Municipal Sewage Sludge as a Resource in the Circular Economy
Abstract
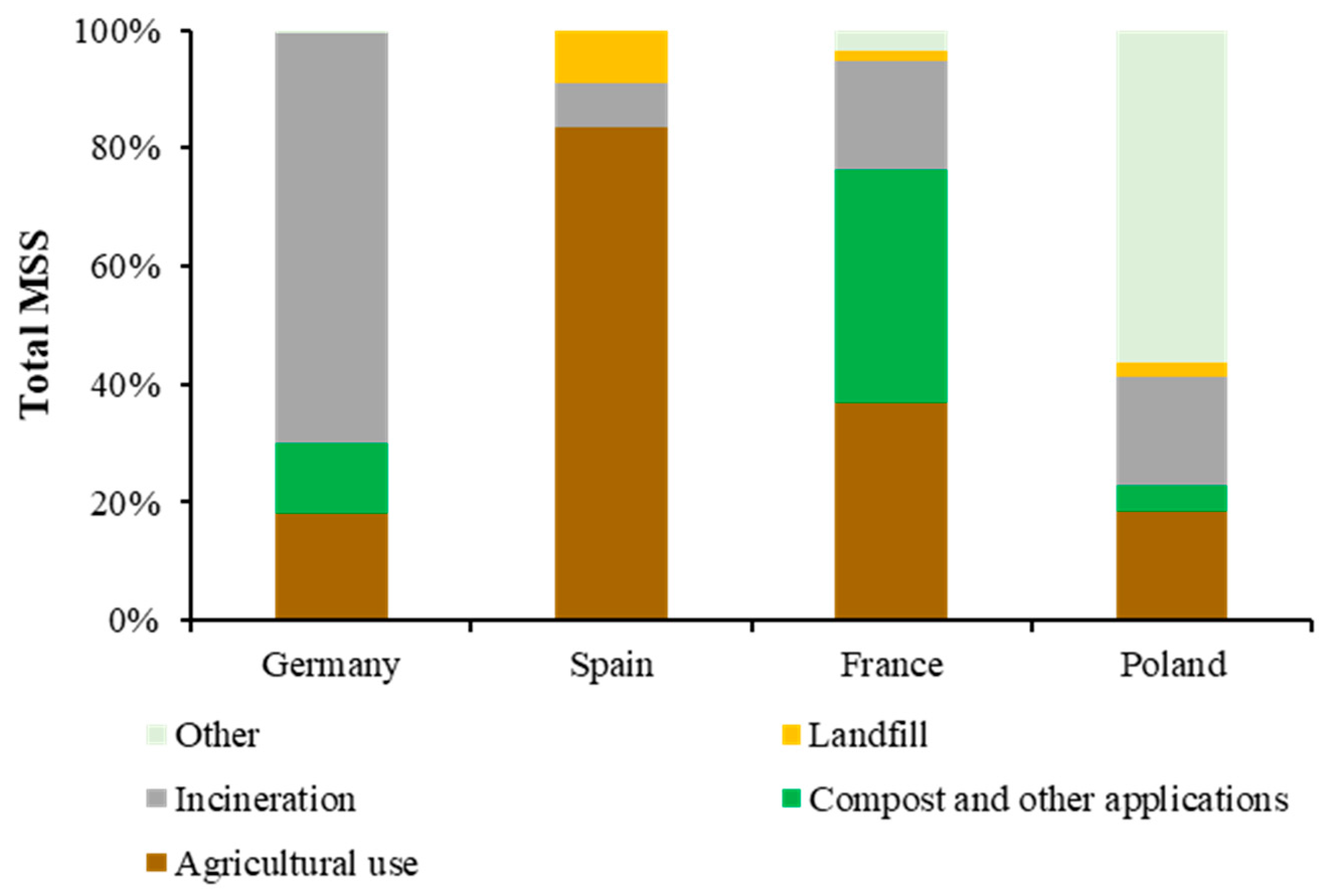
:1. Introduction
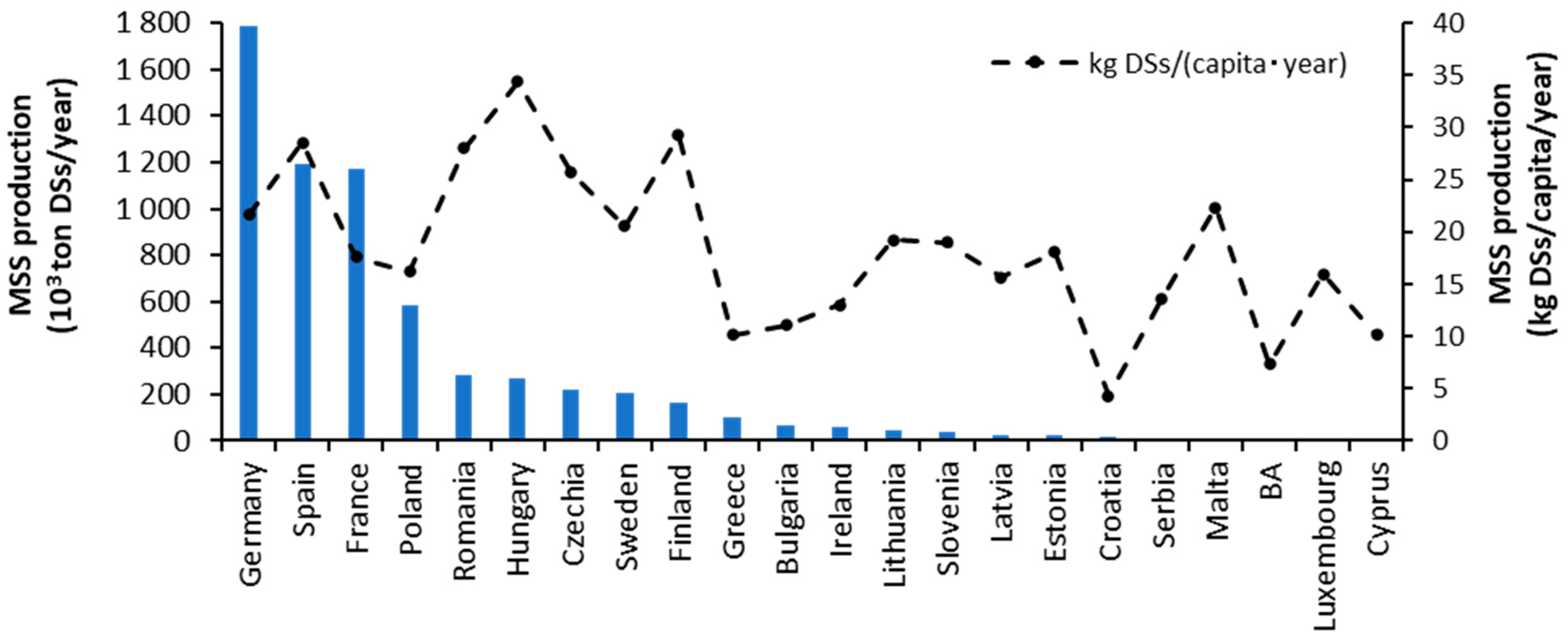
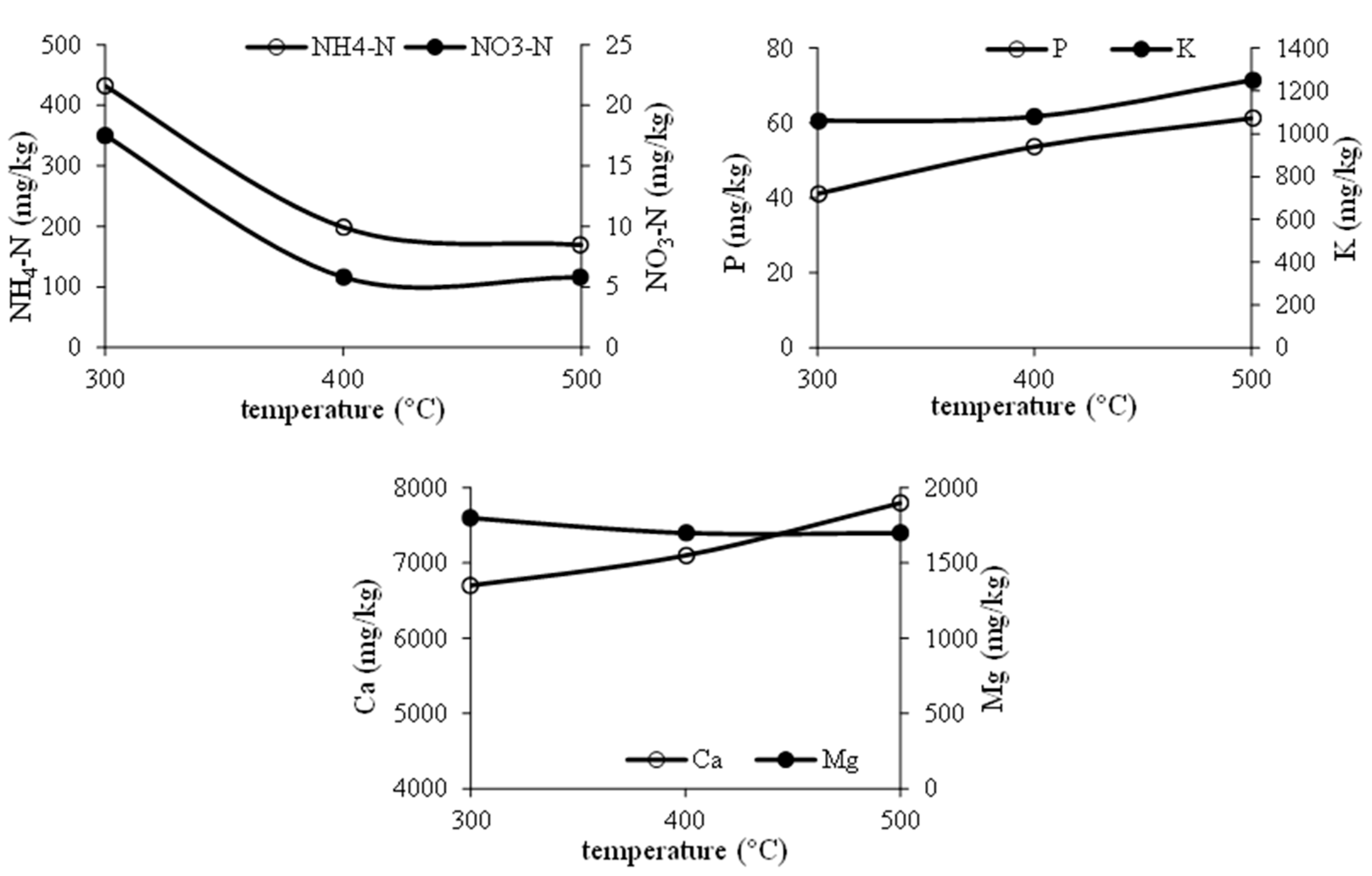
2. Recovery of P and N from MSS
3. MSS as a Source of Alginate-like Exopolymers (ALEs)
4. MSS as a Source of Humic Substances (HSs)
4.1. HS Contents in Raw and Processed MSS
4.2. Characteristics of HSs
4.3. Application of MSS and HSs
5. MSS as Feedstock for Thermochemical Conversion to Biochar
5.1. Properties of MSS-Derived Biochar
| Characteristics | [100] | [101] | [82] | [92] | [102] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSS | MSS-B | MSS | MSS-B | MSS | MSS-B | MSS-B | MSS | MSS-B | MSS-B | MSS | MSS-B | ||
| 300 °C | 500 °C | 500 °C | 300 °C | 500 °C | 300 °C | 500 °C | 500 °C | ||||||
| pH | - | - | - | - | - | - | - | - | 6.82 | 7.02 | 7.70 | - | - |
| Moisture (%) | 7.0 | 2.6 | 3.0 | 6.30 | - | 84.5 | - | - | 85.2 | - | - | 12.3 | 2.3 |
| VM (%) | 72.1 | 49.8 | 26.0 | 54.1 | 26.6 | 73.7 | - | - | - | - | - | 50.9 | 8.9 |
| FC (%) | 11.9 | 11.9 | 23.7 | 7.8 | 22.6 | 0.4 | - | - | - | - | - | 9.1 | - |
| Ash (%) | 16.0 | 38.3 | 50.4 | 31.8 | 50.9 | 25.9 | - | - | 46.6 | 63.9 | 77.4 | 35.5 | 59.6 |
| C (%) | 38.3 | 45.4 | 40.5 | 35.2 | 35.1 | 37.9 | 39.7 | 9.8 | 24.7 | 21.2 | 15.6 | 37.9 | 27.4 |
| H (%) | 5.0 | 4.2 | 2.0 | 5.4 | 3.4 | 5.5 | 4.1 | 0.4 | 4.6 | 2.3 | 0.9 | 4.5 | 0.9 |
| O (%) | 37.3 | 7.3 | 0.7 | 15.6 | 5.8 | - | - | - | 18.6 | 8.2 | 3.3 | 54.1 | 69.9 |
| N (%) | 3.4 | 4.9 | 5.7 | 5.6 | 4.8 | 7.1 | 7.1 | 2.1 | 4.5 | 3.3 | 2.2 | 2.7 | 1.3 |
| S (%) | <0.05 | <0.05 | 0.7 | - | - | - | - | - | 0.9 | 1.0 | 0.6 | 0.7 | 0.5 |
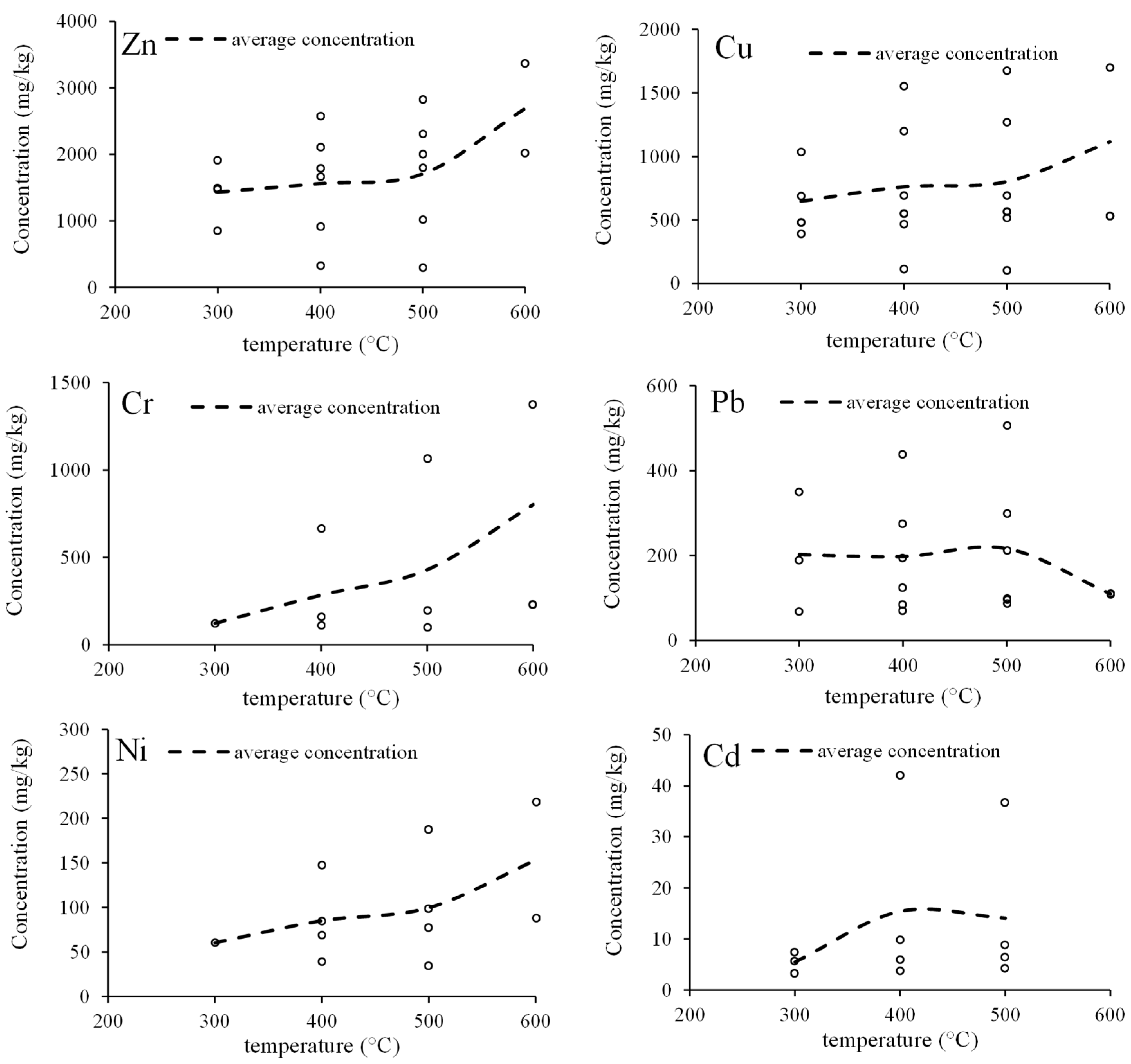
5.2. Potential Environmental Concerns Regarding MSS-Derived Biochar
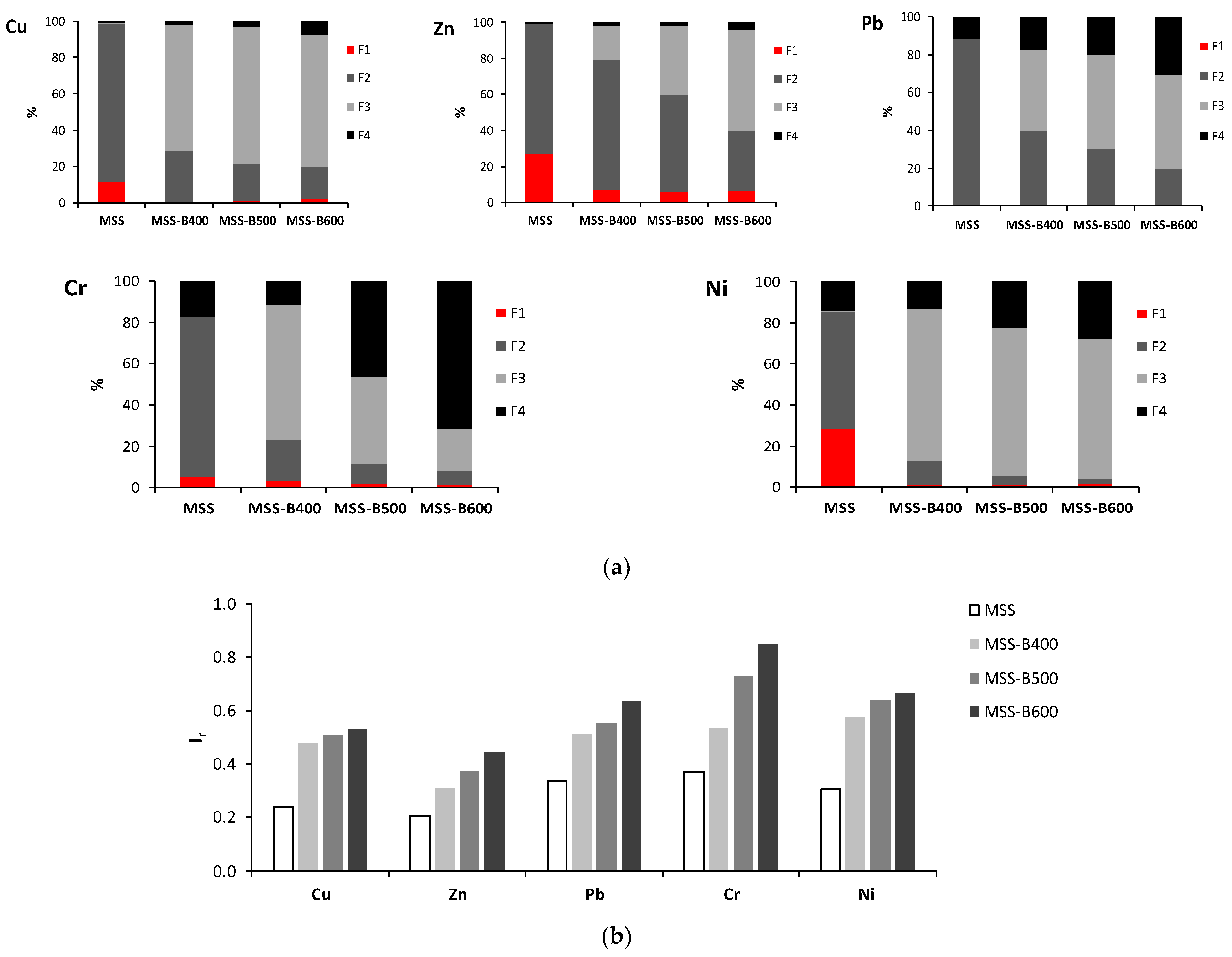
5.2.1. Heavy Metals
5.2.2. Organic Pollutants
6. Main Applications of MSS Biochar
7. Limitations and Future Prospects
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Djandja, O.S.; Yin, L.X.; Wang, Z.C.; Duan, P.G. From Wastewater Treatment to Resources Recovery through Hydrothermal Treatments of Municipal Sewage Sludge: A Critical Review. Process Saf. Environ. Prot. 2021, 151, 101–127. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage Sludge Disposal Strategies for Sustainable Development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Manara, P.; Zabaniotou, A. Towards Sewage Sludge Based Biofuels via Thermochemical Conversion—A Review. Renew Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Eurostat. Sewage Sludge Production and Disposal from Urban Wastewater (in Dry Substance (d.s)). Available online: https://ec.europa.eu/eurostat/databrowser/view/ten00030/default/bar?lang=en (accessed on 29 April 2024).
- Tytła, M. Correction: Tytła, M. Assessment of Heavy Metal Pollution and Potential Ecological Risk in Sewage Sludge from Municipal Wastewater Treatment Plant Located in the Most Industrialized Region in Poland—Case Study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A Review of the Hydrothermal Carbonization of Biomass Waste for Hydrochar Formation: Process Conditions, Fundamentals, and Physicochemical Properties. Renew Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Iyyappan, J.; Pravin, R.; Kadry, S.; Han, J.; Sindhu, R.; Awasthi, M.K.; Rokhum, S.L.; Baskar, G. A Strategic Review on Sustainable Approaches in Municipal Solid Waste Management and Energy Recovery: Role of Artificial Intelligence, Economic Stability and Life Cycle Assessment. Bioresour. Technol. 2023, 379, 129044. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.C.; Eugénio, T.; Branco, M.C. Circular Economy for Cities and Sustainable Development: The Case of the Portuguese City of Leiria. Sustainability 2022, 14, 1726. [Google Scholar] [CrossRef]
