An Overview of Pyrolysis as Waste Treatment to Produce Eco-Energy
Abstract
:1. Introduction
- The Paris Agreement: Adopted in 2015 during the UN Conference of the Parties on Climate Change (COP21), it aims to limit global temperature rise to less than 2 °C above pre-industrial levels. Moreover, it works towards a more ambitious target of 1.5 °C [2].
- Glasgow Accord: This agreement was reached at the UN COP26 on climate change, in 2021, and aims to increase efforts to achieve carbon neutrality by 2050, reducing greenhouse gas emissions in all sectors [3].
- Environment and Economy Partnership Agreement: Reached at COP26, this is a partnership between governments, businesses, and non-governmental organizations. It promotes collaboration and commitment to address climate change and accelerate the transition to a low-carbon economy [4].
- Cellulose: A complex carbohydrate that is the main structural component of plants’ cell walls.
- Hemicellulose: A complex carbohydrate found in plants’ cell walls, often found alongside cellulose.
- Lignin: An amorphous phenol-based organic polymer, which provides strength and rigidity to wood [14].
- Lipids: Compounds present in plants and animals, insoluble in water but soluble in organic solvents. They include gums, oils, and waxes, and their structures may consist of long, straight hydrocarbon chains or isoprene units [1].
- Sugars: A class of simple carbohydrates that include glucose, fructose, and sucrose, which can be found in many plants [15].
2. Raw Materials
- Agricultural and forestry industry residues: Pyrolysis can be used to convert them into biofuels [21,22]. These feedstocks include pine pruning waste, rice straw, corn stover, sunflower waste, and olive waste, among others. Chen et al. [23] carried out pyrolysis at different temperatures with pine needles. The pine needles were pre-treated by cleaning, air-drying, and baking for subsequent grinding. In this case, the authors sought to avoid burning and charring. Garcia-Perez et al. [24] used two types of pine chips, of different origins and sizes, in different pyrolysis reactors. This allowed for a comparative analysis, finding similar results, with slightly better liquid fraction yields in the auger-type reactor compared to the batch one. Another work, by Yildiz et al. [25], analyzed the product composition of catalytic and non-catalytic fast pyrolysis of pine wood, itself a low-ash feedstock. The ash that accumulates can affect the catalyst efficiency by influencing the composition of the resulting pyrolysis vapors. The authors concluded that ash accumulation has an impact comparable to that of other catalyst problems that can affect the pyrolysis process, e.g., catalyst deactivation. Nam et al. [17] experimented with the possibilities of rice residue pyrolysis with different reactors. The rice residue was subjected to pre-treatment by air drying, and then it was chopped into smaller particles. The authors emphasized the importance of the moisture content, and they observed better yields in slow processes (auger and batch) for biochar, in addition to higher bio-oil quantity in fluidized bed reactors. Huang et al. [26] discussed the recovery of rice straw into resources and energy, using microwave-induced pyrolysis. They sought constant moisture by tanning the rice residue for 10 days, before it was crushed and sieved. The efficiency of this process depended on the microwave power and the size of the rice straw particles. They concluded that, for a satisfactory result using very small particles, a lower microwave power would be necessary. Zabaniotou et al. [27] compared the results of pyrolysis of different agricultural-based materials, i.e., maize, sunflower, and olive residues. Using fixed-bed reactors with and without catalysis, cellulose- and hemicellulose-based wastes produced higher amounts of hydrogen-rich gas than those based on lignin. Ren et al. [28] investigated the integration of microwave torrefaction and pyrolysis of corn stover. Torrefaction oils are noted for their high-value-added chemicals (furans and phenols), making them potentially interesting as a fuel source. The authors highlighted the use of torrefaction as a pre-treatment, combined with pyrolysis, to improve bio-oil quality. Colantoni et al. [29] based their study on the pyrolysis of grape and sunflower residues in the search for sustainable alternative fuels. The results demonstrated that torrefaction and pyrolysis of pelletized agricultural residues was an effective method to produce high-calorific-value biochar. Lajili et al. [30] studied olive residue pyrolysis, implementing biomass gasification, although the results were inconclusive. Kabakci et al. [31], in addition to studying the characteristics of olive residue pyrolysis, also investigated pyrolysis kinematics. The results were compared with those from refuse-derived fuel (RDF) pyrolysis, finding that olive residue decomposition started at lower temperatures but showed a higher maximum temperature; the temperature range of olive residue devolatilization was larger than that for RDF.
- Old and discarded furniture can also be processed by pyrolysis to produce biofuels and other chemicals. Uzun and Kanmaz [32] found that pine sawdust was a promising feedstock for bio-oil production, with maximum production rates of 42% (w/w). The importance of particle size is highlighted in both the above study and the one carried out by Heo et al. [33]. In the latter study, with respect to furniture sawdust pyrolysis, it was found that a higher gas flow, together with a higher feed rate, was favorable for bio-oil production, as vapor residence times were reduced.
- Plastic waste is an important source of feedstock for pyrolysis, as it contains carbons and can be converted into fuels, e.g., diesel fuel and natural gas [10]. In fact, liquids with a high calorific value can be obtained from the pyrolysis of plastics. In other words, they can be useful as fuels. Some plastics commonly used for pyrolysis include polyethylene (PE), low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), high-density polyethylene (HDPE), polystyrene (PS), and polypropylene (PP) [34]. Gaurch and Pramanik [35] studied the aromatization of PE plastic waste with fly ash (FA) as a catalyst. From this work, potential interest in and application of the catalytic pyrolysis process as an option to produce aromatics (benzene, toluene, ethylbenzene, and xylene (BTEX)) can be extracted. LDPE has been used as a raw material in numerous works, from which various conclusions can be drawn. Aguado et al. [36] highlighted the increase in the conversion rate by integrating catalysts in LDPE pyrolysis in a continuous screw reactor. Marcilla et al. [37] also analyzed the impact of catalysts. In their study, the authors investigated the polymer structure of products obtained from thermal and catalytic pyrolysis of LDPE and HDPE, in the presence of HZSM5 and HUSY zeolites. They focused on analyzing the composition of the gaseous and liquid fractions and found that the liquid fraction contained higher amounts of 1-olefins and n-paraffins. Investigations by Alonso-Morales et al. [38], on LDPE pyrolysis in batch feed reactors with slow and fast heating, did not provide optimal results in terms of solid char production, despite the use of activation additives and pyrolysis materials. However, the use of a semi-continuous feed reactor with fast heating achieved a high yield (15–52%, w/w) of solid coals due to the longer residence times of the pyrolysis products. In addition, pyrolysis in a metal-free quartz reactor produced very high solid carbon yields (15–43%, w/w). Fan et al. [39] focused their investigation on LLDPE conversion using both continuous-stirred microwave pyrolysis (CSMP) and batch microwave pyrolysis (BMP) systems. Reactions took place in the presence and absence of an ex situ catalytic bed with HZSM-5. The authors observed significant differences in product yields for the non-catalytic processes, where CSMP produced a higher condensate yield and a lower gas yield compared to BMP. Pyrolysis of HDPE was studied by Sogancioglu et al. [40], together with pyrolysis of LDPE, characterizing the resulting fractions. The char obtained was analyzed for its use as an additive in epoxy composites. Epoxy composites with HDPE carbon additives at 300 °C showed improved elongation at break and tensile strength performance. Kim et al. [41] performed kinetics tests on the pyrolysis of a mixture of waste automobile lubricating oil (WALO) and PS using thermogravimetric analysis (TGA). From this study, the analysis of the carbon number distribution of the oil produced at different heating rates is noteworthy. Decreasing the heating rates resulted in a slight shift in the carbon number of the produced oil towards light hydrocarbons. Park et al. [42] studied PP pyrolysis with a novel activator-assisted process. Increasing the activator temperature and the bubbling zone significantly increased the gas and oil yields, respectively. The use of nitrogen and a short residence time were found to increase the olefin yield. Degradation of activated PP molecules took place through different mechanisms. In this context, Kasar and Ahmaruzzaman [43] found that co-pyrolysis of crude oil with PP produced 80% pyrolytic oil. Furthermore, homogeneous catalysis has been proposed as an alternative for plastic waste treatment and high-value chemical production [44]. Aside from everything mentioned so far, plastics are ideal for pyrolysis because of their abundance, low density, and calorific value, among other properties. In summary, plastic waste, once considered to be an environmental problem, has become a valuable pyrolysis raw material.
