Experimental Investigation of the Impact of CO2 Injection Strategies on Rock Wettability Alteration for CCS Applications
Abstract
1. Introduction
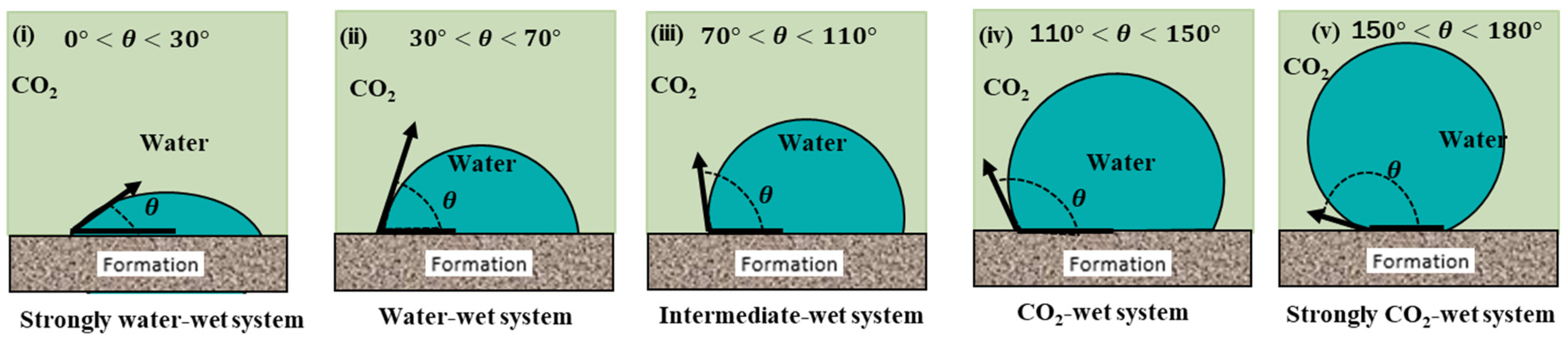
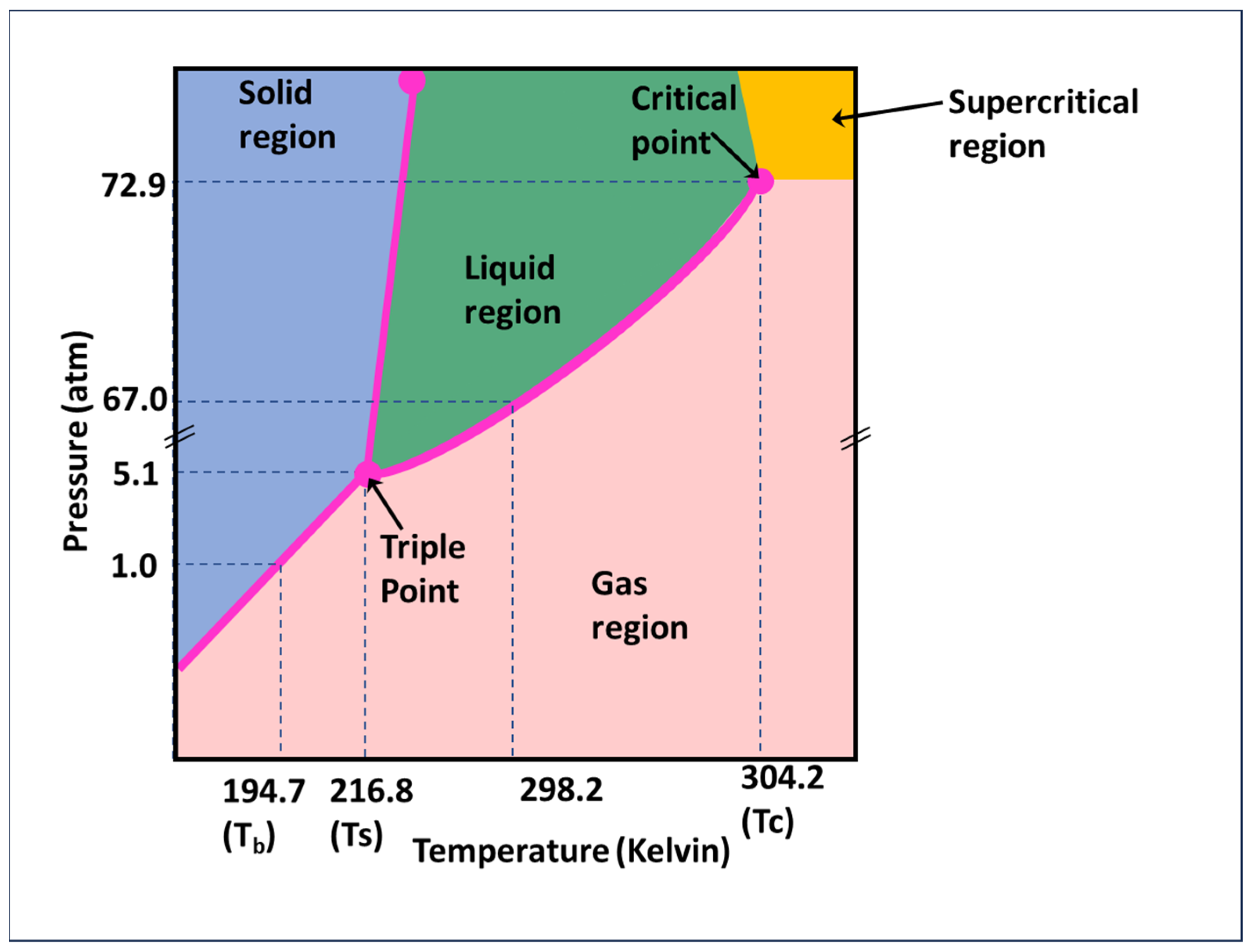
1.1. Wettability Alteration
1.2. Impact of Wettability Alteration (WA) on CO2 Storage
1.3. Injection Strategy as a Means of Reducing WA
2. Materials and Methods
2.1. Description of Sample and Preparation
2.2. Pretest and Post-Test Experimental Setup
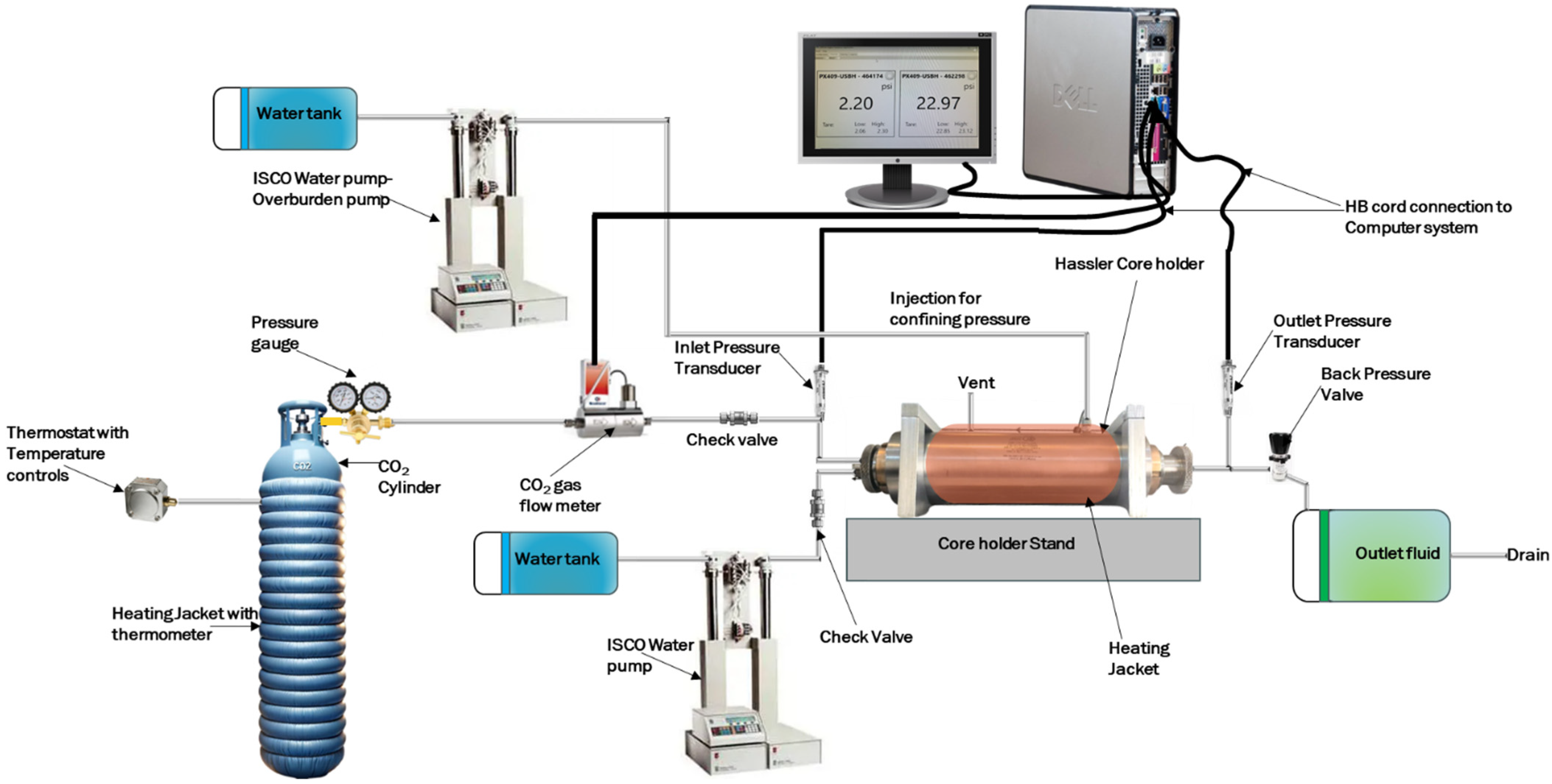
2.3. Core Flooding Experimental Setup and Conditions
2.4. Experimental Procedure
2.4.1. Continuous scCO2 Injection
2.4.2. Alternate scCO2–Water Injection (WAG)
2.4.3. Simultaneous scCO2–Water Aquifer Injection (SAI)
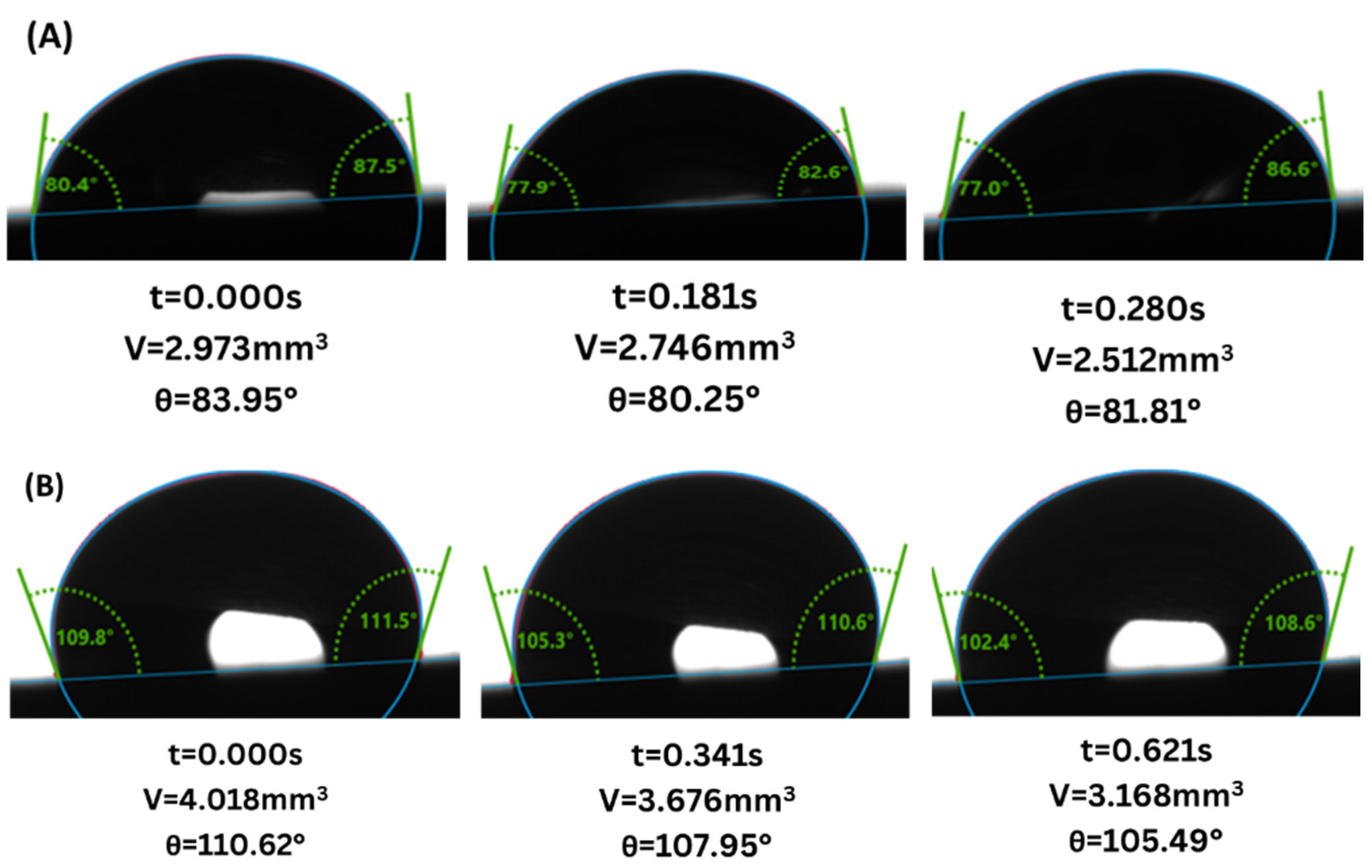
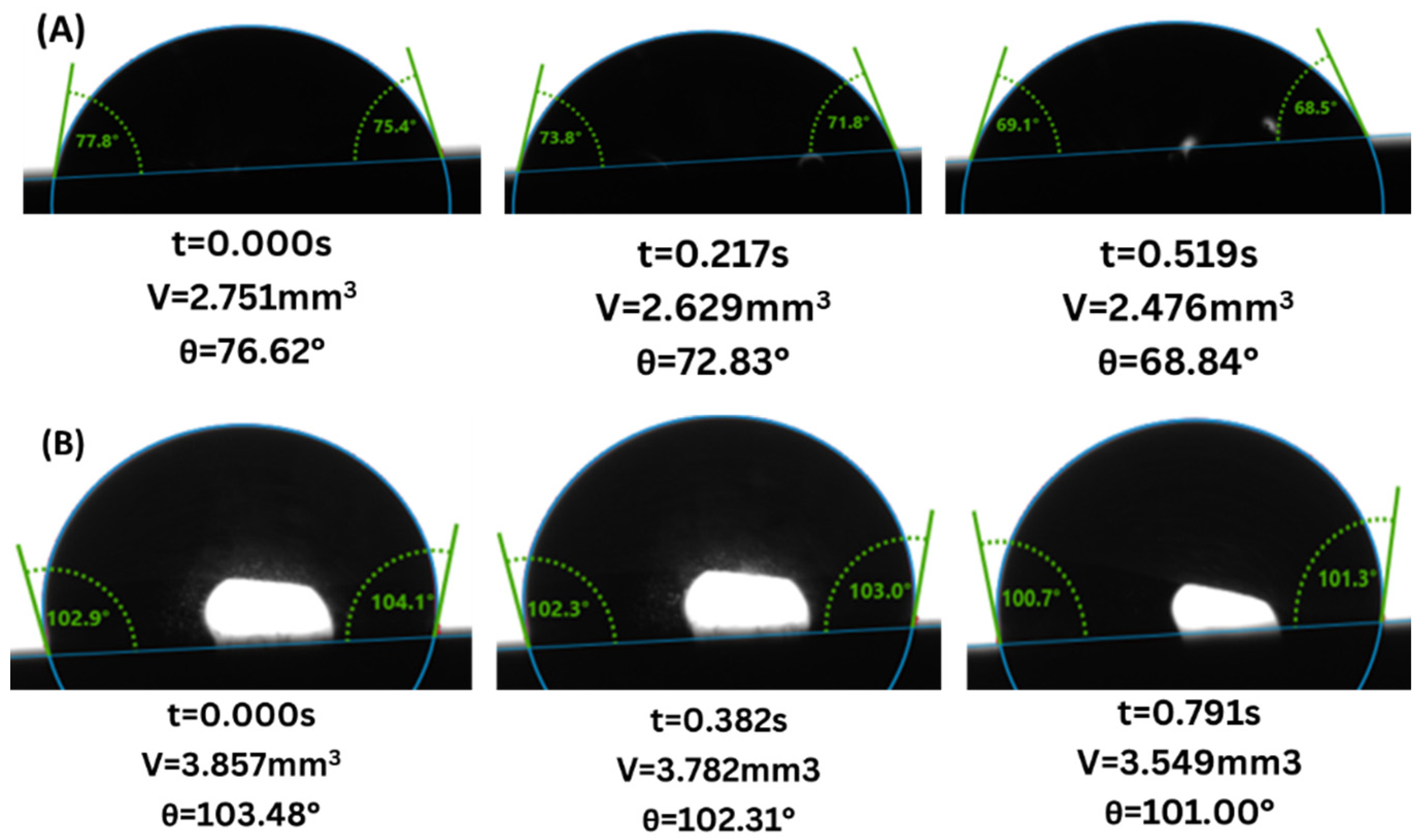
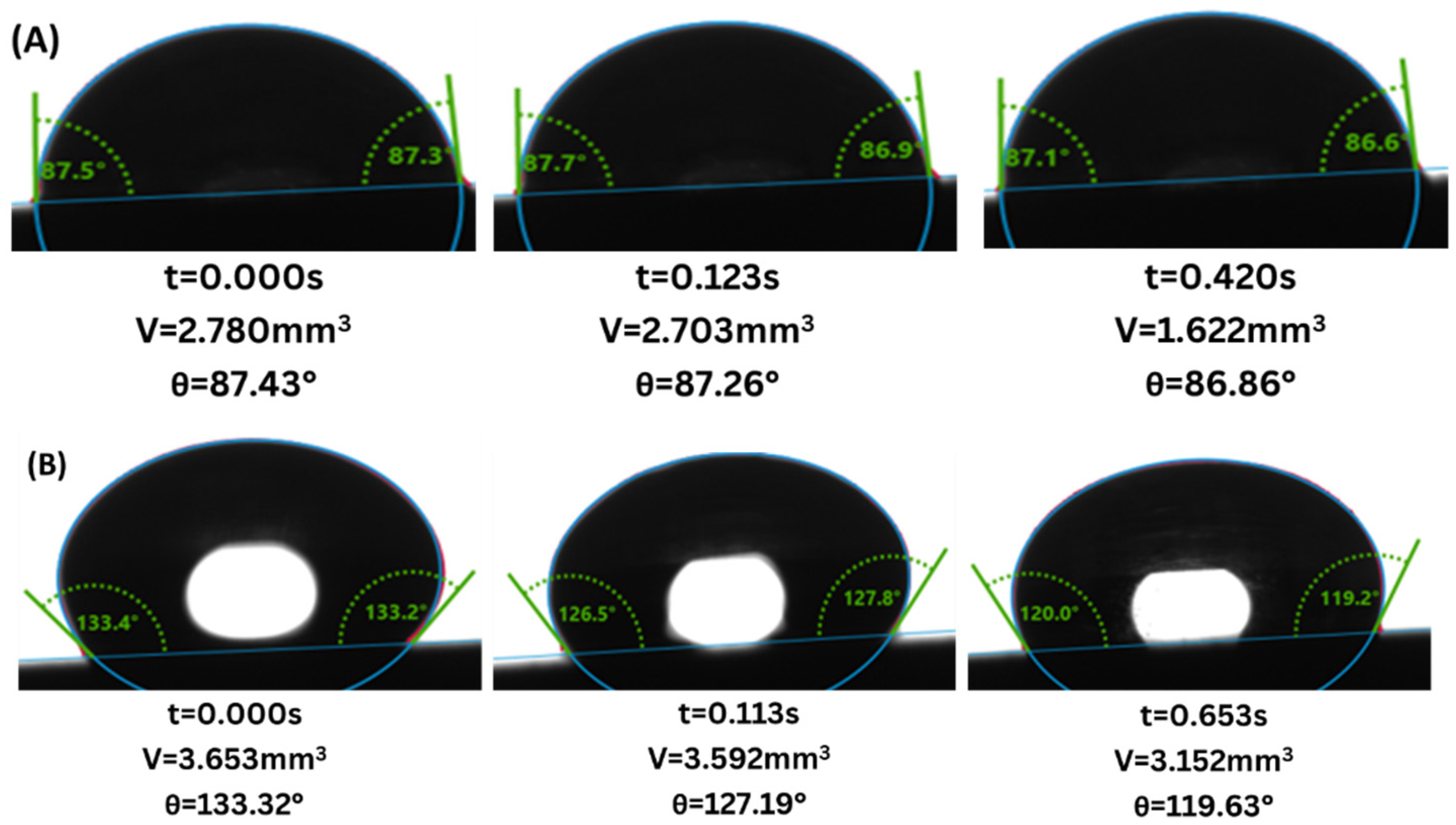
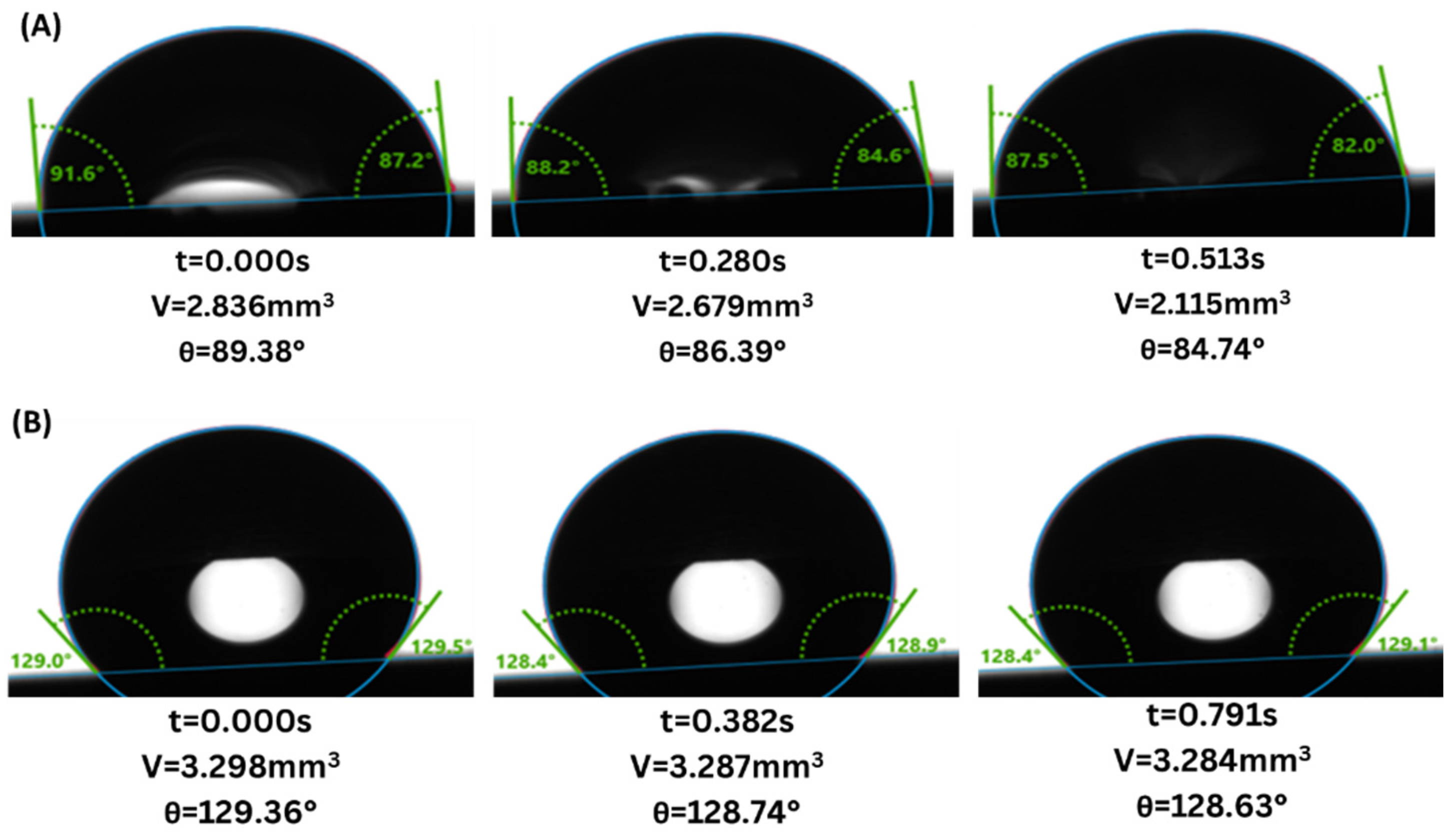
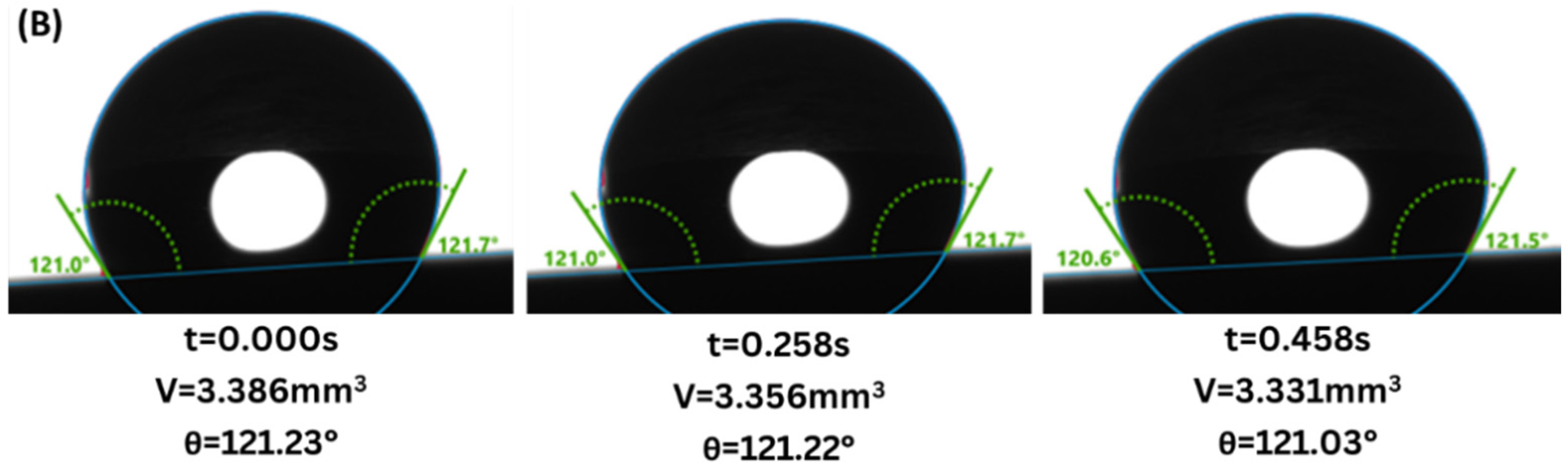
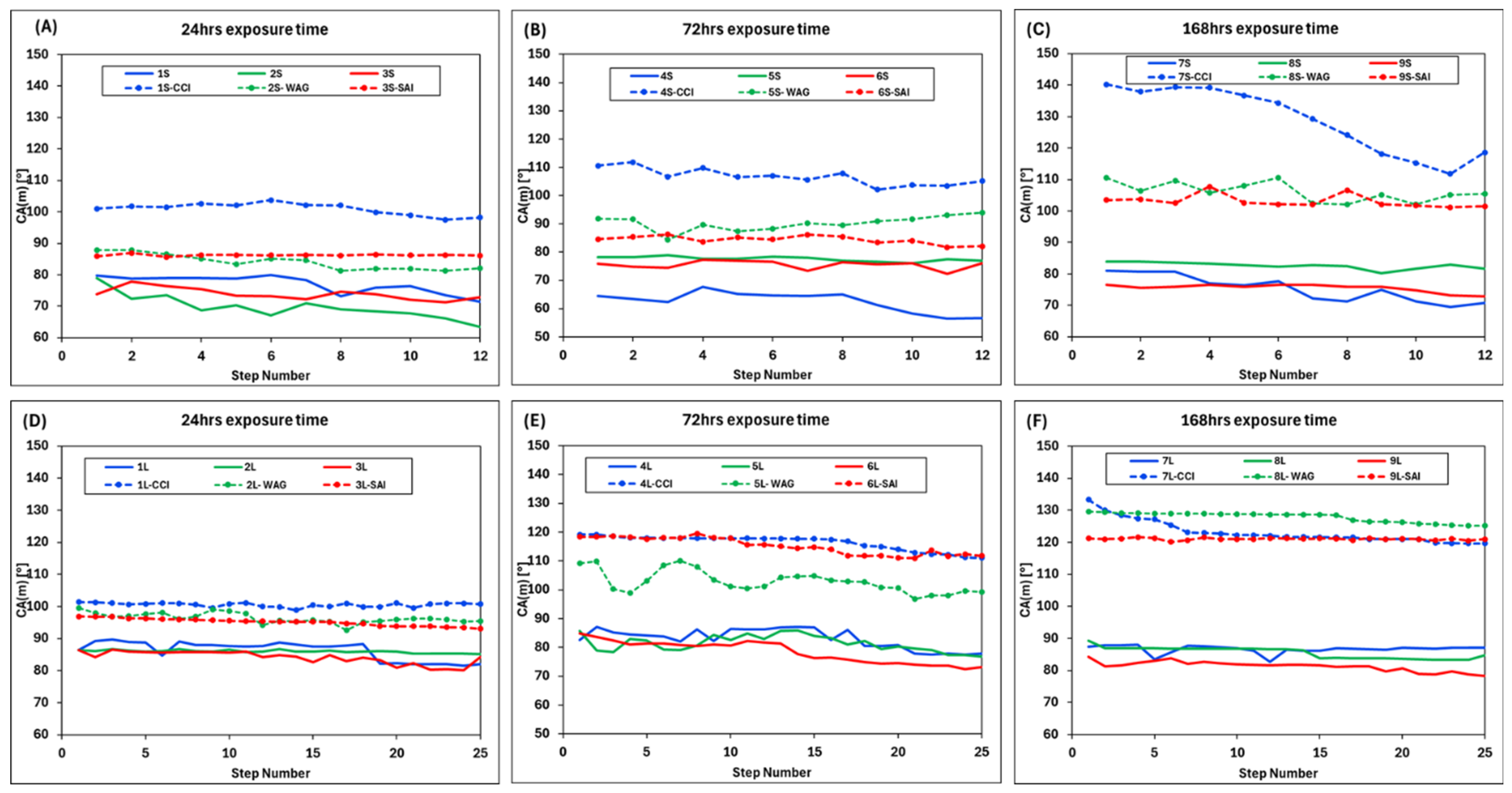
3. Results
4. Discussion
4.1. Gray Berea Sandstone
4.2. Indiana Limestone
4.3. Significance of Wettability Alteration in CCS
5. Conclusions
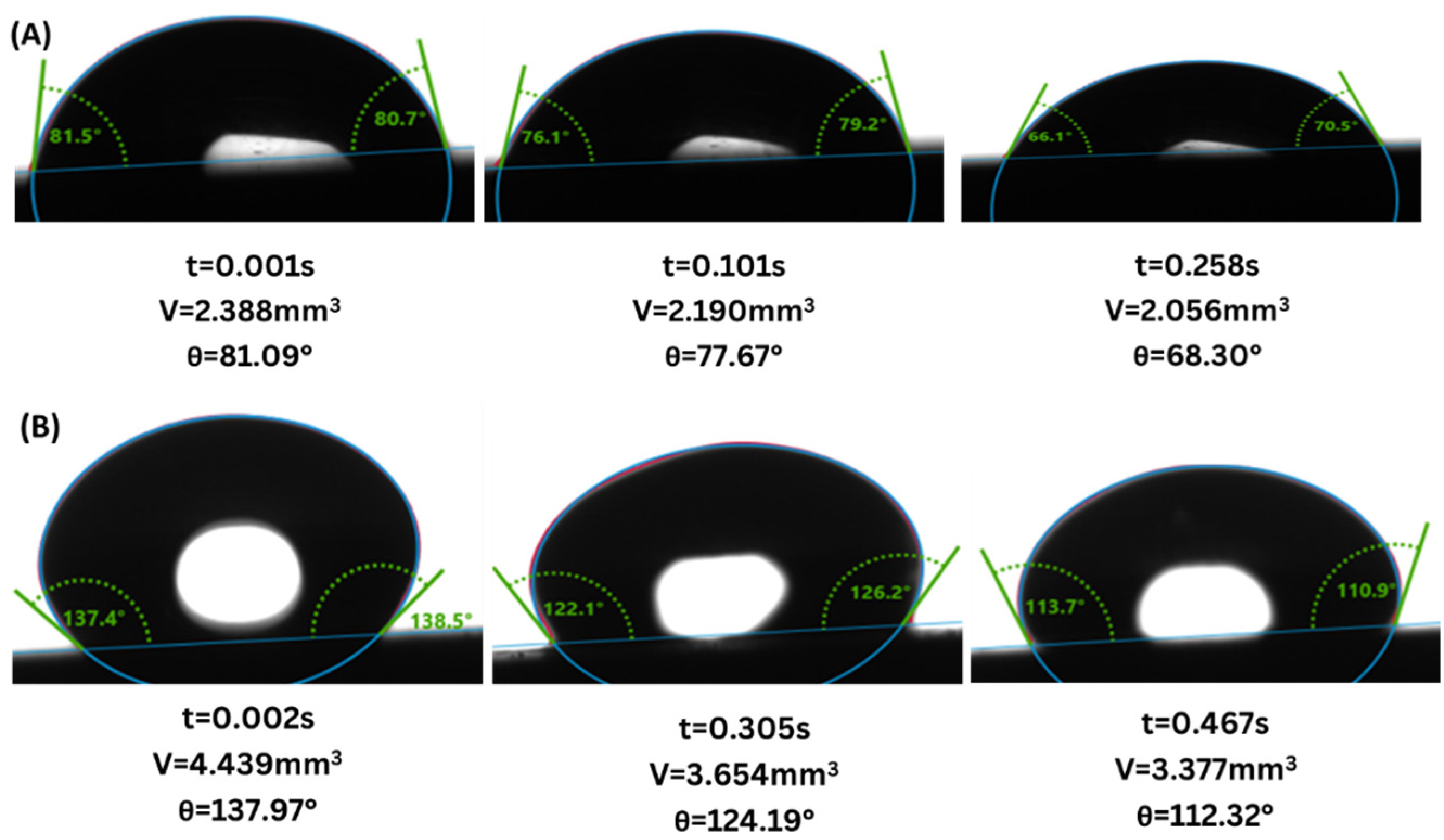
