The Effect of an Electric Field on the Sliding Friction of the Silicone Rubber against Selected Metals in Motor Base Oils
Abstract
1. Introduction
2. Properties of the Material Tested
Lubricants
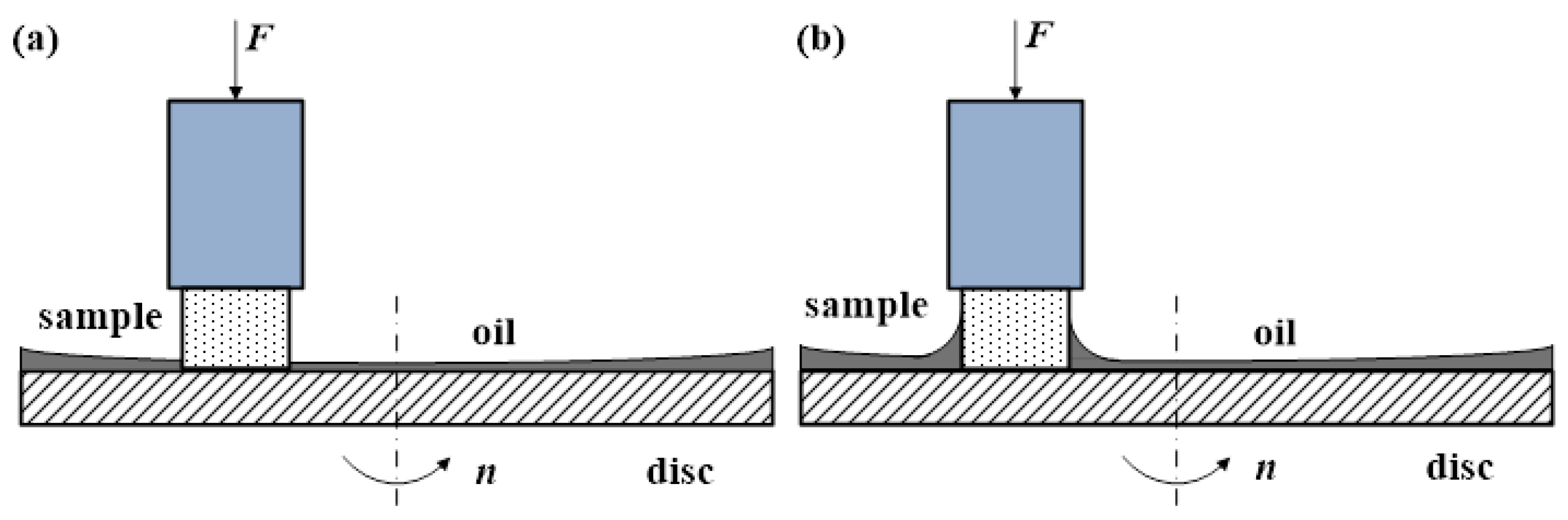
3. Details of the Tribological Measurement
3.1. Measurement Procedure
3.2. Numerical Investigation of External DC Electric Field
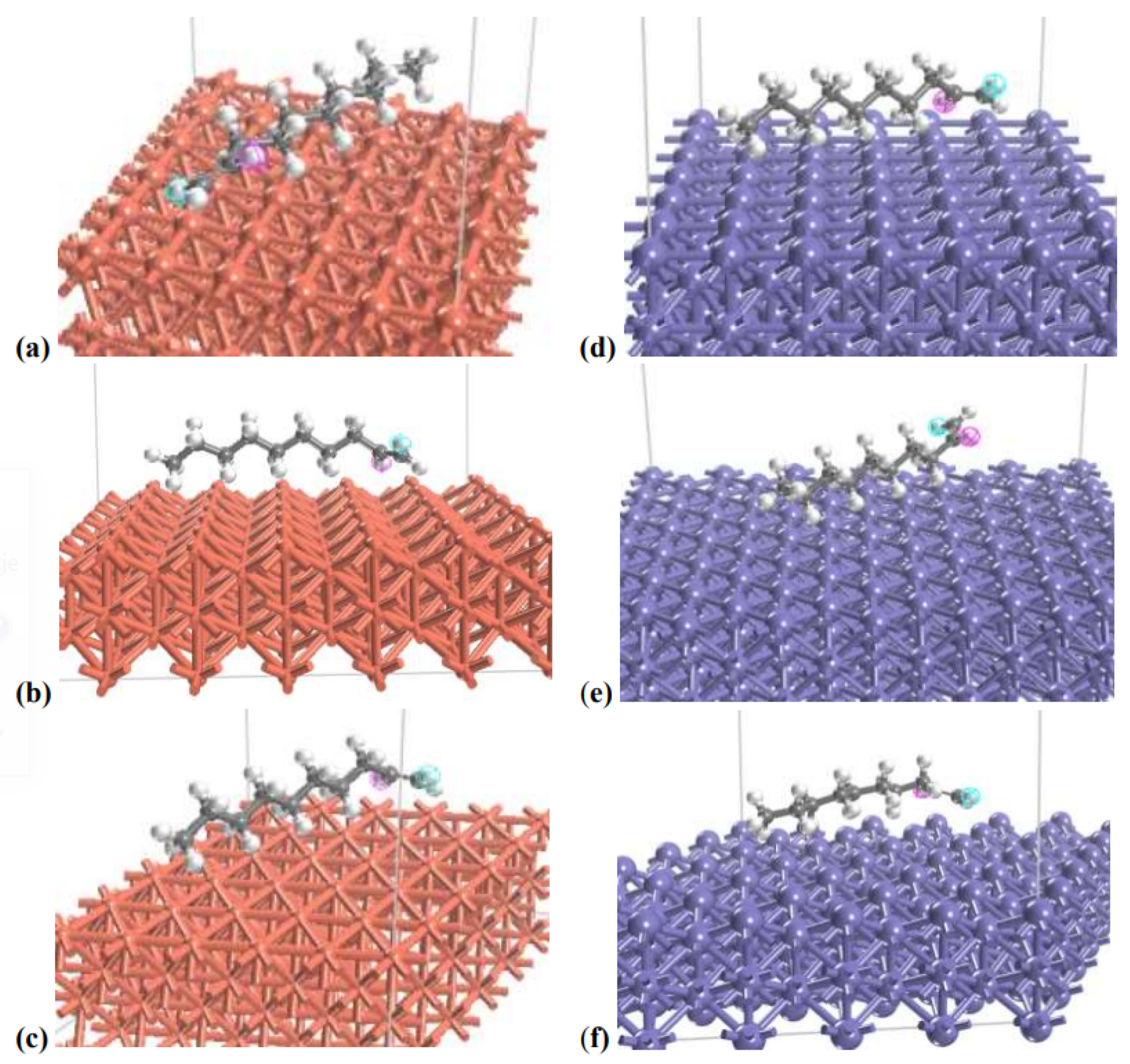
4. Molecular Simulation
5. Results
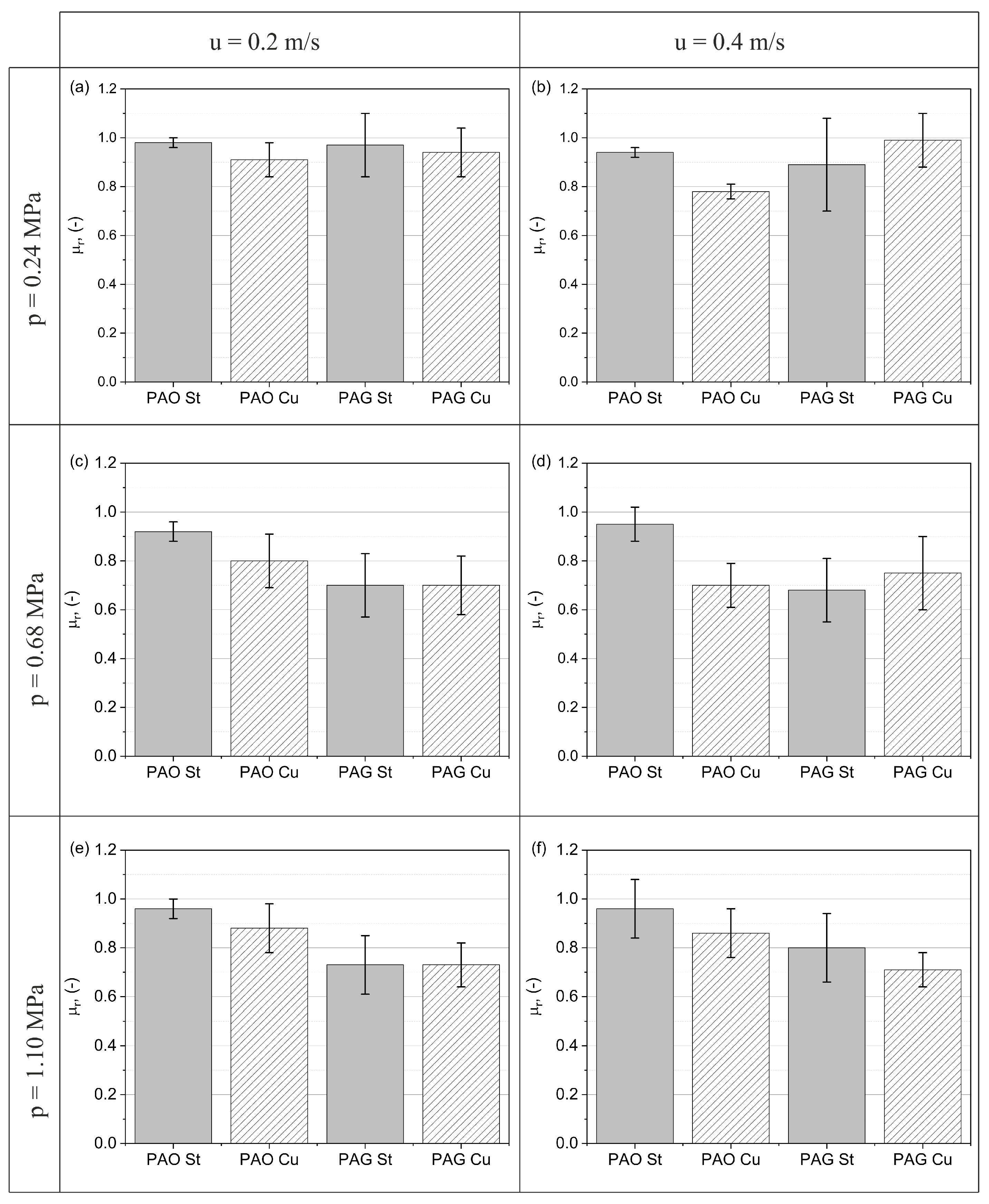
5.1. Experimental Results
5.2. Computational Results
6. Conclusions
- 1
- All the results presented indicate that the application of a DC voltage between the sample holder and the metal disc generates an electric field which causes a reduction in friction in almost all the cases analysed. The highest observed decrease in friction coefficient due to an external constant electric field was for the PAG-St pair and amounted to 32% and the lowest decrease in friction was for the PAO-St pair and was a minimal change.
- 2
- The adsorption energies obtained from molecular modeling simulations show a correlation with those obtained from the experiment.
- (a)
