The Fuel Flexibility of Gas Turbines: A Review and Retrospective Outlook
Abstract
1. Introduction
2. Essential Technical Considerations
2.1. The Value of Fuel Flex in the Power Generation Market
- -
- Peak-shaving service is characterized by few operation hours but a constraint of permanent “dispatchability”, which is the ability to respond to changes in power demand over time, and which requires faultless startups and fast access to the targeted load; here, the plant operator’s attention is essentially focused on maintenance costs;
- -
- Base-load or “semi-base-load” service implies longer operation periods, during which the operator focuses on fuel cost which represents up to 60% of his O&M expenses.
2.2. Inherent Strengths of Gas Turbines Favoring Fuel Flex
2.3. Technologies and Designs Underlying Fuel Flex
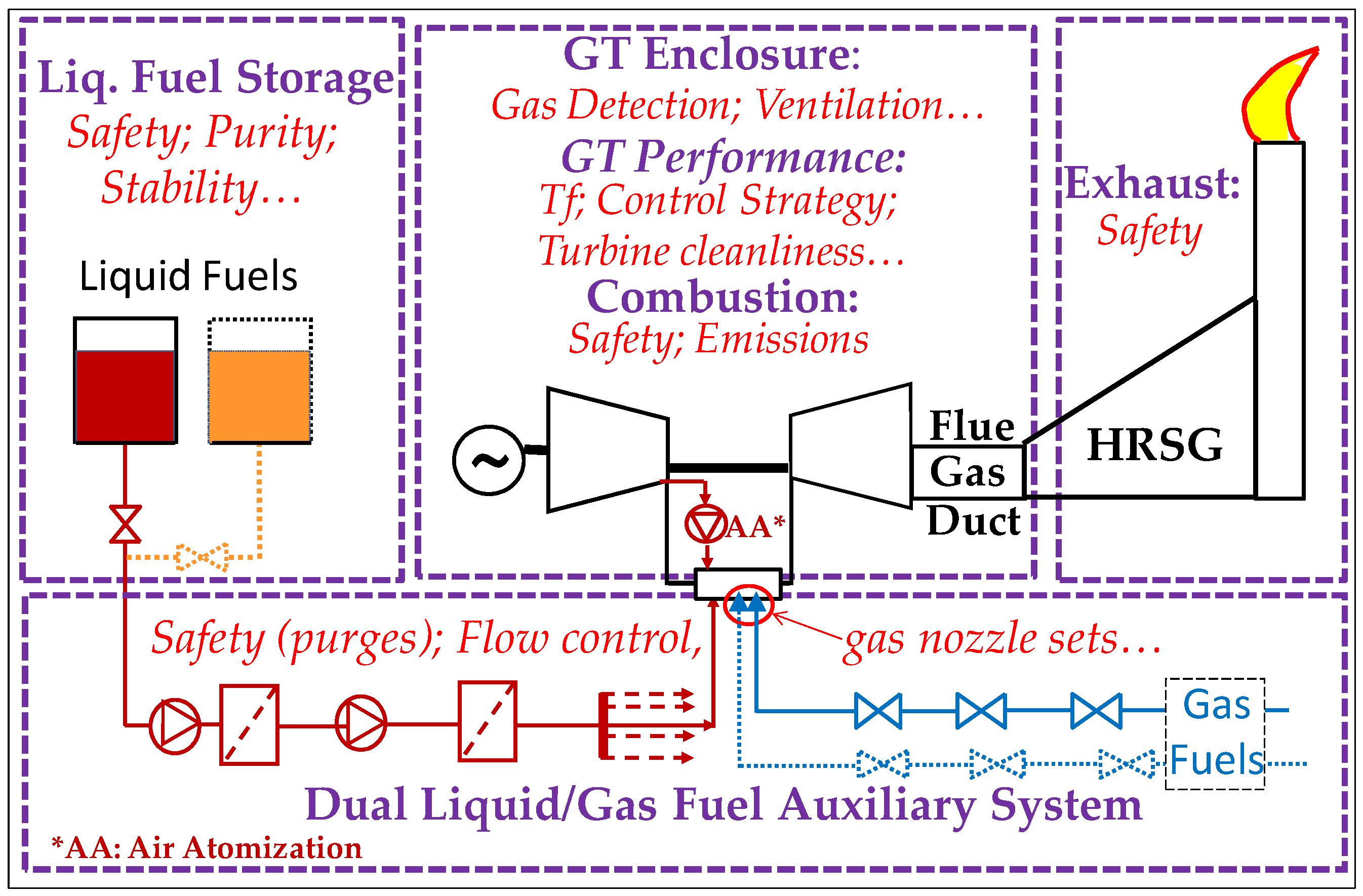
2.3.1. Reliable Operation with Dual Fuel Systems
- Dual Gas Systems
- Liquid Fuel (LF) Systems [39]
2.3.2. Ensuring Operation Safety
- Essential safety measures
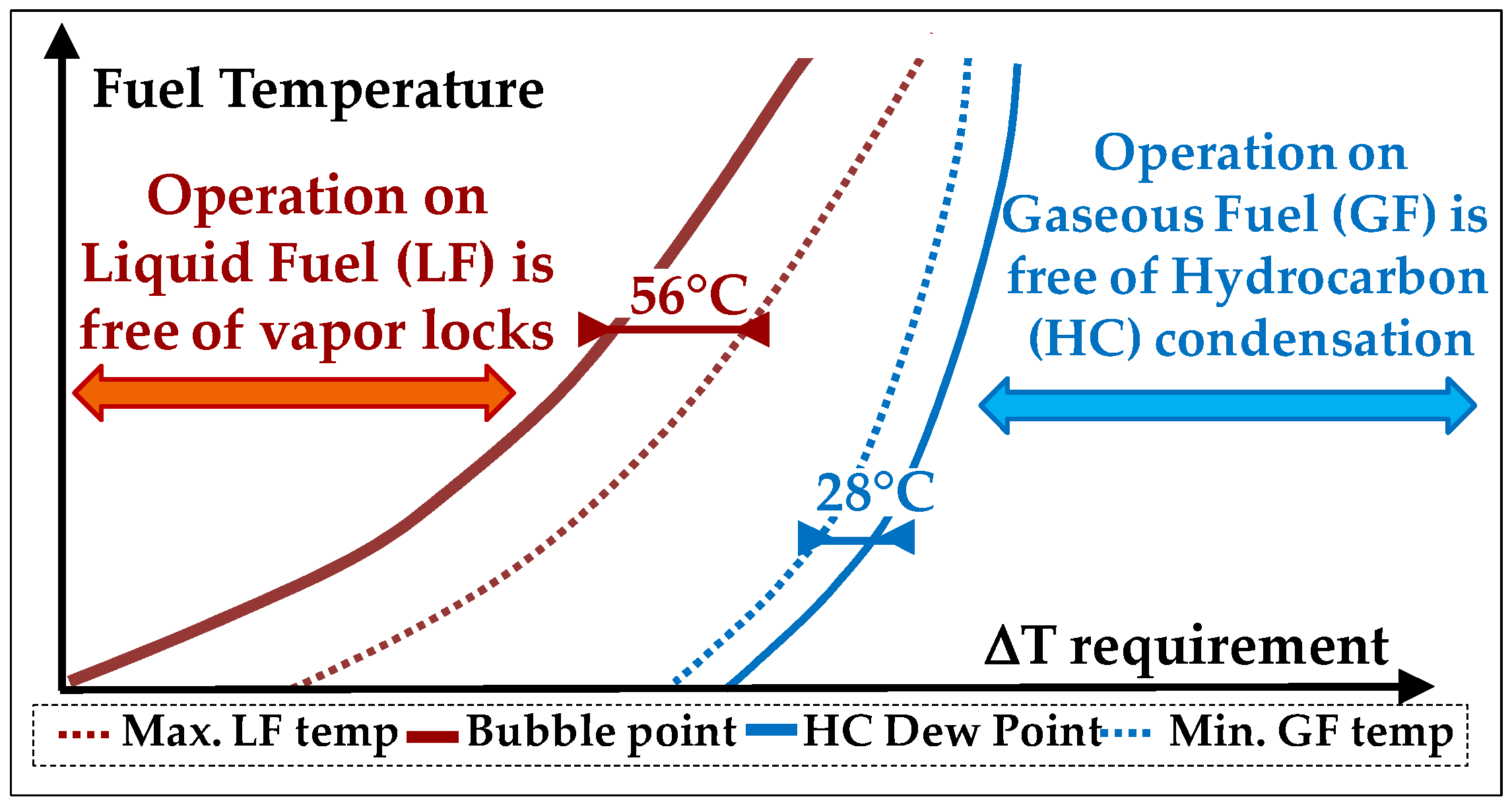
