Pollutant Emissions and Heavy Metal Migration in Co-Combustion of Sewage Sludge and Coal
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Thermogravimetric Analysis
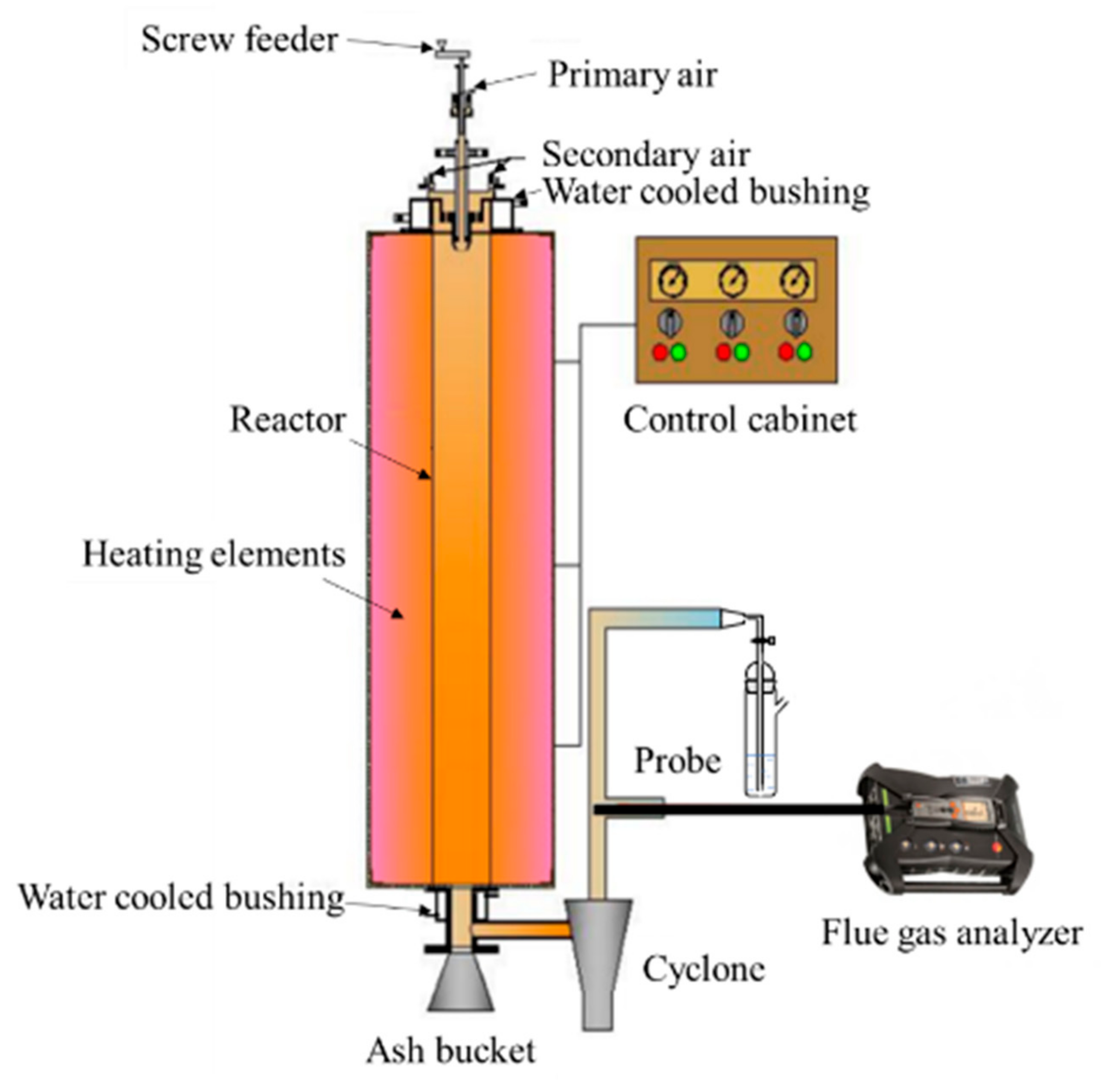
2.3. Combustion Facility
2.4. Heavy Metal Analysis
2.5. Simulation Calculation of Heavy Metals Migration
3. Results and Discussion
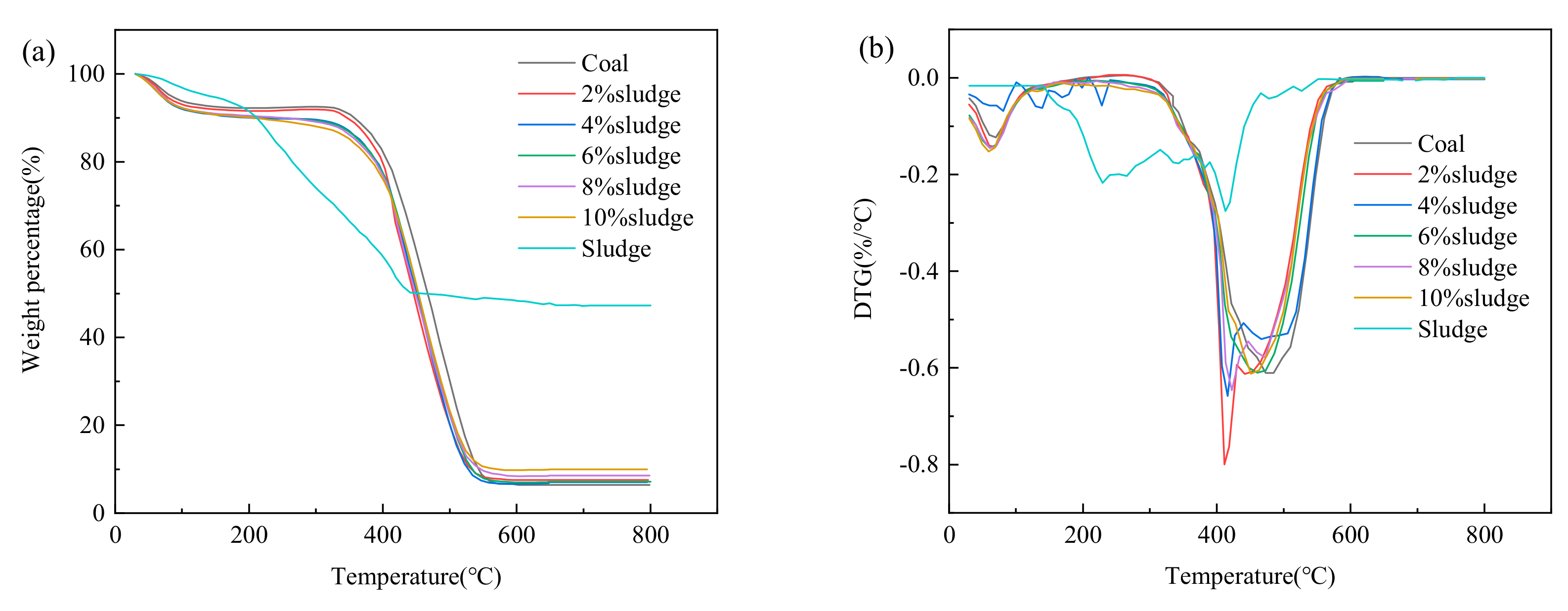
3.1. TG and DTG Profiles of Coal, Sewage Sludge, and Mixtures
3.2. SO2 and NOx Emission
3.3. HCl and HF Emission
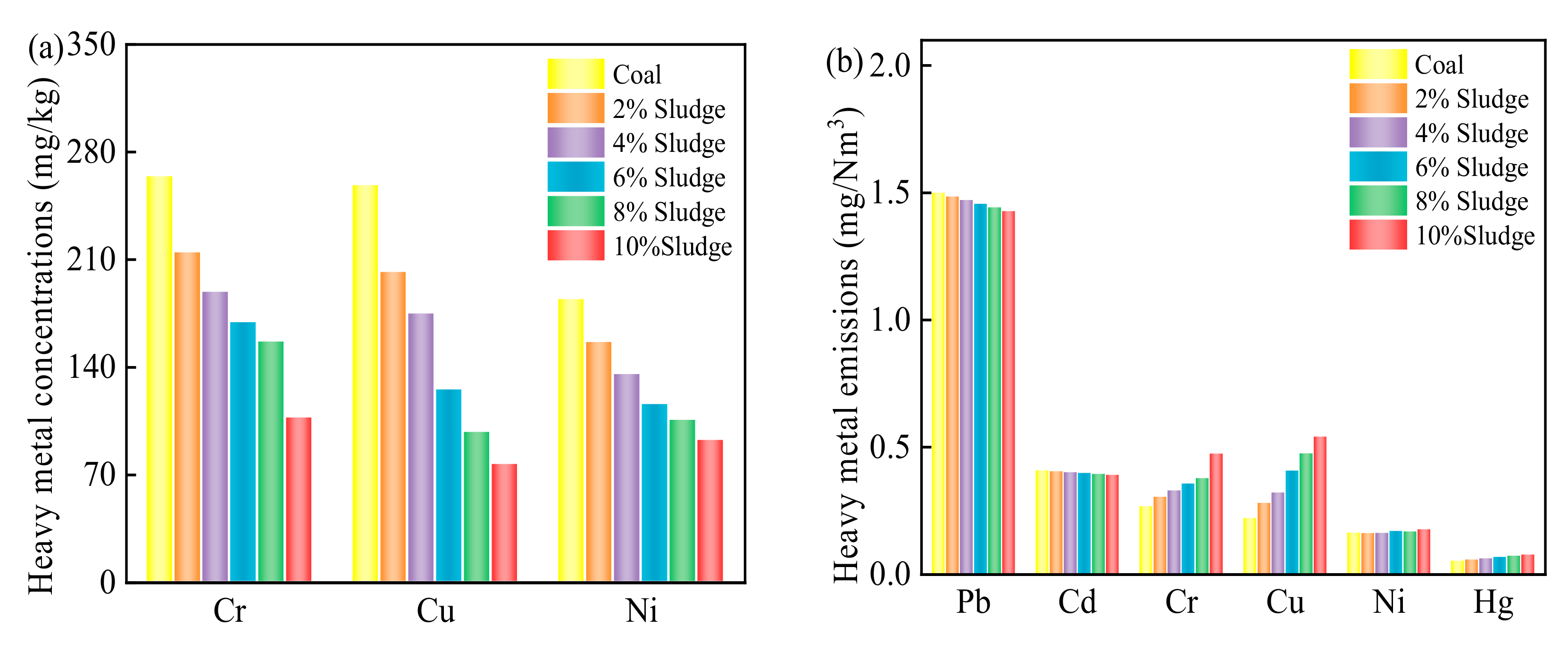
3.4. Heavy Metal Content in Bottom Ash and Flue Gas
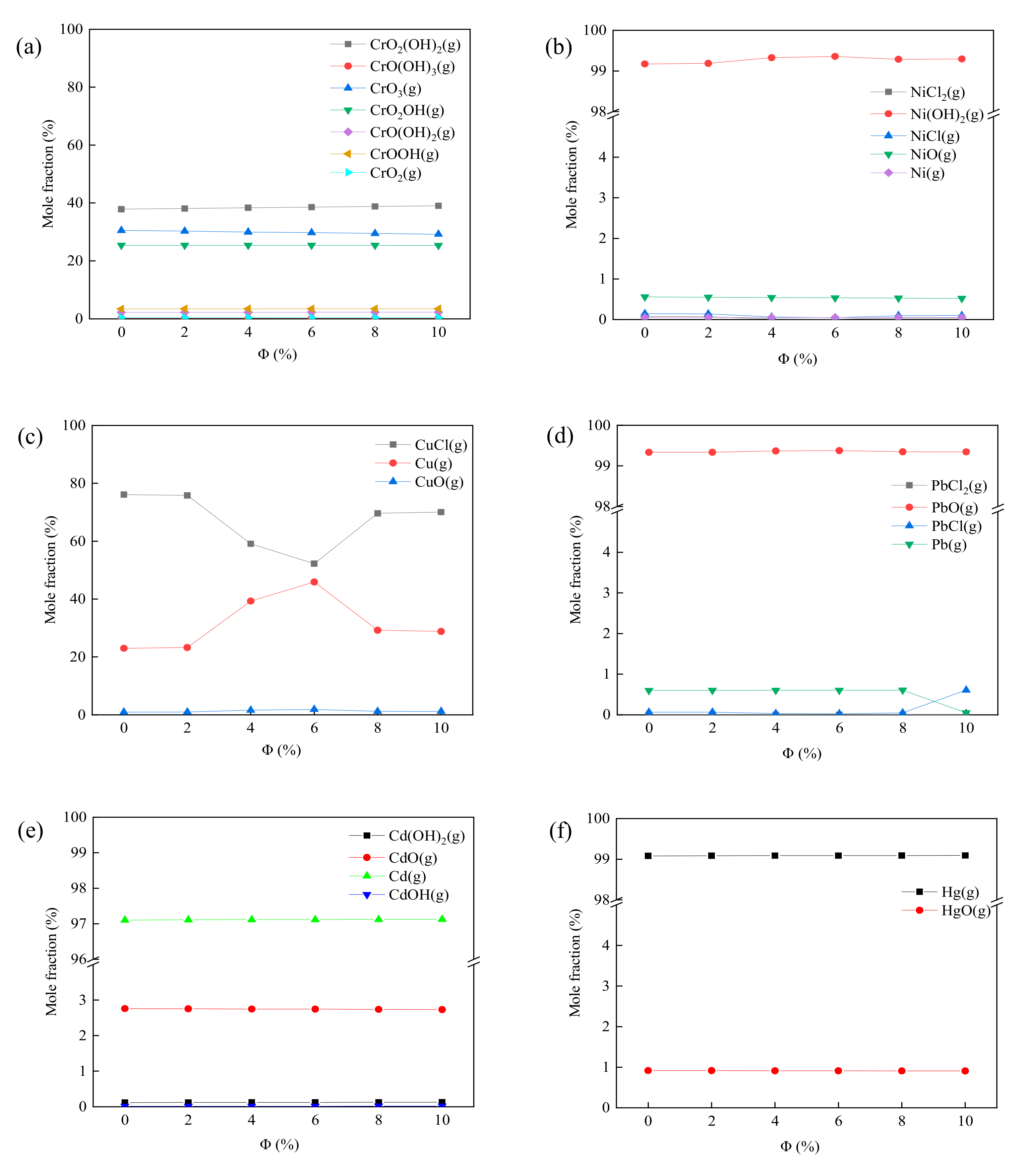
3.5. Simulation Calculation of Heavy Metals Migration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, B.; Liu, G.J.; Mian, M.M.; Zhou, C.C.; Sun, M.; Wu, D.; Liu, Y. Co-combustion of industrial coal slurry and sewage sludge: Thermochemical and emission behavior of heavy metals. Chemosphere 2019, 233, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.L.; Li, X.Y.; Feng, J.; Zhang, R.; Bai, H.; Bu, D.; Dan, Z.; Li, W.; Lu, X.B. Short-Chain Fatty Acids Production from Anaerobic Fermentation of Sewage Sludge: The Effect of Higher Levels Polyaluminium Chloride. Int. J. Environ. Res. Public Health 2022, 19, 2806. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fan, M.H.; Zhang, G.M. Emerging contaminants in surface waters in China—A short review. Environ. Res. Lett. 2014, 9, 074018. