Nonfullerene Small Molecular Acceptor Acting as a Solid Additive Enables Highly Efficient Pseudo-Bilayer All-Polymer Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
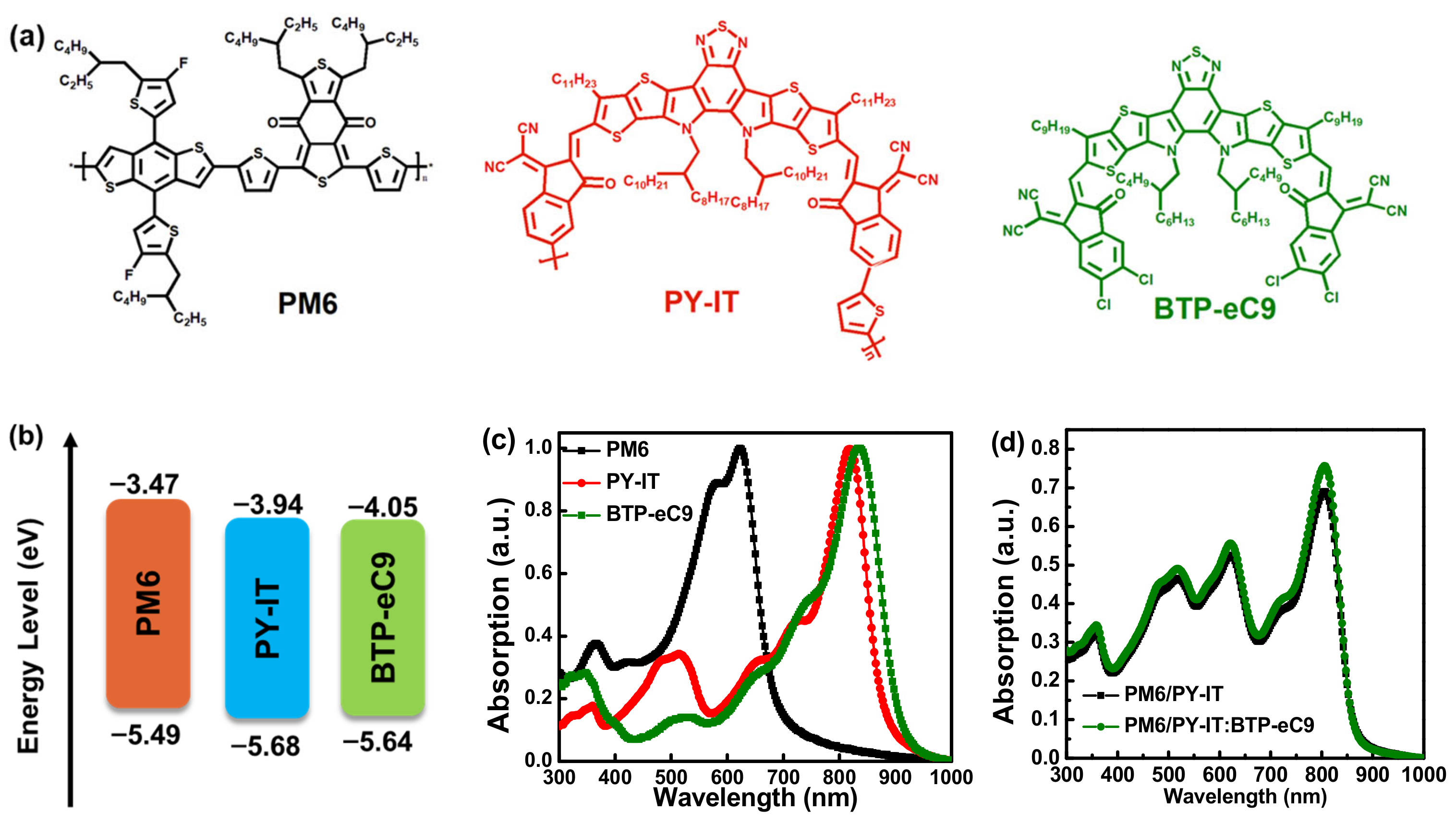
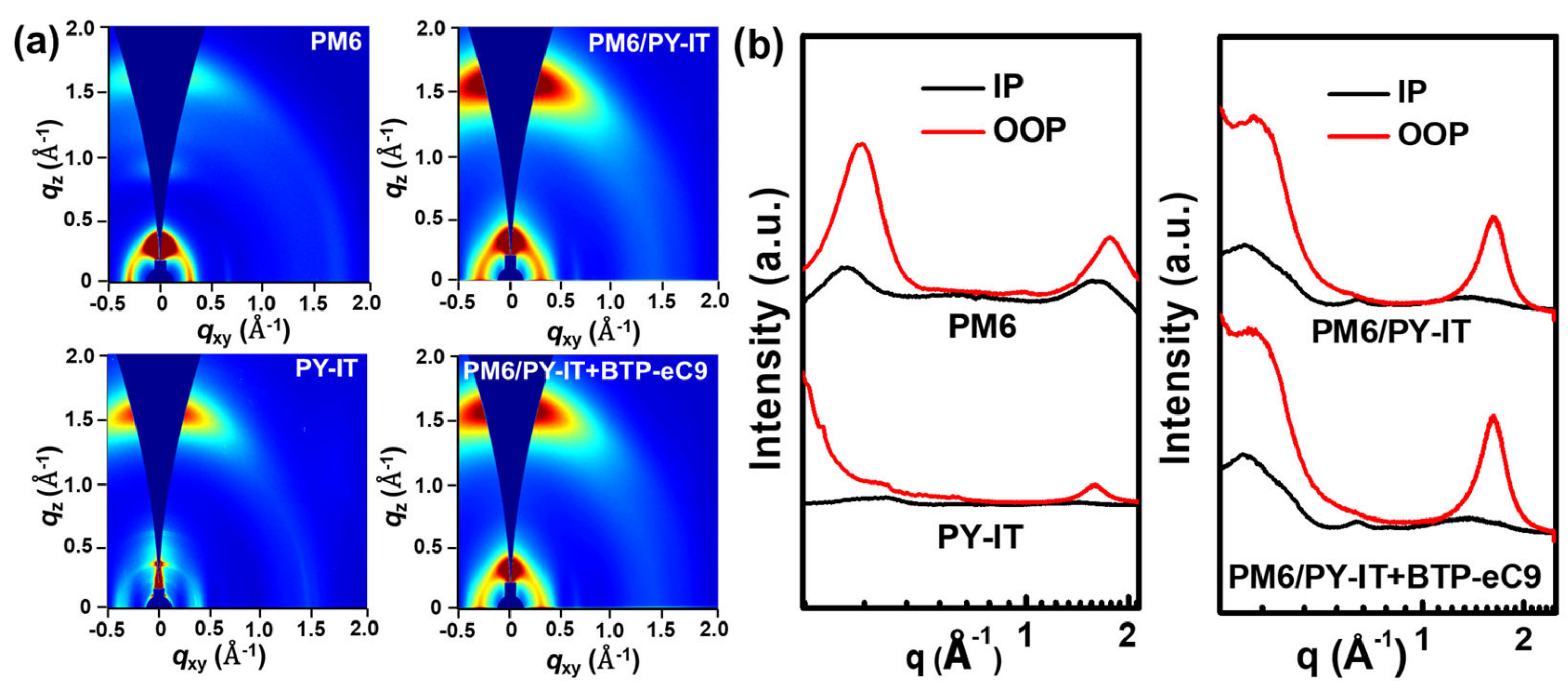
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, P.; Duan, Y.; Li, Y.; Xu, X.; Li, R.; Yu, L.; Peng, Q. 18.6% Efficiency All-Polymer Solar Cells Enabled by a Wide Bandgap Polymer Donor Based on Benzo[1,2-d:4,5-d′]bisthiazole. Adv. Mater. 2024, 36, 2306990. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Zhang, T.; Cui, Y.; Wang, J.; Qiao, J.; Xian, K.; Chua, X.W.; Chen, Z.; Goh, W.P.; Ye, L.; et al. High-Performance Binary All-Polymer Solar Cells with Efficiency Over 18.3% Enabled by Tuning Phase Transition Kinetics. Adv. Energy Mater. 2023, 13, 2302252. [Google Scholar] [CrossRef]
- Ma, R.; Li, H.; Dela Peña, T.A.; Xie, X.; Fong, P.W.-K.; Wei, Q.; Yan, C.; Wu, J.; Cheng, P.; Li, M.; et al. Tunable Donor Aggregation Dominance in a Ternary Matrix of All-Polymer Blends with Improved Efficiency and Stability. Adv. Mater. 2024, 36, 2304632. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Zhu, L.; Zhang, M.; Zhong, W.; Zhou, G.; Zhuang, J.; Hao, T.; Zhou, Z.; Zhou, L.; Hartmann, N.; et al. All-polymer organic solar cells with nano-to-micron hierarchical morphology and large light receiving angle. Nat. Commun. 2023, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Qiao, J.; Li, Y.; Song, J.; Zhang, C.; Fu, Z.; Jee, M.H.; Hao, X.; Woo, H.Y.; Sun, Y. Over 18% Efficiency of All-Polymer Solar Cells with Long-Term Stability Enabled by Y6 as a Solid Additive. Adv. Mater. 2023, 35, 2301906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, M.; Xu, J.; Li, C.; Yan, J.; Zhou, G.; Zhong, W.; Hao, T.; Song, J.; Xue, X.; et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 2022, 21, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Zhang, L.; Jeong, S.Y.; Geng, S.; Woo, H.Y.; Zhang, J.; Zhang, F.; Ma, X. Over 19.1% efficiency for sequentially spin-coated polymer solar cells by employing ternary strategy. Chem. Eng. J. 2023, 471, 144711. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Fu, Y.; Guo, C.; Li, D.; Cheng, J.; Sun, W.; Gan, Z.; Sun, Y.; Zhou, B.; et al. Donor–acceptor mutually diluted heterojunctions for layer-by-layer fabrication of high-performance organic solar cells. Nat. Energy 2024, 9, 208–218. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, H.; Hu, D.; Zhang, C.E.; Xu, X.; Lu, H.; Wu, Y.; Yang, C.; Bo, Z. Improving the Performance of Layer-by-Layer Processed Organic Solar Cells via Introducing a Wide-Bandgap Dopant into the Upper Acceptor Layer. Adv. Mater. 2023, 35, 2211372. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, W.; Liu, Z.; Xie, Y.; Zhang, W.; Xu, Y.; Jeong, S.Y.; Zhang, F.; Weng, N.; Zhang, Z.; et al. Over 18.8% Efficiency of Layer-By-Layer Organic Photovoltaics Enabled by Ameliorating Exciton Utilization in Acceptor Layer. Adv. Funct. Mater. 2024, 34, 2313751. [Google Scholar] [CrossRef]
- Wen, L.; Mao, H.; Zhang, L.; Zhang, J.; Qin, Z.; Tan, L.; Chen, Y. Achieving Desired Pseudo-Planar Heterojunction Organic Solar Cells via Binary-Dilution Strategy. Adv. Mater. 2024, 36, 2308159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chung, S.; Zhao, J.; Sun, Y.; Zhao, B.; Zhao, Z.; Kim, S.; Cho, K.; Kan, Z. Vertical Phase Regulation with 1,3,5-Tribromobenzene Leads to 18.5% Efficiency Binary Organic Solar Cells. Adv. Sci. 2023, 10, 2303150. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, M.; Liu, Z.; Tian, H.; Zhang, W.; Sun, S.; Jeong, S.Y.; Zhang, F.; Li, X.; Sun, Q.; et al. A nonfullerene acceptor as a solid additive realizing a record efficiency of 17.74% in quasi-layered all-polymer solar cells. J. Mater. Chem. A 2024, 12, 4077–4085. [Google Scholar] [CrossRef]