- Smol, M. Circular Economy in Wastewater Treatment Plant—Water, Energy and Raw Materials Recovery. Energies 2023, 16, 3911. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A. Energy and Resources Recovery from Excess Sewage Sludge: A Holistic Analysis of Opportunities and Strategies. RCR Adv. 2023, 19, 200184. [Google Scholar] [CrossRef]
- Racek, J.; Sevcik, J.; Chorazy, T.; Kucerik, J.; Hlavinek, P. Biochar—Recovery Material from Pyrolysis of Sewage Sludge: A Review. Waste Biomass Valoriz. 2020, 11, 3677–3709. [Google Scholar] [CrossRef]
- Đurđević, D.; Blecich, P.; Jurić, Ž. Energy Recovery from Sewage Sludge: The Case Study of Croatia. Energies 2019, 12, 1927. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D.; Klik, B. Suitability of Humic Substances Recovered from Sewage Sludge to Remedy Soils from a Former as Mining Area—A Novel Approach. J. Hazard. Mater. 2017, 338, 160–166. [Google Scholar] [CrossRef]
- Kulikowska, D.; Klik, B.K.; Gusiatin, Z.M.; Jabłoński, R. Sewage Sludge Can Provide a Washing Agent for Remediation of Soil from a Metallurgical Area. Catena 2019, 173, 22–28. [Google Scholar] [CrossRef]
- Di Fraia, S.; Di Meglio, A.; Massarotti, N.; Vanoli, L.; Bentivoglio, R.; Volpecina, V. Energy Recovery and Waste Valorization in a Frozen Food Processing Facility: A Case Study from Lazio, Italy. Energy Effic. 2024, 17, 13. [Google Scholar] [CrossRef]
- Dawson, C.J.; Hilton, J. Fertiliser Availability in a Resource-Limited World: Production and Recycling of Nitrogen and Phosphorus. Food Policy 2011, 36, S14–S22. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective: Increasing Accumulation of Phosphorus in Soil Threatens Rivers, Lakes, and Coastal Oceans with Eutrophication. Bioscience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Bekunda, M.; Grizzetti, B.; de Vries, W.; van Grinsven, H.J.M.; Abrol, Y.P.; Adhya, T.K.; Billen, G.; et al. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution. Global Overview of Nutrient Management. In Centre for Ecology and Hydrology, Edinburgh on Behalf of the Global Partnership on Nutrient Management and the International Nitrogen Initiative; Springer Nature Singapure Pte Ltd.: Singapore, 2013. [Google Scholar]
- Mulder, A. The Quest for Sustainable Nitrogen Removal Technologies. Proc. Water Sci. Technol. 2003, 48, 67–75. [Google Scholar] [CrossRef]
- Wu, X.; Modin, O. Ammonium Recovery from Reject Water Combined with Hydrogen Production in a Bioelectrochemical Reactor. Bioresour. Technol. 2013, 146, 530–536. [Google Scholar] [CrossRef]
- Yeoman, S.; Stephenson, T.; Lester, J.N.; Perry, R. The Removal of Phosphorus during Wastewater Treatment: A Review. Environ. Pollut. 1988, 49, 183–233. [Google Scholar] [CrossRef]
- Cañas, J.; Álvarez-Torrellas, S.; Hermana, B.; García, J. Phosphorus Recovery from Sewage Sludge as Struvite. Water 2023, 15, 2382. [Google Scholar] [CrossRef]
- Ohtake, H.; Tsuneda, S. Phosphorus Recovery and Recycling; Springer Nature Singapure Pte Ltd.: Singapore, 2019. [Google Scholar]
- Huygens, D.; Saveyn, H.G.M.; European Commission, Joint Research Centre. Technical Proposals for by-Products and High Purity Materials as Component Materials for EU Fertilising Products; Publications Office of the European Union: Luxembourg, 2019; ISBN 9789276501169. [Google Scholar]