- Wettability Change: Both sandstone and limestone changed towards more CO2-wet conditions across all injection strategies due to changes in CA from the range of 61.6–83.4° to 77.6–87.9° and from 81.5–124.2° to 94.6–128.0° for sandstone and limestone respectively. The decreased water-wetness suggests that capillary trapping of CO2 is limited and crucial for the long-term security and efficacy of geological carbon storage.
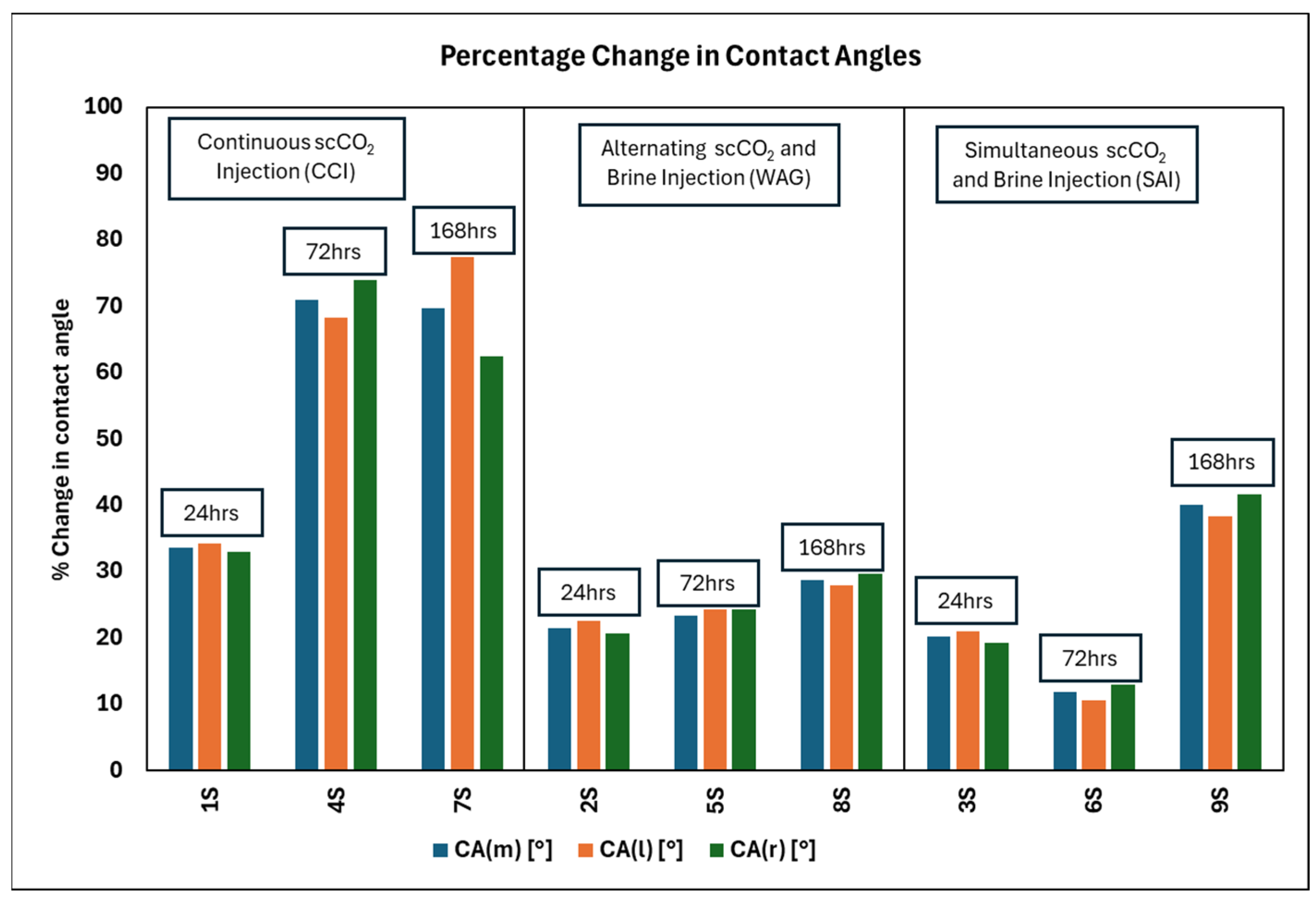
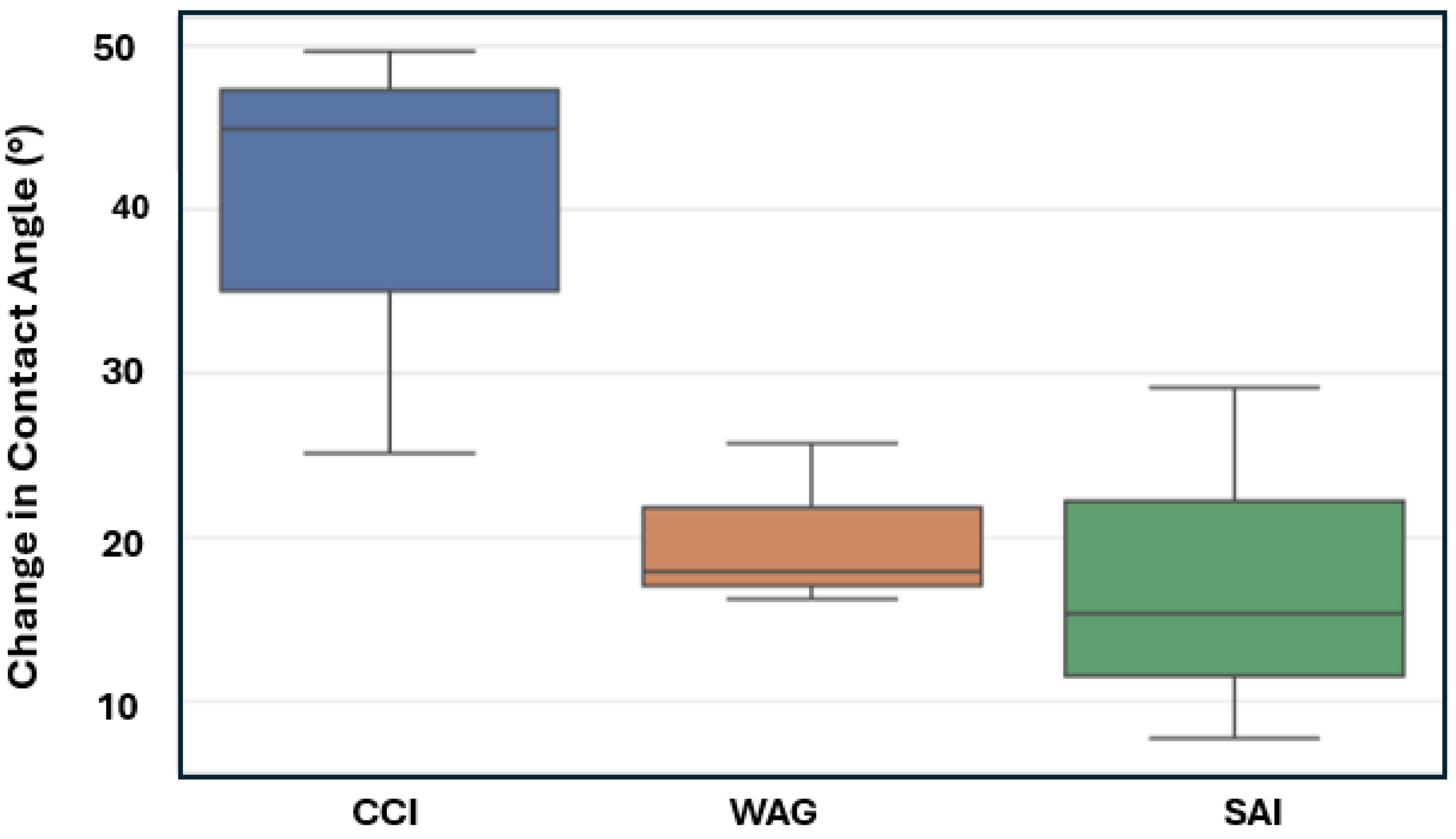
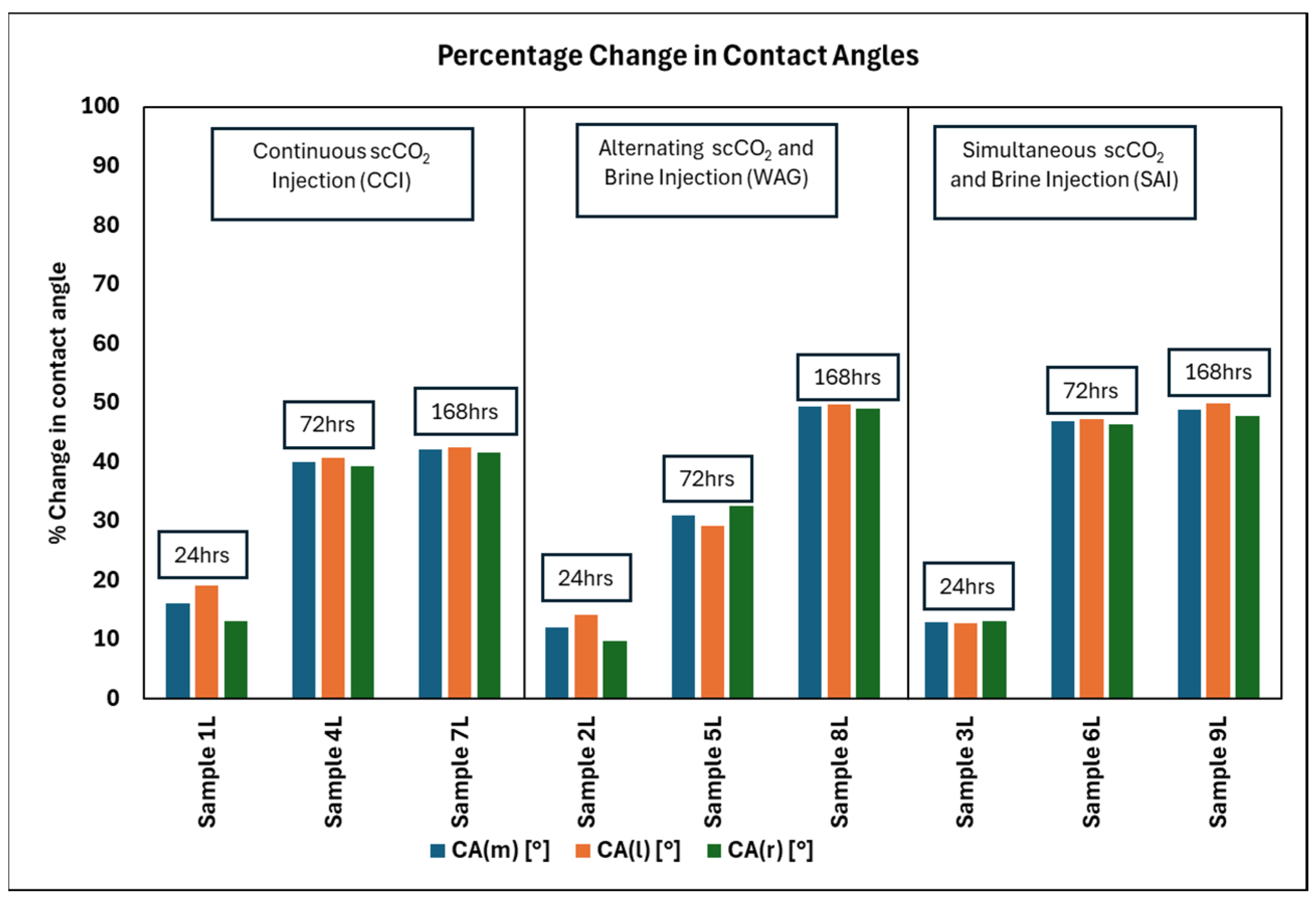
- Injection Strategy Efficiency: The results show variability in surface response depending on the injection strategy employed. Sandstone displayed more substantial changes in wettability for CCI, with a 77.4% change in CA. In comparison, WAG was more effective in reducing the WA with the least change of 29.6%, followed by the SAI strategy with a change of 41.7% maximum. Changes in CA and wettability were similar across strategies in limestone, with changes of 42.5%, 49.7%, and 49.9% in CCI, WAG, and SAI methods, highlighting its chemical reactivity with scCO2 and water. This suggests that WAG and SAI may enhance the interaction between CO2, brine, and rock surfaces, potentially leading to better CO2 trapping mechanisms in sandstone formations.
- Exposure duration: Across all samples, CA gradually increased as the exposure time increased, except for the SAI process when sample 6S was exposed for 72 h. This is related to the permeability variability of these samples.