- Safe Fuel temperature range
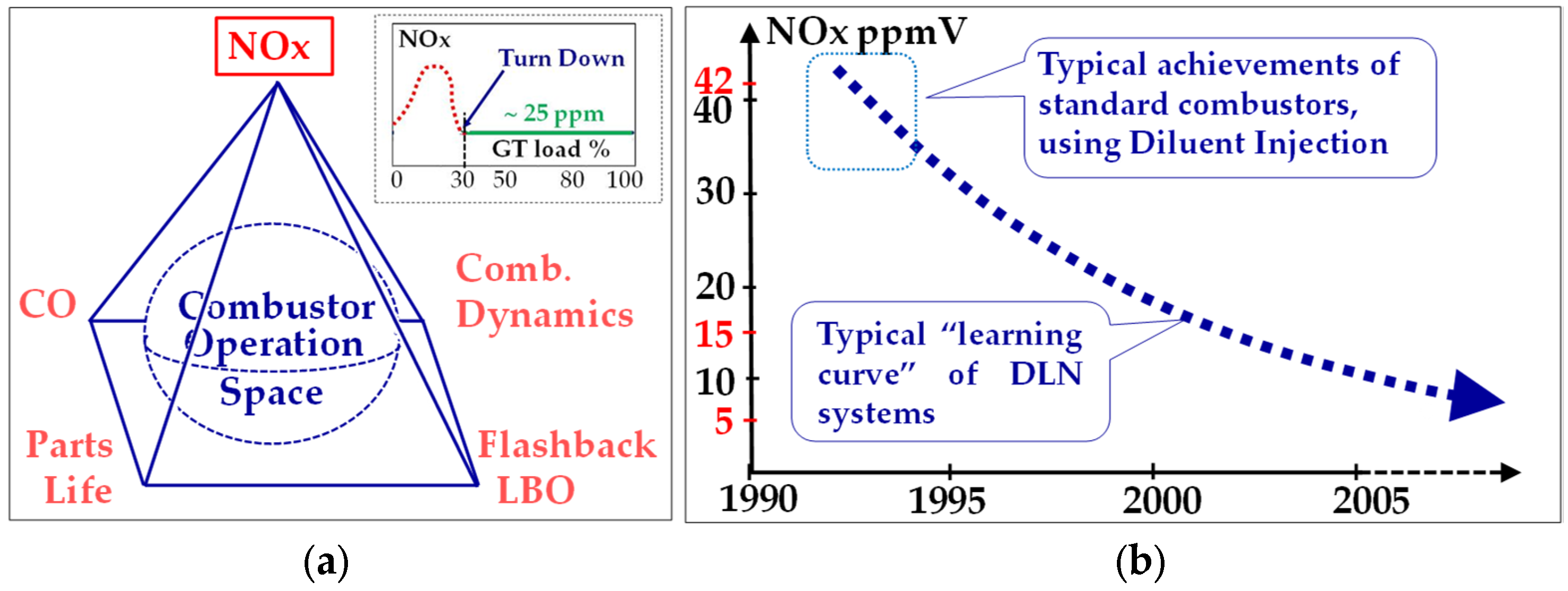
2.3.3. Staying Emission Compliant
2.3.4. Ensuring Machine Integrity
2.3.5. Measures Specific to the Operation on Hydrogen-Rich Gases
3. Creation and Growth of the Fuel Portfolio
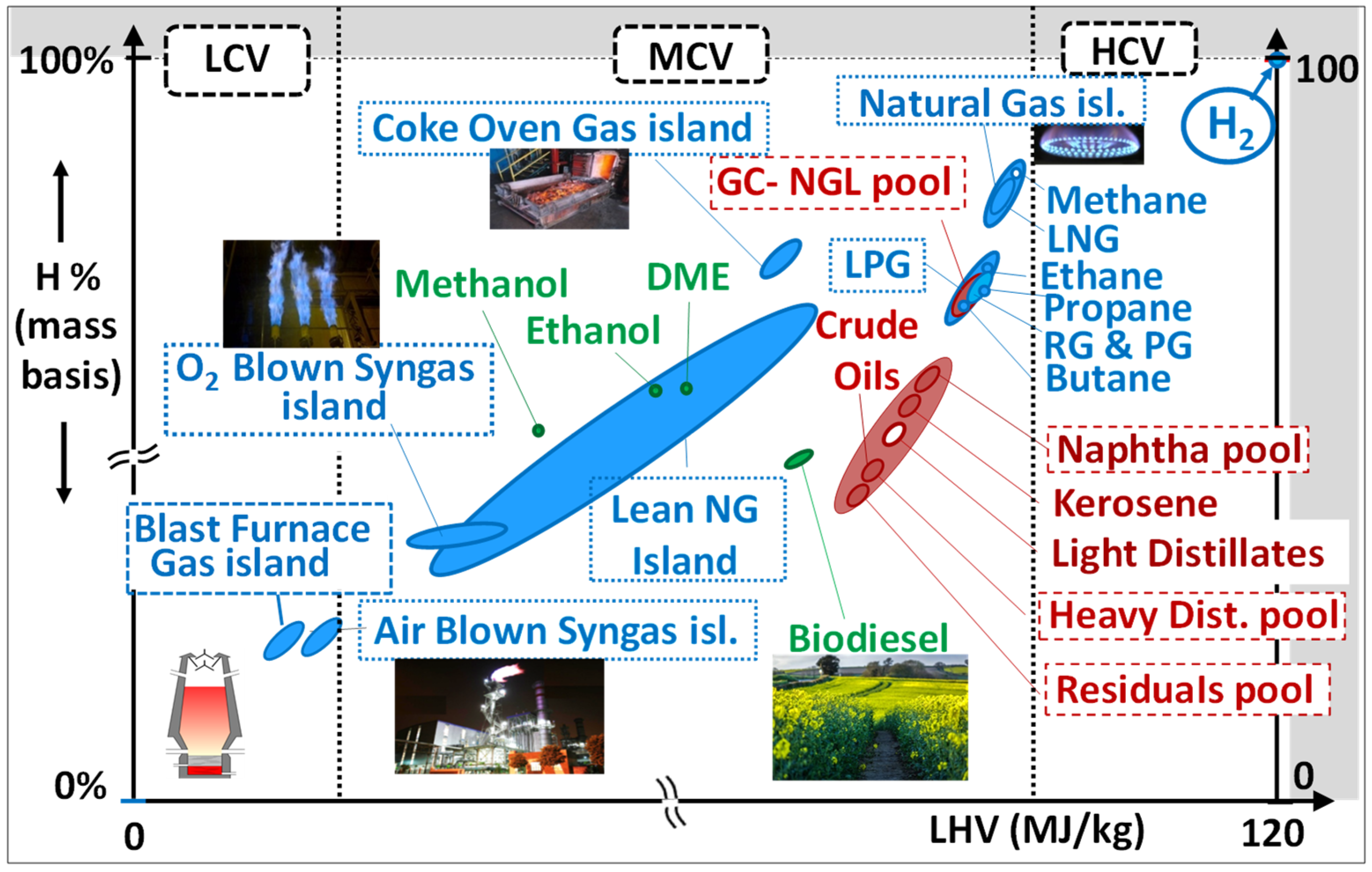
3.1. General Overview
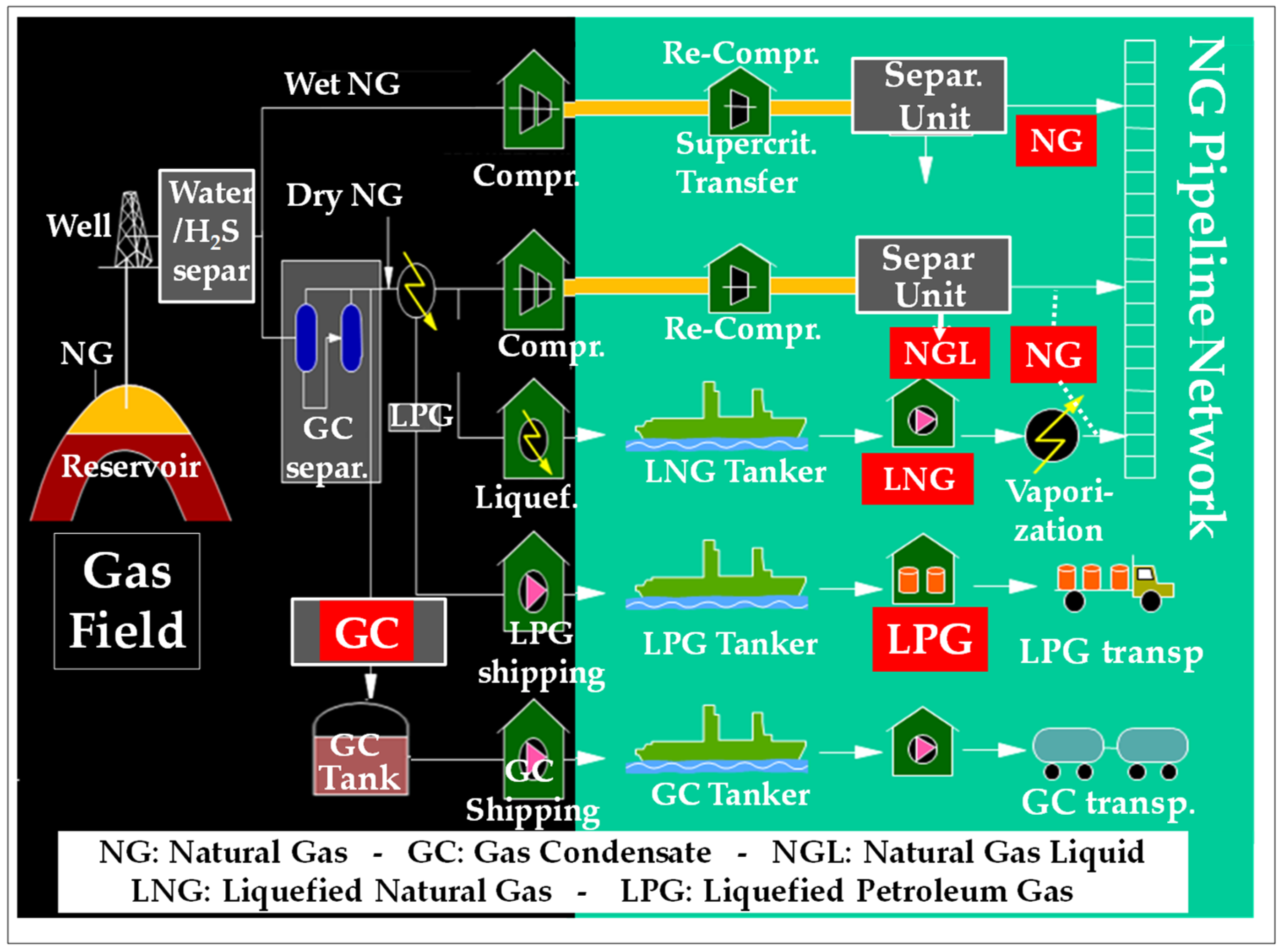
3.2. The Gas Fuel Portfolio
- Expansion towards high-calorific fuels:
- -
- Commercial LPGs (propane, butane, and mixtures thereof) mainly come from the processing of NG and the atmospheric distillation of crudes. They are generally burned after vaporization. Their combustion in variable amounts in GTs is interesting as it allows matching the offer to the demand of LPGs namely in summer [87];
- -
- Refinery fuel gases (RFG) [88] as well as petrochemical net gases (PNG) [87,88,89,90] mainly contain C2 to C4 alkanes and/or olefins, in addition to variable amounts of H2; they stem from fluid catalytic cracking (FCC) or steam catalytic cracking (SCC) units. They are produced continuously and yield high GT performances.