- Jee, M.H.; Ryu, H.S.; Lee, D.; Lee, W.; Woo, H.Y. Recent Advances in Nonfullerene Acceptor-Based Layer-by-Layer Organic Solar Cells Using a Solution Process. Adv. Sci. 2022, 9, 2201876. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Chen, T.; Wang, M.; Xia, X.; He, C.; Zheng, X.; Li, Y.; Zhou, D.; Lu, X.; Zuo, L.; et al. Solid Additive-Assisted Layer-by-Layer Processing for 19% Efficiency Binary Organic Solar Cells. Nano-Micro Lett. 2023, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, L.-M.; Liu, S.; Guo, L.; Dong, S.; Ma, G.; Cao, Z.; Zhan, X.; Gu, X.; Zhu, T.; et al. Vertical Composition Distribution and Crystallinity Regulations Enable High-Performance Polymer Solar Cells with >17% Efficiency. ACS Energy Lett. 2020, 5, 3637–3646. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Hu, L.; Xie, Z.; Mao, H.; Tan, L.; Zhang, Y.; Chen, Y. High-Efficiency (16.93%) Pseudo-Planar Heterojunction Organic Solar Cells Enabled by Binary Additives Strategy. Adv. Funct. Mater. 2021, 31, 2102291. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Cai, Y.; Zhang, R.; Wang, S.; Xin, J.; Han, L.; Wei, D.; Ma, W.; Gao, F.; et al. Solid additive engineering enables high-efficiency and eco-friendly all-polymer solar cells. Matter 2022, 5, 4047–4059. [Google Scholar] [CrossRef]
- Ma, R.; Yu, J.; Liu, T.; Zhang, G.; Xiao, Y.; Luo, Z.; Chai, G.; Chen, Y.; Fan, Q.; Su, W.; et al. All-polymer solar cells with over 16% efficiency and enhanced stability enabled by compatible solvent and polymer additives. Aggregate 2022, 3, e58. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Yao, J.; Xue, L.; Chen, S.; Li, X.; Morrison, W.; Yang, C.; Li, Y. Constructing a Strongly Absorbing Low-Bandgap Polymer Acceptor for High-Performance All-Polymer Solar Cells. Angew. Chem. Int. Ed. 2017, 129, 13688. [Google Scholar] [CrossRef]
- Wang, G.; Melkonyan, F.S.; Facchetti, A.; Marks, T.J. All-Polymer Solar Cells: Recent Progress, Challenges, and Prospects. Angew. Chem. Int. Ed. 2019, 58, 4129. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 32, 1908205. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, T.; Ma, R.; Xiao, Y.; Zhan, L.; Zhang, G.; Sun, H.; Ni, F.; Chai, G.; Wang, J.; et al. Precisely Controlling the Position of Bromine on the End Group Enables Well-Regular Polymer Acceptors for All-Polymer Solar Cells with Efficiencies over 15%. Adv. Mater. 2020, 32, 2005942. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Qiao, J.; Cui, F.; Zhang, W.; Wang, L.; Lu, P.; Yin, H.; Du, X.; Qin, W.; Hao, X. π–π Stacking Modulation via Polymer Adsorption for Elongated Exciton Diffusion in High-Efficiency Thick-Film Organic Solar Cells. Adv. Mater. 2024, 36, 2313532. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, W.; Liu, Z.; Jeong, S.Y.; Xu, C.; Zhang, J.; Woo, H.Y.; Zhou, Z.; Zhang, F. Over 18.1% Efficiency of Layer-by-Layer Polymer Solar Cells by Enhancing Exciton Utilization near the ITO Electrode. ACS Appl. Mater. Interfaces 2023, 15, 7247. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, J.; Liu, L.; Xie, L.; Zhou, H.; Zhang, J.; Zhang, K.; Xiao, M.; Huang, F. Layer-by-Layer Processed PM6:Y6-Based Stable Ternary Polymer Solar Cells with Improved Efficiency over 18% by Incorporating an Asymmetric Thieno[3,2-b]indole-Based Acceptor. Adv. Funct. Mater. 2022, 32, 2200629. [Google Scholar] [CrossRef]
- Sun, Y.; Nian, L.; Kan, Y.; Ren, Y.; Chen, Z.; Zhu, L.; Zhang, M.; Yin, H.; Xu, H.; Li, J.; et al. Rational control of sequential morphology evolution and vertical distribution toward 17.18% efficiency all-small-molecule organic solar cells. Joule 2022, 6, 2835–2848. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Z.; Lu, G.; Yu, N.; Li, C.; Gao, J.; Gu, X.; Hao, X.; Lu, G.; Tang, Z.; et al. Binary Organic Solar Cells Breaking 19% via Manipulating the Vertical Component Distribution. Adv. Mater. 2022, 34, 2204718. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, H.; Qiu, D.; Zhao, T.; Zhu, Y.; Lai, X.; Pu, M.; Li, Y.; Li, H.; Chen, H.; et al. Quasiplanar Heterojunction All-Polymer Solar Cells: A Dual Approach to Stability. Adv. Funct. Mater. 2022, 32, 2201828. [Google Scholar] [CrossRef]