- Other industrial waste, such as paper and wood, can also be processed by pyrolysis to produce biofuels and other chemicals. Potential landfill waste can include newsprint and cardboard, which contain nitrogen, sulfur, and oxygen, as highlighted by Fekhar et al. [45], who also highlighted the difference in moisture content between plastics and paper waste. The latter contains considerably more moisture than plastics, which hardly include any moisture at all. It is important to take this characteristic into account before a pre-treatment process is selected. After pyrolysis, the authors noted that the liquid product from newsprint and cardboard resulted in water and various oxygenated compounds. Ahmed and Gupta [46] investigated the gasification and pyrolysis of paper, underlining that gasification offers better results in terms of higher material destruction, hydrogen production, and chemical energy. Yao et al. [47] focused on the treatment of paper sludge, emphasizing the problems that it poses in terms of industrial pollution in China. Moreover, sludges do not come with paper alone; they also contain heterocyclic compounds, polycyclic aromatic hydrocarbons, amino acids, and organic fluorinated compounds. The authors proposed pyrolysis treatment of this waste to reduce air pollution and carbon emissions, compared to direct burning of waste. Determining the pyrolysis temperature is important for optimal results. Kim et al. [48] sought the optimal temperature to achieve the maximum bio-oil yield from the pyrolysis of construction wood waste. Carlson et al. [49], in their study on the production of aromatics and olefins from wood in a fluidized bed reactor, showed that propylene is more reactive than ethylene and produces higher quantities of aromatics. They also noted that the lower the temperature, the lower the methane production. The study of experimental kinetics is interesting in pyrolysis studies. Slopiecka et al. [50] conducted a kinetics study of the devolatilization of aspen wood, finding that its thermal decomposition proceeds in three stages.
- Waste tires are an important source of raw materials for pyrolysis, as they contain rubber and steel, which can be recycled into fuels and other products. In the research of Berrueco et al. [51] on tire pyrolysis, it was pointed out that gas production is favored by long residence times at high temperatures. Tires are notable for their contents of hydrocarbons and gaseous fractions, i.e., H2, CO, and CO2. However, the calorific value of the gas obtained from the pyrolysis was lower than expected, although it was still valid for use in gas engines. According to Williams [52], the oil from tire pyrolysis is chemically complex and contains aliphatic, aromatic, heteroatomic, and polar compounds. This oil’s properties allow its use as a fuel, as its properties are similar to those of diesel fuel or light fuel oil.
3. Pyrolysis Reactors
- Gas: The gaseous fraction, like all fractions, depends on the composition of the pyrolyzed material and the type of reactor. Biomass pyrolysis results in gases such as CO2, CO, or hydrocarbons, but gases like acetic acid, methanol, furfural, acetaldehyde, ethanol, propane, or hydroxymethylfural (HMF) can also be released. The increase in CO2 content indicates further degradation of cellulosic and hemicellulosic components. Also, the presence of CH4 and CO suggests secondary cracking of the volatile compounds released during the process. Nam et al. [17] demonstrated that the composition of the resulting gases depends on the reactor. Hydrogen formation is characteristic of biomass containing paper and cardboard, whereas in the case of pyrolysis of plastics, mainly hydrocarbons can be identified [45]. In the study by Marcilla et al. [37], pyrolysis was carried out with LDPE and HDPE waste, resulting in the production of 1-olefins, n-paraffins, olefins, iso-paraffins, and aromatics.
- Liquid: The liquid fraction resulting from pyrolysis depends on the type of pyrolyzed material and other parameters, such as temperature or the type of pyrolysis reactor. As highlighted in their research, Uzun and Kanmaz [32] found that the liquid product from biomass pyrolysis was a mixture of multiple organic compounds. It consisted of two phases: an aqueous phase, containing low-molecular-weight oxygenated organic compounds (acetic acid, methanol, and acetone), and a non-aqueous phase, containing aromatic hydrocarbons and organic compounds (aliphatic alcohols, carbonyls, acids, phenols, cresols, benzenediols, guaiacol, and its alkylated derivatives). Aromatic hydrocarbons include single-ring aromatic compounds (such as benzene, toluene, indene, and alkylated derivatives) and polycyclic aromatic hydrocarbons (PAH, such as naphthalene, furans, phenanthrene, and their alkylated derivatives). Water is also released from biomass pyrolysis [33]. On the other hand, the liquid fraction resulting from the pyrolysis of polymers results in aromatic hydrocarbons as the main compound [42]. In the liquid fraction resulting from the pyrolysis of polymers, iso-paraffins, aromatics, n-paraffins, and 1-olefins were also observed. The n-paraffins are more frequent at low temperatures, while 1-olefins are more frequent at high temperatures [37].
- Solid: The solid fraction of pyrolysis mostly consists of charcoal and other residues, e.g., ash. If the pyrolyzed residue is biomass, it is called vegetable charcoal. As with the other fractions, the pyrolysis temperature influences this final fraction. It has been found that the higher the temperature in the pyrolysis of plastics, the higher the amount of charcoal [38]. Secondary repolymerization reactions are responsible for carbon formation. Slight hydrogen production is also observed. In all cases, pyrolysis solids are carbon-rich materials with a high calorific value that can be used as a substitute for solid fossil fuels [57]. The pyrolysis of cellulose-containing biomass results in the formation of dehydrated saccharides, furans, furanones, benzenes, and cyclopentanones. The highest yield in cellulose pyrolysis is obtained from the saccharides [58].