- Rahaman, M.S.; Mavinic, D.S.; Meikleham, A.; Ellis, N. Modeling Phosphorus Removal and Recovery from Anaerobic Digester Supernatant through Struvite Crystallization in a Fluidized Bed Reactor. Water Res. 2014, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Latifian, M.; Liu, J.; Mattiassona, B. Struvite-Based Fertilizer and Its Physical and Chemical Properties. Environ. Technol. 2012, 33, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Burton, F.; Stensel, D.H. Wastewater Engineering: Treatment and Reuse (Book), 4th ed.; Metcalf & Eddy Inc. McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Rodrigues, M.; De Mattos, T.T.; Sleutels, T.; Ter Heijne, A.; Hamelers, H.V.M.; Buisman, C.J.N.; Kuntke, P. Minimal Bipolar Membrane Cell Configuration for Scaling up Ammonium Recovery. ACS Sustain. Chem. Eng. 2020, 8, 17359–17367. [Google Scholar] [CrossRef] [PubMed]
- Koskue, V.; Rinta-Kanto, J.M.; Freguia, S.; Ledezma, P.; Kokko, M. Optimising Nitrogen Recovery from Reject Water in a 3-Chamber Bioelectroconcentration Cell. Sep. Purif. Technol. 2021, 264, 118428. [Google Scholar] [CrossRef]
- Desloover, J.; De Vrieze, J.; Van De Vijver, M.; Mortelmans, J.; Rozendal, R.; Rabaey, K. Electrochemical Nutrient Recovery Enables Ammonia Toxicity Control and Biogas Desulfurization in Anaerobic Digestion. Environ. Sci. Technol. 2015, 49, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Ledezma, P.; Jermakka, J.; Keller, J.; Freguia, S. Recovering Nitrogen as a Solid without Chemical Dosing: Bio-Electroconcentration for Recovery of Nutrients from Urine. Environ. Sci. Technol. Lett. 2017, 4, 119–124. [Google Scholar] [CrossRef]
- Felz, S.; Neu, T.R.; van Loosdrecht, M.C.M.; Lin, Y. Aerobic Granular Sludge Contains Hyaluronic Acid-like and Sulfated Glycosaminoglycans-like Polymers. Water Res. 2020, 169, 115291. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Paoletti, S. Material Properties of Alginates. In Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–53. [Google Scholar]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial Alginate Production, Modification and Its Applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef]
- Lin, Y.M.; Sharma, P.K.; van Loosdrecht, M.C.M. The Chemical and Mechanical Differences between Alginate-like Exopolysaccharides Isolated from Aerobic Flocculent Sludge and Aerobic Granular Sludge. Water Res. 2013, 47, 57–65. [Google Scholar] [CrossRef]
- de Carvalho, C.D.A.; dos Santos, A.F.; Ferreira, T.J.T.; Lira, V.N.S.A.; Barros, A.R.M.; dos Santos, A.B. Resource Recovery in Aerobic Granular Sludge Systems: Is It Feasible or Still a Long Way to Go? Chemosphere 2021, 274, 129881. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial Alginates: From Biosynthesis to Applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Rollemberg, S.L.D.S.; Dos Santos, A.F.; Ferreira, T.J.T.; Firmino, P.I.M.; Dos Santos, A.B. Evaluation of the Production of Alginate-like Exopolysaccharides (ALE) and Tryptophan in Aerobic Granular Sludge Systems. Bioprocess. Biosyst. Eng. 2021, 44, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; van Loosdrecht, M.C.M.; Saikaly, P.E. Gradual Adaptation to Salt and Dissolved Oxygen: Strategies to Minimize Adverse Effect of Salinity on Aerobic Granular Sludge. Water Res. 2017, 124, 702–712. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Rollemberg, S.L.; De Oliveira, L.Q.; De Barros, A.N.; Firmino, P.I.M.; Dos Santos, A.B. Pilot-Scale Aerobic Granular Sludge in the Treatment of Municipal Wastewater: Optimizations in the Start-up, Methodology of Sludge Discharge, and Evaluation of Resource Recovery. Bioresour. Technol. 2020, 311, 123467. [Google Scholar] [CrossRef]
