The Trade-Off between Combustion and Partial Oxidation during Chemical Looping Conversion of Methane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
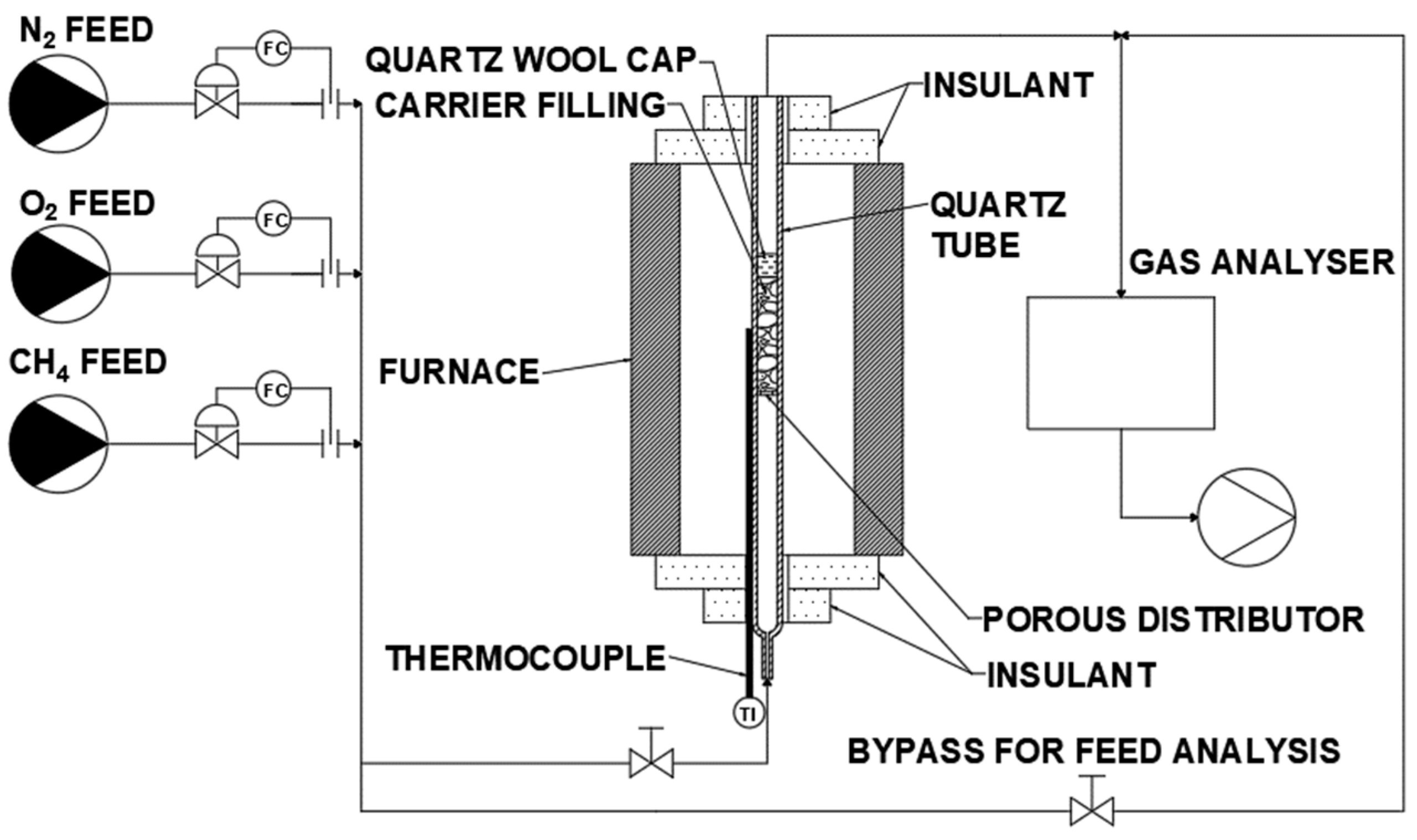
2.2. Experimental Apparatus
2.3. Data Elaboration
3. Results
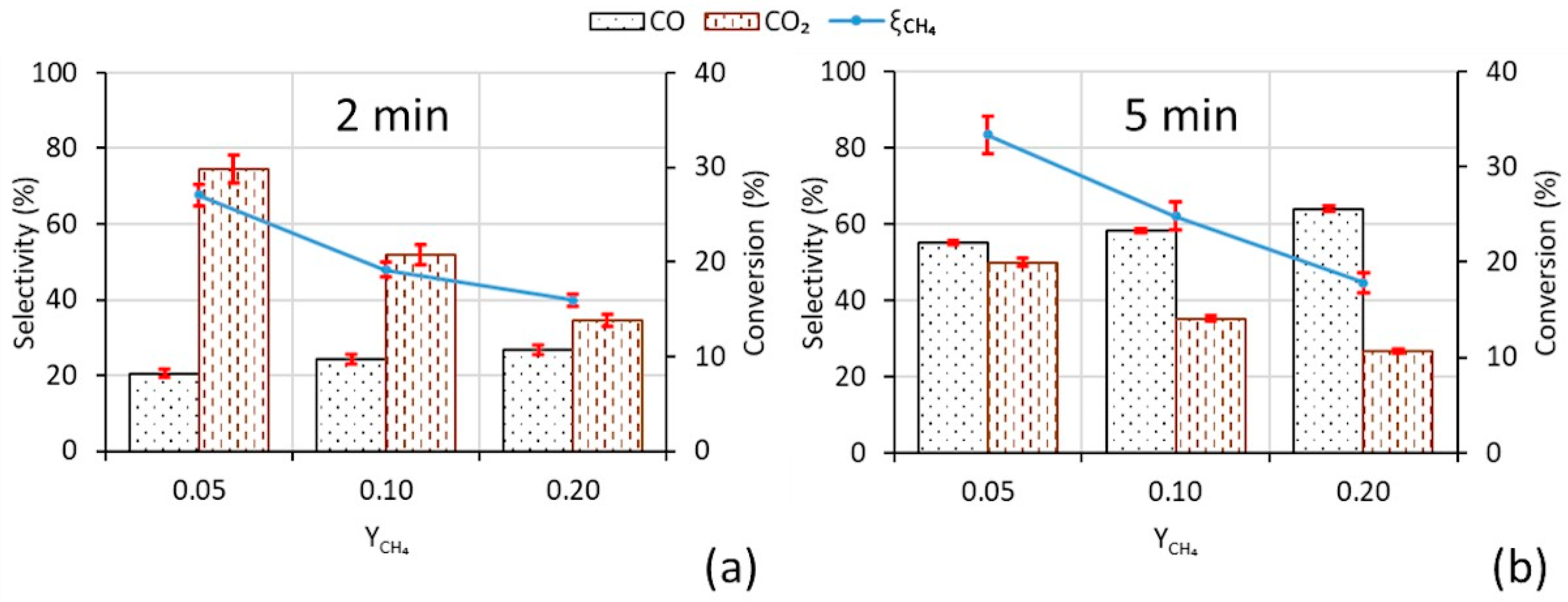
3.1. Reaction Test Results
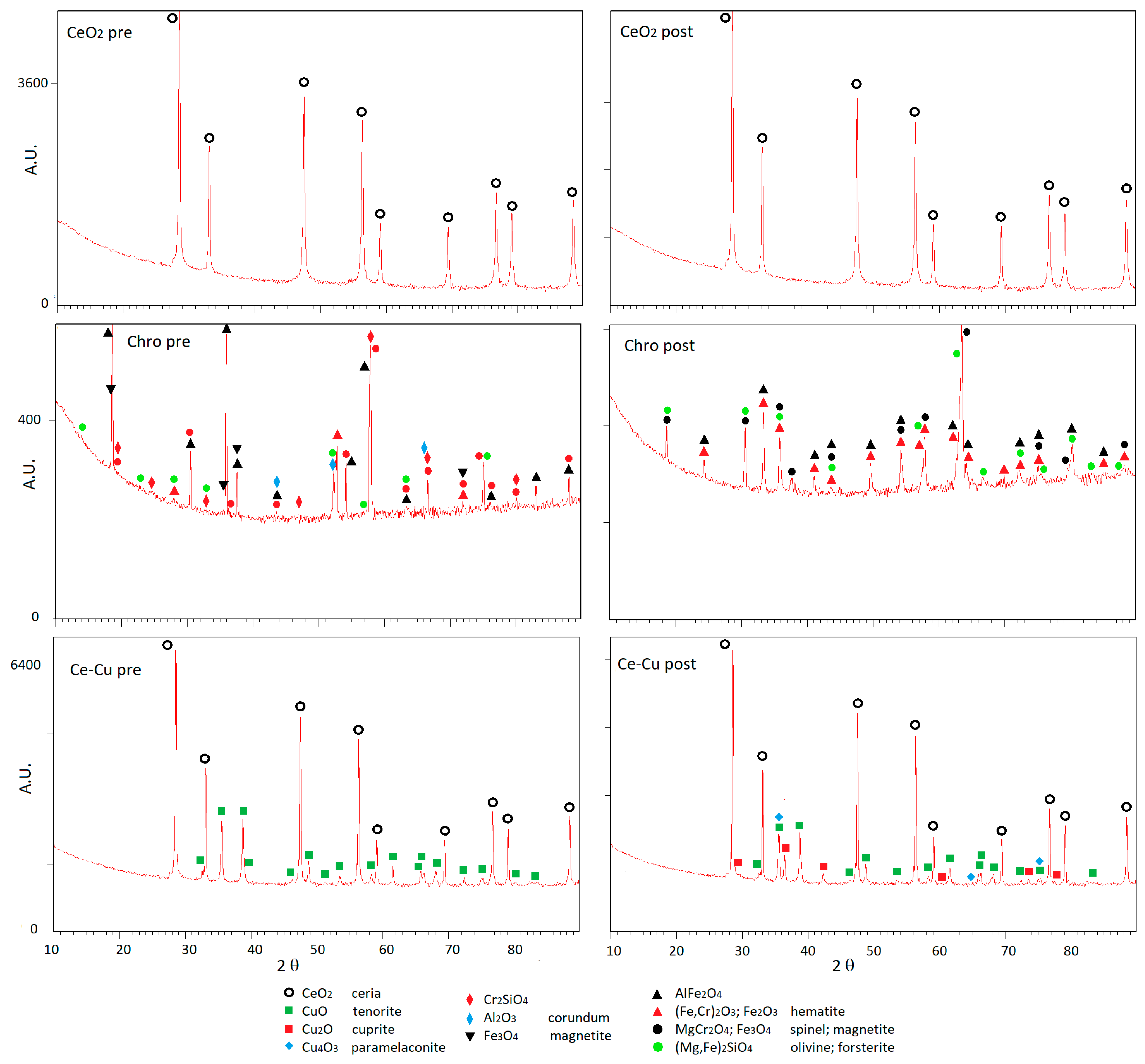
3.2. Characterization of the Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | Meaning | Units |
| q | Molar flow rate | mmol/min |
| yi | Molar fraction of compound i | dimensionless |
| t | time | Min |
| mi | Mass of carrier i | Mg |
| Mi | Molar mass of compound i | g/mol |
| e | Equivalence factor for methane oxidation | dimensionless |
| nO2 stoich, CH4 | Oxygen amount for stoichiometric combustion | mmol |
| nO2 av,carrieri | Oxygen availability in carrier | mmol |
| ξCH4 | Methane conversion | dimensionless |
| ξcarrier | Conversion of carrier i | dimensionless |
| ηi | Selectivity for compound i | dimensionless |
| ωi | Mass fraction of compound i | dimensionless |
| Subscripts | ||
| in | Reactor inlet | - |
| out | Reactor outlet | - |
| c | Combustion step | - |
| r | Regeneration step | - |
References
- Abanades, J.C.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.E.; Li, H.; Ho, M.T.; Mangano, E.; Brandani, S. Emerging CO2 Capture Systems. Int. J. Greenh. Gas. Control. 2015, 40, 126–166. [Google Scholar] [CrossRef]
- Boscherini, M.; Storione, A.; Minelli, M.; Miccio, F.; Doghieri, F. New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies 2023, 16, 6375. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Hedayati, A.; Augustsson, E. Fate of NO and Ammonia in Chemical Looping Combustion—Investigation in a 300 W Chemical Looping Combustion Reactor System. Energy Fuels 2022, 36, 9628–9647. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming Technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Lu, H.; Shi, X.; Costa, M.; Huang, C. Carcinogenic Effect of Nickel Compounds. Mol. Cell Biochem. 2005, 279, 45–67. [Google Scholar] [CrossRef]