- Xu, W.; Ma, X.; Son, J.H.; Jeong, S.Y.; Niu, L.; Xu, C.; Zhang, S.; Zhou, Z.; Gao, J.; Woo, H.Y.J.S. Smart ternary strategy in promoting the performance of polymer solar cells based on bulk-heterojunction or layer-by-layer structure. Small 2022, 18, 2104215. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zeng, L.; Liao, X.; Chen, Z.; Liu, S.; Zhu, P.; Zhu, H.; Chen, Y. All-Green Solvent-Processed Planar Heterojunction Organic Solar Cells with Outstanding Power Conversion Efficiency of 16%. Adv. Funct. Mater. 2022, 32, 2107567. [Google Scholar] [CrossRef]
- Ma, L.; Cui, Y.; Zhang, J.; Xian, K.; Chen, Z.; Zhou, K.; Zhang, T.; Wang, W.; Yao, H.; Zhang, S.; et al. High-Efficiency and Mechanically Robust All-Polymer Organic Photovoltaic Cells Enabled by Optimized Fibril Network Morphology. Adv. Mater. 2022, 34, 2208926. [Google Scholar]
- Li, X.; Yang, H.; Du, X.; Lin, H.; Yang, G.; Zheng, C.; Tao, S. High-Performance Layer-by-Layer organic solar cells enabled by Non-Halogenated solvent with 17.89% efficiency. Chem. Eng. J. 2023, 452, 139496. [Google Scholar] [CrossRef]
- Dou, Y.; Hong, L.; Jing, J.; Jia, T.; Zhang, J.; Zhang, K.; Huang, F. High Efficiency Layer-by-Layer All-Polymer Solar Cell Enabled by Bottom-Layer Optimization. Sol. RRL 2023, 7, 2300599. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, M.; Ma, X.; Zhu, X.; Jeong, S.Y.; Woo, H.Y.; Zhang, J.; Du, W.; Wang, J.; Liu, X.; et al. Over 17.4% Efficiency of Layer-by-Layer All-Polymer Solar Cells by Improving Exciton Utilization in Acceptor Layer. Adv. Funct. Mater. 2023, 33, 2215204. [Google Scholar] [CrossRef]
- Zhuo, H.; Li, X.; Zhang, J.; Qin, S.; Guo, J.; Zhou, R.; Jiang, X.; Wu, X.; Chen, Z.; Li, J.; et al. Giant Molecule Acceptor Enables Highly Efficient Organic Solar Cells Processed Using Non-halogenated Solvent. Angew. Chem. Int. Ed. 2023, 62, e202303551. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, J.; Qin, L.; Chen, H.; Wu, X.; Wei, Z.; Zhang, X.; Ding, L.; Gao, F.; Huang, H. A universal method for constructing high efficiency organic solar cells with stacked structures. Energy Environ. Sci. 2021, 14, 2314–2321. [Google Scholar] [CrossRef]