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, F.; Mei, Z.Y.; Lv, L.K.; Chi, Y. Status and development of sludge incineration in China. Waste Biomass- Valorization 2021, 12, 3541–3574. [Google Scholar] [CrossRef]
- Guo, S.; Yu, S.X.; Che, D.Y.; Liu, H.P.; Sun, B.Z. Migration characteristics of heavy metals during co-combustion of dehydrated sludge with straw. J. Fuel Chem. Technol. 2022, 50, 283–294. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.H.; Chai, X.L. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R. Organic contaminants in sewage sludge (biosolids) and their significance for agricultural recycling. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 4005–4041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, H.; Zhang, X.J.; Xing, H.X.; Hu, H.Y.; Yao, H. Novel utilization of conditioner CaO for gas pollutants control during co-combustion of sludge and coal. Fuel 2017, 206, 541–545. [Google Scholar] [CrossRef]
- Hušek, M.; Muško, J.; Pohořelý, M. Sewage sludge treatment methods and P-recovery possibilities: Current state-of-the-art. J. Environ. Manag. 2022, 315, 115090. [Google Scholar] [CrossRef]
- Raclavská, H.; Růžičková, J.; Šafář, M.; Kucbel, M.; Slamová, K.; Švédová, B.; Juchelková, D.; Kantor, P. Municipal sludges as sources of energy or nutrients—What is the best? Energy 2023, 275, 127469. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture—The effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef] [PubMed]
- Sorinolu, A.J.; Tyagi, N.; Kumar, A.; Munir, M. Antibiotic resistance development and human health risks during wastewater reuse and biosolids application in agriculture. Chemosphere 2021, 265, 129032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, L.; Mao, X.D.; Hong, G.J.; Jiang, S. Deep dewatering of stocked sludge and incineration characteristics of dehydrated sludge cake. Res. Environ. Sci. 2021, 34, 1015–1022. (In Chinese) [Google Scholar]
- Yang, X.X.; Kan, T.; Kheradmand, A.; Xu, H.M.; Strezov, V.; Yu, A.B.; Jiang, Y.Y. Tunable syngas production from two-stage sorption-enhanced steam gasification of sewage sludge. Chem. Eng. J. 2021, 404, 126069. [Google Scholar] [CrossRef]
- Xue, Y.J.; Zhou, Y.; Liu, J.; Xiao, Y.; Wang, T. Comparative analysis for pyrolysis of sewage sludge in tube reactor heated by electromagnetic induction and electrical resistance furnace. Waste Manag. 2021, 120, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, S.H.; Ye, Z.L.; Meng, H.J.; Zhu, Y.G. Utilization of urban sewage sludge: Chinese perspectives. Environ. Sci. Pollut. Res. 2012, 19, 1454–1463. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T.M. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Oleszek, S.; Shiota, K.; Oshita, K.; Takaoka, M. Comparison of sewage sludge mono-incinerators: Mass balance and distribution of heavy metals in step grate and fluidized bed incinerators. Waste Manag. 2020, 105, 575–585. [Google Scholar] [CrossRef]
- Wu, H.; Glarborg, P.; Frandsen, F.J.; Dam-Johansen, K.; Jensen, P.A.; Sander, B. Co-combustion of pulverized coal and solid recovered fuel in an entrained flow reactor—General combustion and ash behaviour. Fuel 2011, 90, 1980–1991. [Google Scholar] [CrossRef]
- Zhang, S.R.; Jiang, X.G.; Lv, G.J.; Wu, L.; Li, W.; Wang, Y.F.; Fang, C.Q.; Jin, Y.Q.; Yan, J.H. Co-combustion of Shenmu coal and pickling sludge in a pilot scale drop-tube furnace: Pollutants emissions in flue gas and fly ash. Fuel Process. Technol. 2019, 184, 57–64. [Google Scholar] [CrossRef]
- Stelmach, S.; Wasielewski, R. Co-combustion of dried sewage sludge and coal in a pulverized coal boiler. J. Mater. Cycles Waste Manag. 2008, 10, 110–115. [Google Scholar] [CrossRef]
- Otero, M.; Gomez, X.; Garcia, A.I.; Moran, A. Effects of sewage sludge blending on the coal combustion: A thermogravimetric assessment. Chemosphere 2007, 69, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Ma, L.; Xia, J.; Fang, Q.Y.; Zhang, C.; Chen, G. Co-firing sludge in a pulverized coal-fired utility boiler: Combustion characteristics and economic impacts. Energy 2017, 119, 392–399. [Google Scholar] [CrossRef]