- The higher values of the friction coefficient can be correlated with the higher values of the lubricant on the metal adsorption energy of the metal. Additionally, the effect of friction coefficient change under the action of an electric field can be explained by the resultant effect of the polarity/nonpolarity of the lubricant molecule with the lubricant-metal interaction energy.
- (b)
- In the case of nonpolar PAO 6 molecules, the electric field does not generate significant forces in the system, which would affect the orientation of molecules and/or the reduction of oil molecule–surface interactions.
- (c)
- Polar PAG molecules are sensitive to the external electric field as a result of their dipole moment. In this case, an external electric field generates new forces that act on the polar molecule and reduce its interaction with the metal surface. In this way, the strong interaction between PAG and the steel surface, which contributes significantly to friction forces, is reduced by the electric field. As a result, the application of the electric field dramatically reduces the friction coefficient.
- (d)
- In contrast, when the electric field is applied to the PAG—copper system, where the oil molecule—metal interactions are relatively weak, their removal by the electric field has a moderate effect on the friction forces.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| a | coefficient |
| A | coefficient |
| b | coefficient |
| B | coefficient |
| adsorption energy | |
| the system composed of a molecule adsorbed on the metal surface energy | |
| single molecule energy | |
| metal surface energy | |
| bond stretching energy | |
| plane angles energy | |
| dihedral angles energy | |
| van der Waals energy | |
| electrostatic interaction energy | |
| cross-terms energy | |
| R-Square | |
| T | absolute temperature |
| U | DC voltage |
| dynamic viscosity | |
| coefficient of friction under the action of the DC electric field applied | |
| coefficient of friction without external DC electric field | |
| kinematic viscosity | |
| density | |
| p | contact pressure |
| u | sliding velocity |
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef][Green Version]
- Loehle, S. Understanding of Adsorption Mechanism and Tribological Behaviors of C18 Fatty Acids on Iron-Based Surfaces: A Molecular Simulation Approach. Ecole Centrale de Lyon. 2014. Available online: https://tel.archives-ouvertes.fr/tel-00999372/document (accessed on 8 March 2022).