- Liu, M.; Yao, Q.; Li, S.; Qin, Y.; Jeong, S.Y.; Ma, Y.; Shen, L.; Ma, X.; Yang, K.; Yuan, G.J.A.O.M. Interfacial Engineering for Photomultiplication Type Organic Photodetectors with Signal-Noise-Ratio Over 89 000. Adv. Opt. Mater. 2024, 12, 2303216. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Zou, X.; Yin, J.; Shi, X.; Li, Y.; Zhao, H.; Wang, L.; Ng, H.M.; Zou, B.; et al. Improved photovoltaic performance and robustness of all-polymer solar cells enabled by a polyfullerene guest acceptor. Nat. Commun. 2023, 14, 2323. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Liu, M.; Zhao, Z.; Yang, K.; Liu, P.; Zhang, H.; Li, J.; Ma, X.; Yao, Q.; et al. Photomultiplica-tion-Type All-Polymer Photodetectors and Their Applications in Photoplethysmography Sensor. Acta Phys. Chim. Sin. 2024, 40, 2311021. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Yao, H.; Zhang, T.; Zhang, J.; Ma, L.; Wang, J.; Wei, Z.; Hou, J. A New Conjugated Polymer that Enables the Integration of Photovoltaic and Light-Emitting Functions in One Device. Adv. Mater. 2021, 33, 2101090. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, J.; Dong, Y.; Jin, H.; Xin, J.; Wang, S.; Cai, Y.; Jiang, L.; Ma, W.; Tang, Z.J.A.M. Polymerized small molecular acceptor with branched side chains for all polymer solar cells with efficiency over 16.7%. Adv. Mater. 2022, 34, 2110155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Wu, X.; Zhao, D.; Zhang, S.; Zou, X.; Li, B.; Gao, D.; Li, Z.; Xia, X.; et al. Boosting the Fill Factor through Sequential Deposition and Homo Hydrocarbon Solvent toward Efficient and Stable All-Polymer Solar Cells. Adv. Energy Mater. 2022, 12, 2202729. [Google Scholar] [CrossRef]
