxicity and Potential of Halophytic Plants in Heavy Metal Extraction. Afr. J. Bot. 2017, 111, 99–110. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogo, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The Implications of Exoenzyme Activity on Microbial Carbon and Nitrogen Limitation in Soil: A Theoretical Model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil Microbial Communities and Activities Under Intensive Organic and Conventional Vegetable Farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112120. [Google Scholar] [CrossRef]
- Guangming, L.; Xuechen, Z.; Xiuping, W.; Hongbo, S.; Jingsong, Y.; Xiangping, W. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, J.; Shi, D.; Li, S.; Zhang, F.; Zhang, F. Syntheses, Urease Inhibition Activities, and Fluorescent Properties of Transition Metal Complexes. J. Coord. Chem. 2013, 66, 1616–1625. [Google Scholar] [CrossRef]
- Eka Safitri, E.; Heng, L.Y.; Musa Ahmad, M.; Ling, T.L. Fluorescence bioanalytical method for urea determination based on water soluble ZnS quantum dots. Sens. Actuators B Chem. 2017, 240, 763–769. [Google Scholar] [CrossRef]
- Klose, S.; Tabatabai, M.A. Urease Activity of Microbial Biomass in Soils. Soil Biol. Biochem. 1999, 31, 205–211. [Google Scholar] [CrossRef]
- Gomes de Melo, B.A.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace Elements in The Soil-plant Interface: Phytoavailability, Translocation, and Phytoremediation-a Review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The Role of Biochar and Biochar-compost in Improving Soil Quality and Crop Performance: A Review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]




 — cases.
— cases.
 — cases.
— cases.
| Plant | Calorific Value MJ kg−1 | Reference |
|---|---|---|
| Bromus inermis Leyss. | 17.231 | [98] |
| Calamagrostis epigejos L. (Roth) | 18.037 | [98] |
| Camelina sativa | 18.500 | [99] |
| Crambe abyssinica | 17.940 | [99] |
| Euphorbia nerrifolia | 21.487 | [100] |
| Elymus elongatus | 15.052 | [12] |
| Festuca rubra | 16.306 | [13] |
| Holcus lanatus L. | 16.029 | [98] |
| Mimusops elengi L. | 19.217 | [100] |
| Miscanthus sinensis | 17.840 | [101] |
| Nerium indicum | 18.443 | [100] |
| Populus × euramericana | 17.980 | [102] |
| Robinia pseudoacacia L. | 17.550 | [102] |
| Salix trianda L. × Salis viminalis L. | 17.930 | [102] |
| Salix viminalis L. * | 8.600–19.500 | [103] |
| Sida hermaphrodita | 17.430 | [101] |
| Silphium perfoliatum L. | 16.610 | [104] |
| Zea mays—corn cob cores | 16.190–16.530 | [105] |
| Zea mays—BBCH 51 phase | 14.799 | Own research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Calorific Value of Zea mays Biomass Derived from Soil Contaminated with Chromium (VI) Disrupting the Soil’s Biochemical Properties. Energies 2023, 16, 3788. https://doi.org/10.3390/en16093788
Wyszkowska J, Borowik A, Zaborowska M, Kucharski J. Calorific Value of Zea mays Biomass Derived from Soil Contaminated with Chromium (VI) Disrupting the Soil’s Biochemical Properties. Energies. 2023; 16(9):3788. https://doi.org/10.3390/en16093788
Chicago/Turabian StyleWyszkowska, Jadwiga, Agata Borowik, Magdalena Zaborowska, and Jan Kucharski. 2023. "Calorific Value of Zea mays Biomass Derived from Soil Contaminated with Chromium (VI) Disrupting the Soil’s Biochemical Properties" Energies 16, no. 9: 3788. https://doi.org/10.3390/en16093788