- Expansion towards low-calorific fuels:
- -
- “Lean natural gases” stem from some NG wells or coal mines and contain CH4, N2, and CO2 [37]; they are medium- or low-BTU fuels; their combustion after compression is interesting if the sale of the kWhs exceeds the cost of compression that is only partly compensated by the extra power produced by the expansion in the turbine;
- -
- -
- -
- Air-blown syngases (SG) are very lean gases which contain mainly CO, N2, H2O, and H2 and come from the gasification of coal/lignite [98,99,100,101,102], or biomass [103,104,105], or even solid municipal wastes [106,107,108]; oxygen-blow gasification is applied to very difficult solids (HFOs and bituminous coals) and yield syngases with higher LHVs [101]. Syngases from coals and HFOs must undergo drastic clean-up processes to remove particles, sulfur, nitrogen, and heavy metals;
- -
- Expansion towards higher H2 contents:
3.3. The Liquid Fuel Portfolio
3.3.1. Fossil Liquids
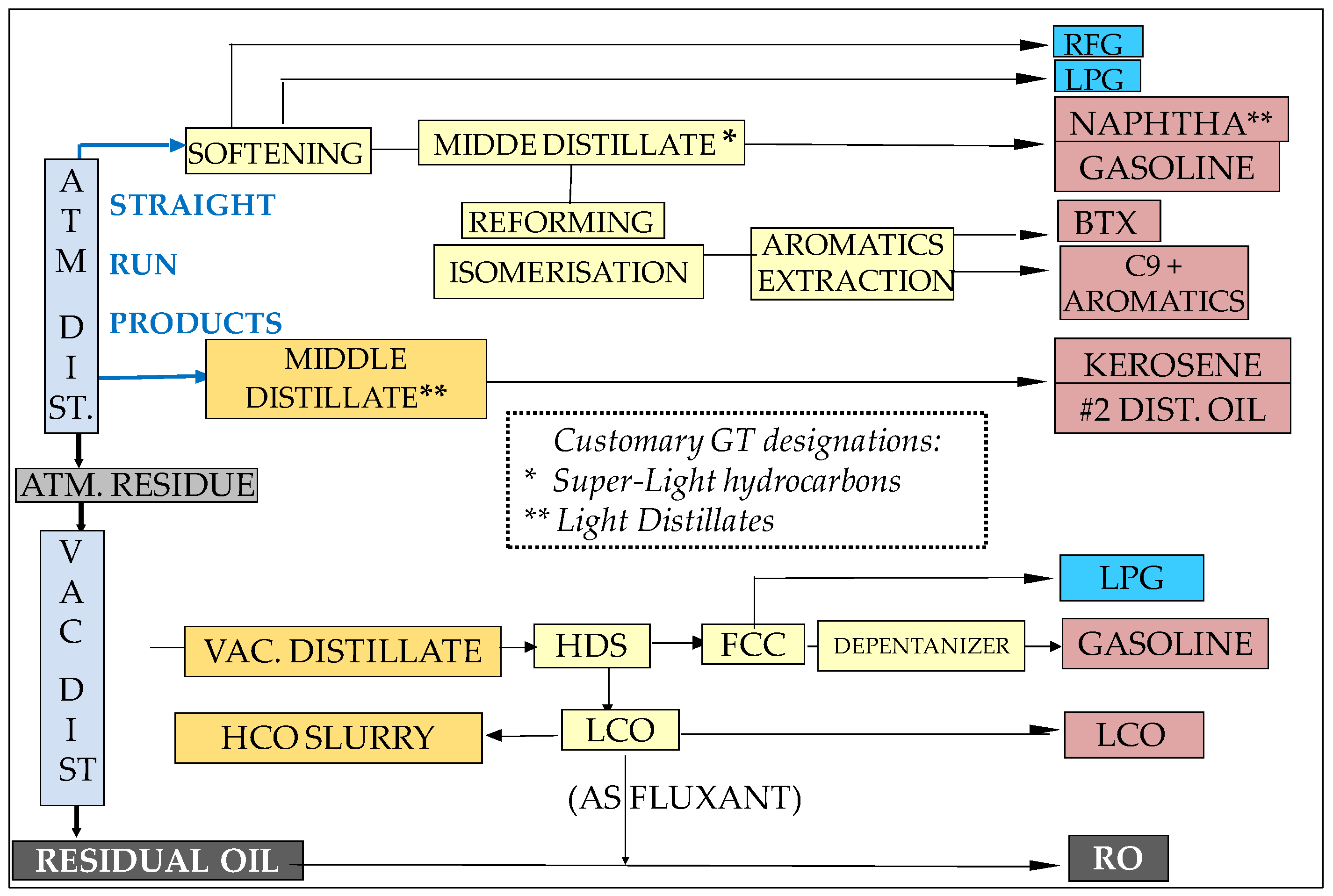
- “Super-Light Distillates” (SLHCs) include three main products:
- Light distillates (LDs) are classified as “middle distillates” by the oil business. They consist of kerosene and #2 distillate oil; their use in GTs does not require the additives package that is necessary for aircraft and diesel cars. Kerosene’s and No. 2 distillate oils are “straight-run” refinery products that originate from the atmospheric distillation of crudes (Figure 11); they are prime-quality, expensive GT fuels. While No. 2 distillate is the conventional liquid fuel for heavy-duty GTs, kerosene is the preferred one for aeroderivatives in consideration of its still-higher purity. However, the expensive kerosene is generally used only on some rare islands where No. 2 diesel is not demanded as car fuel. Finally, another refinery cut called “light cycle oils” (LCOs) can be integrated in this group although it is of much lower grade; LCOs are not straight-run products as they come from the cracking of vacuum distillates (Figure 11) and are rich in aromatics and polyaromatics. Their possible use in gas turbines is described below in the paragraph devoted to aromatic fuels;
- Heavy distillate Oils originate from the vacuum distillation of crudes [87]. They are available from refineries that do not have “deep conversion” units; they must be burned after heating to reduce their viscosity for proper atomization;
- Crude oils are the raw hydrocarbons extracted from oil fields where they may be associated with C1-C4 gases (LPGs) or C5+ condensable gases (gas condensates). Their composition is variable as they can contain heavy hydrocarbon ends. Heavy crudes, especially those extracted in remote areas, do not interest refiners as they do not yield light cuts; they can be cleanly burned in GTs on the spots, using—if necessary—a combustion catalyst additive, e.g., cerium derivatives [114], to avoid a slight smoking trend;
- Residual oils (RO) are very-low-grade refinery cuts. These ash-forming fuels can no longer be distilled and represent the “bottom of the barrel”. Due to their high SOx, NOx, and PM emissions, they are disappearing from the market, being converted into “pet-cokes” or gasified in oxygen-blown gasifiers to produce syngases (SG). Nevertheless, the combustion of HFOs in GT combined cycles generates significantly fewer polluting emissions than if using diesel engines. As mentioned in Section 2.3.4, both crudes and ROs need to be cleaned before combustion. Some low-sulfur waxy residual fuels, called “LSWR”, have been used in the Far East (Japan, Korea, Singapore…) for power generation. Efforts have also been made to alleviate the adverse effects of smoke and ash deposition on the performances of gas turbines [114,115];
- Aromatic fuels belong either to the group of super light hydrocarbons (“BTX” and “C9+ aromatics”) or to that of light distillates (Light Cycle Oil or “LCO”):
- -