- Hutchings, I.M. Tribology Friction and Wear of Engineering Materials; Elsevier Butterworth Heinemann Ltd.: London, UK, 1992. [Google Scholar]
- Fischer, D.A.; Hu, Z.S.; Hsu, S.M. Molecular orientation and bonding of monolayer stearic acid on a copper surface prepared in air. Tribol. Lett. 1997, 3, 41–45. [Google Scholar] [CrossRef]
- Spikes, H. Tribology research in the twenty-first century. Tribol. Int. 2001, 34, 789–799. [Google Scholar] [CrossRef]
- Granick, S. Molecular Tribology. MRS Bull. 1991, 16, 33–35. [Google Scholar] [CrossRef]
- Pawlak, R.; Kawai, S.; Meier, T.; Glatzel, T.; Baratoff, A.; Meyer, E. Single-molecule manipulation experiments to explore friction and adhesion. J. Phys. D Appl. Phys. 2017, 50, 113003. [Google Scholar] [CrossRef]
- Bhushan, B. Modern Tribology Handbook; Two Volume Set; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Bouyer, J.; Fillon, M.; Helene, M.; Beaurain, J.; Giraudeau, C. Behavior of a two-lobe journal bearing with a scratched shaft: Comparison between theory and experiment. J. Tribol. 2019, 141, 021702. [Google Scholar] [CrossRef]
- Scaraggi, M.; Mezzapesa, F.P.; Carbone, G.; Ancona, A.; Sorgente, D.; Lugarà, P.M. Minimize friction of lubricated laser-microtextured-surfaces by tuning microholes depth. Tribol. Int. 2014, 75, 123–127. [Google Scholar] [CrossRef]
- Cuia, S.; Gu, L.; Fillon, M.; Wang, L.; Zhang, C. The effects of surface roughness on the transient characteristics of hydrodynamic cylindrical bearings during startup. Tribol. Int. 2018, 128, 421–428. [Google Scholar] [CrossRef]
- Vakis, A.I.; Yastrebov, V.A.; Scheibert, J.; Nicola, L.; Dini, D.; Minfray, C.; Almqvist, A.; Paggi, M.; Lee, S.; Limbert, G.; et al. Modeling and simulation in tribology across scales: An overview. Tribol. Int. 2018, 125, 169–199. [Google Scholar] [CrossRef]
- Cagin, W.A., III; Qi, Y.; Zhou, Y.; Che, J. First Principles Multiscale Modeling of Physico-Chemical Aspects of Tribology. Tribol. Ser. 2001, 39, 15–33. Available online: https://www.academia.edu/40023380 (accessed on 8 March 2022).
- Gao, G.T.; Mikulski, P.T.; Harrison, J.A. Molecular-scale tribology of amorphous carbon coating: Effects of film thickness, adhesion, and long-range interactions. J. Am. Chem. Soc. 2002, 124, 7202–7209. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Seo, K.-J.; Kang, K.H.; Kim, D.-E. Nano-lubrication: A review. Int. J. Precis. Eng. Manuf. 2016, 17, 829–841. [Google Scholar] [CrossRef]
- Zhang, L.C.; Mylvaganam, K. Nano-tribological analysis by molecular dynamics simulation—A review. J. Comput. Theor. Nanosci. 2006, 3, 167–188. [Google Scholar] [CrossRef]
- Hsu, S.M. Molecular basis of lubrication. Tribol. Int. 2004, 37, 553–559. [Google Scholar] [CrossRef]
- Gajewski, J.B.; Głogowski, M.J. How do the temperature, angular velocity and electric fields affect mechanical and electrokinetic phenomena in a friction junction? Tribol. Int. 2015, 87, 139–144. [Google Scholar] [CrossRef]
- Gajewski, J.B.; Głogowski, M.J.; Hałuszka, N. Effect of an electric field on friction of silicone rubber against steel in the motor base oil’s environment. Tribol. Int. 2017, 88, 214–217. [Google Scholar] [CrossRef]
- Gajewski, J.; Głogowski, M.; Paszkowski, M. Effect of rheological properties of PAG and PAO oils on the braking torque in a friction junction. In Proceedings of the 20th International Symposium on Surfactants in Solution, Coimbra, Portugal, 22–27 June 2014. [Google Scholar]
- Gajewski, J.B.; Głogowski, M.J.; Gatner, K.; Gawliński, M. ZDDP content in mineral and synthetic motor base oils and its effect on electrostatic and tribological phenomena in a rotating shaft–oil–lip seal system. Tribol. Int. 2010, 43, 1012–1016. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, X.; Li, W.; Tian, Y.; Meng, Y. On-Line Feedback Control of Sliding Friction of Metals Lubricated by Adsorbed Boundary SDS Films. Lubricants 2022, 10, 148. [Google Scholar] [CrossRef]
- Gajewski, J.B.; Głogowski, M.J.; Wieleba, W. The effect of an electric field on the friction of selected elastomers against steel in a motor base oil’s environment. Tribologia 2015, 3, 21–31. Available online: https://bibliotekanauki.pl/articles/188744 (accessed on 8 March 2022).