- de Sousa Rollemberg, S.L.; Ferreira, T.J.T.; Firmino, P.I.M.; Dos Santos, A.B. Impact of Cycle Type on Aerobic Granular Sludge Formation, Stability, Removal Mechanisms and System Performance. J. Environ. Manag. 2020, 256, 109970. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, K.; de Vries, E.; Koop, S.; Roest, K. The Energy & Raw Materials Factory: Role and Potential Contribution to the Circular Economy of the Netherlands. Environ. Manag. 2018, 61, 786–79543. [Google Scholar]
- Ferreira, T.J.T.; de Sousa Rollemberg, S.L.; de Barros, A.N.; de Lima, J.P.M.; Dos Santos, A.B. Integrated Review of Resource Recovery on Aerobic Granular Sludge Systems: Possibilities and Challenges for the Application of the Biorefinery Concept. J. Environ. Manag. 2021, 291, 112718. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xie, G.J.; Nie, W.B.; Xing, D.F.; Liu, B.F.; Ding, J.; Ren, N.Q. High Value-Added Biomaterials Recovery from Granular Sludge Based Wastewater Treatment Process. Resour. Conserv. Recycl. 2021, 169, 105481. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A. Biopolymers in Aerobic Granular Sludge—Their Role in Wastewater Treatment and Possibilities of Re-Use in Line with Circular Economy. Energies 2021, 14, 7219. [Google Scholar] [CrossRef]
- Hamza, R.; Rabii, A.; Ezzahraoui, F.Z.; Morgan, G.; Iorhemen, O.T. A Review of the State of Development of Aerobic Granular Sludge Technology over the Last 20 Years: Full-Scale Applications and Resource Recovery. Case Stud. Chem. Environ. Eng. 2022, 5, 100173. [Google Scholar] [CrossRef]
- Zeng, R.G.; Shi, C.; Hao, L.T.; Huang, A.; Yuan, T.; Zhang, N. A Review of Alginate-like Extracellular Polymers from Excess Sludge: Extraction, Characterization, and Potential Application. J. Water Process Eng. 2023, 56, 104346. [Google Scholar] [CrossRef]
- Michalska, J.; Turek-Szytow, J.; Dudło, A.; Kowalska, K.; Surmacz-Górska, J. Evaluation of the Applicability of Selected Analytical Techniques for Determining the Characteristics of Humic Substances Sourced from by-Products of the Wastewater Treatment Process. Sci. Total Environ. 2023, 888, 164237. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.J.; Hu, L.F.; Mahmood, Q.; Long, Y.; Shen, D.S. Study on Biosorption of Humic Acid by Activated Sludge. Biochem. Eng. J. 2008, 39, 478–485. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, C. Evolution of Humic Substances during Anaerobic Sludge Digestion. Environ. Eng. Manag. J. 2017, 16, 1577–1582. [Google Scholar] [CrossRef]
- Filip, Z.; Pecher, W.; Berthelin, J. Microbial Utilization and Transformation of Humic Acid-like Substances Extracted from a Mixture of Municipal Refuse and Sewage Sludge Disposed of in a Landfill. Environ. Pollut. 2000, 109, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Li, C. Characterization of Humic Acids and Fulvic Acids Derived from Sewage Sludge. Asian J. Chem. 2013, 25, 10087–10091. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Tabatabai, M.A. Decomposition of Different Organic Materials in Soils. Biol. Fertil. Soils 1994, 18, 175–182. [Google Scholar] [CrossRef]
- Riffaldi, R.; Sartori, F.; Levi-Minzi, R. Humic Substances in Sewage Sludges. Environ. Pollut. Ser. B Chem. Physic. 1982, 3, 139–146. [Google Scholar] [CrossRef]