- BTXs (Benzene–Toluene–Xylene) are C6–C8 mono-aromatics. Huge volumes are also used as petrochemical feedstock for the synthesis of a large variety of commodities, including polymers, paints, and solvents. They have also been used, along with “C9+ aromatics”, as RON improvers for gasoline’s, owing to the high AITs of aromatics. However, due to the ban of aromatics in automotive fuels, they may be produced in surpluses in refineries deprived of petrochemical units; moreover, the exportation of such sensitive cuts represents a financial burden and creates EHS difficulties that can lead refiners to envisage other usages. This is where on-site power generation becomes an interesting option. A stream of such aromatic hydrocarbons, called BHC (for “Benzene Heart Cut”), with a BTX composition, have been successfully tested in a 40 MWe heavy-duty GT [84]. Subsequently, based on this successful test, a C9+ aromatic cut found an industrial application in Korea [116];
- -
- LCO (light cycle oil) tends to be produced in increasing amounts in modern refineries. Indeed, fluid catalytic crackers (“FCC”) crack heavy distillate feedstocks into lighter ones, thereby helping convert the original crude to more middle distillates and gasolines with higher RON indices. LCOs contain up to 70% aromatics (essentially in the form of di-aromatics). Their very poor cetane number and high smoking propensity strongly limits the proportion that can be added to diesel fuels and to domestic heating oil. In contrast, the combustion of LCO has been successfully tested, also in a 40 MW heavy-duty GT [84,85].
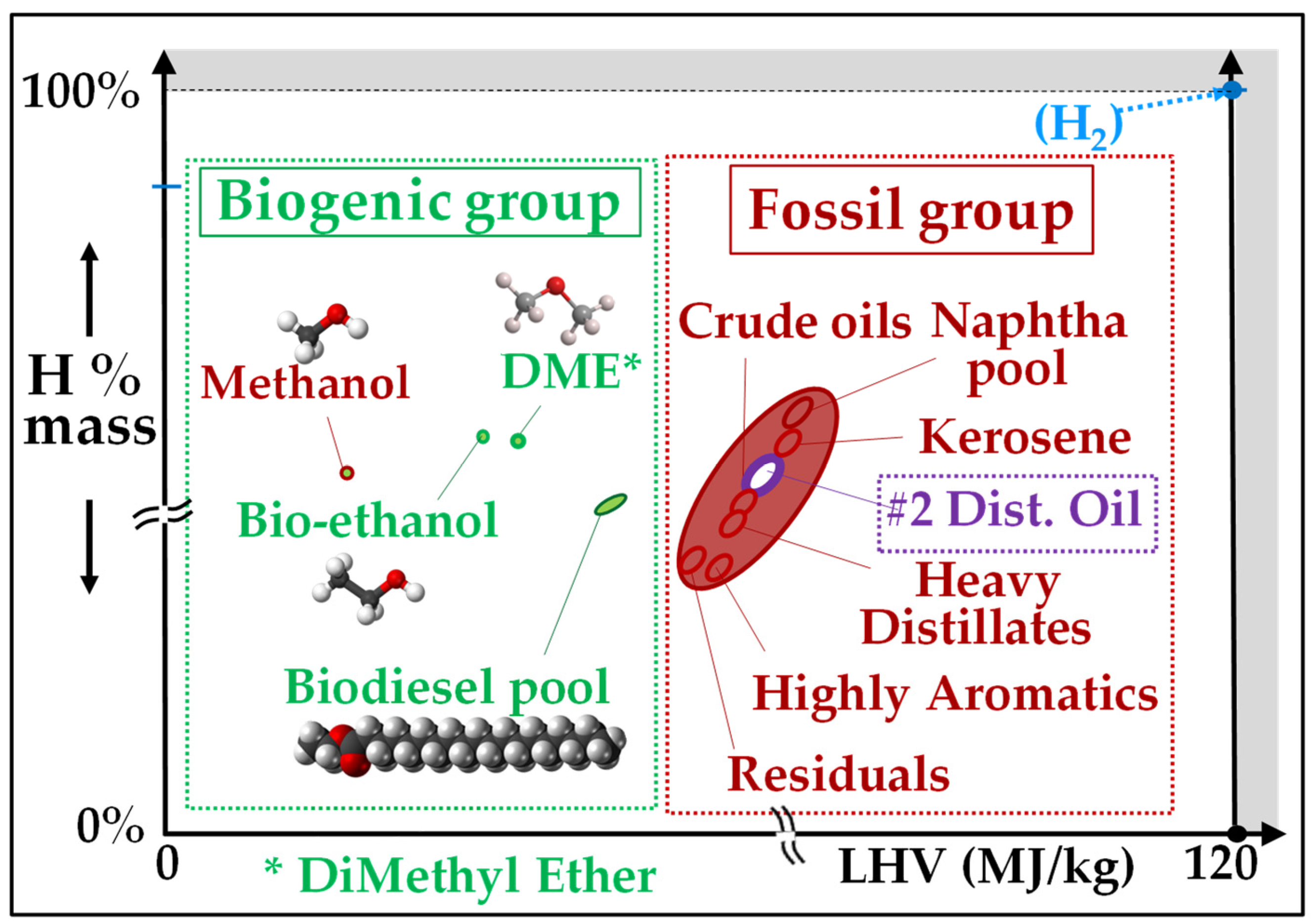
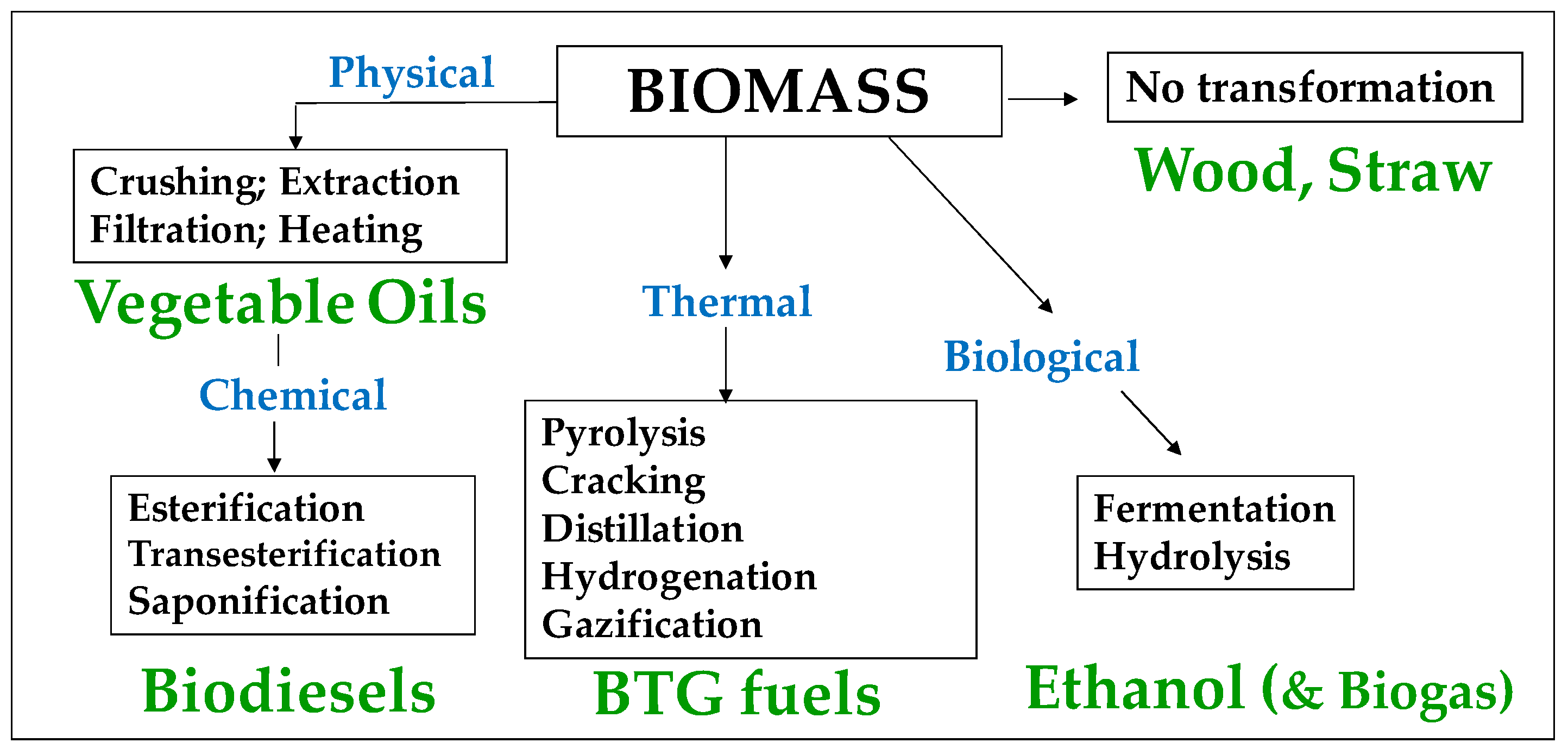
3.3.2. Liquid Biofuels
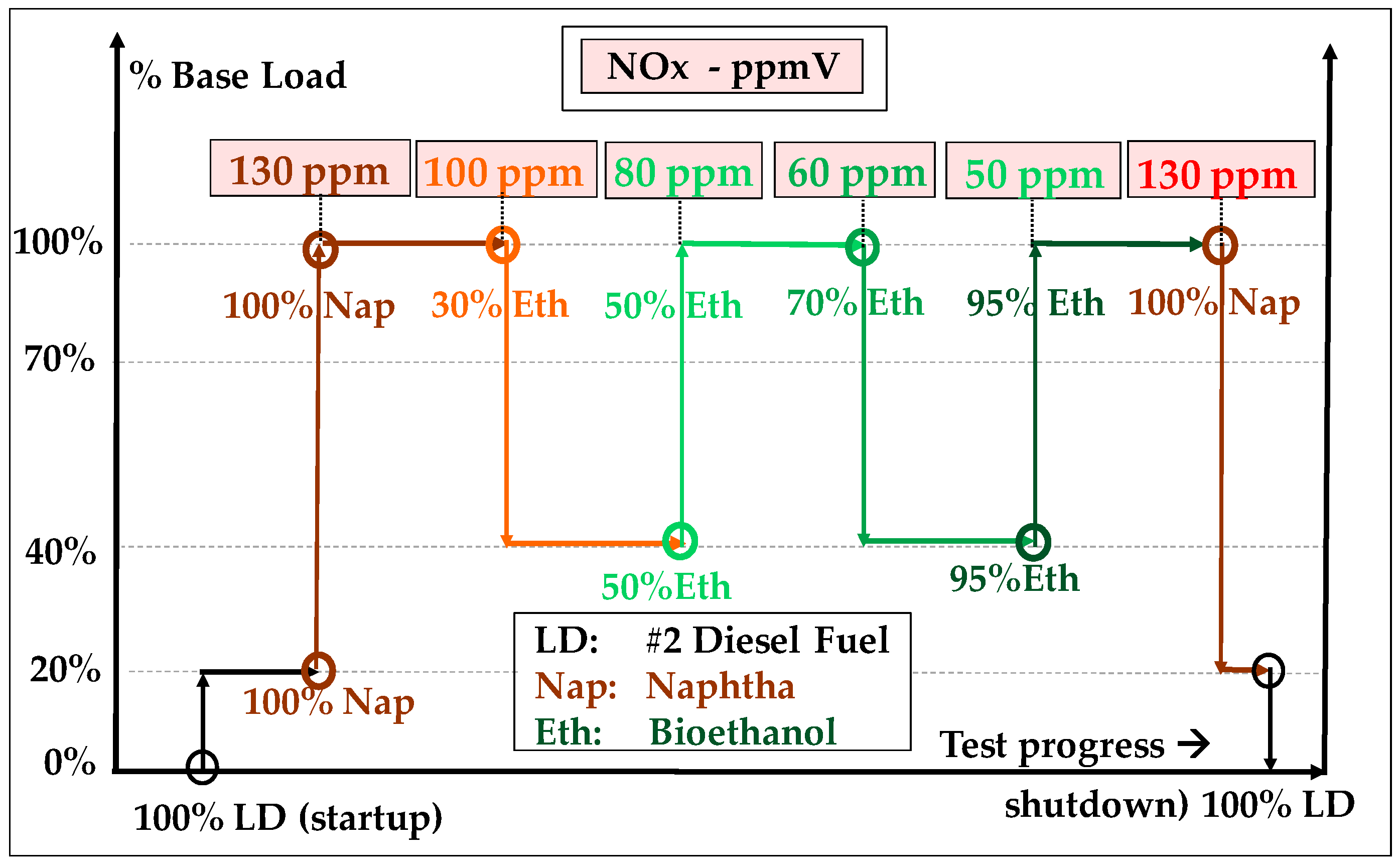
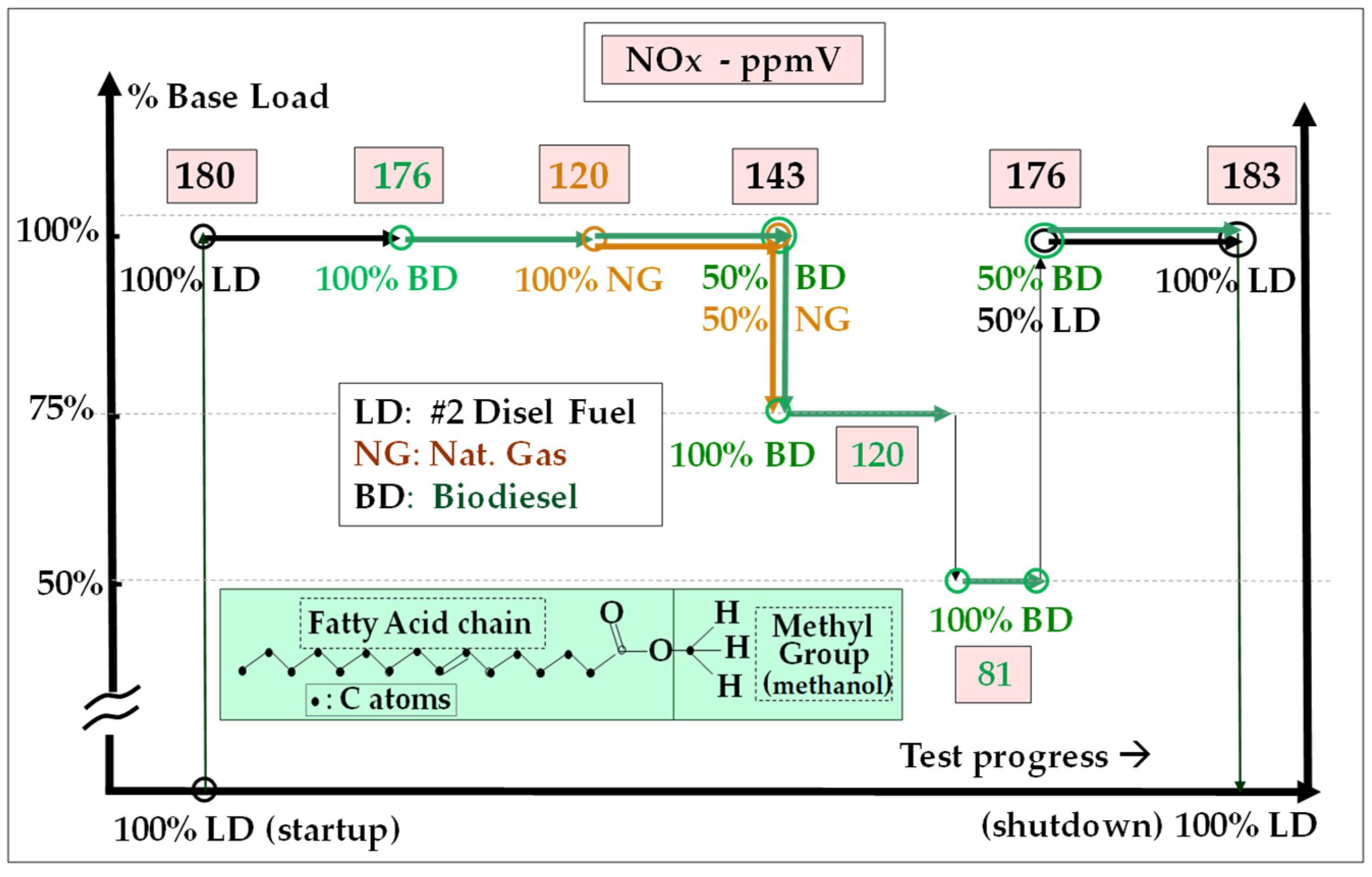
- Bioethanol comes from the anaerobic fermentation of natural sugars (grape, sugar beet, sugar cane, etc.) (Figure 12) and are often produced in surplus in some agricultural sectors and some countries. Dehydrated ethanol is fully miscible with gasoline; this property has been exploited in a bioethanol combustion test that was carried out on a 20 MW GT in India and in which naphtha was used as starting fuel and gradually blended with increasing proportions of bioethanol, up to 95% [126]. This field test demonstrated that ethanol can be burned without any trouble with NOx emissions lower than those of LD and close to those of NG (Figure 13). Bioethanol from sugar cane has also been recently burned in a 40 MW GT at Saint Pierre de La Réunion (a French ultramarine territory), although this experience is not well documented [127].
- Methanol was formerly prepared by the distillation/carbonization of wood (charcoal industry) and was named “wood alcohol”. However, currently, 98% of methanol comes from the reforming of methane and has thus a non-bio, fossil origin. It can also be produced through the coal-to-liquid or biomass-to-liquid (CTL/BTL) routes. It is a feedstock to produce FAME biodiesel, as discussed below. Kinetics simulations show that it could be burned in GTs [130]. However, its high vapor pressure (13 kPa at 20 °C) and acute toxicity are major obstacles for this application.
- Dimethyl-Ether
- Biodiesels
- Vegetable Oils
3.3.3. Similarities between Biofuels and Fossils
- -
- Bioethanol versus naphtha: Anhydrous ethanol is fully miscible with naphtha, i.e., with gasoline. This is interesting in countries such as India where naphtha is often used as base-load GT fuel due to the scarcity of natural gas and distillate fuels and, on another hand, the fact that bioethanol is available through the fermentation of discarded cotton [136];
- -
- Biodiesel versus light distillate: Biodiesel is fully miscible with light distillate oils, i.e., with diesel fuels. It is more lubricious than diesel fuels but, due to its higher power as solvent, it can be aggressive against certain elastomeric seals used in fuel circuits;
- -
- Vegetable oils versus crude oils: VOs are partly miscible with crude oils [137] and have several similarities with them: both are unprocessed liquids that contain long molecules with many chemical functions and are contaminated with metals. However, there are important differences. Indeed, contrary to crude oils, vegetable oils contain no heavy metals (vanadium, nickel) and very little sulfur and nitrogen; their metallic contaminants are partly oil-soluble and, when they are heated, they do not distil but tend to degrade more rapidly than petroleum cuts by pyrolysis. For these reasons, VOs are not currently used as a GT fuel; they can be burned in boilers with some precautions due to the generation of corrosive low-melting point salts (potassium and calcium chlorides).