- Fu, H.; Peng, Z.; Fan, Q.; Lin, F.R.; Qi, F.; Ran, Y.; Wu, Z.; Fan, B.; Jiang, K.; Woo, H.Y.; et al. A Top-Down Strategy to Engineer ActiveLayer Morphology for Highly Efficient and Stable All-Polymer Solar Cells. Adv. Mater. 2022, 34, 2202608. [Google Scholar] [CrossRef] [PubMed]


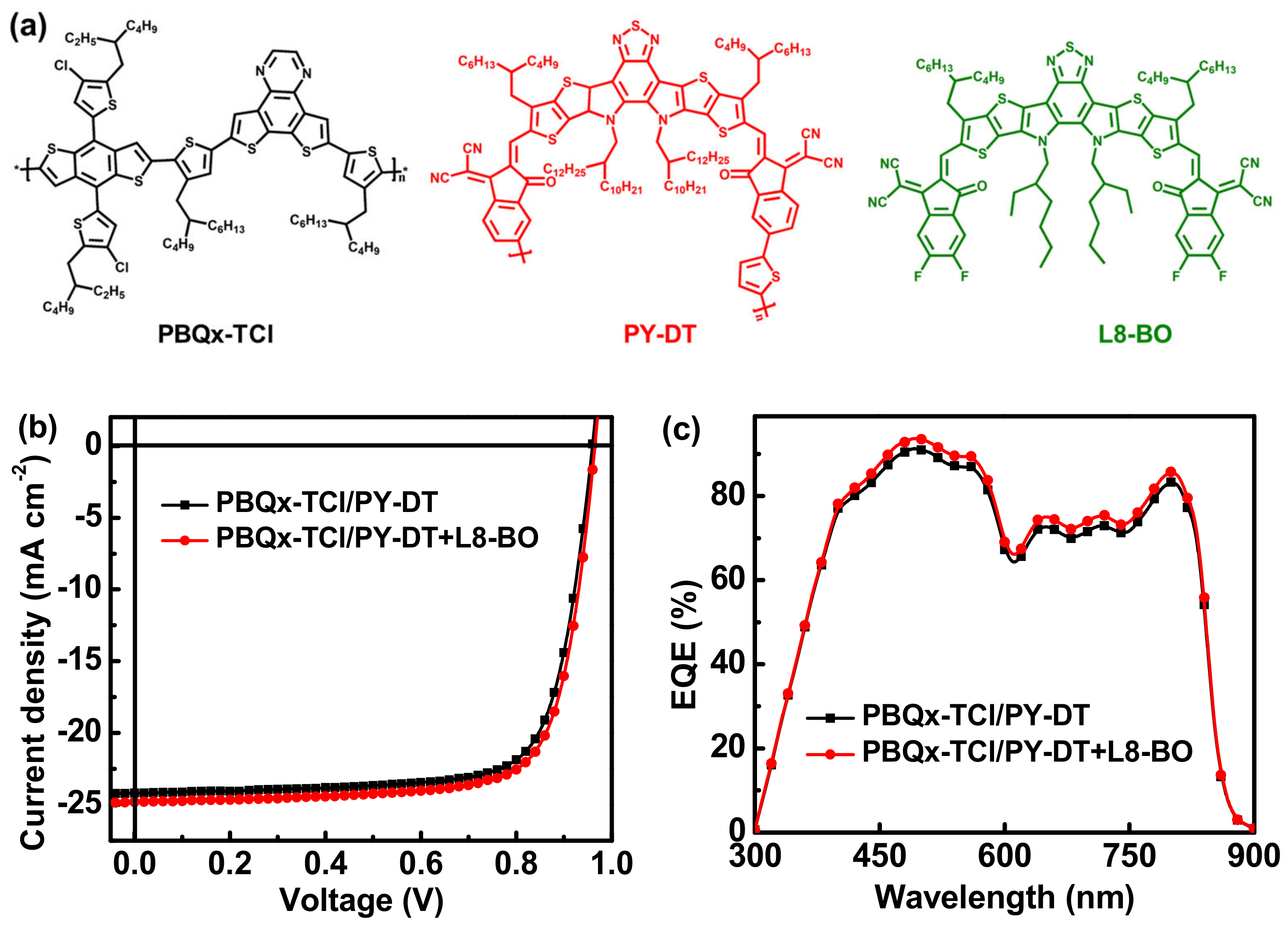
| Donor Layer | Acceptor Layer | JSC | Cal. JSC | VOC | FF | PCE |
|---|---|---|---|---|---|---|
| (avg. ± dev.) a (mA cm−2) | (mA cm−2) | (avg. ± dev.) a (V) | (avg. ± dev.) a (%) | (avg. ± dev.) a (%) | ||
| PM6 | PY-IT | 23.36 (23.16 ± 0.32) | 22.86 | 0.94 (0.937 ± 0.007) | 68.83 (68.92 ± 0.91) | 15.11 (14.95 ± 0.12) |
| PM6 | PY-IT+ BTP-eC9 | 24.08 (23.87 ± 0.15) | 23.58 | 0.94 (0.938 ± 0.006) | 72.76 (73.02 ± 0.45) | 16.47 (16.35 ± 0.09) |
| Donor Layer | Acceptor Layer | JSC | Cal. JSC | VOC | FF | PCE |
|---|---|---|---|---|---|---|
| (avg. ± dev.) a (mA cm−2) | (mA cm−2) | (avg. ± dev.) a (V) | (avg. ± dev.) a (%) | (avg. ± dev.) a (%) | ||
| PBQx-TCl | PY-DT | 24.19 (23.97 ± 0.24) | 23.08 | 0.96 (0.968 ± 0.004) | 75.38 (74.69 ± 0.89) | 17.51 (17.33 ± 0.13) |
| PBQx-TCl | PY-DT+ L8-BO | 24.80 (24.59 ± 0.16) | 23.73 | 0.96 (0.965 ± 0.005) | 75.88 (75.82 ± 0.25) | 18.07 (17.99 ± 0.08) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ni, Y.; Zhang, J.; Zhao, Y.; Xu, W.; Ma, X.; Zhang, F. Nonfullerene Small Molecular Acceptor Acting as a Solid Additive Enables Highly Efficient Pseudo-Bilayer All-Polymer Solar Cells. Energies 2024, 17, 2623. https://doi.org/10.3390/en17112623
Liu J, Ni Y, Zhang J, Zhao Y, Xu W, Ma X, Zhang F. Nonfullerene Small Molecular Acceptor Acting as a Solid Additive Enables Highly Efficient Pseudo-Bilayer All-Polymer Solar Cells. Energies. 2024; 17(11):2623. https://doi.org/10.3390/en17112623
Chicago/Turabian StyleLiu, Jiayin, Yuheng Ni, Jiaqi Zhang, Yijun Zhao, Wenjing Xu, Xiaoling Ma, and Fujun Zhang. 2024. "Nonfullerene Small Molecular Acceptor Acting as a Solid Additive Enables Highly Efficient Pseudo-Bilayer All-Polymer Solar Cells" Energies 17, no. 11: 2623. https://doi.org/10.3390/en17112623