- Stanciu, I. A new mathematical model for the viscosity of vegetable oils based on freely sliding molecules. Grasas y Aceites 2019, 70, e318. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, M.; Nakada, K.; Ishii, A. Density functional theory calculations for Pd adsorption on SO4 adsorbed on h-BN. Comput. Mater. Sci. 2014, 82, 231–236. [Google Scholar] [CrossRef][Green Version]
- Karimadom, B.R.; Meyerstein, D.; Kornweitz, H. Calculating the adsorption energy of a charged adsorbent in a periodic metallic system—The case of BH4- hydrolysis on the Ag(111) surface. Phys. Chem. Chem. Phys. 2021, 23, 25667–25678. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.B.; Rodrigues, G.L.S.; Santos, E.S.d.; Pettersson, L.G.M. Adsorption energies on transition metal surfaces: Towards an accurate and balanced description. Nat. Commun. 2022, 13, 6853. [Google Scholar] [CrossRef] [PubMed]
- Sittiwong, J.; Hiruntrakool, K.; Rasrichai, A.; Opasmongkolchai, O.; Srifa, P.; Nilwanna, K.; Maihom, T.; Probst, M.; Limtrakul, J. Insights into glyphosate adsorption on Lewis acidic zeolites from theoretical modelling. Microporous Mesoporous Mater. 2022, 41, 112083. [Google Scholar] [CrossRef]
- Lu, C.; Chen, P.; Li, C.; Wang, J. Study of Intermolecular Interaction between Small Molecules and Carbon Nanobelt: Electrostatic, Exchange, Dispersive and Inductive Forces. Catalysts 2022, 12, 561. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Mueller, J.E.; van Duin, A.C.T.; Goddard, W.A. Development and validation of ReaxFF Reactive Force Field for hydrocarbon chemistry catalyzed by nickel. J. Phys. Chem. C 2010, 114, 4939–4949. [Google Scholar] [CrossRef]