4. The Contribution of Gas Turbines to the Hydrogen Energy Move
4.1. The “Hydrogen Rainbow”
4.2. The Possible Role of Gas Turbines
- -
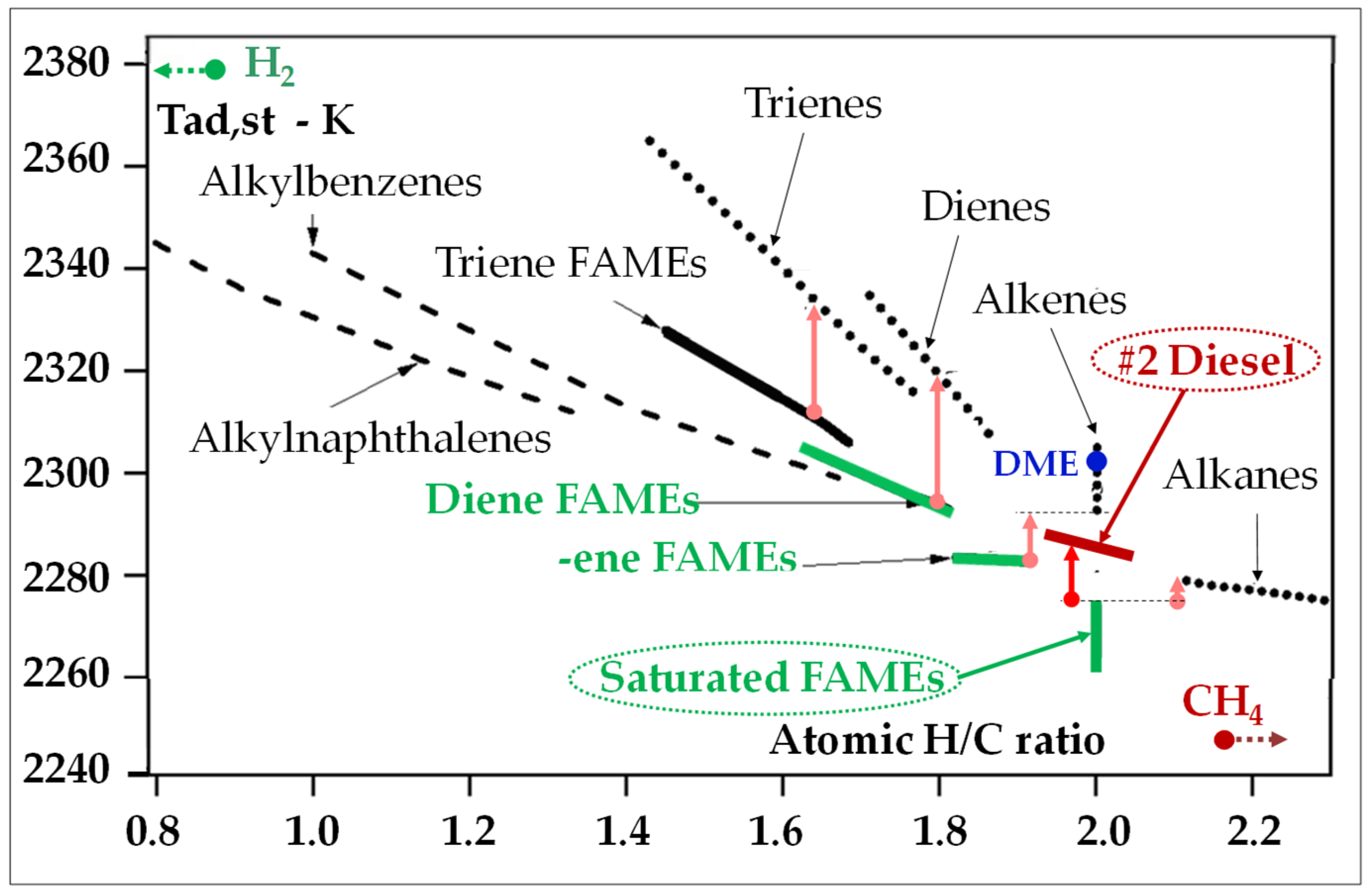
- Hydrogen flames are very hot (Figure 18a) [147]. Indeed, one molecule of H2 generates three times less molecules of combustion products than does CH4 (Table 3); therefore, its stoichiometric combustion temperature (at the flame front) is much higher (2120 °C versus 1950 °C, in E-class GT conditions) since its combustion heat is transferred to one molecule instead of three. This is why hydrogen generates much higher NOx in diffusion flames than methane;
- -
- Conventional Dry Low NOx systems based on the current fuel/air premix devices are defeated due to the very fast flame speed (Figure 18b) [148] that results in very short flames (Figure 18a) but causes very difficult flashback issues (see Section 2.3.3).

4.3. Exploiting the Experience Gained with H2-rich fuels
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hainsch, K.; Hainsch, K.; Löffler, K.; Burandt, T.; Auer, H.; del Granado, P.C.; Pisciella, P.; Zwickl, S. Energy transition scenarios: What policies, societal attitudes, and technology developments will realize the EU Green Deal? Energy 2022, 239, 122067. [Google Scholar] [CrossRef]
- IEA On-Line Report, World Energy Outlook 2022. Available online: https://iea.blob.core.windows.net/assets/830fe099-5530-48f2-a7c1-11f35d510983/WorldEnergyOutlook2022.pdf (accessed on 17 March 2023).
- Farhat, H.; Farhat, H.; Salvini, C. Novel Gas Turbine Challenges to Support the Clean Energy Transition. Energies 2022, 15, 5474. [Google Scholar] [CrossRef]
- IEA. Power Systems in Transition, Section: Maintaining Reliability; IEA Report; IEA: Paris, France, 2020; pp. 25–27. [Google Scholar]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- EPRI. Report: Conversion to Dual Fuel Capability in Combustion Turbine Plants—Addition of Distillate Oil Firing for Combined Cycles; EPRI: Washington, DC, USA, 2001. [Google Scholar]
- Power Engineering International On-Line Article, Flexibility Brings Economies, 18 September 2013. Available online: https://www.powerengineeringint.com/coal-fired/equipment-coal-fired/flexibility-brings-economies/ (accessed on 17 March 2023).
- Boyce, P.B. Theoretical and Actual Cycle Analysis. In Gas Turbine Engineering Handbook, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2017; Chapter 2; pp. 57–60. [Google Scholar]
- Correa, M.S. A Review of NOx Formation Under Gas-Turbine Combustion Conditions. Combust. Sci. Technol. 1993, 87, 329–362. [Google Scholar] [CrossRef]
- Yeong, J.K.; Jae, K.Y.; Keun, L.D.; Yongmo, K. Experimental study on combustion instability and attenuation characteristics in the lab-scale gas turbine combustor. J. Mech. Sci. Technol. 2018, 32, 1879–1887. [Google Scholar]
- Basu, P.; Kefa, C.; Jestin, L. General Design Considerations. In Boiler and Burner Deign and Theory; Springer: Berlin/Heidelberg, Germany, 2000; Chapter 2; pp. 15–20. [Google Scholar]
- Langston, L.S. Single-Crystal Turbine Blades Earn ASME Milestone Status, On-Line Publication. Available online: https://cdn.machinedesign.com/files/base/ebm/machinedesign/document/2019/04/machinedesign_14966_engineblades.pdf (accessed on 17 March 2023).
- Dexin, M.A. Novel casting processes for single-crystal turbine blades of superalloys. Front. Mech. Eng. 2018, 13, 3–16. [Google Scholar]
- Cao, X.Q. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Han, J.C.; Wright, L.M. Enhanced Internal Cooling of Turbine Blades and Vanes, NETL DOE On-Line Publication. Available online: https://netl.doe.gov/sites/default/files/gas-turbine-handbook/4-2-2-2.pdf (accessed on 17 March 2023).
- Gavali, S.; Dipali; Garad, D.S.; Yadav, J.N. Review of Cooling of Gas Turbine Blades. Int. J. Appl. Eng. Res. 2018, 13, 43–48. [Google Scholar]
- Takeishi, K. Evolution of Turbine Cooled Vanes and Blades Applied for Large Industrial Gas Turbines and Its Trend toward Carbon Neutrality. Energies 2022, 15, 8935. [Google Scholar]
- Harada, H. High Temperature Materials for Gas Turbines: The Present and Future. In Proceedings of the International Gas Turbine Congress, Tokyo, Japan, 2–7 November 2003. [Google Scholar]
- Jansohn, P. Modern Gas Turbine Systems: High Efficiency, Low Emission, Fuel Flexible Power Generation; Woodhead Publishing Series in Energy: Cambridge, UK, 2013. [Google Scholar]
- Ghojel, J. Practical Cycles for Reciprocating Engines. In Fundamentals of Heat Engines: Reciprocating and Gas Turbine Internal Combustion Engines; John Wiley and Sons: Hoboken, NJ, USA, 2020; Chapter 6. [Google Scholar]
- Smith, J.R.; Green, R.M.; Westbrook, C.K.; Pitz, W.J. An experimental and modeling study of engine knock. Symp. Int. Combust. 1985, 20, 91–100. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Liu, H.; Reitz, R.D. Knocking combustion in spark-ignition engines. Prog. Energy Combust. Sci. 2017, 61, 78–112. [Google Scholar] [CrossRef]
- Tree, D.R.; Svensson, K.I. Soot processes in compression ignition engines. Prog. Energy Combust. Sci. 2007, 33, 273–278. [Google Scholar] [CrossRef]
- Omidvarborna, H. Recent studies on soot modeling for diesel combustion. Renew. Sustain. Energy Rev. 2015, 48, 635–647. [Google Scholar] [CrossRef]
- Kholod, N.; Evans, M.; Kuklinski, T. Black carbon emissions: Focus on diesel sources. Atmos. Chem. Phys. 2016, 16, 11267–11281. [Google Scholar] [CrossRef]
- Cipriano, D.; Sacchi, C.; Negri, A.; FIani, E.; Morelli, M.; Poulleau, J.; Lelouer, E. Balance of Micropollutants Emitted by Gas Fired Combustion Turbines. In Proceedings of the Continuous Emission Monitoring (CEM) Conference, Stresa, Italy, 25 January 2009. [Google Scholar]
- Boyce, M.P. Rotor Dynamics; Gulf Professional Publishing: Houston, TX, USA, 2017; Chapter 5; pp. 176–218. [Google Scholar]
- Bogard, D.G.; Thole, K.A. Gas turbine film cooling. J. Propuls. Power 2006, 22, 249–270. [Google Scholar] [CrossRef]
- Wills, J.R.; Reuben, B.G. New local diesel power stations: An economic assessment. Util. Policy 1992, 2, 108–119. [Google Scholar] [CrossRef]
- Kanoglu, M.; Işık, S.K.; Abuşogolu, A. Performance characteristics of a Diesel engine power plant. Energy Convers. Manag. 2005, 46, 1692–1702. [Google Scholar] [CrossRef]
- Jun, L.; Laredj, A.; Tian, G. A case study of a CHP system and its energy use mapping. Energy Procedia 2017, 105, 1526–1531. [Google Scholar]
- Horlock, J.H. The Combined Cycle Gas Turbine. In Advanced Gas Turbine Cycles—A Brief Review of Power Generation Thermodynamics; Pergamon Press: Oxford, UK, 2013; Chapter 7; pp. 109–129. [Google Scholar]
- Flin, D. Why Use Cogeneration? In Cogeneration—A User’s Guide; Institution of Engineering and Technology: London, UK, 2010; Chapter 3; pp. 25–35. [Google Scholar]
- Otsuka, H.; Hiroshi, T.; Syouichi, H.; Satoshi, T.; Junji, O.; Xuewen, C. Construction and Operation Experience of 300 MW Blast Furnace Gas Firing Combined Cycle Power Plant. MHI Tech. Rev. 2007, 44, 1–6. [Google Scholar]
- Yang, W.H.; Yang, W.H.; Xu, T.; Li, W.; Chen, G.; Jia, L.Y.; Guo, Y.J. Waste Gases Utilization and Power Generation in Iron and Steel Works. In Proceedings of the Third International Symposium on Intelligent Information Technology Application Workshops, Nanchang, China, 21–22 November 2009; pp. 246–249. [Google Scholar]
- Molière, M. Benefitting from the Wide Fuel Capability of Gas Turbines: A Review of Application Opportunities. In Proceedings of the ASME Turbo Expo, Amsterdam, The Netherlands, 3–6 June 2002. Paper GT2002-30017. [Google Scholar]
- Molière, M. The omnivorous gas turbine: Robust designs adapt to a wide range of fuels. Turbomach. Int. 2005, 45, 21–28. [Google Scholar]
- Soares, C. Gas Turbines, Gas Turbine Fuel Systems and Fuels. In A Handbook of Air, Land, and Sea Applications; Butterworth-Heinemann: Oxford, UK, 2008; Chapter 7; pp. 213–291. [Google Scholar]
- Feng, L.; Qiang, Y.; Xiao, L.; Ming-Jia, L.; Jun-Hui, X.; Ya-Un, L. Experimental Study on Fuel-Switching of Dual-Fuel Gas Turbine Combustor. Front. Energy Res. 2022, 9, 792620. [Google Scholar] [CrossRef]
- Meher-Homji, C.B.; Zachary, J.C.B.; Bromley, A. Gas Turbine Fuels—System Design, Combustion and Operability. In Proceedings of the 39th Turbomachinery Symposium, Houston, TX, USA, 4–7 October 2010; pp. 155–187. [Google Scholar]
- Klimstra, J. Interchangeability of Gaseous Fuels—The Importance of the Wobbe-Index. In Proceedings of the SAE Conference, Warrendale, PA, USA, 10 January 1986. Technical Paper 861578. [Google Scholar]
- Specification for Fuel Gases for Combustion in GE Heavy-Duty Gas; GEI 41040 G; GE Library: Singapore, 2002.