3.1. Batch Reactor
3.2. Continuous Reactors
3.3. Other Types of Reactors
| Reactor Type | Feedstock | Sample (g) | Particle Size (mm) | Heating Rate (°C/min) | Pyrolysis Temperature (°C) | Solid (%, w/w) | Liquid (%, w/w) | Gas (%, w/w) | References |
|---|---|---|---|---|---|---|---|---|---|
| Wire mesh | PVC | 0.02–0.20 | 0.106–0.150 | n.r. | 500 | n.r. | 6.13 | n.r. | [80] |
| 600 | 17.50 | ||||||||
| 700 | 22.50 | ||||||||
| 800 | 27.79 | ||||||||
| Cellulose | n.r. | n.r. | n.r. | 500 | 2.00 | 80.00 | 18.00 * | [81] | |
| Vacuum | Palm oil decanter cake (PDC) | n.r. | 0.850–2.000 | 15.0 | 400 | 48.07 | 34.52 | 17.41 | [82] |
| 500 | 38.57 | 41.31 | 20.06 | ||||||
| Drop-tube reactor | Coal (Naomachu sub-bituminous) | n.r. | 0.200–1.000 | n.r. | 500 | 94.00 | 3.00 * | 3.00 | [88] |
| 550 | 79.00 | 16.00 * | 5.00 | ||||||
| 600 | 68.00 | 25.50 * | 6.50 | ||||||
| 700 | 59.00 | 28.00 * | 13.00 | ||||||
| Coal | n.r. | 0.200–0.400 | n.r. | 600 | 80.10 | 6.40 | 4.00 | [87] | |
| 600 | 71.70 | 7.00 | 9.70 | ||||||
| 600 | 68.90 | 6.20 | 13.10 | ||||||
| Horizontal tubular reactor | Municipal plastic waste | 750.00 | 5.000 | 8.0 | 550–560 | 5.0–6.0 | 85.0–87.0 | 8.0–9.0 | [86] |
4. Types of Pyrolysis Depending on the Operating Conditions
4.1. Slow Pyrolysis
4.2. Fast Pyrolysis
4.3. Soft Pyrolysis or Dry Torrefaction
5. Catalytic Pyrolysis
6. Pyrolysis Composition
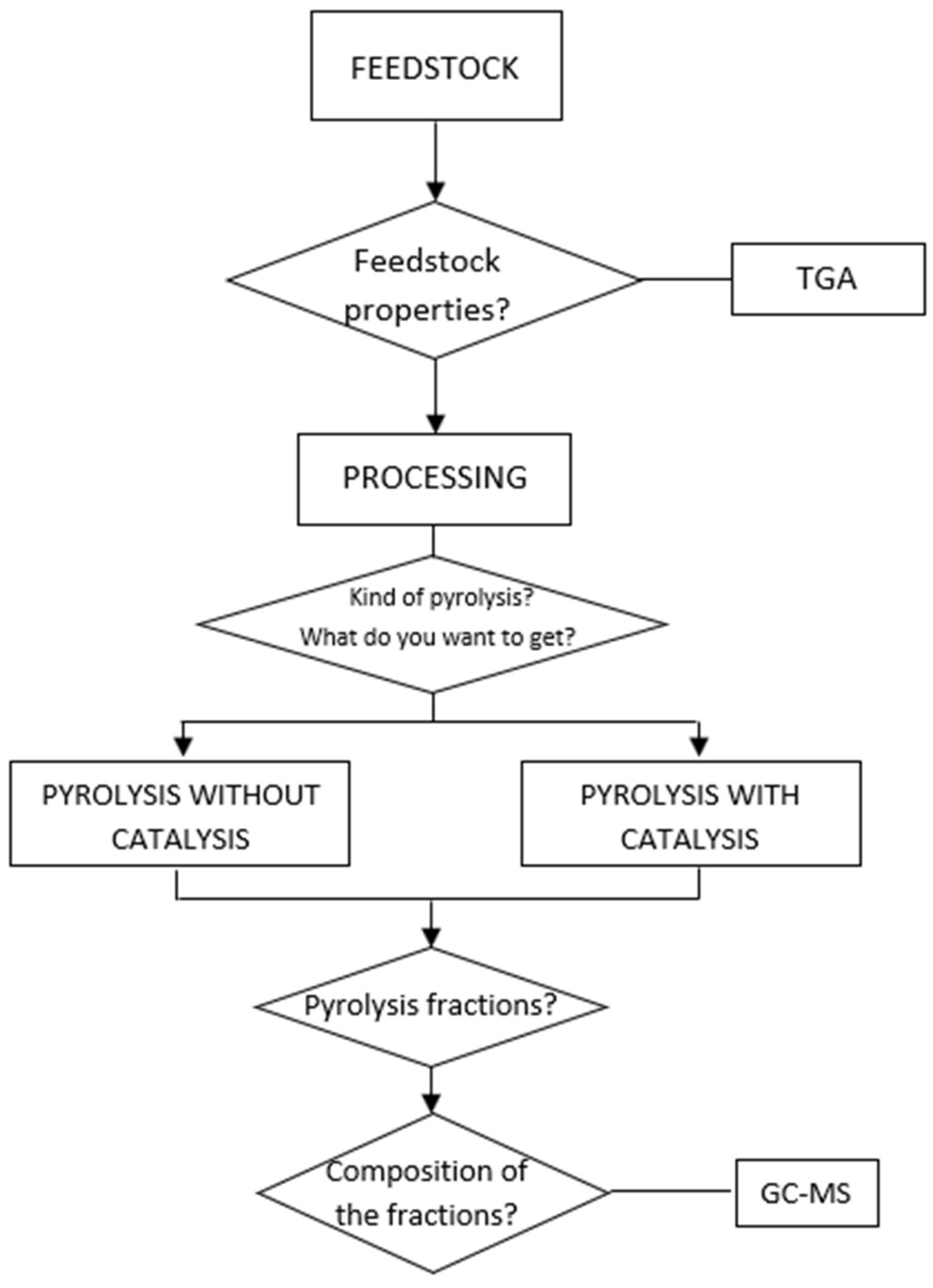
7. Workflow for Pyrolysis Experimentation
8. Conclusions
Funding
Conflicts of Interest
References
- Metzger, P.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, J.; Schroeder, H.; Linnér, B.-O. The Evolution of the UNFCCC. Annu. Rev. Environ. Resour. 2018, 43, 343–368. [Google Scholar] [CrossRef]
- Lennan, M.; Morgera, E. Current legal developments climate change the Glasgow climate conference (COP26). Int. J. Mar. Coast. Law 2022, 37, 137–151. [Google Scholar] [CrossRef]
- Chaytor, B. Environmental Issues in Economic Partnership Agreements: Implications for Developing Countries; International Centre for Trade and Sustainable Development (ICTSD): Geneva, Switzerland, 2009; p. 64. [Google Scholar]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 16. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.A. European environment policy for the circular economy: Implications for business and industry stakeholders. Sustain. Dev. 2020, 28, 1804–1812. [Google Scholar] [CrossRef]
- Watrobski, J. Temporal PROMETHEE II-New multi-criteria approach to sustainable management of alternative fuels consumption. J. Clean. Prod. 2023, 413, 26. [Google Scholar] [CrossRef]
- Bieniek, A.; Jerzak, W.; Gajek, M.; Magdziarz, A. Numerical investigations of biomass pyrolysis with partial oxidation in a drop tube reactor. J. Clean. Prod. 2023, 401, 136774. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 11. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Zhao, C.P.; Liu, M.R.; Du, H.Z.; Gong, Y. The evolutionary trend and impact of global plastic waste trade network. Sustainability 2021, 13, 3662. [Google Scholar] [CrossRef]
- Wen, Z.G.; Xie, Y.L.; Chen, M.H.; Dinga, C.D. China’s plastic import ban increases prospects of environmental impact mitigation of plastic waste trade flow worldwide. Nat. Commun. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Annual, R. Plastics in the marine environment. In Annual Review of Marine Science; Annual Reviews: Palo Alto, CA, USA, 2017; Volume 9, pp. 205–229. [Google Scholar]
- Han, X.W.; Guo, Y.; Liu, X.H.; Xia, Q.N.; Wang, Y.Q. Catalytic conversion of lignocellulosic biomass into hydrocarbons: A mini review. Catal. Today 2019, 319, 2–13. [Google Scholar] [CrossRef]
- Davis, R.; Tao, L.; Tan, E.C.D.; Biddy, M.J.; Beckham, G.T.; Scarlata, C.; Jacobson, J.; Cafferty, K.; Ross, J.; Lukas, J.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons; Idaho National Lab. (INL): Idaho Falls, ID, USA, 2013. [Google Scholar]
- Lechleitner, A.E.; Schubert, T.; Hofer, W.; Lehner, M. Lumped kinetic modeling of polypropylene and polyethylene co-pyrolysis in tubular reactors. Processes 2021, 9, 34. [Google Scholar] [CrossRef]
- Nam, H.; Capareda, S.C.; Ashwath, N.; Kongkasawan, J. Experimental investigation of pyrolysis of rice straw using bench-scale auger, batch and fluidized bed reactors. Energy 2015, 93, 2384–2394. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis. A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Mennell James, A.; Despen Daniel, J. Systems and Apparatus for the Production of High-Carbon Bioreagents; WIPO: Geneva, Switzerland, 2022. [Google Scholar]
- Fu, P.; Hu, S.; Xiang, J.; Li, P.S.; Huang, D.; Jiang, L.C.; Zhang, A.Y.; Zhang, J. FTIR study of pyrolysis products evolving from typical agricultural residues. J. Anal. Appl. Pyrolysis 2010, 88, 117–123. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjostrom, K.; Bjornbom, E. Rapid pyrolysis of agricultural residues at high temperature. Biomass Bioenergy 2002, 23, 357–366. [Google Scholar] [CrossRef]