- Mueller, J.E.; van Duin, A.C.T.; Goddard, W.A. Application of the ReaxFF Reactive Force Field to reactive dynamics of hydrocarbon chemisorption and decomposition. J. Phys. Chem. C 2010, 114, 5675–5685. [Google Scholar] [CrossRef][Green Version]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef][Green Version]

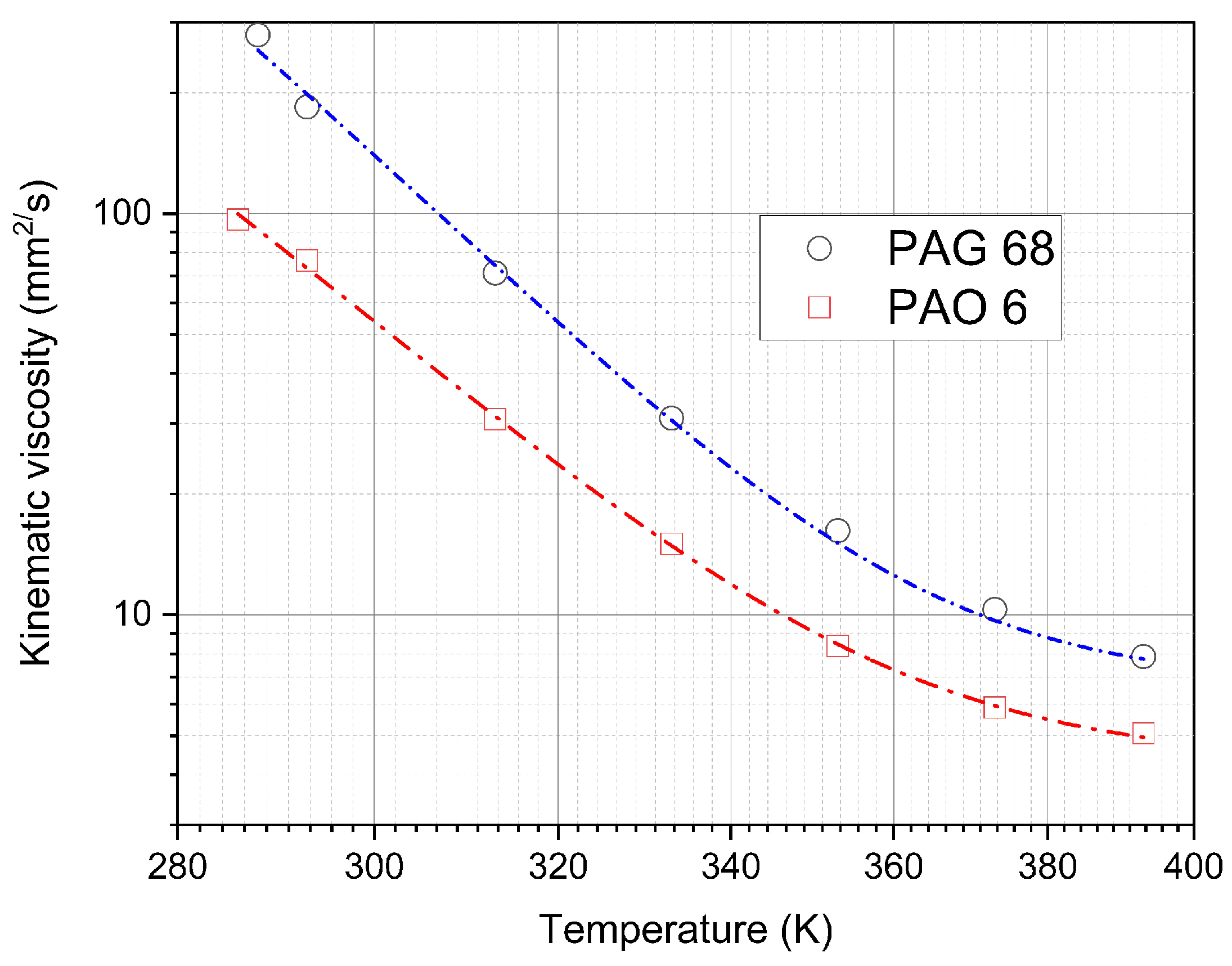



| Oil | ||||
|---|---|---|---|---|
| PAG 68 | PAO 6 | |||
| Temperature | Slope of Line | Ordinate | Slope of Line | Ordinate |
| K | 0.1 Pa· s | 0.1 Pa | 0.1 Pa· s | 0.1 Pa |
| 286 | – | – | 0.7992 | 0.5071 |
| 288 | 2.7643 | 1.8073 | – | – |
| 293 | 1.8293 | 1.9469 | 0.6315 | 0.3662 |
| 313 | 0.7051 | 0.7961 | 0.2543 | 0.7716 |
| 333 | 0.3065 | 0.3959 | 0.1244 | 0.7993 |
| 353 | 0.1608 | 0.9405 | 0.0692 | 0.9640 |
| 373 | 0.1023 | 0.0112 | 0.0486 | 0.5998 |
| 393 | 0.0632 | 0.1615 | 0.0419 | 0.0871 |
| Oil | A | B | ||
|---|---|---|---|---|
| mm/s | K | mm/s | – | |
| PAO 6 | 6.35117 × | 21.3325 | 4.32622 | 0.99992 |
| PAG 68 | 8.24265 × | 19.18416 | 6.71995 | 0.99988 |
| Oil | Temperature | Kinematic Viscosity | |
|---|---|---|---|
| Catalogue | Experiment | ||
| K | mm/s | mm/s | |
| PAO 6 | 313 | 30.8 | 30.76 |
| PAO 6 | 373 | 5.9 | 5.88 |
| PAG 68 | 313 | 68 | 71.1 |
| PAG 68 | 373 | 12.4 | 10.32 |
| Oil | ||
|---|---|---|
| Resistivity at at 313 K for DC Voltage | Relative Permittivity at 313 K for DC Voltage | |