- Welch, M.; Igoe, B. Gas Turbine Fuel and Fuel Quality Requirements for use in Siemens Industrial Gas Turbine Combustion. In Proceedings of the Second Middle East Turbomachinery Symposium, Doha, Qatar, 17–21 March 2013. [Google Scholar]
- Abbot, D.J. The Impact of Natural Gas Composition Variations on the Operation of Gas Turbines for Power Generation. In Proceedings of the 6th International Conference on the Future of Gas Turbine Technology, Brussels, Belgium, 17–18 October 2012. [Google Scholar]
- Liu, K.; Sanderson, V. The influence of changes in fuel calorific value to combustion performance for Siemens SGT-300 dry low emission combustion system. Fuel 2013, 103, 239–246. [Google Scholar] [CrossRef]
- Jones, R.M. IGCC Gas Turbines for Refinery Applications; GER 4219; GE Library: Singapore, 2003. [Google Scholar]
- Nakamura, S.; McDonell, V.; Samuelssen, S. The Effect of Liquid-Fuel Preparation on Gas Turbine Emissions. In Proceedings of the GT2006 ASME Turbo Expo, Barcelona, Spain, 6–11 May 2006. Paper GT2006-90730. [Google Scholar]
- Agarwal, S.; Chibber, V.K.; Bhatnagar, A.K. Tribological behavior of diesel fuels and the effect of anti-wear additives. Fuel 2013, 106, 21–29. [Google Scholar] [CrossRef]
- Stalder, J.P.; Roberts, P. Firing Low Viscosity Liquid Fuels in Heavy Duty Gas Turbines. In Proceedings of the ASME Turbo Expo, Atlanta, Georgia, 16–19 June 2003. Paper GT2003-38691. [Google Scholar]
- Nolan, P.D. Physical Properties of Hydrocarbons and Petrochemicals. In Handbook of Fire and Explosion Protection Engineering Principles, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2014; Chapter 4; pp. 33–48. [Google Scholar]
- Machuga, C.V.; Crowl, D.A. Derivation of Le Chatelier’s mixing rule for flammable limits. Process Saf. Prog. 2017, 19, 112–117. [Google Scholar]
- Bounaceur, R.; Glaude, P.A.; Sirjean, B.; Fournet, R.; Montagne, P.; Vierling, M.; Molière, M. Prediction of Flammability Limits of Gas Mixtures Containing Inert Gases Under Variable Temperature and Pressure Conditions. In Proceedings of the ASME Turbo Expo, Charlotte, NC, USA, 26–30 June 2017. Paper GT2017-64172. [Google Scholar]
- Molière, M.; Cozzarin, P.; Bouchet, S.; Rech, P. Catalytic Detection of Fuel Leaks in Gas turbine Units: 1. Gaseous and Volatile, Hydrocarbon based fuels. In Proceedings of the ASME Turbo Expo, Reno, NV, USA, 6–9 June 2005. [Google Scholar]
- Molière, M.; Cozzarin, P.; Bouchet, S.; Rech, P. Catalytic Detection of Fuel Leaks in Gas turbine Units: 2. Gas Fuels Containing Hydrogen, Carbon Monoxide and Inert. In Proceedings of the ASME Turbo Expo, Barcelona, Spain, 6–11 May 2006. [Google Scholar]
- Bounaceur, R.; Heymes, R.; Glaude, P.A.; Sirjean, B.; Fournet, R.; Montagne, P.; Auvray, A.; Impellizzeri, E.; Biehler, P.; Picard, A.M.; et al. Development of an Artificial Intelligence Model to Predict Combustion Properties with a Focus on Auto-Ignition Delays. In Proceedings of the ASME Turbo Expo, Boston, MA, USA, 26–30 June 2023. Paper GT2023-102128 (paper accepted). [Google Scholar]
- Alonso, D.; Genin, G.; Heller, D.; Chabrier, B.; Molière, M. Evaporation of Volatile Liquid Pools under Forced Convection. In Proceedings of the ASME Turbo Expo, Vancouver, BC, Canada, 6–10 June 2011. [Google Scholar]
- ISO 21789:2022; Gas Turbine Applications—Safety, 2nd issue, §§ 5:13, 5:17 and 5:19. International Organization for Standardization: Geneva, CH, USA, 2022.
- Gas Turbine Liquid Fuel Specifications; GEI 41047K; GE Library; Singapore, 2003.
- Beychock, M.R. Comparative Economics of Advanced Regenerable Flue Gas Desulfurization Processes, EPRI Report, 1980. Available online: https://www.osti.gov/servlets/purl/5451340 (accessed on 17 March 2022).
- Nouali, H.; Dorge, S.; Brilhac, J.F.; Habermacher, D.; Lebeau, B.; Vierling, M.; Geiger, F.; Patarin, J.; Soulard, M.; Molière, M. Novel Regenerative Desulfurization of Power Plant Flue Gas Streams. In Proceedings of the 29th TIChE Conference, Virtual, 1–2 June 2020. [Google Scholar]
- Pavri, R.; Moore, G.D. Gas Turbine Emissions and Control; GER 4211; GE Library: Singapore, 2001. [Google Scholar]
- Lefebvre, A.H.; Ballal, D.R. Oxides of Nitrogen. In Gas Turbine Combustion: Alternative Fuels and Emissions, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010; Chapter 9; pp. 374–381. [Google Scholar]
- Welch, M.; Igoe, B.; Samuelsson, M. Gas Fuel Flexibility in: DLE Combustion Systems. In Proceedings of the 8th International Gas Turbine Conference, Brussels, Belgium, 12–13 October 2016. [Google Scholar]
- Bender, B. Lean Pre-Mixed Combustion (Section 3.2.1.2) in: Gas Turbine Handbook, NETL-DOE Publication. Available online: https://netl.doe.gov/carbon-management/turbines/handbook (accessed on 17 March 2023).
- Davis, L.B.; Black, S.H. Dry Low NOx Combustion Systems for Heavy-Duty Gas Turbines; GER-3568G; GE Library; Singapore, 2000. [Google Scholar]
- Igoe, B.M. Dry Low Emissions Experience across the Range of Siemens Small Industrial Gas Turbines. Available online: https://www.academia.edu/35139417/Dry_Low_Emissions_Experience_across_the_range_of_Siemens_Small_Industrial_Gas_Turbines_BM_Igoe_Product_Manager_SGT-100_SGT-200_Siemens_Industrial_Turbomachinery_Limited (accessed on 3 May 2023).
- Tanimura, S.; Nose, M.; Ishizaka, K.; Takiguchi, S.; Rodriguez, J. Advanced Dry Low NOx Combustor for Mitsubishi G Class Gas Turbines. In Proceedings of the ASME Turbo Expo, Berlin, Germany, 9–13 June 2008. Paper GT2008-50819. [Google Scholar]
- Lieuwen, T.C.; Yang, V. Combustion Dynamics Principles. In Combustion Instabilities in Gas Turbine Engines: Operational Experience, Fundamental Mechanisms and Modeling; AIAA Publications: Reston, VA, USA, 2005; Chapter 1; pp. 3–26. [Google Scholar]
- Benim, A.C.; Syed, K.J. An Overview of Flashback Mechanisms. In Flashback Mechanisms in Lean Premixed Gas Turbine Combustion; Elsevier: Amsterdam, The Netherlands, 2015; Chapters 4–8; pp. 25–71. [Google Scholar]
- Candel, S. Combustion dynamics and control: Progress and challenges. Proc. Combust. Inst. 2002, 29, 1–28. [Google Scholar] [CrossRef]
- Thomas, L.L. E-class DLN Technology Advancements, DLN1+. In Proceedings of the ASME Turbo Expo, Vancouver, BC, Canada, 6–10 June 2011. GT2011-45944. [Google Scholar]
- Rapp, R.A. Hot Corrosion by Fused Salts. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Pergamon Press: Oxford, UK, 2001; pp. 3834–3835. [Google Scholar]
- Huth, M. Fuel Flexibility in Gas Turbine Systems: Impact on Burner Design and Performance. In Modern Gas Turbine Systems—High Efficiency—Low Emission-Fuel Flexible Power Generation; Woodhead Publishing Series in Energy: Cambridge, UK, 2013; pp. 635–684. [Google Scholar]
- Campbell, A.; Goldmeer, J.; Healy, T.; Washam, R.; Molière, M.; Citeno, J. Heavy Duty Gas Turbines Fuel Flexibility. In Proceedings of the ASME Turbo Expo, Berlin, Germany, 9–13 June 2008. Paper 98GT-231. [Google Scholar]
- Rahm, S.; Goldmeer, J.; Molière, M.; Eranki, A. Addressing Gas Turbine Fuel Flexibility; GER 4601; GE Library: Singapore, 2009. [Google Scholar]
- Asai, T.; Miura, K.; Matsubara, Y.; Akiyama, Y.; Karishuku, M.; Dodo, S.; Okazaki, T.; Tanimura, S. Development of Gas Turbine Combustors for Fuel Flexibility. In Proceedings of the 8th International Gas Turbine Conference, Brussels, Belgium, 12–13 October 2016. [Google Scholar]
- Moliere, M. Expanding Fuel Flexibility of Gas Turbines. Proc. Inst. Mech. Eng. Part A J. Power Energy 2005, 219, 109–119. [Google Scholar] [CrossRef]
- Molière, M. Mobilizing Gas Turbine Units to Reduce Flaring. In Proceedings of the Global Forum on Flaring Reduction and Associated Gas Utilization, Paris, France, 13–16 December 2006. [Google Scholar]
- European Commission Publication On-Line Publication, Cogeneration of Heat and Power. Available online: https://energy.ec.europa.eu/topics/energy-efficiency/cogeneration-heat-and-power_en (accessed on 17 March 2023).
- Demirbas, A. Biofuels. In Biofuels—Securing the Planet’s Future Energy Needs; Springer: Berlin/Heidelberg, Germany, 2009; Chapter 3; pp. 87–101. [Google Scholar]
- USEPA Article, EPA History: Clean Air Act of 1970/1977. Available online: https://www.epa.gov/history/epa-history-clean-air-act-19701977 (accessed on 17 March 2023).