- Rock Type Specificity: The results underscored the importance of considering rock type when selecting CO2 injection strategies, as a rock’s chemical composition and physical properties significantly influence the outcome of wettability changes. The differential response between sandstone and limestone to the same injection strategies indicates that tailoring the injection approach based on specific geological settings of a storage site could optimize sequestration results. In this case, the WAG method with a sandstone formation gave the most favorable result for CO2 storage. This prolongs WA and hence maintains the water-wet nature of formation for longer than CCI.
Future Research
- Study surface morphology of the rock samples before and after injection, providing visual evidence of physical changes at the microstructural level using the SEM. In addition, X-ray diffraction patterns will also show mineralogical changes post-injection, particularly in limestone, supporting the observed wettability results.
- Future studies should focus on the long-term stability of wettability changes and their implications over the lifespan of a CO2 storage project (for instance, 6 months to 1 year). Understanding how these changes persist under varying geological and operational conditions is crucial.
- Exploring the role of different brine compositions and additives that might enhance wettability changes or react chemically with CO2 and rock could further optimize injection strategies.
- Developing comprehensive models that integrate fluid dynamics, rock chemistry, and wettability changes would help predict outcomes more accurately and design better injection protocols.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, T. Reservoir Engineering Handbook, 4th ed.; Gulf Professional Publishing: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Iglauer, S.; Al-yaseri, A.Z.; Rezaee, R.; Lebedev, M. CO2 wettability of caprocks: Implications for structural storage capacity and containment security. Geophys. Res. Lett. 2015, 42, 9279–9284. [Google Scholar] [CrossRef]
- Iglauer, S.; Wülling, W.; Pentland, C.H.; Al-Mansoori, S.K.; Blunt, M.J. Capillary-trapping capacity of sandstones and sandpacks. SPE J. 2011, 16, 778–783. [Google Scholar] [CrossRef]
- Tudek, J.; Crandall, D.; Fuchs, S.; Werth, C.J.; Valocchi, A.J.; Chen, Y.; Goodman, A. In situ contact angle measurements of liquid CO2, brine, and Mount Simon sandstone core using micro X-ray CT imaging, sessile drop, and Lattice Boltzmann modeling. J. Pet. Sci. Eng. 2017, 155, 3–10. [Google Scholar] [CrossRef]