- Réveillé, V.; Mansuy, L.; Jardé, É.; Garnier-Sillam, É. Characterisation of Sewage Sludge-Derived Organic Matter: Lipids and Humic Acids. Org. Geochem. 2003, 34, 615–627. [Google Scholar] [CrossRef]
- Giulio, C.; Camelin, E.; Ottone, C.; Fraterrigo Garofalo, S.; Jorquera, L.; Castro, M.; Fino, D.; Schiappacasse, M.C.; Tommasi, T. Recovery of Humic Acids from Anaerobic Sewage Sludge: Extraction, Characterization and Encapsulation in Alginate Beads. Int. J. Biol. Macromol. 2020, 164, 277–285. [Google Scholar]
- Kulikowska, D.; Klik, B.K.; Gusiatin, Z.M.; Hajdukiewicz, K. Characteristic of Humic Substances from Municipal Sewage Sludge: A Case Study. Desalin. Water Treat. 2019, 144, 57–64. [Google Scholar] [CrossRef]
- Paredes, C.; Roig, A.; Bernal, M.P.; Sánchez-Monedero, M.A.; Cegarra, J. Evolution of Organic Matter and Nitrogen during Co-Composting of Olive Mill Wastewater with Solid Organic Wastes. Biol. Fertil. Soils 2000, 32, 222–227. [Google Scholar] [CrossRef]
- Jouraiphy, A.; Amir, S.; Winterton, P.; El Gharous, M.; Revel, J.C.; Hafidi, M. Structural Study of the Fulvic Fraction during Composting of Activated Sludge-Plant Matter: Elemental Analysis, FTIR and 13C NMR. Bioresour. Technol. 2008, 99, 1066–1072. [Google Scholar] [CrossRef]
- Jouraiphy, A.; Amir, S.; El Gharous, M.; Revel, J.C.; Hafidi, M. Chemical and Spectroscopic Analysis of Organic Matter Transformation during Composting of Sewage Sludge and Green Plant Waste. Int. Biodeterior. Biodegrad. 2005, 56, 101–108. [Google Scholar] [CrossRef]
- Kulikowska, D.; Bernat, K. Waste Willow-Bark from Salicylate Extraction Successfully Reused as an Amendment for Sewage Sludge Composting. Sustainability 2021, 13, 6771. [Google Scholar] [CrossRef]
- Kulikowska, D.; Sindrewicz, S. Effect of Barley Straw and Coniferous Bark on Humification Process during Sewage Sludge Composting. Waste Manag. 2018, 79, 207–213. [Google Scholar] [CrossRef]
- Kulikowska, D. Kinetics of Organic Matter Removal and Humification Progress during Sewage Sludge Composting. Waste Manag. 2016, 49, 196–203. [Google Scholar] [CrossRef]
- Sidelko, R.; Walendzik, B.; Smuga-Kogut, M.; Janowska, B.; Szymanski, K.; Glowacka, A.; Lesnianska, A. Impact of Reduced Straw Content on the Sewage Sludge Composting Process. Arch. Environ. Prot. 2020, 46, 70–77. [Google Scholar]
- Kononova, M.M. Organic Matter and Soil Fertility; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 1968. (In Polish) [Google Scholar]
- Kalsom, M.S.U.; Nur, H.; Norlea, A.A.; Ngaspan, S. Characterization of Humic Acid from Humification of Oil Palm Empty Fruit Bunch Fibre Using Trichoderma Viride. J. Trop. Agric. Food Sci. 2006, 34, 165–172. [Google Scholar]
- Amir, S.; Hafidi, M.; Merlina, G.; Revel, J.C. Structural Changes in Lipid-Free Humic Acids during Composting of Sewage Sludge. Int. Biodeterior. Biodegrad. 2005, 55, 239–246. [Google Scholar] [CrossRef]
- Amir, S.; Benlboukht, F.; Cancian, N.; Winterton, P.; Hafidi, M. Physico-Chemical Analysis of Tannery Solid Waste and Structural Characterization of Its Isolated Humic Acids after Composting. J. Hazard. Mater. 2008, 160, 448–455. [Google Scholar] [CrossRef]
- Droussi, Z.; D’Orazio, V.; Hafidi, M.; Ouatmane, A. Elemental and Spectroscopic Characterization of Humic-Acid-like Compounds during Composting of Olive Mill by-Products. J. Hazard. Mater. 2009, 163, 1289–1297. [Google Scholar] [CrossRef]
- Bartoszek, M.; Polak, J.; Sułkowski, W.W. NMR Study of the Humification Process during Sewage Sludge Treatment. Chemosphere 2008, 73, 1465–1470. [Google Scholar] [CrossRef]