- Otsuka, K.; Sunada, E.; Ushiyama, T.; Yamanaka, I. The Production of Synthesis Gas by the Redox of Cerium Oxide. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1997; pp. 531–536. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar Chemical Looping Reforming of Methane Combined with Isothermal H2O/CO2 Splitting Using Ceria Oxygen Carrier for Syngas Production. J. Energy Chem. 2020, 41, 60–72. [Google Scholar] [CrossRef]
- Cheng, Z.; Qin, L.; Fan, J.A.; Fan, L.-S. New Insight into the Development of Oxygen Carrier Materials for Chemical Looping Systems. Engineering 2018, 4, 343–351. [Google Scholar] [CrossRef]
- Warren, K.J.; Scheffe, J.R. Role of Surface Oxygen Vacancy Concentration on the Dissociation of Methane over Nonstoichiometric Ceria. J. Phys. Chem. C 2019, 123, 13208–13218. [Google Scholar] [CrossRef]
- Cheng, Z.; Qin, L.; Guo, M.; Xu, M.; Fan, J.A.; Fan, L.-S. Oxygen Vacancy Promoted Methane Partial Oxidation over Iron Oxide Oxygen Carriers in the Chemical Looping Process. Phys. Chem. Chem. Phys. 2016, 18, 32418–32428. [Google Scholar] [CrossRef]
- Koleli, N.; Demir, A. Chapter 11-Chromite. In Environmental Materials and Waste. Resource Recovery and Pollution Prevention; Academic Press: Cambridge, MA, USA, 2016; pp. 245–263. [Google Scholar] [CrossRef]
- Nurjaman, F.; Subandrio, S.; Ferdian, D.; Suharno, B. Effect of Basicity on Beneficiated Chromite Sand Smelting Process Using Submerged Arc Furnace. In Proceedings of the International Seminar on Metallurgy and Materials (ISMM2017): Metallurgy and Advanced Material Technology for Sustainable Development, Jakarta, Indonesia, 24–25 October 2017; p. 020009. [Google Scholar] [CrossRef]