- Agazzani, A.; Massardo, A.F.; Frangopoulos, C.A. Environmental Influence on the Thermoeconomic Optimization of a Combined Plant with NOx Abatement. J. Eng. Gas Turbines Power 1998, 120, 557–565. [Google Scholar] [CrossRef]
- Molière, M.; Nguyen, B.T.; Lebreton, J.H.; Chapon, D.; Maunand, J.; Onillon, H. The Alliance of Gas Turbines and Alternative Fuels. In Proceedings of the PowerGen Asia Conference, Ho Chi Min City, Vietnam, 25 September 2003. [Google Scholar]
- Geiger, F.; Molière, M. Gas Turbines in Alternative Fuel Applications: The Utilization of Highly Aromatic Fuels in Power Generation. In Proceedings of the ASME TURBo Expo Conference, Vienna, Austria, 4–17 June 2004. Paper GT2007-53272. [Google Scholar]
- Molière, M.; Panarotto, E.; Aboujaib, M.; Bisseaud, J.M.; Campbell, A.; Citeno, J.; Maire, P.A.; Ducrest, L. Gas Turbines in Alternative Fuel Applications: Biodiesel Field Test. In Proceedings of the ASME Turbo Expo Conference, Montreal, QC, Canada, 14–17 May 2007. Paper GT2007-27212. [Google Scholar]
- Olbes, A.; Pujol, M.; Moliere, M.; Colas, M. High Compatibility between Gas Turbine and Refinery Utilities. In Proceedings of the PowerGen Europe Conference, Madrid, Spain, 17–19 June 1997. [Google Scholar]
- Gonzales-Martin, J.M.; Mora, A.; Lafuente-Perez, J.M.; Moliere, M.; Martinez, M. The Achievements of the Frame 6B Gas Turbine in Refinery Cogenerations. In Proceedings of the PowerGen Europe Conference, Amsterdam, The Netherlands, 16–18 May 1995. [Google Scholar]
- Skinner, T.; Powell, D.; Molière, M.; Lasne, D.; Hugonnet, M. Heavy Duty Gas Turbines Give Impetus to Large-Scale Cogens: Fuel Flexibility as Added Value at the ESSO Fawley Plant, UK. In Proceedings of the PowerGen Asia Conference, Shenzhen, China, September 2000. [Google Scholar]
- Jun, T.K.; Si, Y.Z.; Hai, C.F.; Molière, M. Gas Turbines Matching Petrochemists’ Energy Needs: CSPC’s 700 MWth-class cogen unit (China). In Proceedings of the PowerGen Asia 2008 Conference, Kuala Lumpur, Malaysia, 21–23 October 2008. [Google Scholar]
- Pointon, K.; Langan, M. OSTI Publication, Distributed Power Generation Using Biogas Fuelled Microturbines, 2002. Available online: https://www.osti.gov/etdeweb/servlets/purl/20350865 (accessed on 17 March 2023).
- Cenusa, V.E.; Alexe, F. Comparative Analysis on Performances of Micro Gas Turbines Burning Biogas vs. Natural Gas. WSEAS Trans. Environ. Dev. 2007, 3, 72–80. [Google Scholar]
- Rasul, M.G. Bio-gas Mixed Fuel Micro Gas Turbine Co-Generation for Meeting Power Demand in Australian Remote Areas. Energy Procedia 2015, 75, 1065–1071. [Google Scholar] [CrossRef]
- Chia-Chi, C. A Case Study on the Electricity Generation Using a Micro Gas Turbine Fuelled by Biogas from a Sewage Treatment Plant. Energies 2019, 12, 2424. [Google Scholar]
- Bonzani, F.; Pollarolo, G.; Rocca, F. Operating Experience of Ansaldo V94.2 K Gas Turbine Fed by Steelworks Gas. In Proceedings of the ASME Turbo Expo, Amsterdam, The Netherlands, 3–6 June 2002. GT2002-30014. [Google Scholar]
- Weizhu, S. Performance Simulation of Gas Turbine Combined Cycle with Coke Oven Gas as Fuel. In Proceedings of the Asia-Pacific Power and Energy Engineering Conference, Wuhan, China, 28–30 March 2009; pp. 1–4. [Google Scholar]
- Solar Turbines On-Line Brochure: Combined Heat and Power Plant—Coke Oven Gas in Chemical Manufacturing. Available online: https://s7d2.scene7.com/is/content/Caterpillar/CM20160211-68438-36713 (accessed on 3 May 2023).
- Wang, T.; Stiegel, G. The combined cycle power island and IGCC system simulations (Part IV). In Integrated Gasification Combined Cycle (IGCC) Technologies; Elesvier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bučko, Z.; Mutinsky, J.; Carros, P.; Molière, M. Gas Turbines Burning Coal-Derived Fuels: The Lignite-Gasification, Power Generation Plant at Vresova (Czech Republic). In Proceedings of the PowerGen Europe Conference, Budapest, Hungary, 26–28 June 1996. [Google Scholar]
- Takeharu, H. Developments of Gas Turbine Combustors for Air-Blown and Oxygen-Blown IGCC. In Advances in Gas Turbine Technology; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef]
- Huth, M.; Heilos, A.; Gaio, G.; Karg, J. Operation Experiences of Siemens IGCC Gas Turbines Using Gasification Products From Coal and Refinery Residues. In Proceedings of the ASME Turbo Expo, Munich, Germany, 8–11 May 2000. Paper 2000-GT-0026. [Google Scholar]
- Brdar, R.D.; Jones, R.M. GE IGCC Technology and Experience with Advanced Gas Turbines; GER-4207; GE Library: Singapore, 2000. [Google Scholar]
- Williams, R.H.; Larson, E.D. Biomass gasifier gas turbine power generating technology. Biomass Bioenergy 1996, 10, 149–166. [Google Scholar] [CrossRef]
- Paisley, M.A.; Anson, D. Biomass Gasification for Gas Turbine-Based Power Generation. J. Eng. Gas Turbines Power 1998, 120, 284–288. [Google Scholar] [CrossRef]
- Lan, W.; Chen, G.; Zhu, X.; Wang, X.; Liu, X.; Xu, B. Biomass gasification-gas turbine combustion for power generation system model based on ASPEN PLUS. Sci. Total Environ. 2018, 628–629, 1278–1286. [Google Scholar] [CrossRef]
- Yong-Chil, S. Gasification of Municipal Solid Waste. In Gasification for Low-Grade Feedstock; IntechOpen: London, UK, 2018; Chapter 7; pp. 115–142. [Google Scholar]
- Indrawan, N.; Kumar, A.; Moliere, M.; Sallam, A.K.; Huhnke, R.L. Distributed power generation via gasification of biomass and municipal solid waste: A review. J. Energy Inst. 2020, 93, 2293–2313. [Google Scholar] [CrossRef]
- Machin, E.B.; Pedroso, D.T.; Acosta, D.G.; dos Santos, M.I.S.; de Carvalho, F.S.; Machín, A.B.; Ortíz, M.A.N.; Arriagada, R.S.; Fernández, D.A.T.; Maciel, L.B.B.B.; et al. Techno-Economic and Environmental Assessment of Municipal Solid Waste Energetic Valorization. Energies 2022, 15, 8900. [Google Scholar] [CrossRef]
- Bertolotto, E.; Amato, A.; Guoqiang, L. Atmospheric Tests of a Full-Scale Gas Turbine Burner Fed With Blast Furnace Gas and Coke Oven Gas. In Proceedings of the ASME Turbo Expo, Phoenix, AZ, USA, 17–21 June 2019. GT2019-91360. [Google Scholar]
- Komori, T.; Hara, H.; Arimura, H.; Kitauchi, Y. Design for F Class Blast Furnace Gas Firing 300 MW Gas Turbine Combined Cycle Plant. In Proceedings of the International Gas Turbine Congress, Tokyo, Japan, 2–7 November 2003. [Google Scholar]
- Komori, T.; Yamagami, N.; Shimamura, Y. Development of Leading Technology for a Low-BTU Gas-firing Gas-turbine Combined-cycle Plant at a Steelworks. MHI Tech. Rev. 2011, 48, 24–28. [Google Scholar]
- Morel, G.; Molière, M. Naphtha, NGL and Gas Condensates in Gas Turbine Plants: Achievements and Prospects. In Proceedings of the PowerGen Asia Conference, Delhi, India, 18–20 November 1998. [Google Scholar]
- Roy, G.K. Design of Gas Turbine Burning Explosive Fuels. In Proceedings of the ASME Turbo Expo, The Hague, The Netherlands, 13 June 1994. Paper 94-GT-329. [Google Scholar]
- Vierling, M.; Molière, M.; Glaser, P.; Denolle, R.; Nayani, S.; Amadou, N.; Dutta, A.; Aboujaib, M.; Westrup, B.; Sokolov, D.; et al. Efficient Smoke Suppressant Reduces PM Emissions of Crude Oil-Fired Gas Turbines. In Proceedings of the ASME Turbo Expo Conference, Phoenix, AZ, USA, 17–21 June 2019. GT2019-90382. [Google Scholar]
- Marikkar, N.; Jayath, T.; Vierling, M.; Aboujaib, M.; Sokolov, D.; Guichard, J.L. Ash Deposition Control Technology: A Strong Enabler for Performance Upgrades in HFO CCGT Plants. In Proceedings of the PowerGen Asia Conference, Bangkok, Thailand, 13–14 July 2017. [Google Scholar]
- Lee, Y.H.; Ahn, H.Y.; Molière, M. Gas Turbine Fuel Flexibility Serving the Petrochemical Industry: The Case of the Frame 6B at Yeosu, Korea. In Proceedings of the PowerGen Asia Conference, Bangkok, Thailand, 3–5 October 2012. [Google Scholar]
- Lupandin, V.; Thamburaj, R.; Nikolayev, A. Test results of the OGT2500 Gas Turbine Engine Running on Alternative Fuels: BioOil, Ethanol, BioDiesel and Crude Oil, 2005. In Proceedings of the ASME Turbo Expo Conference, Reno, NV, USA, 6–9 June 2005. Paper GT2005-68488. [Google Scholar]
- Gupta, K.K.; Rehman, A.; Sarviya, R.M. Bio-fuels for the gas turbine: A review. Renew. Sustain. Energy Rev. 2010, 14, 2946–2955. [Google Scholar] [CrossRef]
- Do Nascimiento, M.A.R.; Dos Santos, E.C. Biofuel and Gas Turbine Engines. In Advances in Gas Turbine Technology; IntechOpen: London, UK, 2011; Chapter 6; pp. 115–126. [Google Scholar]
- Kallenberg, A. Liquid Bio Fuels for Gas Turbines. Master’s Thesis, Lund University On-Line Publication, Lund, Sweden, 2013. Available online: https://www.lunduniversity.lu.se/lup/publication/3457896 (accessed on 17 March 2023).
- Meng, C.C. Liquid Biofuels Production and Emissions Performance in Gas Turbines: A Review. Energy Convers. Manag. 2018, 173, 640–658. [Google Scholar]
- Reinmart, B.; Arifin, Z. A Fuel Testing Analysis of B20 Biodiesel for Gas Turbines in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 551, 012003. [Google Scholar] [CrossRef]
- Cheng, T.C. Dual-Fuel Operation of Biodiesel and Natural Gas in a Model Gas Turbine Combustor. Energy Fuels 2020, 34, 3788–3796. [Google Scholar] [CrossRef]
- Amri, K.; Kismanto, A.; Solikhah, M.D.; Pratiwi, F.T.; Arisanti, A.G. Study of biodiesel specifications for heavy-duty gas turbine application. J. Phys. Conf. Ser. 2021, 1858, 012040. [Google Scholar] [CrossRef]
- Thompson, P.B. The Agricultural Ethics of Biofuels: The Food vs. Fuel Debate. Agriculture 2012, 2, 339–358. [Google Scholar] [CrossRef]
- Molière, M.; Vierling, M.; Aboujaib, M.; Patil, P.; Eranki, A.; Campbell, A.; Trivedi, R.; Nainani; Subhendu, R.; Pandey, N. Gas Turbines in Alternative Fuel Applications: Bio-Ethanol Field Test. In Proceedings of the ASME Turbo Expo; Orlando, FL, USA, 8–12 June 2009, Paper GT2009-59047.