- Gonet, S.S.; Zawalska, Z. The Effect of Fertilizing with Starch Wastewater on Properties on Humic Acids (in Polish). Zesz. Probl. Postępów Nauk Rol. 1993, 411, 259–268. [Google Scholar]
- Kumada, K. Chemistry of Soil Organic Matter; Japan Scientific Societies Press: Tokyo, Japan; Elsevier Scientific Publishing Company: Amsterdam, Poland; Oxford, UK; New York, NY, USA, 1987. [Google Scholar]
- De Nobili, M.; Chen, Y. Size Exclusion Chromatography of Humic Substances: Limits, Perspectives and Prospectives. Soil Sci. 1999, 164, 825–833. [Google Scholar] [CrossRef]
- Asing, J.; Wong and Lau, N.S.; Wong, N.; Lau, S.; Asing, J.; Nan Chong, W.; Seng, L. Optimization of Extraction Method and Characterization of Humic Acid Derived from Coals and Composts. J. Trop. Agric. Fd Sc 2009, 37, 211–223. [Google Scholar]
- Gusiatin, Z.M.; Kulikowska, D. Behaviors of Heavy Metals (Cd, Cu, Ni, Pb and Zn) in Soil Amended with Composts. Environ. Technol. 2016, 37, 2337–2347. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D. Influence of Soil Aging and Stabilization with Compost on Zn and Cu Fractionation, Stability, and Mobility. Clean 2016, 44, 272–283. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a Soil Amendment to Remediate Heavy Metal-Contaminated Agricultural Soil: Mechanisms, Efficacy, Problems, and Strategies. Water Air Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Klik, B.; Kulikowska, D.; Gusiatin, Z.M.; Pasieczna-Patkowska, S. Washing Agents from Sewage Sludge: Efficiency of Cd Removal from Highly Contaminated Soils and Effect on Soil Organic Balance. J. Soils Sediments 2020, 20, 284–296. [Google Scholar] [CrossRef]
- Klik, B.; Gusiatin, Z.M.; Kulikowska, D. Kinetics of Cu, Pb and Zn Removal during Soil Flushing with Washing Agents Derived from Sewage Sludge. Sci. Rep. 2021, 11, 10067. [Google Scholar] [CrossRef] [PubMed]
- Klik, B.; Gusiatin, Z.M.; Kulikowska, D. A Holistic Approach to Remediation of Soil Contaminated with Cu, Pb and Zn with Sewage Sludge-Derived Washing Agents and Synthetic Chelator. J. Clean Prod. 2021, 311, 127664. [Google Scholar] [CrossRef]
- Klik, B.K.; Kulikowska, D.; Gusiatin, Z.M. Flushing of Soils Highly Contaminated with Cd Using Various Washing Agents Derived from Sewage Sludge. Energies 2022, 15, 349. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar Production by Sewage Sludge Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of Sewage Sludge into Biochar: A Potential Resource in Water and Wastewater Treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, H.; Koukouch, A.; Bakhattar, I.; Asbik, M.; Bonnamy, S.; Bennouna, E.G.; Boushaki, T.; Sarh, B.; Rouboa, A. Physicochemical Characterization, Thermal Behavior, and Pyrolysis Kinetics of Sewage Sludge. Energies 2024, 17, 582. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical Conversion of Sewage Sludge for Energy and Resource Recovery: Technical Challenges and Prospects. Environ. Pollut. Biovail. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Lins, P.V.d.S.; Oliveira, L.M.T.d.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage Sludge-Derived Biochar for the Adsorptive Removal of Wastewater Pollutants: A Critical Review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, H.; Ko, J.H.; Wu, H.; Xu, Q. Structure Characteristics of Bio-Char Generated from Co-Pyrolysis of Wooden Waste and Wet Municipal Sewage Sludge. Fuel Process Technol. 2019, 183, 48–54. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Zhao, D.; Huang, H.; Noriyuki, K.; Chen, Y. Influence of Temperature on Product Distribution and Biochar Properties by Municipal Sludge Pyrolysis. J. Mater. Cycles Waste Manag. 2013, 15, 357–361. [Google Scholar] [CrossRef]