- Xu, T.; Wang, C.B.; Hong, D.K.; Li, S.; Yue, S. The synergistic effect during co-combustion of municipal sludge and coal: Experimental and ReaxFF molecular dynamic study. Energy 2023, 262, 125553. [Google Scholar] [CrossRef]
- Yu, L.Y.; Li, P.S. Thermogravimetric analysis of coal and sludge co-combustion with microwave radiation dehydration. J. Energy Inst. 2014, 87, 220–226. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Díaz, R.M.; Xiberta, J.; Prieto, I. Thermogravimetric analysis of the co-combustion of coal and sewage sludge. Fuel 2003, 82, 2051–2055. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M. Thermogravimetric study of biomass, sewage sludge and coal combustion. Energy Convers. Manag. 2013, 75, 425–430. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Hong, C.; Xing, Y.; Li, Y.F.; Feng, L.H.; Jia, M.M. Combustion behaviors and kinetics of sewage sludge blended with pulverized coal: With and without catalysts. Waste Manag. 2018, 74, 288–296. [Google Scholar] [CrossRef]
- Zhang, S.R.; Jiang, X.G.; Lv, G.J.; Liu, B.X.; Jin, Y.Q.; Yan, J.H. SO2, NOx, HF, HCl and PCDD/Fs emissions during Co-combustion of bituminous coal and pickling sludge in a drop tube furnace. Fuel 2016, 186, 91–99. [Google Scholar] [CrossRef]