- Chen, B.L.; Zhou, D.D.; Zhu, L.Z. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Adams, T.T.; Goodrum, J.W.; Geller, D.P.; Das, K.C. Production and fuel properties of pine chip Bio-oil/Biodiesel blends. Energy Fuels 2007, 21, 2363–2372. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; Venderbosch, R.; van Duren, R.; Kersten, S.R.A.; Prins, W. Effect of biomass ash in catalytic fast pyrolysis of pine wood. Appl. Catal. B Environ. 2015, 168, 203–211. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Total recovery of resources and energy from rice straw using microwave-induced pyrolysis. Bioresour. Technol. 2008, 99, 8252–8258. [Google Scholar] [CrossRef] [PubMed]
- Zabaniotou, A.; Ioannidou, O.; Antonakou, E.; Lappas, A. Experimental study of pyrolysis for potential energy, hydrogen and carbon material production from lignocellulosic biomass. Int. J. Hydrogen Energy 2008, 33, 2433–2444. [Google Scholar] [CrossRef]
- Ren, S.J.; Lei, H.W.; Wang, L.; Yadavalli, G.; Liu, Y.P.; Julson, J. The integrated process of microwave torrefaction and pyrolysis of corn stover for biofuel production. J. Anal. Appl. Pyrolysis 2014, 108, 248–253. [Google Scholar] [CrossRef]
- Colantoni, A.; Evic, N.; Lord, R.; Retschitzegger, S.; Proto, A.R.; Gallucci, F.; Monarca, D. Characterization of biochars produced from pyrolysis of pelletized agricultural residues. Renew. Sustain. Energy Rev. 2016, 64, 187–194. [Google Scholar] [CrossRef]
- Lajili, M.; Guizani, C.; Sanz, F.J.E.; Jeguirim, M. Fast pyrolysis and steam gasification of pellets prepared from olive oil mill residues. Energy 2018, 150, 61–68. [Google Scholar] [CrossRef]
- Kabakci, S.B.; Aydemir, H. Pyrolysis of olive pomace and copyrolysis of olive pomace with refuse derived fuel. Environ. Prog. Sustain. Energy 2014, 33, 649–656. [Google Scholar] [CrossRef]
- Uzun, B.B.; Kanmaz, G. Effect of operating parameters on bio-fuel production from waste furniture sawdust. Waste Manag. Res. 2013, 31, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.S.; Park, H.J.; Park, Y.K.; Ryu, C.; Suh, D.J.; Suh, Y.W.; Yim, J.H.; Kim, S.S. Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed. Bioresour. Technol. 2010, 101, S91–S96. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. Production of benzene/toluene/ethyl benzene/xylene (BTEX) via multiphase catalytic pyrolysis of hazardous waste polyethylene using low cost fly ash synthesized natural catalyst. Waste Manag. 2018, 77, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Garagorri, E. Catalytic conversion of low-density polyethylene using a continuous screw kiln reactor. Catal. Today 2002, 75, 257–262. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltran, M.I.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Alonso-Morales, N.; Gilarranz, M.A.; Heras, F.; Eser, S.; Rodriguez, J.J. Effects of reactor configuration on the yield of solid carbon from pyrolysis of low-density polyethylene. Energy Fuels 2009, 23, 6095–6101. [Google Scholar] [CrossRef]
- Fan, L.L.; Liu, L.; Xiao, Z.G.; Su, Z.Y.; Huang, P.; Peng, H.Y.; Lv, S.; Jiang, H.W.; Ruan, R.; Chen, P.; et al. Comparative study of continuous-stirred and batch microwave pyrolysis of linear low-density polyethylene in the presence/absence of HZSM-5. Energy 2021, 228, 120612. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, J.; Jeon, J.K.; Park, Y.K.; Park, C.J. Non-isothermal pyrolysis of the mixtures of waste automobile lubricating oil and polystyrene in a stirred batch reactor. Renew. Energy 2013, 54, 241–247. [Google Scholar] [CrossRef]
- Park, K.B.; Jeong, Y.S.; Kim, J.S. Activator-assisted pyrolysis of polypropylene. Appl. Energy 2019, 253, 10. [Google Scholar] [CrossRef]
- Kasar, P.; Ahmaruzzaman, M. Characterization of liquid products obtained from catalytic binary co-cracking of residual fuel oil with various waste plastics. Sci. Rep. 2022, 12, 10987. [Google Scholar] [CrossRef]
- Chen, X.R.; Cheng, L.L.; Gu, J.; Yuan, H.R.; Chen, Y. Chemical recycling of plastic wastes via homogeneous catalysis: A review. Chem. Eng. J. 2024, 479, 147853. [Google Scholar] [CrossRef]
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Thermo-catalytic co-pyrolysis of waste plastic and paper in batch and tubular reactors for in-situ product improvement. J. Environ. Manag. 2020, 269, 110741. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Gupta, A.K. Syngas yield during pyrolysis and steam gasification of paper. Appl. Energy 2009, 86, 1813–1821. [Google Scholar] [CrossRef]
- Yao, Z.L.; Ma, X.Q.; Wu, Z.D.; Yao, T.T. TGA-FTIR analysis of co-pyrolysis characteristics of hydrochar and paper sludge. J. Anal. Appl. Pyrolysis 2017, 123, 40–48. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.W.; Lee, I.G.; Jeon, J.K.; Ryu, C.; Park, S.H.; Jung, S.C.; Park, Y.K. Influence of reaction conditions on bio-oil production from pyrolysis of construction waste wood. Renew. Energy 2014, 65, 41–48. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011, 4, 145–161. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.J.; Ceamanos, J.; Garcia-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J. Anal. Appl. Pyrolysis 2005, 74, 245–253. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Inayat, A.; Klemencova, K.; Grycova, B.; Sokolova, B.; Lestinsky, P. Thermo-catalytic pyrolysis of polystyrene in batch and semi-batch reactors: A comparative study. Waste Manag. Res. 2021, 39, 260–269. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Inguanzo, M.; Dominguez, A.; Menendez, J.A.; Blanco, C.G.; Pis, J.J. On the pyrolysis of sewage sludge: The influence of pyrolysis conditions on solid, liquid and gas fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Chen, L.M.; Liao, Y.F.; Guo, Z.G.; Cao, Y.W.; Ma, X.Q. Products distribution and generation pathway of cellulose pyrolysis. J. Clean. Prod. 2019, 232, 1309–1320. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, G.C.; Bae, S.Y.; Yi, S.C.; Moon, S.K.; Kumazawa, H. Pyrolysis of polystyrene in a batch-type stirred vessel. Korean J. Chem. Eng. 1999, 16, 161–165. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.; Yang, W. Catalytic pyrolysis of demineralized lignocellulosic biomass. Fuel 2019, 252, 200–209. [Google Scholar] [CrossRef]