| m | – | |
| PAO 6 | 2.6 × 10 | 1.9 |
| PAG 68 | 1.3 × 10 | 5.8 |
| Sample | ||
| rubber based on methyl-vinyl-silicone (MVQ), diameter 9 mm | ||
| Rotating disc | ||
| steel X5CrNi18-10 and copper (97.4% of Cu), roughness = 0.3 m, for both, diameter 140 mm | ||
| Parameters | Unit | Value |
|---|---|---|
| Contact load | N | 15.6–43.5–70.2 |
| Contact pressure | MPa | 0.24–0.68–1.10 |
| Diameter of the rubber pin | mm | 9 |
| Diameter of the metal discs | mm | 140 |
| Diameter of the pin’s metal holder | mm | 13 |
| Radius of the test track | mm | 45 |
| The length of the sliding distance of each test | m | 1000 |
| Parameters | Model |
|---|---|
| Triangular elements | 7021 |
| Edge elements | 655 |
| Minimum element quality | 0.6602 |
| Average element quality | 0.9465 |
| Maximum growth rate | 2.372 |
| Average growth rate | 1.284 |
| Parameters | Value |
|---|---|
| Electric potential, steel holder | −5000 V |
| Electric potential, copper disc | 0 V |
| Rubber pin | MVQ |
| Thickness of oil film (PAO 6) | 0.5 mm |
| Resistivity of oil | 2.6 × 10 m |
| Relative permittivite of oil | 1.9 |
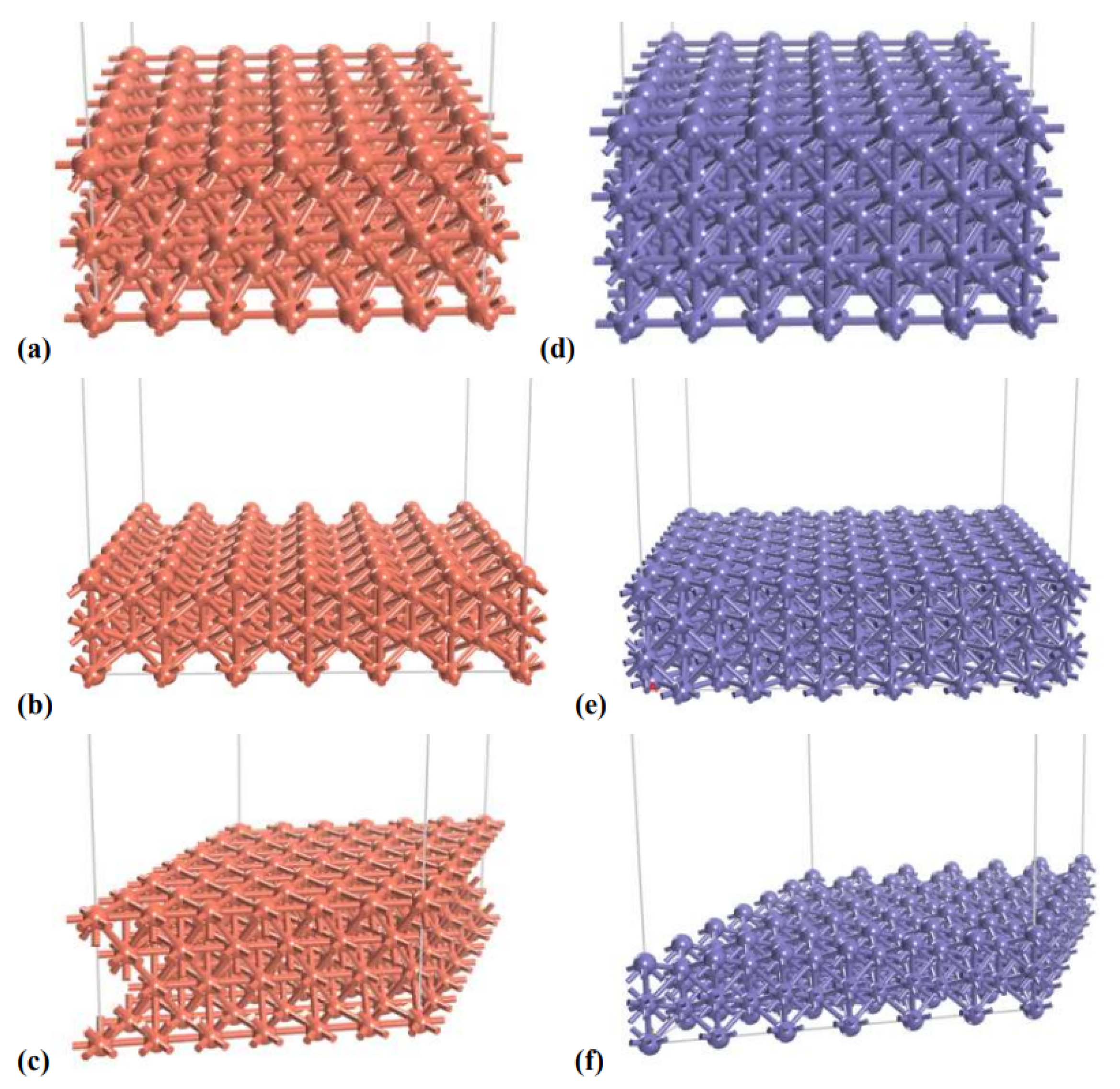
| Model | Cell size A × B × C, Å | Cell Angles: , , |
|---|---|---|
| Cu[100] | 15.336 × 15.336 × 27.229 | = = = 90 |
| Cu[110] | 15.336 × 21.688 × 25.112 | = = = 90 |
| Cu[111] | 15.336 × 15.336 × 26.261 | = = 90, = 120 |
| Fe[100] | 17.198 × 17.198 × 28.599 | = = = 90 |
| Fe[110] | 17.198 × 24.322 × 26.081 | = = = 90 |
| Fe[111] | 24.322 × 24.322 × 24.965 | = = 90, = 120 |