- Miccio, F.; Ruoppolo, G.; Russo, S.; Urciuolo, M.; De Riccardis, A. Fluidized Bed Combustion of Wet Biomass Fuel (Olive Husks). Chem. Eng. Trans. 2014, 37, 1–6. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A Review on CO Oxidation Over Copper Chromite Catalyst. Catal. Rev. 2012, 54, 224–279. [Google Scholar] [CrossRef]
- Ammendola, P.; Chirone, R.; Lisi, L.; Ruoppolo, G.; Russo, G. Copper Catalysts for H2 Production via CH4 Decomposition. J. Mol. Catal. A Chem. 2007, 266, 31–39. [Google Scholar] [CrossRef]
- Warren, K.J.; Carrillo, R.J.; Greek, B.; Hill, C.M.; Scheffe, J.R. Solar Reactor Demonstration of Efficient and Selective Syngas Production via Chemical-Looping Dry Reforming of Methane over Ceria. Energy Technol. 2020, 8, 2000053. [Google Scholar] [CrossRef]
- Storione, A.; Boscherini, M.; Miccio, F.; Landi, E.; Minelli, M.; Doghieri, F. Improvement of Process Conditions for H2 Production by Chemical Looping Reforming. Energies 2024, 17, 1544. [Google Scholar] [CrossRef]
- Miccio, F.; Natali Murri, A.; Landi, E. Synthesis and Characterization of Geopolymer Oxygen Carriers for Chemical Looping Combustion. Appl. Energy 2017, 194, 136–147. [Google Scholar] [CrossRef]
- Miccio, F.; Landi, E.; Murri, A.N.; Minelli, M.; Doghieri, F.; Storione, A. Fluidized Bed Reforming of Methane by Chemical Looping with Cerium Oxide Oxygen Carriers. Chem. Eng. Res. Des. 2023, 191, 568–577. [Google Scholar] [CrossRef]
- Liu, G.; Lisak, G. Cu-Based Oxygen Carriers for Chemical Looping Processes: Opportunities and Challenges. Fuel 2023, 342, 127828. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Johansson, M.; Jerndal, E.; Mattisson, T. The Use of Ilmenite as an Oxygen Carrier in Chemical-Looping Combustion. Chem. Eng. Res. Des. 2008, 86, 1017–1026. [Google Scholar] [CrossRef]