- Shadangi, K.P.; Singh, R.K. Thermolysis of polanga seed cake to bio-oil using semi batch reactor. Fuel 2012, 97, 450–456. [Google Scholar] [CrossRef]
- Celikgogus, C.; Karaduman, A. Thermal-catalytic pyrolysis of polystyrene waste foams in a semi-batch reactor. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 2507–2513. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. A novel approach of solid waste management via aromatization using multiphase catalytic pyrolysis of waste polyethylene. Waste Manag. 2018, 71, 86–96. [Google Scholar] [CrossRef]
- Xue, Q.; Heindel, T.J.; Fox, R. A CFD model for biomass fast pyrolysis in fluidized-bed reactors. Chem. Eng. Sci. 2011, 66, 2440–2452. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martinez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Marmur, B.L.; Heindel, T.J. Effect of particle size, density, and concentration on granular mixing in a double screw pyrolyzer. Powder Technol. 2016, 302, 222–235. [Google Scholar] [CrossRef]
- Park, H.C.; Lee, B.K.; Yoo, H.S.; Choi, H.S. Influence of operating conditions for fast pyrolysis and pyrolysis oil production in a conical spouted-bed reactor. Chem. Eng. Technol. 2019, 42, 2493–2504. [Google Scholar] [CrossRef]
- Fernandez-Akarregi, A.R.; Makibar, J.; Lopez, G.; Amutio, M.; Olazar, M. Design and operation of a conical spouted bed reactor pilot plant (25 kg/h) for biomass fast pyrolysis. Fuel Process. Technol. 2013, 112, 48–56. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, H.C.; Choi, H.S. Numerical study on fast pyrolysis of lignocellulosic biomass with varying column size of bubbling fluidized bed. ACS Sustain. Chem. Eng. 2017, 5, 2196–2204. [Google Scholar] [CrossRef]
- Hastaoglu, M.A.; Hassam, M.S. Application of a general gas-solid reaction model to flash pyrolysis of wood in a circulating fluidized bed. Fuel 1995, 74, 697–703. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Gelinger, V.; Cacho, F. Pyrolysis of automobile shredder residue in a laboratory scale screw type reactor. J. Environ. Chem. Eng. 2016, 4, 965–972. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Dudas, J. Pyrolysis of aseptic packages (tetrapak) in a laboratory screw type reactor and secondary thermal/catalytic tar decomposition. Waste Manag. 2013, 33, 1136–1141. [Google Scholar] [CrossRef]
- Martinez, J.D.; Murillo, R.; Garcia, T.; Veses, A. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef]
- Efika, C.E.; Wu, C.F.; Williams, P.T. Syngas production from pyrolysis-catalytic steam reforming of waste biomass in a continuous screw kiln reactor. J. Anal. Appl. Pyrolysis 2012, 95, 87–94. [Google Scholar] [CrossRef]
- Yang, Y.; Brammer, J.G.; Mahmood, A.S.N.; Hornung, A. Intermediate pyrolysis of biomass energy pellets for producing sustainable liquid, gaseous and solid fuels. Bioresour. Technol. 2014, 169, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Sirijanusorn, S.; Sriprateep, K.; Pattiya, A. Pyrolysis of cassava rhizome in a counter-rotating twin screw reactor unit. Bioresour. Technol. 2013, 139, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kang, B.S.; Kim, J.S. Production of bio-oil from rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed and a char separation system. J. Anal. Appl. Pyrolysis 2008, 82, 240–247. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhao, B.F.; Liu, S.X.; Zhu, D.; Huang, F.Y.; Yang, H.J.; Guan, H.B.; Song, A.G.; Xu, D.; Sun, L.Z.; et al. Catalytic pyrolysis of biomass with Ni/Fe-CaO-based catalysts for hydrogen-rich gas: DFT and experimental study. Energy Convers. Manag. 2022, 254, 115246. [Google Scholar] [CrossRef]
- Gui, B.; Qiao, Y.; Wan, D.; Liu, S.; Han, Z.N.; Yao, H.; Xu, M.H. Nascent tar formation during polyvinylchloride (PVC) pyrolysis. Proc. Combust. Inst. 2013, 34, 2321–2329. [Google Scholar] [CrossRef]
- Gong, X.; Yu, Y.; Gao, X.P.; Qiao, Y.; Xu, M.H.; Wu, H.W. Formation of anhydro-sugars in the primary volatiles and solid residues from cellulose fast pyrolysis in a wire-mesh reactor. Energy Fuels 2014, 28, 5204–5211. [Google Scholar] [CrossRef]
- Dewayanto, N.; Isha, R.; Nordin, M.R. Use of palm oil decanter cake as a new substrate for the production of bio-oil by vacuum pyrolysis. Energy Convers. Manag. 2014, 86, 226–232. [Google Scholar] [CrossRef]
- Ruan, J.J.; Huang, J.X.; Qin, B.J.; Dong, L.P. Heat transfer in vacuum pyrolysis of decomposing hazardous plastic wastes. ACS Sustain. Chem. Eng. 2018, 6, 5424–5430. [Google Scholar] [CrossRef]
- Lehto, J. Determination of kinetic parameters for Finnish milled peat using drop tube reactor and optical measurement techniques. Fuel 2007, 86, 1656–1663. [Google Scholar] [CrossRef]