- Ma, M.Y.; Liang, Y.; Xu, D.H.; Sun, S.Y.; Zhao, J.; Wang, S.Z. Gas emission characteristics of sewage sludge co-combustion with coal: Effect of oxygen atmosphere and feedstock mixing ratio. Fuel 2022, 322, 124102. [Google Scholar] [CrossRef]
- Bartoňová, L.; Raclavská, H.; Čech, B.; Kucbel, M. Behavior of Cd during coal combustion: An overview. Processes 2020, 8, 1237. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.J.; Chen, J. Transformation of Zn and Cr during co-combustion of sewage sludge and coals: Influence of coal and steam. Environ. Sci. Pollut. Res. 2023, 30, 98351–98361. [Google Scholar] [CrossRef] [PubMed]
- GB 18484-2020; Standard for Pollution Control on Hazardous Waste Incineration. China Environment Publishing Group: Beijing, China, 2020.
- GB/T 212-2008; Proximate Analysis of Coal. Standards of Press of China: Beijing, China, 2008.
- GB/T 31391-2015; Ultimate Analysis of Coal. Standards of Press of China: Beijing, China, 2015.
- GB/T 213-2008; Determination of Calorific Value of Coal. Standards of Press of China: Beijing, China, 2008.
- GB/T 3558-2014; Determination of Chlorine of Coal. Standards of Press of China: Beijing, China, 2014.
- GB/T 4633-2014; Determination of Fluorine in Coal. Standards of Press of China: Beijing, China, 2014.
- GB/T 1574-2007; Test Method for Analysis of Coal Ash. Standards of Press of China: Beijing, China, 2007.