- Jing, D.; Arjmand, M.; Mattisson, T.; Rydén, M.; Snijkers, F.; Leion, H.; Lyngfelt, A. Examination of Oxygen Uncoupling Behaviour and Reactivity towards Methane for Manganese Silicate Oxygen Carriers in Chemical-Looping Combustion. Int. J. Greenh. Gas. Control. 2014, 29, 70–81. [Google Scholar] [CrossRef]
- Živković, A.; Sheehama, J.; Warwick, M.E.A.; Jones, D.R.; Mitchel, C.; Likius, D.; Uahengo, V.; Dzade, N.Y.; Meenakshisundaram, S.; Dunnill, C.W.; et al. Structural and Electronic Properties of Cu4O3 (Paramelaconite): The Role of Native Impurities. Pure Appl. Chem. 2021, 93, 1229–1244. [Google Scholar] [CrossRef]
- Perrot, P. Chromium–Iron–Oxygen. Ternary Alloy Systems: Phase Diagrams; Crystallographic and Thermodynamic Data. In Ternary Alloy Systems: Phase Diagrams, Crystallographic and Thermodynamic Data Critically Evaluated by MSIT®; Springer: Berlin/Heidelberg, Germany, 2009; Volume 11, pp. 250–276. [Google Scholar] [CrossRef]
- Jacob, A.; Povoden-Karadeniz, E.; Kozeschnik, E. Revised Thermodynamic Description of the Fe-Cr System Based on an Improved Sublattice Model of the σ Phase. Calphad 2018, 60, 16–28. [Google Scholar] [CrossRef]
- Otsuka, K.; Wang, Y.; Sunada, E.; Yamanaka, I. Direct Partial Oxidation of Methane to Synthesis Gas by Cerium Oxide. J. Catal. 1998, 175, 152–160. [Google Scholar] [CrossRef]
- Fosheim, J.R.; Hathaway, B.J.; Davidson, J.H. High Efficiency Solar Chemical-Looping Methane Reforming with Ceria in a Fixed-Bed Reactor. Energy 2019, 169, 597–612. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Chen, S.; Li, M.; Zhang, L.; Xiang, W. Chemical Looping Dry Reforming of Methane with Hydrogen Generation on Fe2O3/Al2O3 Oxygen Carrier. Chem. Eng. J. 2019, 368, 812–823. [Google Scholar] [CrossRef]