- Chalbaud, C.; Robin, M.; Lombard, J.M.; Martin, F.; Egermann, P.; Bertin, H. Interfacial tension measurements and wettability evaluation for geological CO2 storage. Adv. Water Resour. 2009, 32, 98–109. [Google Scholar] [CrossRef]
- Chalbaud, C.; Robin, M.; Bekri, S.; Egermann, P. Wettability Impact on CO2 Storage in Aquifers: Visualisation and Quantification Using Micromodel Tests, Pore Network Model and Reservoir Simulations. In Proceedings of the International Symposium of the Society of Core Analysts, Calgary, AB, Canada, 10–12 September 2007; pp. 1–12. [Google Scholar]
- Plug, W.J.; Bruining, J. Capillary pressure for the sand-CO2-water system under various pressure conditions. Application to CO2 sequestration. Adv. Water Resour. 2007, 30, 2339–2353. [Google Scholar] [CrossRef]
- Lauren, S. Enhanced oil Recovery: How to Measure the Wettability of Carbonate Reservoir Core? Biolin Sci. Surf. Sci. Blog 2019, 1–11. Available online: https://www.biolinscientific.com/blog/enhanced-oil-recovery-how-to-measure-the-wettability-of-carbonate-reservoir-core#:~:text=The-USBM-test-compares-the,spins-with-stepwise-increasing-speed (accessed on 3 March 2023).