- Lin, Y.; Liao, Y.F.; Yu, Z.S.; Fang, S.W.; Ma, X.Q. The investigation of co-combustion of sewage sludge and oil shale using thermogravimetric analysis. Thermochim. Acta 2017, 653, 71–78. [Google Scholar] [CrossRef]
- Calvo, L.F.; Otero, M.; Jenkins, B.M.; García, A.I.; Morán, A. Heating process characteristics and kinetics of sewage sludge in different atmospheres. Thermochim. Acta 2004, 409, 127–135. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Díaz, R.M.; Xiberta, J. Pyrolysis of blends of different types of sewage sludge with one bituminous coal. Energy 2005, 30, 1079–1091. [Google Scholar] [CrossRef]
- Lin, Y.S.; Ma, X.Q.; Yu, Z.S.; Cao, Y.W. Investigation on thermochemical behavior of co-pyrolysis between oil-palm solid wastes and paper sludge. Bioresour. Technol. 2014, 166, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Chen, J.B.; Yao, P.K.; Zhou, D.P.; Zhao, L.; Yin, H.C. Evaluation of co-pyrolysis petrochemical wastewater sludge with lignite in a thermogravimetric analyzer and a packed-bed reactor: Pyrolysis characteristics, kinetics, and products analysis. Bioresour. Technol. 2016, 221, 147–156. [Google Scholar] [CrossRef]
- Vamvuka, D.; Salpigidou, N.; Kastanaki, E.; Sfakiotakis, S. Possibility of using paper sludge in co-firing applications. Fuel 2009, 88, 637–643. [Google Scholar] [CrossRef]
- Liao, Y.F.; Ma, X.Q. Thermogravimetric analysis of the co-combustion of coal and paper mill sludge. Appl. Energy 2010, 87, 3526–3532. [Google Scholar] [CrossRef]
- Tarelho, L.A.C.; Matos, M.A.A.; Pereira, F. The influence of operational parameters on SO2 removal by limestone during fluidised bed coal combustion. Fuel Process. Technol. 2005, 86, 1385–1401. [Google Scholar] [CrossRef]
- Weber, R.; Kupka, T.; Zajac, K. Jet flames of a refuse derived fuel. Combust. Flame 2009, 156, 922–927. [Google Scholar] [CrossRef]
- Jin, Y.Y.; Li, Y.Y.; Liu, F.Q. Combustion effects and emission characteristics of SO2, CO, NOx and heavy metals during co-combustion of coal and dewatered sludge. Front. Environ. Sci. Eng. 2016, 10, 201–210. [Google Scholar] [CrossRef]
- Pu, G.; Zan, H.F.; Du, J.T.; Zhang, X. Study on NO emission in the oxy-fuel combustion of co-firing coal and biomass in a bubbling fluidized bed combustor. BioResources 2017, 12, 1890–1902. [Google Scholar] [CrossRef]
- Jiang, X.G.; Xu, X.; Yan, J.H.; He, J.; Chi, Y.; Cen, K.F. Experimental study on the release characteristic of chlorine in coal combustion process. J. China Coal Soc. 2002, 27, 398–401. (In Chinese) [Google Scholar]
- Li, W.; Lu, H.L.; Chen, H.K.; Li, B.Q. Volatilization behavior of fluorine in coal during fluidized-bed pyrolysis and CO2-gasification. Fuel 2005, 84, 353–357. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, G.; Liu, J.Y.; Evrendilek, F.; Buyukada, M.; Xie, W.M. Thermal behaviors of fluorine during (co-)incinerations of spent potlining and red mud: Transformation, retention, leaching and thermodynamic modeling analyses. Chemosphere 2020, 249, 126204. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, G.; Chen, Z.H.; Evrendilek, F.; Liu, J.Y. Water-soluble fluorine detoxification mechanisms of spent potlining incineration in response to calcium compounds. Environ. Pollut. 2020, 266, 115420. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. China Environment Publishing Group: Beijing, China, 2018.
- GB 36600-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Development Land. China Environment Publishing Group: Beijing, China, 2018.