- Saddiq, H.A.; Muhammed-Dabo, I.A.; Hamza, A.; Waziri, S.M. Kinetic Modeling of CuO/CeO2 and CuO/Nb2O₅ as Oxygen Carriers in the Production of Syngas. React. Kinet. Mech. Catal. 2021, 134, 727–742. [Google Scholar] [CrossRef]
- He, F.; Wei, Y.; Li, H.; Wang, H. Synthesis Gas Generation by Chemical-Looping Reforming Using Ce-Based Oxygen Carriers Modified with Fe, Cu, and Mn Oxides. Energy Fuels 2009, 23, 2095–2102. [Google Scholar] [CrossRef]
- Elgarni, M.M.; Tijani, M.M.; Mahinpey, N. Characterization, Kinetics and Stability Studies of NiO and CuO Supported by Al2O3, ZrO2, CeO2 and Their Combinations in Chemical Looping Combustion. Catal. Today 2022, 397–399, 206–219. [Google Scholar] [CrossRef]
- Tijani, M.M.; Aqsha, A.; Mahinpey, N. Synthesis and Study of Metal-Based Oxygen Carriers (Cu, Co, Fe, Ni) and Their Interaction with Supported Metal Oxides (Al2O3, CeO2, TiO2, ZrO2) in a Chemical Looping Combustion System. Energy 2017, 138, 873–882. [Google Scholar] [CrossRef]




| Chro | CeO2 | Ce–Cu | |
|---|---|---|---|
| Size (mm) | 0.20–0.40 | 0.60–0.84 | 0.60–0.84 |
| Density (kg/m3) | 4170 | 7220 | 6930 |
| O2 capacity (mmol/g) | 0.90 | 1.45 | 2.98 |
| Major phases | |||
| CeO2, wt. % | - | >99 | 68 |
| CuO, wt. % | - | - | 32 |
| Cr2O3, wt. % | 47 | - | - |
| FeO, wt. % | 26 | - | - |
| Al2O3, wt. % | 15 | - | - |
| MgO, wt. % | 10 | - | - |
| SiO2, wt. % | 1 | - | - |
| Time, min | e | ξCH4 | ηCO | ηCO2 | |
|---|---|---|---|---|---|
| Chro | 2 | 0.81 | 0.13 | 0.12 | 0,29 |
| 5 | 0.32 | 0.37 | 0.45 | 0,00 | |
| CeO2 | 2 | 1.25 | 0.19 | 0.24 | 0.78 |
| 5 | 0.50 | 0.25 | 0.58 | 0.54 | |
| Ce–Cu | 2 | 2.48 | 0.60 | 0.00 | 1.00 |
| 5 | 0.99 | 0.83 | 0.00 | 1.00 |
| yCH4 | 0.05 | 0.05 | 0.10 | 0.10 | 0.20 | 0.20 |
| Time, min | 2 | 5 | 2 | 5 | 2 | 5 |