- Sagbana, P.I.; Sarkodie, K.; Nkrumah, W.A. A critical review of carbonate reservoir wettability modification during low salinity waterflooding. Petroleum 2022, 9, 317–330. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability Literature Survey—Part 2: Wettability Measurement. JPT J Pet. Technol. 1986, 38, 1246–1262. [Google Scholar] [CrossRef]
- Chaudhary, K.; Guiltinan, E.J.; Cardenas, M.B.; Maisano, J.A.; Ketcham, R.A.; Bennett, P.C. Wettability measurement under high P-T conditions using X-ray imaging with ap- plication to the brine-supercritical CO2 system. Geochem. Geophys. Geosystems 2015, 16, 2858–2864. [Google Scholar] [CrossRef]
- Kassa, A.M.; Gasda, S.E.; Landa-Marbán, D.; Sandve, T.H.; Kumar, K. Field-scale impacts of long-term wettability alteration in geological CO2 storage. Int. J. Greenh. Gas Control 2022, 114, 103556. [Google Scholar] [CrossRef]
- Kassa, A.M.; Gasda, S.E.; Kumar, K.; Radu, F.A. Impact of time-dependent wettability alteration on the dynamics of capillary pressure. Adv. Water Resour. 2020, 142, 103631. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, X.; Chen, Z.; Mu, L.; Wang, T. An analytical approach for evaluating the capillary trapping during CO2 storage in aquifers. In Society of Petroleum Engineers—SPE/IATMI Asia Pacific Oil and Gas Conference and Exhibition; SPE: Lumpur, Malaysia, 2017; pp. 1–14. [Google Scholar]
- Javanbakht, G.; Sedghi, M.; Welch, W.; Goual, L. Molecular dynamics simulations of CO2/water/quartz interfacial properties: Impact of CO2 dissolution in water. Langmuir 2015, 31, 5812–5819. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Abu-Khamsin, S.A.; Iglauer, S. Wettability of rock/CO2/brine and rock/oil/CO2-enriched-brine systems:Critical parametric analysis and future outlook. Adv. Colloid Interface Sci. 2019, 268, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Liu, Y.; Wang, Z.; Liu, S.; Jiang, L.; Chen, J.; Song, Y. In Situ Local Contact Angle Measurement in a CO2-Brine-Sand System Using Microfocused X-ray CT. Langmuir 2017, 33, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Al-yaseri, A.Z.; Lebedev, M.; Barifcani, A.; Iglauer, S. Receding and advancing (CO2 + brine + quartz) contact angles as a function of pressure, temperature, surface roughness, salt type and salinity. J. Chem. Thermodyn. 2015, 93, 416–423. [Google Scholar] [CrossRef]
- Sarmadivaleh, M.; Al-Yaseri, A.Z.; Iglauer, S. Influence of temperature and pressure on quartz-water-CO2 contact angle and CO2-water interfacial tension. J. Colloid Interface Sci. 2015, 441, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Alnili, F.; Al-Yaseri, A.; Roshan, H.; Rahman, T.; Verall, M.; Lebedev, M.; Sarmadivaleh, M.; Iglauer, S.; Barifcani, A. Carbon dioxide/brine wettability of porous sandstone versus solid quartz: An experimental and theoretical investigation. J. Colloid Interface Sci. 2018, 524, 188–194. [Google Scholar] [CrossRef]
- Al-hadhrami, H.S.; Blunt, M.J. Thermally Induced Wettability Alteration to Improve Oil Recovery in Fractured Reservoirs. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 22–26 April 2000; pp. 1–9. [Google Scholar]
- Arif, M.; Al-Yaseri, A.Z.; Barifcani, A.; Lebedev, M.; Iglauer, S. Impact of pressure and temperature on CO2-brine-mica contact angles and CO2-brine interfacial tension: Implications for carbon geo-sequestration. Colloid Interface Sci. 2015, 462, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Botto, J.; Fuchs, S.J.; Fouke, B.W.; Clarens, A.F.; Freiburg, J.T.; Berger, P.M.; Werth, C.J. Effects of Mineral Surface Properties on Supercritical CO2 Wettability in a Siliciclastic Reservoir. Energy Fuels 2017, 31, 5275–5285. [Google Scholar] [CrossRef]
- Mutailipu, M.; Liu, Y.; Jiang, L.; Zhang, Y. Measurement and estimation of CO2–brine interfacial tension and rock wettability under CO2 sub- and super-critical conditions. J. Colloid Interface Sci. 2019, 534, 605–617. [Google Scholar] [CrossRef]
- Jaeger, P.T.; Alotaibi, M.B.; Nasr-El-Din, H.A. Influence of compressed carbon dioxide on the capillarity of the gas-crude oil-reservoir water system. J. Chem. Eng. Data 2010, 55, 5246–5251. [Google Scholar] [CrossRef]
- Saraji, S.; Piri, M.; Goual, L. The effects of SO2 contamination, brine salinity, pressure, and temperature on dynamic contact angles and interfacial tension of supercritical CO2/brine/quartz systems. Int. J. Greenh. Gas Control 2014, 28, 147–155. [Google Scholar] [CrossRef]
- Liu, Y.; Mutailipu, M.; Jiang, L.; Zhao, J.; Song, Y.; Chen, L. Interfacial Tension and Contact Angle Measurements for the Evaluation of CO2-Brine Two-Phase Flow Characteristics in Porous Media. Environ. Prog. Sustain. Energy 2015, 34, 1756–1762. [Google Scholar] [CrossRef]
- Ameri, A.; Shojai Kaveh, N.; Rudolph, E.S.J.; Wolf, K.H.; Farajzadeh, R.; Bruining, J. Investigation on interfacial interactions among crude oil-brine-sandstone rock-CO2 by contact angle measurements. Energy Fuels 2013, 27, 1015–1025. [Google Scholar] [CrossRef]
- Drummond, C.; Israelachvili, J. Fundamental studies of crude oil-surface water interactions and its relationship to reservoir wettability. J. Pet. Sci. Eng. 2004, 45, 61–81. [Google Scholar] [CrossRef]
- Chiquet, P.; Broseta, D.; Thibeau, S. Capillary Alteration of Shaly Caprocks by Carbon Dioxide. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid, Spain, 13–16 June 2005; pp. 1–10. [Google Scholar] [CrossRef]
- Kaveh, N.S.; Rudolph, E.S.J.; Van Hemert, P.; Rossen, W.R.; Wolf, K.H. Wettability evaluation of a CO2/water/bentheimer sandstone system: Contact angle, dissolution, and bubble size. Energy Fuels 2014, 28, 4002–4020. [Google Scholar] [CrossRef]
- Wang, S.; Tao, Z.; Persily, S.M.; Clarens, A.F. CO2 adhesion on hydrated mineral surfaces. Environ. Sci. Technol. 2013, 47, 11858–11865. [Google Scholar] [CrossRef]
- Iglauer, S.; Pentland, C.H.; Busch, A. CO2 wettability of seal and reservoir rocks and the implications for carbon geo-sequestration. Water Resour. Res. 2014, 51, 729–774. [Google Scholar] [CrossRef]
- Daryasafar, A.; Keykhosravi, A.; Shahbazi, K. Modeling CO2 wettability behavior at the interface of brine/CO2/mineral: Application to CO2 geo-sequestration. J. Clean. Prod. 2019, 239, 118101. [Google Scholar] [CrossRef]
- Chiquet, P.; Broseta, D.; Thibeau, S. Wettability alteration of caprock minerals by carbon dioxide. Geofluids 2007, 7, 112–122. [Google Scholar] [CrossRef]
- Bikkina, P.K. Contact angle measurements of CO2-water-quartz/calcite systems in the perspective of carbon sequestration. Int. J. Greenh. Gas Control 2011, 5, 1259–1271. [Google Scholar] [CrossRef]
- Mills, J.; Riazi, M.; Sohrabi, M. Wettability of common rock-forming minerals in a CO2-brine system at reservoir conditions. In Proceedings of the International Symposium of the Society of Core Analysts, Austin, TX, USA, 21 August–1 September 2011; pp. 1–12. [Google Scholar]
- Kim, Y.; Wan, J.; Kneafsey, T.J.; Tokunaga, T.K. Dewetting of Silica Surfaces upon Reactions with Supercritical CO2 and Brine: Pore-Scale Studies in Micromodels. Environ. Sci. Technol. 2012, 46, 4228–4235. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Puerto, M. Recent Advances in Surfactant EOR. SPE J. 2011, 16, 889–907. [Google Scholar] [CrossRef]
- Underschultz, J.; Boreham, C.; Dance, T.; Stalker, L.; Freifeld, B.; Kirste, D.; Ennis-King, J. CO2 storage in a depleted gas field: An overview of the CO2CRC Otway Project and initial results. Int. J. Greenh. Gas Control 2011, 5, 922–932. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, J.; Kamali, F.; Othman, F.; Wang, Y.; Le-Hussain, F. Wettability of sandstone rocks and their mineral components during CO2 injection in aquifers: Implications for fines migration. J. Nat. Gas Sci. Eng. 2020, 73, 103050. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, L.; Xiao, T.; McPherson, B.; Zhang, X.; Zheng, L.; Dong, S.; Yang, Z.; Soltanian, M.R.; Yang, C.; et al. Reactive chemical transport simulations of geologic carbon sequestration: Methods and applications. Earth-Sci. Rev. 2020, 208, 103265. [Google Scholar] [CrossRef]