- Turbomachinery International Article: Combustion Turbine Fueled by Sugarcane-Based Bioethanol, 27 February 2019. Available online: https://www.turbomachinerymag.com/view/albioma-commissioning-of-the-first-peak-load-combustion-turbine-fueled-by-sugarcane-based-bioethanol-in-la-reunion (accessed on 17 March 2023).
- De Souza-Abud, A.K. Bioethanol Production from Food Crops: Sustainable Sources, Interventions and Challenges; Academic Press: London, UK, 2019; Chapter 21; pp. 417–443. [Google Scholar]
- Chandel, A.K.; Forte, M.B.S.; Gonçalves, I.S.; Milessi, T.S.; Arruda, P.V.; Carvalho, W.; Mussatto, S.I. Brazilian biorefineries from second generation biomass: Critical insights from industry and future perspectives, Brazilian biorefineries from second generation biomass: Critical insights from industry and future perspectives. Biofuels Biorefin. Bioprod. 2021, 15, 1190–1208. [Google Scholar] [CrossRef]
- Glaude, P.A.; Sirjean, B.; Fournet, R.; Bounaceur, R.; Molière, M. Combustion and Oxidation Kinetics of Alternative Gas Turbines Fuels. In Proceedings of the ASME Turbo Expo Conference, Düsseldorf, Germany, 16–20 June 2014. Paper 2014GT25070. [Google Scholar]
- Basu, A.; Gradassi, M.; Sills, R.; Fleisch, T.; Pur, R. Use of DME as Gas Turbine Fuel. In Proceedings of the ASME Turbo Expo Conference, New Orleans, LA, USA, 4–7 June 2001. Paper 2001-GT-0003. [Google Scholar]
- Haining, Z.; Lin, L. Biodiesel: An Alternative to Conventional Fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar]
- Siemens On-Line Brochure: Green Biofuels: A Proven Fuel for Gas Turbines, 2022. Available online: https://assets.siemens-energy.com/siemens/assets/api/uuid:f15b4a76-64df-4b83-affe-80fe072d7233/biofuels-all.pdf (accessed on 17 March 2023).
- Glaude, P.A.; Fournet, R.; Bounaceur, R.; Molière, M. Adiabatic flame temperature from biofuels and fossil fuels and derived effect on NOx emissions. Fuel Process. Technol. 2010, 91, 229–235. [Google Scholar] [CrossRef]
- Ram, S.D.; Prakash, J.; Dhimanc, S.K.; Lald, A.; Kumar, A. Study of the Bulk Modulus of Compressibility of Biodiesel and its Impact on Exhaust Emissions. Int. J. Emerg. Technol. Innov. Eng. 2015, 1, 1–7. [Google Scholar]
- Baig, M.Z.; Dharmadhikari, S.M. Bioethanol Production from Enzymatically Hydrolyzed Cotton Stalk: One Approach towards Sustainable Energy Development. Curr. World Environ. 2014, 9, 940. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; Chen, J. Hydroprocessing of Biomass-Derived Oils and Their Blends with Petroleum Feedstocks: A Review. Energy Fuels 2012, 26, 5373–5399. [Google Scholar] [CrossRef]
- Truche, L.; Bazarkina, E.F. Natural Hydrogen the Fuel of the 21st Century. In Proceedings of the E3S Web of Conferences, Jember, Indonesia, 31 July–2 August 2019; Volume 98, p. 03006. [Google Scholar]
- ClimaTalk Association On-Line Publication. Available online: https://climatalk.org/2022/04/18/the-hydrogen-rainbow/ (accessed on 17 March 2023).
- Vautrin, A.; Bossmann, T.; Beaussant, O. METIS Study on Costs and Benefits of a Pan-European Hydrogen Infrastructure, On-Line Publication of the EU Commission, 2021. Available online: https://op.europa.eu/fr/publication-detail/-/publication/c50a12fc-5eeb-11ec-9c6c-01aa75ed71a1/ (accessed on 17 March 2023).
- Conrad, R.; Rheinardt, T. Hydrogen Strategy Enabling A Low-Carbon Economy, On-Line DOE Publication, 2020. Available online: https://netl.doe.gov/sites/default/files/2020-12/USDOE_FE_Hydrogen_Strategy_July2020.pdf (accessed on 17 March 2023).
- UK Government On-Line Publication, UK Hydrogen Strategy Update to the Market, 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1123751/hydrogen-strategy-update-to-the-market-december-2022.pdf (accessed on 17 March 2023).
- Hord, J. Is Hydrogen a Safe Fuel? Int. J. Hydrogen Energy 1978, 3, 157–176. [Google Scholar] [CrossRef]
- Rigas, F.; Amiotte, P. Hydrogen Properties Associated with Hazards. In Hydrogen Safety; CRC Press: Boca Raton, FL, USA, 2013; Chapter 3; pp. 19–25. [Google Scholar]
- Patel, M.; Roy, S.; Roskilly, A.P.; Smallbone, H. The techno-economics potential of hydrogen interconnectors for electrical energy transmission and storage. J. Clean. Prod. 2022, 335, 130045. [Google Scholar] [CrossRef]
- Molière, M.; Hugonnet, H. Hydrogen-Fueled Gas Turbines: Experience and Prospects. In Proceedings of the PowerGen Asia Conference, Bangkok, Thailand, 22–26 November 2004. [Google Scholar]
- Tomczak, H.J.; Benelli, G. Investigation of Gas Turbine combustion system fired with mixtures of natural gas and hydrogen. IFRF Combust. J. 2002, 19, 200–207. [Google Scholar]
- Glaude, P.A.; Fournet, R.; Bounaceur, R.; Moliere, M. Kinetic study of the combustion of hydrogen under pressure. In Proceedings of the Conference at Ecole Nationale Supérieure des Industries Chimiques (ENSIC), Nancy, France, 5 October 2022. [Google Scholar]
- Ayed, A.H.; Kusterer, K.; Funke, H.H.-W.; Keinz, J.; Striegan, C.; Bohn, D. Experimental and numerical investigations of the dry-low-NOx hydrogen micromix combustion chamber of an industrial gas turbine. Propuls. Power Res. 2015, 4, 123–131. [Google Scholar]
- Funke, H.H.-W.; Beckmann, N.; Abanteriba, S. An overview on dry low NOx micromix combustor development for hydrogen-rich gas turbine applications. Int. J. Hydrogen Energy 2019, 44, 6978–6990.7. [Google Scholar] [CrossRef]
- Noble, D.; Wu, D.; Emerson, B.; Sheppard, S.; Lieuwen, T.; Angello, L. Assessment of Current Capabilities and Near-Term Availability of Hydrogen-Fired Gas Turbines Considering a Low-Carbon Future. J. Eng. Gas Turbines Power 2021, 143, 041002. [Google Scholar] [CrossRef]
- Kim, D. Review on the Development Trend of Hydrogen Gas Turbine Combustion Technology. J. Korean Soc. Combust. 2019, 24, 1–10. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Molière, M.; Lallemand, R.; Chun, M.K.; Son, H.K. Heavy-Duty Gas Turbines in Petrochemical Plants: Samsung’s Daesan Plant (Korea) Beats Fuel Flexibility Records with over 95% Hydrogen in Process Gas. In Proceedings of the PowerGen Asia Conference, Bangkok, Thailand, 21 September 1999. [Google Scholar]







| Economy Branches | Origin Process | Fuel Name | Acronym | State (L/G) | Main Features | Ash-Less or Ash-Forming |
|---|---|---|---|---|---|---|
| Oil Production & refining | Extraction Distillation Catalytic Cracking | Crude oil Liq. Petr. Gas Naphtha Kerosene Light Distil’te Heavy Dist’te Heavy Oil Light Cycle oil Ref Fuel Gas | CO LPG - - LD - HFO LCO RFG | L L/G1 L L L L L L G | Light to Heavy HCV2 Volatile Ultra Pure Conventional Liq. Viscous Impurities Aromatic H2 & Cn | AL AL AL AL AL Slight. AF Strong. AF AL AL |
| Gas Exploration & processing | NG extraction NG reforming | Natural Gas Gas Cond’sate Nat. Gas Liq Hydrogen | NG GC NGL H2 | G L L G | Rich/weak/Soft/sour Light to heavy Low viscosity Highly flamm. | AL AL AL AL |
| Coal Mining & Processing | Coal mining Coal gasification | Coalbed gas Syngas Substitute NG Methanol Hydrogen | CBG SG SNG MeOH H2 | G G G G G | LCV3 MCV4/LCV MCV/LCV Highly volatile Highly flamm. | Slight. AF AF AL AL AL |
| Metallurgy | Coal pyrolysis Metal oxide reduction | Coke Oven Gas Blast Furnace Gas | COG BFG | G G | MCV LCV | AF AF |
| Petrochemistry | Naphtha Cracking Aromatisation Butadiene unit | Olefin-rich Pet. Gas H2 Pet Net G. C3/C4 Pet Net G | PNG PNG PNG | G G G | Var. olefin % Var. H2 content Var. C3 & C4% | AL AL AL |
| Farming | Aerobic fermentation | Biogas | BG | G | Var. N2-CO2% | AL/sligh AF |
| Biomass | Vegetables processing | Biodiesel Bioethanol Dimethyl Ether | BD EtOH DME | L L L | Analog. to LD Analog. to naph Analog. to LPG | AL AL AL |
| Biofuel | Similar Fossil Fuel | Commonalities | Differences (Bio Versus Fossil) |
|---|---|---|---|
| GASES | |||
| Biogas (raw) | Weak Natural Gas (raw) | - Comparable values of %C1 & WI | - Higher CO2 & H2S contents - siloxane contaminants |
| LIQUIDS | |||
| DiMethyl-Ether (DME) | LPG | - High Vapor pressure - High vapor density | - Narrower distillation range - burned as liquid (LPG as gas) |
| Bioethanol-(biomethanol) | Naphtha - Gasoline | - Intermiscible - Good purity - Highly volatile - Poor lubricity | - Narrower distillation range - Lower NOx emission |
| Biodiesel | Light Distillate Fuel (#2 DF) | - Intermiscible - Good to fair purity - limited volatility | - Better lubricity - Aggressive to gaskets |
| Vegetable Oil (VO) * | Crude oils | - Wide distillation range - Partly intermiscible - Metallic impurities - Highly viscous | - No light hydrocarbon - No aromatic species - Less heat resistant - oil soluble contaminants |
| DATA − FUEL | No of Molecules of Combustion Products | LHV Mass Volume MJ/kg MJ/Nm3 | Diffusion Coefficient 1 atm; 25 °C − 10−6 m2s−1 | LFL – UFL Low/Up. Flam. Lim. − % vol | Lamin. Flame Speed − cm.s−1 | Min. Ignition Energy − mJ |
|---|---|---|---|---|---|---|
| H2 | 1 (H2O) | 120 10.7 | 7.9 | 4.0 - 75 | 265 | 0.018 |
| CH4 | 3 (CO2 + 2 H2O) | 50 35.8 | 0.2 | 5.0 - 15 | 33 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molière, M. The Fuel Flexibility of Gas Turbines: A Review and Retrospective Outlook. Energies 2023, 16, 3962. https://doi.org/10.3390/en16093962
Molière M. The Fuel Flexibility of Gas Turbines: A Review and Retrospective Outlook. Energies. 2023; 16(9):3962. https://doi.org/10.3390/en16093962
Chicago/Turabian StyleMolière, Michel. 2023. "The Fuel Flexibility of Gas Turbines: A Review and Retrospective Outlook" Energies 16, no. 9: 3962. https://doi.org/10.3390/en16093962
APA StyleMolière, M. (2023). The Fuel Flexibility of Gas Turbines: A Review and Retrospective Outlook. Energies, 16(9), 3962. https://doi.org/10.3390/en16093962