| Chro | 3.5 | 0.7 | 1.3 | 11.9 | - | - |
| CeO2 | 7.7 | 2.4 | 4.4 | 1.1 | 1.0 | 0.7 |
| Ce–Cu | 0.8 | 0.1 | 0.5 | 1.4 | 4.6 | 2.6 |
| yCH4 | 0.05 | 0.05 | 0.10 | 0.10 | 0.20 | 0.20 | |
| ξcarrier | 0.10 | 0.20 | 0.10 | 0.20 | 0.10 | 0.20 | |
| Chro | ξCH4 | 0.18 | (-) | 0.21 | 0.14 | (-) | (-) |
| ηCO | 0.15 | (-) | 0.39 | 0.58 | (-) | (-) | |
| ηCO2 | 0.85 | (-) | 0.36 | 0.35 | (-) | (-) | |
| CeO2 | ξCH4 | 0.29 | 0.28 | 0.22 | 0.21 | 0.17 | 0.16 |
| ηCO | 0.47 | 0.33 | 0.59 | 0.74 | 0.63 | 0.80 | |
| ηCO2 | 0.53 | 0.77 | 0.44 | 0.27 | 0.39 | 0.24 | |
| Ce–Cu | ξCH4 | 0.73 | 0.73 | 0.74 | 0.74 | 0.74 | 0.74 |
| ηCO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| ηCO2 | 1.00 | 1.00 | 1.00 | 1.00 | 0.87 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miccio, F.; Mazzocchi, M.; Boscherini, M.; Storione, A.; Minelli, M.; Doghieri, F. The Trade-Off between Combustion and Partial Oxidation during Chemical Looping Conversion of Methane. Energies 2024, 17, 2764. https://doi.org/10.3390/en17112764
Miccio F, Mazzocchi M, Boscherini M, Storione A, Minelli M, Doghieri F. The Trade-Off between Combustion and Partial Oxidation during Chemical Looping Conversion of Methane. Energies. 2024; 17(11):2764. https://doi.org/10.3390/en17112764
Chicago/Turabian StyleMiccio, Francesco, Mauro Mazzocchi, Mattia Boscherini, Alba Storione, Matteo Minelli, and Ferruccio Doghieri. 2024. "The Trade-Off between Combustion and Partial Oxidation during Chemical Looping Conversion of Methane" Energies 17, no. 11: 2764. https://doi.org/10.3390/en17112764
APA StyleMiccio, F., Mazzocchi, M., Boscherini, M., Storione, A., Minelli, M., & Doghieri, F. (2024). The Trade-Off between Combustion and Partial Oxidation during Chemical Looping Conversion of Methane. Energies, 17(11), 2764. https://doi.org/10.3390/en17112764