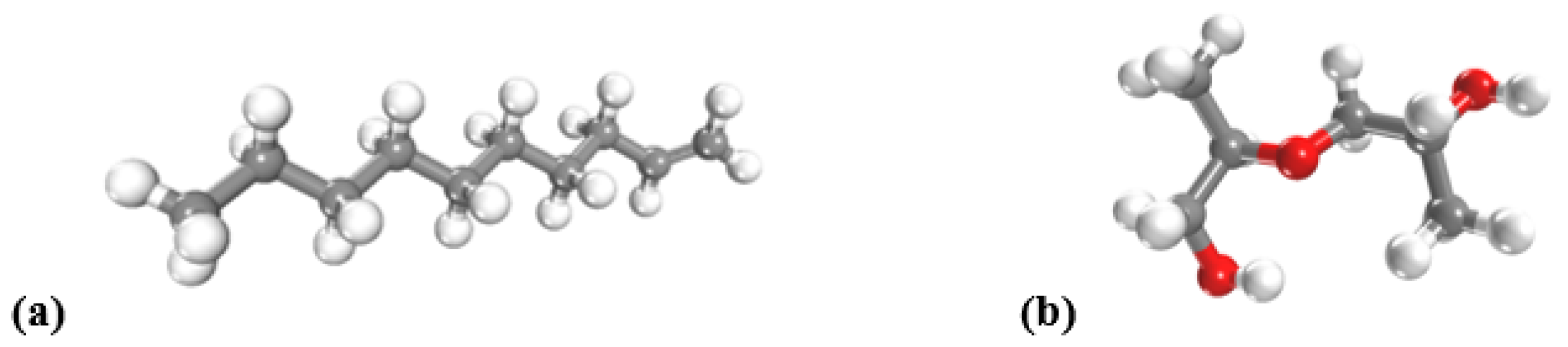
| Surface Type | 1-Decene (ReaxFF), | 1-Decene (COMPASS), | PPG (ReaxFF), | PPG (COMPASS), |
|---|---|---|---|---|
| kJ/mol | kJ/mol | kJ/mol | kJ/mol | |
| Cu[100] | –54.2 | –210.0 | –85.7 | –182.9 |
| Cu[110] | –44.6 | –181.9 | –42.1 | –121.4 |
| Cu[111] | –50.8 | –206.8 | –46.3 | –108.3 |
| Fe[100] | –155.4 | –381.5 | –290.2 | –265.3 |
| Fe[110] | –125.4 | –293.8 | –400.8 | –203.1 |
| Fe[111] | –136.2 | –435.4 | –246.9 | –153.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głogowski, M.; Smykowski, D.; Pietrowicz, S. The Effect of an Electric Field on the Sliding Friction of the Silicone Rubber against Selected Metals in Motor Base Oils. Energies 2023, 16, 3954. https://doi.org/10.3390/en16093954
Głogowski M, Smykowski D, Pietrowicz S. The Effect of an Electric Field on the Sliding Friction of the Silicone Rubber against Selected Metals in Motor Base Oils. Energies. 2023; 16(9):3954. https://doi.org/10.3390/en16093954
Chicago/Turabian StyleGłogowski, Marek, Daniel Smykowski, and Sławomir Pietrowicz. 2023. "The Effect of an Electric Field on the Sliding Friction of the Silicone Rubber against Selected Metals in Motor Base Oils" Energies 16, no. 9: 3954. https://doi.org/10.3390/en16093954
APA StyleGłogowski, M., Smykowski, D., & Pietrowicz, S. (2023). The Effect of an Electric Field on the Sliding Friction of the Silicone Rubber against Selected Metals in Motor Base Oils. Energies, 16(9), 3954. https://doi.org/10.3390/en16093954