- Kończak, M.; Oleszczuk, P.; Rózyło, K. Application of Different Carrying Gases and Ratio between Sewage Sludge and Willow for Engineered (Smart) Biochar Production. J. CO2 Util 2019, 29, 20–28. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Q.; Liu, X.; Wang, Y. Influence of Pyrolysis Temperature on Characteristics and Environmental Risk of Heavy Metals in Pyrolyzed Biochar Made from Hydrothermally Treated Sewage Sludge. Chemosphere 2019, 216, 698–706. [Google Scholar] [CrossRef]
- Januševičius, T.; Mažeikienė, A.; Danila, V.; Paliulis, D. The Characteristics of Sewage Sludge Pellet Biochar Prepared Using Two Different Pyrolysis Methods. Biomass. Convers. Biorefin. 2024, 14, 891–900. [Google Scholar] [CrossRef]
- Trabelsi, A.B.H.; Zaafouri, K.; Friaa, A.; Abidi, S.; Naoui, S.; Jamaaoui, F. Municipal Sewage Sludge Energetic Conversion as a Tool for Environmental Sustainability: Production of Innovative Biofuels and Biochar. Environ. Sci. Pollut. Res. 2021, 28, 9777–9791. [Google Scholar] [CrossRef] [PubMed]
- Velli, P.; Manolikaki, I.; Diamadopoulos, E. Effect of Biochar Produced from Sewage Sludge on Tomato (Solanum lycopersicum L.) Growth, Soil Chemical Properties and Heavy Metal Concentrations. J. Environ. Manag. 2021, 297, 113325. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.; Terradillos, M.; Gascó, G. Physicochemical and Agronomic Properties of Biochar from Sewage Sludge Pyrolysed at Different Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Udayanga, W.C.; Veksha, A.; Giannis, A.; Lim, T.T. Pyrolysis Derived Char from Municipal and Industrial Sludge: Impact of Organic Decomposition and Inorganic Accumulation on the Fuel Characteristics of Char. Waste Manag. 2019, 83, 131–141. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of Sewage Sludge-Derived Biochars from Different Feedstocks and Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- International Biochar Initiative (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil; 2015; IBI Biochar Standards. Version 2.1. Available online: https://biochar-international.org/wp-content/uploads/2018/04/IBI_Biochar_Standards_V2.1_Final.pdf (accessed on 1 January 2023).
- Barry, D.; Barbiero, C.; Briens, C.; Berruti, F. Pyrolysis as an Economical and Ecological Treatment Option for Municipal Sewage Sludge. Biomass Bioenergy 2019, 122, 472–480. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical Conversion of Sewage Sludge: A Critical Review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Pandey, J.K.; Chen, W.H.; Patel, A.; Ashokkumar, V. Pyrolysis of Sewage Sludge for Sustainable Biofuels and Value-Added Biochar Production. J. Environ. Manag. 2021, 298, 113450. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of Pyrolysis Temperature on Chemical and Physical Properties of Biochar from Sewage Sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of Pyrolysis Temperature on Properties and Environmental Safety of Heavy Metals in Biochars Derived from Municipal Sewage Sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- European Biochar Certificate (EBC). Guidelines for a Sustainable Production of Biochar; European Biochar Certificate (EBC): Frick, Switzerland, 2023; Available online: https://www.european-biochar.org/media/doc/2/version_en_10_3.pdf (accessed on 5 April 2023).