- Juanes, R.; Spiteri, E.J.; Orr, F.M.; Blunt, M.J. Impact of relative permeability hysteresis on geological CO2 storage. Water Resour. Res. 2006, 42, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Han, J.; Ling, K.; Wang, H.; Jia, B. Investigation of properties alternation during super-critical CO2 injection in shale. Appl. Sci. 2019, 9, 1686. [Google Scholar] [CrossRef]
- Li, Z.; Dong, M.; Li, S.; Huang, S. CO2 sequestration in depleted oil and gas reservoirs-caprock characterization and storage capacity. Energy Convers. Manag. 2006, 47, 1372–1382. [Google Scholar] [CrossRef]
- Li, S.; Dong, M.; Li, Z.; Huang, S.; Qing, H.; Nickel, E. Gas breakthrough pressure for hydrocarbon reservoir seal rocks: Implications for the security of long-term CO2 storage in the Weyburn field. Geofluids 2005, 5, 326–334. [Google Scholar] [CrossRef]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Atkins’ Physical Chemistry, 8th ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Brendan, J. Lowering Ethanol Carbon Intensity with CO2 Capture and Enhanced Oil Recovery; Great Plains Institute: Minneapolis, MN, USA, 2017. [Google Scholar]
- Sampaio, M.A.; de Mello, S.F.; Schiozer, D.J. Impact of physical phenomena and cyclical reinjection in miscible CO2-WAG recovery in carbonate reservoirs. J. Pet. Explor. Prod. Technol. 2020, 10, 3865–3881. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.; Will, R.; Cather, M.; Gunda, D.; Dai, Z. Co-optimization of CO2-EOR and Storage Processes under Geological Uncertainty. Energy Procedia 2017, 114, 6928–6941. [Google Scholar] [CrossRef]
- Kashkooli, S.B.; Gandomkar, A.; Riazi, M.; Tavallali, M.S. Coupled optimization of carbon dioxide sequestration and CO2 enhanced oil recovery. J. Pet. Sci. Eng. 2022, 208, 109257. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Gai, Z.; Tao, F.; Tang, H.; Xu, P. The plasticity of indigenous microbial community in a full-scale heavy oil-produced water treatment plant. J. Hazard. Mater. 2018, 358, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Pawar, R.J. Capacity assessment and co-optimization of CO2 storage and enhanced oil recovery in residual oil zones. J. Pet. Sci. Eng. 2019, 182, 106342. [Google Scholar] [CrossRef]
- Azzolina, N.A.; Nakles, D.V.; Gorecki, C.D.; Peck, W.D.; Ayash, S.C.; Melzer, L.S.; Chatterjee, S. CO2 storage associated with CO2 enhanced oil recovery: A statistical analysis of historical operations. Int. J. Greenh. Gas Control 2015, 37, 384–397. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Eftekhari, A.A.; Dafnomilis, G.; Lake, L.W.; Bruining, J. On the sustainability of CO2 storage through CO2—Enhanced oil recovery. Appl. Energy 2020, 261, 114467. [Google Scholar] [CrossRef]
- AlRassas, A.M.; Vo Thanh, H.; Ren, S.; Sun, R.; Al-Areeq, N.M.; Kolawole, O.; Hakimi, M.H. CO2 Sequestration and Enhanced Oil Recovery via the Water Alternating Gas Scheme in a Mixed Transgressive Sandstone-Carbonate Reservoir: Case Study of a Large Middle East Oilfield. Energy Fuels 2022, 36, 10299–10314. [Google Scholar] [CrossRef]
- Jia, K.; Zeng, J.; Wang, X.; Li, B.; Gao, X.; Wang, K. Wettability of Tight Sandstone Reservoir and Its Impacts on the Oil Migration and Accumulation: A Case Study of Shahejie Formation in Dongying Depression, Bohai Bay Basin. Energies 2022, 15, 4267. [Google Scholar] [CrossRef]
- Khoshsima, A.; Sedighi, M.; Mohammadi, M. Enhanced oil recovery by water alternating gas injection. In Gas Injection Methods: A Volume in Enhanced Oil Recovery Series; Gulf Professional Publishing: Amsterdam, The Netherlands, 2023; pp. 295–316. [Google Scholar] [CrossRef]
- Arzhilovsky, A.V.; Afonin, D.G.; Ruchkin, A.A.; Kobyashev, A.V.; Morozovskiy, N.A.; Toropov, K.V. Express assessment of the increase in the oil recovery as a result of water-alternating-gas technology application. Neft. Khozyaystvo-Oil Ind. 2022, 2022, 63–67. [Google Scholar] [CrossRef]
- Lima, R.O.; de L. Cunha, A.; Santos, J.A.O.; Garcia, A.J.V.; dos Santos, J.P.L. Assessment of continuous and alternating CO2 injection under Brazilian-pre-salt-like conditions. J. Pet. Explor. Prod. Technol. 2020, 10, 2947–2956. [Google Scholar] [CrossRef]
- Nordic CCS Competence Centre Report. The Nordic CO2 Storage Atlas; Nordic CCS Competence Centre Report: Copenhagen, Denmark, 2009. [Google Scholar]
- Okamoto, I.; Li, X.; Ohsumi, T. Effect of supercritical CO2 as the organic solvent on cap rock sealing performance for underground storage. Energy 2005, 30, 2344–2351. [Google Scholar] [CrossRef]
- Sohrabi, M.; Fatemi, S.M.; Farzaneh, S.A.; Jami, M.; Ireland, S.; Ahmed, K. Performance of Swag Injection Versus Alternating and Continuous Injection of Water and Gas in Low Gas-Oil Ift and Mixed-Wet Systems. In Proceedings of the 26th International Symposium of the Society of Core Analysts, Aberdeen, UK, 27–30 August 2012; pp. 1–12. [Google Scholar]
- Drozdov, A.; Gorbyleva, Y.; Drozdov, N.; Gorelkina, E. Perspectives of application of simultaneous water and gas injection for utilizing associated petroleum gas and enhancing oil recovery in the Arctic fields. IOP Conf. Ser. Earth Environ. Sci. 2021, 678, 012039. [Google Scholar] [CrossRef]
- Heidari, P.; Kharrat, R.; Alizadeh, N.; Ghazanfari, M.H. A comparison of WAG and SWAG processes: Laboratory and simulation studies. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 2225–2232. [Google Scholar] [CrossRef]
- Chen, P.; Sowaidi, A.K.A.; Patel, H.; Brantferger, K.; Bin Buang, K.A.; Syed, F.I.; Shehhi, R. Al Assessment of simultaneous water and gas injection swag pilot in a giant offshore carbonate reservoir. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016; pp. 1–10. [Google Scholar] [CrossRef]
- Shetty, S. Evaluation of Simultaneous Water and Gas Injection Using CO2; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2011. [Google Scholar]
- Donaldson, E.C.; Chilingarin, G.V.; Yen, T.F. (Eds.) Enhanced Oil Recovery, II process and operations; Elsevier: Amsterdam, The Netherlands,, 1989; Volume 17B, pp. 1–607. [Google Scholar]
- Teklu, T.W. Experimental and Numerical Study of Carbon Dioxide Injection Enhanced Oil Recovery in Low-Permeability Reservoirs; Colorado School of Mines: Golden, CO, USA, 2015; Volume 1. [Google Scholar]
- Haeri, F.; Myshakin, E.M.; Sanguinito, S.; Moore, J.; Crandall, D.; Gorecki, C.D.; Goodman, A.L. Simulated CO2 storage efficiency factors for saline formations of various lithologies and depositional environments using new experimental relative permeability data. Int. J. Greenh. Gas Control 2022, 119, 103720. [Google Scholar] [CrossRef]
- Christensen, M.; Tanino, Y. Waterflood oil recovery from mixed-wet limestone: Dependence on contact anle. Energy Fuels 2015, 31, 1529–1535. [Google Scholar] [CrossRef]
- dos Santos Lucas, C.R.; Aum, Y.K.P.G.; de Andrade Araújo, E.; de Castro Dantas, T.N.; Araújo, E.A.; Sousa, T.N.; Aum, P.T.P. Investigating the fluid-solid interaction of acid nonionic nanoemulsion with carbonate porous media. Molecules 2020, 25, 1475. [Google Scholar] [CrossRef]
- Feldmann, F.; Al-Shalabi, E.W.; AlAmeri, W. Carbonate mineral effect on surface charge change during low-salinity imbibition. In SPE Annual Technical Conference and Exhibition; SPE: Lumpur, Malaysia, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Anfinsen, B.; Hashemian, Y.; Yuan, Z. Evaluating Subsea Capping Stack Usage for CO2 Blowouts Lei. In Offshore Technology Conference; OTC: Springfield, MO, USA, 2024; pp. 1–18. [Google Scholar] [CrossRef]
- Wang, S.; Edwards, I.M.; Clarens, A.F. Wettability Phenomena at the CO2–Brine–Mineral Interface: Implications for Geologic Carbon Sequestration. Environ. Sci. Technol. 2013, 47, 234–241. [Google Scholar] [CrossRef]
- Eyitayo, S.I.; Arbad, N.; Kolawole, O.; Watson, M.C.; Eyitayo, S.I.; Arbad, N.; Kolawole, O.; Marshall, C. Optimizing CO2 storage in deep saline formations: A comprehensive review of enhancing pore space utilization through simultaneous or alternate aquifer injection through simultaneous or alternate aquifer injection ABSTRACT. Energy Sources Part A Recover. Util. Environ. Eff. 2024, 46, 6513–6536. [Google Scholar] [CrossRef]
- Dickson, J.L.; Gupta, G.; Horozov, T.S.; Binks, B.P.; Johnston, K.P. Wetting Phenomena at the CO2/Water/Glass Interface. Langmuir 2006, 22, 2161–2170. [Google Scholar] [CrossRef]