- Matsuoka, K.; Ma, Z.X.; Akiho, H.; Zhang, Z.G.; Tomita, A.; Fletcher, T.H.; Wojtowicz, M.A.; Niksa, S. High-pressure coal pyrolysis in a drop tube furnace. Energy Fuels 2003, 17, 984–990. [Google Scholar] [CrossRef]
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Value added hydrocarbons obtained by pyrolysis of contaminated waste plastics in horizontal tubular reactor: In situ upgrading of the products by chlorine capture. J. Clean. Prod. 2019, 241, 118166. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, D.M.; Li, C.M.; Yang, H.; Wang, D.L.; Lai, D.G.; Yu, J.; Gao, S.Q. A tandem pyrolysis-upgrading strategy in an integrated reactor to improve the quality of coal tar. Energy Convers. Manag. 2020, 220, 113065. [Google Scholar] [CrossRef]
- Wang, D.M.; Chen, Z.H.; Li, C.M.; Wang, D.L.; Li, Y.J.; Yang, H.; Liu, Z.E.; Yu, J.; Gao, S.Q. High-quality tar production from coal in an integrated reactor: Rapid pyrolysis in a drop tube and downstream volatiles upgrading over char in a moving bed. Fuel 2021, 285, 119156. [Google Scholar] [CrossRef]
- Cai, N.; Zhang, H.L.; Nie, J.P.; Deng, Y.M.; Baeyens, J. Biochar from biomass slow pyrolysis. In Proceedings of the 2nd International Conference on Environment Sciences and Renewable Energy (ESRE), Vienna, Austria, 18–21 May 2020. [Google Scholar]
- Yuan, T.; He, W.J.; Yin, G.J.; Xu, S. Comparison of bio-chars formation derived from fast and slow pyrolysis of walnut shell. Fuel 2020, 261, 116450. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stahl, R.; Yanik, J. The slow and fast pyrolysis of cherry seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef]
- Snyder, B.F. Costs of biomass pyrolysis as a negative emission technology: A case study. Int. J. Energy Res. 2019, 43, 1232–1244. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Wu, N.N.; Niu, Q.; Pieters, J.; Ronsse, F. Influence of torrefaction as pretreatment on the fast pyrolysis of sugarcane trash. Energy Convers. Manag. 2023, 291, 117291. [Google Scholar] [CrossRef]
- Bach, Q.V.; Trinh, T.N.; Tran, K.Q.; Ngoc, B.D.T. Pyrolysis characteristics and kinetics of biomass torrefied in various atmospheres. Energy Convers. Manag. 2017, 141, 72–78. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Processing biomass-derived oxygenates in the oil refinery: Catalytic cracking (FCC) reaction pathways and role of catalyst. J. Catal. 2007, 247, 307–327. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manag. 2019, 182, 143–153. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.L.; Liu, Z.; Ma, Q.L.; Zhao, C.; Ma, C.Y. Kinetic study of the pyrolysis of waste printed circuit boards subject to conventional and microwave heating. Energies 2012, 5, 3295–3306. [Google Scholar] [CrossRef]
| Reactor Type | Feedstock | Sample (g) | Particle Size (mm) | Heating Rate (°C/min) | Pyrolysis Temperature (°C) | Solid (%, w/w) | Liquid (%, w/w) | Gas (%, w/w) | References |
|---|---|---|---|---|---|---|---|---|---|
| Batch reactor | Construction waste wood | 5.0 | n.r. | 20.0, 30.0, 40.0 | 400 | 35.00 | 47.00 | 18.00 | [48] |
| 450 | 29.50 | 50.50 | 20.00 | ||||||
| 500 | 25.30 | 54.20 | 20.50 | ||||||
| 550 | 24.70 | 51.70 | 23.60 | ||||||
| LDPE | 0.6 | 0.50 | 5.0 | 550 | n.r. | 93.10 | 14.60 | [37] | |
| HDPE | n.r. | 84.70 | 16.30 | ||||||
| Municipal solid waste | 50.0 | 5.00 | 15.0 | 550 | 28.00 | 49.00 | 23.00 | [45] | |
| Rice straw | 250.0 | 2.00 | 4.0 | 500 | 47.70 | 31.30 | 11.50 | [17] | |
| Pine chips | 1.4 × 103 | n.r. | 10.0 | 500 | 31.20 | 50.40 | 18.40 * | [23] | |
| Softwood sawdust | n.r. | 0.35–0.50 | 4.0 | 400 | 32.90 | 52.70 | 14.40 | [61] | |
| 500 | 21.30 | 61.60 | 17.10 | ||||||
| 600 | 16.30 | 61.50 | 22.20 | ||||||
| PS | 4.0 | 0.63–1.00 | 10.0 | 400 | 21.00 | 64.00 | 15.00 | [53] | |
| 500 | 0.00 | 76.00 | 24.00 | ||||||
| Semi-batch | PS | 4.0 | 0.63–1.00 | 10.0 | 400 | 0.00 | 90.00 | 10.00 | |
| 500 | 0.00 | 87.00 | 13.00 | ||||||
| Polanga (Calophyllum inophyllum) | 15.0 | n.r. | 20.0 | 400 | 39.00 | 40.00 | 21.00 | [62] | |
| 500 | 35.00 | 45.00 | 20.00 | ||||||
| 550 | 32.50 | 45.50 | 22.00 | ||||||
| 600 | 28.30 | 43.40 | 28.30 | ||||||
| PS | 30.0 | n.r. | 5.0 | 400 | 15.00 | 79.10 | 5.90 | [63] | |
| 500 | 5.00 | 89.90 | 5.10 | ||||||
| Plastic mixture | 100.0 | n.r. | 20.0 | 460 | 1.10 | 72.00 | 26.90 | [54] | |
| 500 | 0.80 | 65.20 | 34.00 | ||||||
| 600 | 0.90 | 42.90 | 56.20 | ||||||
| Municipal plastic waste (42.5% PE) | 50.0 | 8.00 | 7.0 | 700 | 15.16 | 68.02 | 16.82 | [64] | |
| Municipal solid waste | 50.0 | 4.00 × 4.00 | n.r. | 700 | 15.16 | 68.02 | 16.82 | [35] | |
| Static-bed batch reactor | Waste tires | 300.0 | n.r. | 12.0 | 400 | 64.00 | 30.00 | 2.40 | [51] |
| 500 | 52.70 | 39.90 | 3.60 | ||||||
| 550 | 52.50 | 39.10 | 3.60 | ||||||
| 700 | 51.30 | 42.80 | 4.40 | ||||||
| Semi-continuous (Hastelloy) | LDPE | 2.0 | n.r. | 10.0 | 750 | 44.00 | n.r. | n.r. | [38] |
| 850 | 52.20 | n.r. | n.r. | ||||||
| Semi-continuous (quartz) | LDPE | 2.0 | n.r. | 10.0 | 750 | 15.30 | n.r. | n.r. | |
| 850 | 32.00 | n.r. | n.r. | ||||||
| Microwave batch reactor | LLDPE | 30.0 | n.r. | n.r. | 500–550 | 4.00 | 46.00 | 50.00 | [39] |
| Batch-type stirred vessel | PS | 100.0 | n.r. | 11.3–12.3 | 370 | n.r. | 96.40 | 3.60 | [59] |
| 380 | n.r. | 96.10 | 3.90 |
| Reactor Type | Feedstock | Sample (g) | Particle Size (mm) | Heating Rate (°C/min) | Pyrolysis Temperature (°C) | Solid (%, w/w) | Liquid (%, w/w) | Gas (%, w/w) | References |