- Zhang, G.; Hai, J.; Ren, M.Z.; Zhang, S.K.; Cheng, J.; Yang, Z.R. Emission, mass balance, and distribution characteristics of PCDD/Fs and heavy metals during cocombustion of sewage sludge and coal in power plants. Environ. Sci. Technol. 2013, 47, 2123–2130. [Google Scholar] [CrossRef]
- Jiao, F.C.; Zhang, L.; Song, W.J.; Meng, Y.; Yamada, N.; Sato, A.; Ninomiya, Y. Effect of inorganic particulates on the condensation behavior of lead and zinc vapors upon flue gas cooling. Proc. Combust. Inst. 2013, 34, 2821–2829. [Google Scholar] [CrossRef]
- Meij, R.; Winkel, B.H.T. Trace elements in world steam coal and their behaviour in Dutch coal-fired power stations: A review. Int. J. Coal Geol. 2009, 77, 289–293. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, Y.Y.; Liu, L.L.; Wang, X.D.; Zhang, Z.T. Environmental investigation on co-combustion of sewage sludge and coal gangue: SO2, NOx and trace elements emissions. Waste Manag. 2016, 50, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, W.D.C.; Veksha, A.; Giannis, A.; Lisak, G.; Chang, V.W.C.; Lim, T.-T. Fate and distribution of heavy metals during thermal processing of sewage sludge. Fuel 2018, 226, 721–744. [Google Scholar] [CrossRef]
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (Integrated Pollution Prevention and Control). Off. J. Eur. Union 2010, 334, 17–119.


| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | Content (mg/kg) | HHV (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | V | FC | C | H | N | O | S | F | Cl | ||
| Coal | 3.63 | 32.75 | 63.61 | 74.11 | 4.13 | 0.87 | 16.62 | 0.64 | 212.37 | 899.50 | 35.11 |
| Sludge | 41.68 | 51.08 | 7.24 | 36.23 | 5.21 | 3.88 | 11.62 | 1.38 | 177.78 | 632.09 | 16.97 |
| Sample | Chemical Composition (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | MnO2 | SO3 | P2O5 | |
| Coal | 14.46 | 10.96 | 3.76 | 0.12 | 54.42 | 0.34 | 0.17 | 2.13 | 1.29 | 8.48 | 0.02 |
| Sludge | 21.61 | 6.20 | 37.10 | 0.79 | 8.65 | 3.54 | 1.82 | 0.97 | 0.20 | 7.57 | 9.80 |
| Sample | Heavy Metal Content (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Cr | Cu | Hg | Ni | Cd | Pb | |
| Coal | 20.06 | 18.05 | 2.01 | 13.04 | 16.05 | 59.18 |
| Sludge | 85.42 | 107.38 | 11.71 | 20.01 | 9.27 | 30.75 |
| Sample | Ti (°C) | Tb (°C) | S × 108 (%2/°C3 min2) |
|---|---|---|---|
| Coal | 385 | 530 | 6.75 |
| 2% sludge | 384 | 530 | 6.62 |
| 4% sludge | 384 | 530 | 6.39 |
| 6% sludge | 384 | 530 | 6.22 |
| 8% sludge | 383 | 530 | 6.03 |
| 10% sludge | 382 | 529 | 5.96 |
| Sludge | 235 | 641 | 1.19 |
| Element | Coal | 2% Sludge | 4% Sludge | 6% Sludge | 8% Sludge | 10% Sludge |
|---|---|---|---|---|---|---|
| Cr | 0.48 | 0.44 | 0.43 | 0.42 | 0.41 | 0.30 |
| Cu | 0.52 | 0.45 | 0.42 | 0.32 | 0.26 | 0.21 |
| Ni | 0.51 | 0.52 | 0.52 | 0.51 | 0.51 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yue, C.; Ma, Y. Pollutant Emissions and Heavy Metal Migration in Co-Combustion of Sewage Sludge and Coal. Energies 2024, 17, 2457. https://doi.org/10.3390/en17112457
Liu C, Yue C, Ma Y. Pollutant Emissions and Heavy Metal Migration in Co-Combustion of Sewage Sludge and Coal. Energies. 2024; 17(11):2457. https://doi.org/10.3390/en17112457
Chicago/Turabian StyleLiu, Chunyu, Changtao Yue, and Yue Ma. 2024. "Pollutant Emissions and Heavy Metal Migration in Co-Combustion of Sewage Sludge and Coal" Energies 17, no. 11: 2457. https://doi.org/10.3390/en17112457