- Yang, D.; Gu, Y.; Tontiwachwuthikul, P. Wettability Determination of the Reservoir Brine−Reservoir Rock System with Dissolution of CO2 at High Pressures and Elevated Temperatures. Energy Fuels 2007, 22, 504–509. [Google Scholar] [CrossRef]
- Tonnet, N.; Broseta, D.F.; Mouronval, G. Evaluation of the Petrophysical Properties of a Carbonate-rich Caprock for CO2 Geological Storage Purposes. In Proceedings of the 72nd European Association of Geoscientists and Engineers Conference and Exhibition 2010 A New Spring for Geoscience, Barcelona, Spain, 14–17 June 2010; Volume 2, pp. 802–814. [Google Scholar]
- Espinoza, D.N.; Santamarina, J.C. Water-CO2-mineral systems: Interfacial tension, contact angle, and diffusion—Implications to CO2 geological storage. Water Resour. Res. 2010, 46. [Google Scholar] [CrossRef]
- Broseta, D.; Tonnet, N.; Shah, V. Are rocks still water-wet in the presence of dense CO2 or H2S? Geofluids 2012, 12, 280–294. [Google Scholar] [CrossRef]
- Jung, J.-W.; Wan, J. Supercritical CO2 and Ionic Strength Effects on Wettability of Silica Surfaces: Equilibrium Contact Angle Measurements. Energy Fuels 2012, 26, 6053–6059. [Google Scholar] [CrossRef]
- Farokhpoor, R.; Bjørkvik, B.J.; Lindeberg, E.; Torsæter, O. CO2 Wettability Behavior During CO2 Sequestration in Saline Aquifer -An Experimental Study on Minerals Representing Sandstone and Carbonate. Energy Procedia 2013, 37, 5339–5351. [Google Scholar] [CrossRef]
- Seyyedi, M.; Sohrabi, M.; Farzaneh, A. Investigation of Rock Wettability Alteration by Carbonated Water through Contact Angle Measurements. Energy Fuels 2015, 29, 5544–5553. [Google Scholar] [CrossRef]
- Iglauer, S.; Mathew, M.; Bresme, F. Molecular dynamics computations of brine–CO2 interfacial tensions and brine–CO2–quartz contact angles and their effects on structural and residual trapping mechanisms in carbon geo-sequestration. J. Colloid Interface Sci. 2012, 386, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Liang, Y.; Kunieda, M.; Takahashi, S.; Matsuoka, T. Molecular Dynamics Simulations of the CO2-Water-silica Interfacial Systems. Energy Procedia 2013, 37, 5435–5442. [Google Scholar] [CrossRef]
- Chen, C.; Dong, B.; Zhang, N.; Li, W.; Song, Y. Pressure and Temperature Dependence of Contact Angles for CO2/Water/Silica Systems Predicted by Molecular Dynamics Simulations. Energy Fuels 2016, 30, 5027–5034. [Google Scholar] [CrossRef]
- Yang, Y.; Nair, A.K.N.; Ruslan, M.F.A.C.; Sun, S. Interfacial properties of the alkane+water system in the presence of carbon dioxide and hydrophobic silica. Fuel 2021, 310, 122332. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, M.; Lin, M.; Peng, B.; Sun, L.; Chen, L. Investigation on Variations in Wettability of Reservoir Rock Induced by CO2-Brine-rock Interactions. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Vienna, Austria, 23–26 May 2011; Volume 5, pp. 4027–4038. [Google Scholar]
- Yang, D.; Gu, Y. Interfacial Interactions of Crude Oil-Brine-CO2 Systems under Reservoir Conditions. SPE Annual Technical Conference and Exhibition. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar]
- McCaughan, J.; Iglauer, S.; Bresme, F. Molecular Dynamics Simulation of Water/CO2-quartz Interfacial Properties: Application to Subsurface Gas Injection. Energy Procedia 2013, 37, 5387–5402. [Google Scholar] [CrossRef]
- Chen, C.; Chai, Z.; Shen, W.; Li, W.; Song, Y. Wettability of Supercritical CO2–Brine–Mineral: The Effects of Ion Type and Salinity. Energy Fuels 2017, 31, 7317–7324. [Google Scholar] [CrossRef]
- Al-Mutairi, S.M.; Abu-Khamsin, S.A.; Okasha, T.M.; Aramco, S.; Hossain, M.E. An experimental investigation of wettability alteration during CO2 immiscible flooding. J. Pet. Sci. Eng. 2014, 120, 73–77. [Google Scholar] [CrossRef]
- Ali, M.; Arif, M.; Sahito, M.F.; Al-Anssari, S.; Keshavarz, A.; Barifcani, A.; Stalker, L.; Sarmadivaleh, M.; Iglauer, S. CO2-wettability of sandstones exposed to traces of organic acids: Implications for CO2 geo-storage. Int. J. Greenh. Gas Control 2019, 83, 61–68. [Google Scholar] [CrossRef]
- da Costa, A.A.; Costa, G.; Embiruçu, M.; Soares, J.B.; Trivedi, J.J.; Rocha, P.S.; Souza, A.; Jaeger, P. The Influence of Rock Composition and pH on Reservoir Wettability for Low-Salinity Water-CO2 Enhanced Oil Recovery Applications in Brazilian Reservoirs. SPE Reserv. Evaluation Eng. 2020, 24, 45–65. [Google Scholar] [CrossRef]
- Ali, M.; Awan, F.U.R.; Ali, M.; Al-Yaseri, A.; Arif, M.; Sánchez-Román, M.; Keshavarz, A.; Iglauer, S. Effect of humic acid on CO2-wettability in sandstone formation. J. Colloid Interface Sci. 2020, 588, 315–325. [Google Scholar] [CrossRef]
- Ali, M.; Al-Yaseri, A.; Awan, F.U.R.; Arif, M.; Keshavarz, A.; Iglauer, S. Effect of water-soluble organic acids on wettability of sandstone formations using streaming zeta potential and NMR techniques: Implications for CO2 geo-sequestration. Fuel 2022, 329. [Google Scholar] [CrossRef]
- Liu, S.; Yang, X.; Qin, Y. Molecular dynamics simulation of wetting behavior at CO2/water/solid interfaces. Chin. Sci. Bull. 2010, 55, 2252–2257. [Google Scholar] [CrossRef]



| Sandstone | Pretest CA Mean ± STD (°) | Post-Test CA Mean ± STD (°) | ||||
|---|---|---|---|---|---|---|
| 1S | 74.5 ± 4.7 | 74.1 ± 5.5 | 74.9 ± 3.9 | 99.6 ± 2.2 | 99.5 ± 2.3 | 99.6 ± 2.5 |
| 2S | 68.2 ± 4.0 | 66.5 ± 4.1 | 69.9 ± 4.3 | 82.9 ± 3.1 | 81.5 ± 4.4 | 84.4 ± 3.1 |
| 3S | 71.6 ± 3.1 | 70.4 ± 3.2 | 72.9 ± 3.0 | 86.1 ± 0.5 | 85.2 ± 0.8 | 86.9 ± 0.4 |
| 4S | 62.3 ± 3.1 | 63.0 ± 2.9 | 61.6 ± 4.4 | 106.5 ± 2.8 | 105.9 ± 3.0 | 107.2 ± 3.9 |
| 5S | 73.7 ± 2.9 | 73.7 ± 2.7 | 73.7 ± 3.1 | 90.9 ± 2.3 | 91.6 ± 2.9 | 91.6 ± 2.9 |
| 6S | 75.4 ± 1.4 | 77.2 ± 1.6 | 73.6 ± 1.3 | 84.3 ± 1.5 | 85.4 ± 2.5 | 83.1 ± 1.8 |
| 7S | 72.65 ± 5.2 | 70.0 ± 6.3 | 75.3 ± 5.5 | 123.3 ± 12.4 | 124.2 ± 14.4 | 122.3 ± 11.1 |
| 8S | 82.4 ± 1.2 | 81.4 ± 1.9 | 83.4 ± 1.9 | 106.1 ± 3.1 | 104.1 ± 4.8 | 108.1 ± 2.3 |
| 9S | 73.5 ± 2.4 | 74.7 ± 2.5 | 72.4 ± 2.4 | 103.0 ± 2.1 | 103.3 ± 3.9 | 102.6 ± 0.9 |
| Limestone | Pretest CA Mean ± STD (°) | Post-Test CA Mean ± STD (°) | ||||
|---|---|---|---|---|---|---|
| 1L | 86.3 ± 2.9 | 84.7 ± 2.9 | 87.9 ± 3.5 | 100.3 ± 0.9 | 100.9 ± 0.8 | 99.6 ± 1.1 |
| 2L | 85.9 ± 0.5 | 83.9 ± 0.4 | 87.9 ± 0.6 | 96.3 ± 1.6 | 95.9 ± 1.7 | 96.6 ± 1.5 |
| 3L | 84.1 ± 2.0 | 83.8 ± 1.6 | 84.5 ± 3.2 | 95.1 ± 1.2 | 94.6 ± 1.3 | 95.5 ± 1.1 |
| 4L | 83.1 ± 3.4 | 82.4 ± 3.4 | 83.7 ± 3.4 | 116.4 ± 2.6 | 116.1 ± 2.1 | 116.7 ± 3 |
| 5L | 78.5 ± 3.6 | 79.5 ± 3.3 | 77.6 ± 4.0 | 102.8 ± 3.9 | 102.8 ± 3.9 | 102.8 ± 3.8 |
| 6L | 78.4 ± 3.8 | 78.2 ± 3.4 | 78.7 ± 4.5 | 115.2 ± 2.9 | 115.2 ± 2.7 | 115.2 ± 3.4 |
| 7L | 86.6 ± 1.3 | 86.2 ± 1.3 | 87.0 ± 1.4 | 123.1 ± 3.5 | 122.9 ± 3.9 | 123.3 ± 3.2 |
| 8L | 85.5 ± 1.7 | 85.2 ± 3.4 | 85.9 ± 2.4 | 127.8 ± 1.5 | 127.6 ± 1.5 | 128.0 ± 1.6 |
| 9L | 81.3 ± 1.5 | 80.4 ± 1.6 | 82.1 ± 2.2 | 121.0 ± 0.3 | 120.6 ± 0.3 | 121.5 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyitayo, S.I.; Talal, G.; Kolawole, O.; Okere, C.J.; Ispas, I.; Arbad, N.; Emadibaladehi, H.; Watson, M.C. Experimental Investigation of the Impact of CO2 Injection Strategies on Rock Wettability Alteration for CCS Applications. Energies 2024, 17, 2600. https://doi.org/10.3390/en17112600
Eyitayo SI, Talal G, Kolawole O, Okere CJ, Ispas I, Arbad N, Emadibaladehi H, Watson MC. Experimental Investigation of the Impact of CO2 Injection Strategies on Rock Wettability Alteration for CCS Applications. Energies. 2024; 17(11):2600. https://doi.org/10.3390/en17112600
Chicago/Turabian StyleEyitayo, Stella I., Gamadi Talal, Oladoyin Kolawole, Chinedu J. Okere, Ion Ispas, Nachiket Arbad, Hossein Emadibaladehi, and Marshall C. Watson. 2024. "Experimental Investigation of the Impact of CO2 Injection Strategies on Rock Wettability Alteration for CCS Applications" Energies 17, no. 11: 2600. https://doi.org/10.3390/en17112600
APA StyleEyitayo, S. I., Talal, G., Kolawole, O., Okere, C. J., Ispas, I., Arbad, N., Emadibaladehi, H., & Watson, M. C. (2024). Experimental Investigation of the Impact of CO2 Injection Strategies on Rock Wettability Alteration for CCS Applications. Energies, 17(11), 2600. https://doi.org/10.3390/en17112600