|---|---|---|---|---|---|---|---|---|---|
| Fluidized bed | Construction waste wood | 120.0 | n.r. | 20.0, 30.0, 40.0 | 400 | 27.00 | 55.00 | 18.00 | [48] |
| 450 | 23.20 | 56.80 | 20.00 | ||||||
| 500 | 20.60 | 56.90 | 22.50 | ||||||
| 550 | 18.00 | 54.00 | 28.00 | ||||||
| Waste furniture sawdust | 1000.0 | 0.04 | n.r. | 400 | 35.80 | 51.50 | 12.70 | [32] | |
| 450 | 28.80 | 58.10 | 13.10 | ||||||
| 500 | 23.00 | 56.00 | 21.00 | ||||||
| 550 | 21.30 | 42.50 | 36.20 | ||||||
| Rice straw | 200.0 | 2.00 | 4.0 | 500 | 26.80 | 43.40 | 23.00 | [17] | |
| Auger | Rice straw | 200.0 | 2.00 | 4.0 | 500 | 44.90 | 25.70 | 13.20 | |
| Packages (Tetra Pak) | 100.0 | 5.00 × 5.00 | n.r. | 650 | 24.00 | 16.00 | 60.00 | [73] | |
| 850 | 19.00 | 5.00 | 76.00 | ||||||
| Automobile shredder residue (ASR) | 10.04 | 20.00–50.00 | 10.0 | 550 | 30.00 | 28.00 | 42.00 | [72] | |
| 600 | 34.00 | 18.00 | 48.00 | ||||||
| 800 | 28.00 | 7.00 | 65.00 | ||||||
| Pine pellets | n.r. | L: 12.80 ø: 6.46 | 10.0 | 500 | 30.00 | 57.80 | 12.20 * | [23] | |
| Waste tires | 5.6 × 105 | 2.00–4.00 | 5.0, 10.0, 20.0 | 550 | 40.50 | 42.60 | 16.90 | [74] | |
| Wood pellets | n.r. | L: 15.00 ø: 6.00 | n.r. | 500 | 21.50 | 38.00 | 40.50 | [75] | |
| Barley straw pellets | n.r. | L: 15.00–25.00 ø: 6.00 | 20.0 | 450 | 30.10 | 49.00 | 20.90 | [76] | |
| Twin co-axial | Wood pellets | n.r. | L: 15.00–25.00 ø: 6.00 | 20.0 | 450 | 28.50 | 54.30 | 17.70 | |
| Twin screw | Cassava rhizome | 300.0 | 0.30–0.40 | n.r. | 500 | 22.00 | 40.00 | 38.00 | [77] |
| 550 | 23.00 | 50.00 | 27.00 | ||||||
| 650 | 13.00 | 45.00 | 42.00 | ||||||
| 700 | 18.00 | 34.00 | 48.00 | ||||||
| Conical spouted bed | Pinewood sawdust | n.r. | n.r. | n.r. | 450 | 15.00 | 22.80 | 62.20 | [69] |
| 500 | 20.60 | 14.50 | 64.90 | ||||||
| 550 | 24.10 | 13.60 | 62.30 | ||||||
| Sawdust (Larix leptolepis) | n.r. | n.r. | n.r. | 400 | 29.60 | 44.20 | 26.20 | [68] | |
| 500 | 23.20 | 50.80 | 26.00 | ||||||
| 550 | 17.20 | 43.90 | 38.80 | ||||||
| Bubbling fluidized bed | Rice straw | n.r. | 5.00 | n.r. | 491 | 18.00 | 68.00 | 14.00 | [78] |
| n.r. | 10.00 | n.r. | 490 | 21.00 | 60.00 | 19.00 | |||
| Bamboo | n.r. | 0.60 | n.r. | 405 | 15.00 | 72.00 | 13.00 | ||
| n.r. | 0.85 | n.r. | 410 | 20.00 | 67.00 | 13.00 | |||
| Continuous-stirred microwave | Plastic waste, LLDPE | 30.0 | n.r. | n.r. | 500–550 | 5.00 | 84.00 | 11.00 | [39] |
| Fixed-bed reactor | Corncob | 0.3 | n.r. | 20.0 | 500 | 37.31 | 40.22 | 16.16 | [26] |
| Corn stalk | 32.67 | 42.22 | 14.47 | ||||||
| Sunflower residues | 48.21 | 34.79 | 9.49 | ||||||
| Olive pruning | 36.02 | 37.95 | 9.99 | ||||||
| Olive kernels | 28.03 | 41.45 | 11.65 | ||||||
| Sawdust | 4.0 | 0.42–0.60 | 10.0 | 650 | 25.26 | 51.58 | 23.16 | [79] |
| Catalysis Information | Feedstock | Sample (g) | Particle Size (mm) | Heating Rate (°C/min) | Pyrolysis Temperature (°C) | Solid (%, w/w) | Liquid (%, w/w) | Gas (%, w/w) | Coke (%, w/w) | Reactor Type | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HZSM-5 | LLDPE | 30.0 | n.r. | n.r. | 500–550 | 4.70 | 33.90 | 61.40 | n.r. | Microwave batch reactor | [39] |
| 3.80 | 29.20 | 67.00 | n.r. | Continuous-stirred microwave | |||||||
| Construction waste wood | 5.0 | n.r. | 20.0, 30.0, 40.0 | 500 | 25.00 | 50.00 | 25.00 | n.r. | Batch reactor | [48] | |
| LDPE | 0.6 | 0.50 | 5.0 | 550 | n.r. | 18.30 | 70.70 | 0.50 | Batch reactor | [37] | |
| HDPE | n.r. | 17.30 | 72.60 | 0.70 | |||||||
| HUSY | LDPE | 0.6 | 0.50 | 5.0 | 550 | n.r. | 61.60 | 34.50 | 1.90 | ||
| HDPE | n.r. | 41.00 | 39.50 | 1.90 | |||||||
| ZSM-5 catalysis in vapor phase | Municipal plastic waste (42.5% PE) | 50.0 | 8.00 | 7.0 | 700 | 6.06 | 72.72 | 21.22 | n.r. | Semi-batch | [64] |
| ZSM-5 catalysis in liquid phase | 4.76 | 58.68 | 36.56 | n.r. | |||||||
| ZSM-5 catalysis in liquid and vapor phase | 4.18 | 45.36 | 49.46 | n.r. | |||||||
| HZSM-5 catalysis in vapor phase | Softwood sawdust | n.r. | 0.35–0.50 | 4.0 | 400 | 32.40 | 45.40 | 12.00 | 10.20 | Batch reactor | [61] |
| 500 | 22.20 | 56.20 | 15.90 | 5.70 | |||||||
| 600 | 16.50 | 40.30 | 37.00 | 6.20 | |||||||
| Catalysis using FAN (liquid phase) | Municipal solid waste | 50.0 | 4.00 × 4.00 | n.r. | 700 | 13.20 | 68.20 | 18.60 | n.r. | Semi-batch | [35] |
| Catalysis using FAN (liquid and vapor phase) | 12.40 | 71.70 | 15.90 | n.r. | |||||||
| Catalysis using FA-600 (liquid phase) | 11.36 | 70.24 | 18.4 | n.r. | |||||||
| Catalysis using FA-600 (liquid and vapor phase) | 9.90 | 76.14 | 13.96 | n.r. | |||||||
| Catalysis using FA-700 (liquid phase) | 10.86 | 71.36 | 17.78 | n.r. | |||||||
| Catalysis using FA-700 (liquid and vapor phase) | 7.16 | 70.96 | 21.88 | n.r. | |||||||
| Catalysis using FA-800 (liquid phase) | 1.76 | 78.20 | 20.04 | n.r. | |||||||
| Catalysis using FA-800 (liquid and vapor phase) | 1.66 | 73.12 | 25.22 | n.r. | |||||||
| MgO catalysis in gas phase | PS | 4.0 | 0.63–1.00 | 10.0 | 400 | 17.00 | 74.00 | 9.00 | n.r. | Batch | [53] |
| 500 | 1.50 | 90.00 | 8.50 | n.r. | |||||||
| 400 | 0.00 | 92.00 | 8.00 | n.r. | Semi-batch | ||||||
| 500 | 0.00 | 89.00 | 11.00 | n.r. | |||||||
| 33.33% Ni/ZSM-5 | Municipal solid waste | 50.0 | 5.00 | 15 | 550 | 31.00 | 37.00 | 32.00 | n.r. | Batch | [45] |
| 50.00% Ni/ZSM-5 | 27.00 | 35.00 | 38.00 | n.r. | |||||||