- Raj, A.; Yadav, A.; Arya, S.; Sirohi, R.; Kumar, S.; Rawat, A.P.; Thakur, R.S.; Patel, D.K.; Bahadur, L.; Pandey, A. Preparation, Characterization and Agri Applications of Biochar Produced by Pyrolysis of Sewage Sludge at Different Temperatures. Sci. Total Environ. 2021, 795, 148722. [Google Scholar] [CrossRef]
- Regkouzas, P.; Diamadopoulos, E. Adsorption of Selected Organic Micro-Pollutants on Sewage Sludge Biochar. Chemosphere 2019, 224, 840–851. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Klimiuk, E. Metal (Cu, Cd and Zn) Removal and Stabilization during Multiple Soil Washing by Saponin. Chemosphere 2012, 86, 383–391. [Google Scholar] [CrossRef]
- Xiao, Y.; Raheem, A.; Ding, L.; Chen, W.H.; Chen, X.; Wang, F.; Lin, S.L. Pretreatment, Modification and Applications of Sewage Sludge-Derived Biochar for Resource Recovery- A Review. Chemosphere 2022, 287, 131969. [Google Scholar] [CrossRef]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Huang, H.J.; Yang, T.; Lai, F.Y.; Wu, G.Q. Co-Pyrolysis of Sewage Sludge and Sawdust/Rice Straw for the Production of Biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Dondi, D. Properties and Beneficial Uses of Biochar from Sewage Sludge Pyrolysis. 5th Int. Conf. Sustain. Solid Waste Manag. 2017, 16, 769–775. [Google Scholar]
- Chen, X.; Fu, L.; Yu, Y.; Wu, C.; Li, M.; Jin, X.; Yang, J.; Wang, P.; Chen, Y. Recent Development in Sludge Biochar-Based Catalysts for Advanced Oxidation Processes of Wastewater. Catalysts 2021, 11, 1275. [Google Scholar] [CrossRef]
- Okiemute Akpasi, S.; Michael Smarte Anekwe, I.; Adedeji, J.; Lewis Kiambi, S. Biochar Development as a Catalyst and Its Application. In Biochar—Productive Technologies, Properties and Applications; IntechOpen: London, UK, 2023. [Google Scholar]
- Mian, M.M.; Alam, N.; Ahommed, M.S.; He, Z.; Ni, Y. Emerging Applications of Sludge Biochar-Based Catalysts for Environmental Remediation and Energy Storage: A Review. J. Clean Prod. 2022, 360, 132131. [Google Scholar] [CrossRef]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the Biochar Produced from Sewage Sludge a Good Quality Solid Fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.K.; Chen, W.H. Progress in Green Adsorbent Technologies from Sewage Sludge for Wastewater Remediation and Carbon Capture: A Sustainable Approach towards Clean Environment. Curr. Opin. Green Sustain. Chem. 2024, 46, 100883. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Sun, T. Comparison of Sewage Sludge- and Pig Manure-Derived Biochars for Hydrogen Sulfide Removal. Chemosphere 2014, 111, 296–303. [Google Scholar] [CrossRef]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Tomozei, C.; Panainte-Lehadus, M.; Mosnegutu, E. Evaluation of the Use of Sewage Sludge Biochar as a Soil Amendment—A Review. Sustainability 2022, 14, 5309. [Google Scholar] [CrossRef]
- Junior, A.; Guo, M. Efficacy of Sewage Sludge Derived Biochar on Enhancing Soil Health and Crop Productivity in Strongly Acidic Soil. Front. Soil Sci. 2023, 3, 1066547. [Google Scholar] [CrossRef]

| MSS Resources | Criteria | Limitations and Future Prospects |
|---|---|---|
| Struvite | Recovery | Further optimization of the operating parameters is necessary for maximum efficiency and minimum recovery costs. |
| Cost | A comprehensive economic analysis is needed to evaluate the cost-efficiency of struvite recovery compared to conventional methods. | |
| Quality | A more detailed characterization of recovered struvite is necessary to ensure its suitability as a commercial fertilizer (analysis of impurities, nutrient contents, and compliance with fertilizer quality standards). | |
| Scale | The struvite recovery process should be scaled up to full-scale municipal wastewater treatment. | |
| Environmental impact | A comprehensive lifecycle assessment (LCA) is needed to evaluate the environmental impact of the struvite recovery process. | |
| ALEs | Operating parameters | Further research is needed to optimize the operating parameters (e.g., organic loading rate, COD/N, retention time) to maximize ALE synthesis in MSS. |
| Recovery | Investigation, optimization, and standardization of the extraction methods for ALE recovery is necessary to maximize the yield and quality of the recovered biopolymer not only at the laboratory/pilot scale but also at full scale. | |
| Quality | Further characterization of the recovered ALEs is necessary to ensure their quality and suitability for various applications (pharmaceutical, environmental, agricultural, etc.). | |
| HSs | Synthesis | Identification of pathways for the synthesis of HS during municipal wastewater treatment is needed. |
| Recovery | Research into more efficient and cost-effective extraction techniques is needed to make the process more economically viable. | |
| Quality | Sewage sludge can contain various impurities that can interfere with the extraction and purification process. The analysis of the HS quality enables further HS use. Creating a clear framework for the use of recovered HSs in various applications will provide certainty for producers and consumers. | |
| Biochar | Production | Research into the optimization of the pyrolysis process is needed, including the use of catalysts, additives, and novel reactor designs, to help improve the efficiency and cost-effectiveness of biochar production from MSS. |
| Quality | MSS biochar may contain impurities such as heavy metals and organic pollutants, which must be removed or reduced to ensure the safety and quality of the product. The relationship between the WWTP equivalence population and the quality of MSS biochar should be analyzed. Research into strategies to improve the properties of biochar (pre-treatment, co-pyrolysis, post-treatment) is necessary. | |
| Scale | The commercialization and scaling of MSS biochar should be undertaken. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusiatin, M.Z.; Kulikowska, D.; Bernat, K. Municipal Sewage Sludge as a Resource in the Circular Economy. Energies 2024, 17, 2474. https://doi.org/10.3390/en17112474
Gusiatin MZ, Kulikowska D, Bernat K. Municipal Sewage Sludge as a Resource in the Circular Economy. Energies. 2024; 17(11):2474. https://doi.org/10.3390/en17112474
Chicago/Turabian StyleGusiatin, Mariusz Z., Dorota Kulikowska, and Katarzyna Bernat. 2024. "Municipal Sewage Sludge as a Resource in the Circular Economy" Energies 17, no. 11: 2474. https://doi.org/10.3390/en17112474