| 33.33% Ni/SAPO-11 | 27.00 | 44.50 | 28.50 | n.r. | |||||||
| 50.00% Ni/SAPO-11 | 27.50 | 40.50 | 32.00 | n.r. | |||||||
| 10% Ni/Al2O3 | PS | 30.0 | n.r. | 5 | 400 | 29.00 | 40.00 | 31.00 | n.r. | Semi-batch | [63] |
| 500 | 5.00 | 88.50 | 6.50 | n.r. | |||||||
| 10% La/Al2O3 | 400 | 20.00 | 70.00 | 10.00 | n.r. | ||||||
| 500 | 5.00 | 85.00 | 10.00 | n.r. | |||||||
| Sand (vapor phase) | Wood pellets | n.r. | L: 15.00 ø: 6.00 | 2400 | 500 | 21.80 | 32.90 | 45.30 | n.r. | Auger | [75] |
| NiO/SiO2 | 22.90 | 27.30 | 49.80 | n.r. | |||||||
| Ni/CeO2/Al2O3 | 23.00 | 29.40 | 47.60 | n.r. | |||||||
| NiO/Al2O3 | 23.40 | 27.10 | 49.50 | n.r. | |||||||
| Ni-Fe | Sawdust | 4.0 | 0.42–0.60 | 10 | 650 | 20.28 | 32.61 | 47.11 | n.r. | Fixed-bed reactor | [79] |
| Feedstock | T (°C) | Compounds (%, w/w) | BP | CP | CBP | CCP | References |
|---|---|---|---|---|---|---|---|
| LLDPE | 500–550 | H2 | 8.000 | 4.000 | 12.000 | 10.000 | [39] |
| CH4 | 25.000 | 9.000 | 20.000 | 6.000 | |||
| Ethylene | 30.000 | 29.000 | 16.000 | 11.000 | |||
| Ethane | 8.000 | 10.000 | 10.000 | 6.000 | |||
| Propylene | 18.000 | 22.000 | 22.000 | 36.000 | |||
| Propane | 3.000 | 7.000 | 9.000 | 20.000 | |||
| C4+ | 8.000 | 19.000 | 11.000 | 11.000 | |||
| LDPE | 550 | n-Paraffins | 31.800 | n.r. | 7.700 | n.r. | [37] |
| Is-Paraffins | 2.100 | n.r. | 9.000 | n.r. | |||
| 1-Olefins | 46.300 | n.r. | 15.800 | n.r. | |||
| Olefins | 19.500 | n.r. | 67.300 | n.r. | |||
| Aromatics | 0.300 | n.r. | 0.200 | n.r. | |||
| HDPE | 550 | n-Paraffins | 29.100 | n.r. | 15.200 | n.r. | |
| Is-Paraffins | 1.900 | n.r. | 11.700 | n.r. | |||
| 1-Olefins | 47.300 | n.r. | 21.400 | n.r. | |||
| Olefins | 21.500 | n.r. | 51.400 | n.r. | |||
| Aromatics | 0.200 | n.r. | 0.300 | n.r. | |||
| Plastic mixture | 500 | H2 | 0.400 | n.r. | n.r. | n.r. | [57] |
| CO | 0.700 | n.r. | n.r. | n.r. | |||
| CO2 | 2.900 | n.r. | n.r. | n.r. | |||
| CH4 | 8.300 | n.r. | n.r. | n.r. | |||
| Ethane | 10.000 | n.r. | n.r. | n.r. | |||
| Ethene | 12.200 | n.r. | n.r. | n.r. | |||
| C3 | 29.100 | n.r. | n.r. | n.r. | |||
| C4 | 17.600 | n.r. | n.r. | n.r. | |||
| C5 | 9.500 | n.r. | n.r. | n.r. | |||
| C6 | 9.200 | n.r. | n.r. | n.r. | |||
| Waste tires | 550 | H2 | 0.304 | n.r. | n.r. | n.r. | [51] |
| CO | 0.063 | n.r. | n.r. | n.r. | |||
| CO2 | 0.292 | n.r. | n.r. | n.r. | |||
| CH4 | 0.768 | n.r. | n.r. | n.r. | |||
| C2+ | 0.646 | n.r. | n.r. | n.r. | |||
| Propylene | 0.273 | n.r. | n.r. | n.r. | |||
| Propane | 0.272 | n.r. | n.r. | n.r. | |||
| C4+ | 0.933 | n.r. | n.r. | n.r. | |||
| Cis-pentene | 0.013 | n.r. | n.r. | n.r. | |||
| C6+ | 0.217 | n.r. | n.r. | n.r. | |||
| Waste tires | 550 | H2 | n.r. | 6.500 | n.r. | n.r. | [74] |
| CO | n.r. | 1.300 | n.r. | n.r. | |||
| CO2 | n.r. | 1.600 | n.r. | n.r. | |||
| N2 | n.r. | 35.100 | n.r. | n.r. | |||
| CH4 | n.r. | 10.800 | n.r. | n.r. | |||
| Ethene | n.r. | 4.500 | n.r. | n.r. | |||
| Ethane | n.r. | 4.300 | n.r. | n.r. | |||
| Propane | n.r. | 5.100 | n.r. | n.r. | |||
| Propene | n.r. | 4.800 | n.r. | n.r. | |||
| C4 | n.r. | 12.300 | n.r. | n.r. | |||
| H2S | n.r. | 0.500 | n.r. | n.r. | |||
| Municipal solid waste | 550 | H2 | 8.900 | n.r. | 12.000 | n.r. | [45] |
| CO | 7.300 | n.r. | 6.500 | n.r. | |||
| CO2 | 8.400 | n.r. | 9.500 | n.r. | |||
| CH4 | 7.400 | n.r. | 18.000 | n.r. | |||
| C2–C5 | 68.000 | n.r. | 54.000 | n.r. | |||
| Packages (Tetra Pak) | 650 | H2 | n.r. | 0.160 | n.r. | 0.170 | [73] |
| CO | n.r. | 0.230 | n.r. | 0.260 | |||
| CO2 | n.r. | 0.130 | n.r. | 0.150 | |||
| CH4 | n.r. | 0.120 | n.r. | 0.140 | |||
| HCX | n.r. | 0.190 | n.r. | 0.220 | |||
| 750 | H2 | n.r. | 0.225 | n.r. | 0.255 | ||
| CO | n.r. | 0.235 | n.r. | 0.260 | |||
| CO2 | n.r. | 0.125 | n.r. | 0.135 | |||
| CH4 | n.r. | 0.160 | n.r. | 0.180 | |||
| HCX | n.r. | 0.160 | n.r. | 0.125 | |||
| 850 | H2 | n.r. | 0.300 | n.r. | 0.375 | ||
| CO | n.r. | 0.270 | n.r. | 0.320 | |||
| CO2 | n.r. | 0.085 | n.r. | 0.095 | |||
| CH4 | n.r. | 0.130 | n.r. | 0.130 | |||
| HCX | n.r. | 0.130 | n.r. | 0.650 | |||
| Construction waste wood | 500 | CO | 29.000 | 42.000 | n.r. | n.r. | [48] |
| CO2 | 63.000 | 46.000 | n.r. | n.r. | |||
| C1–C4 | 8.000 | 12.000 | n.r. | n.r. | |||
| Pinewood sawdust | 550 | CO | n.r. | 45.000 | n.r. | n.r. | [69] |
| CO2 | n.r. | 22.000 | n.r. | n.r. | |||
| CH4 | n.r. | 14.500 | n.r. | n.r. | |||
| H2 | n.r. | 12.800 | n.r. | n.r. | |||
| C2–C3 | n.r. | 5.000 | n.r. | n.r. | |||
| Wood pellets | 450 | H2 | n.r. | 2.240 | n.r. | n.r. | [76] |
| O2 | n.r. | n.r. | n.r. | n.r. | |||
| N2 | n.r. | 5.540 | n.r. | n.r. | |||
| CO | n.r. | 34.700 | n.r. | n.r. | |||
| CO2 | n.r. | 50.270 | n.r. | n.r. | |||
| CH4 | n.r. | 7.240 | n.r. | n.r. | |||
| Barley straw | 450 | H2 | n.r. | 1.540 | n.r. | n.r. | |
| O2 | n.r. | 0.420 | n.r. | n.r. | |||
| N2 | n.r. | 4.680 | n.r. | n.r. | |||
| CO | n.r. | 21.740 | n.r. | n.r. | |||
| CO2 | n.r. | 60.130 | n.r. | n.r. | |||
| CH4 | n.r. | 10.480 | n.r. | n.r. | |||
| Waste furniture sawdust | 400 | CO | n.r. | 10.000 | n.r. | n.r. | [32] |
| CO2 | n.r. | 89.500 | n.r. | n.r. | |||
| C1–C4 | n.r. | 0.500 | n.r. | n.r. | |||
| 450 | CO | n.r. | 28.000 | n.r. | n.r. | ||
| CO2 | n.r. | 62.000 | n.r. | n.r. | |||
| C1–C4 | n.r. | 10.000 | n.r. | n.r. | |||
| 500 | CO | n.r. | 34.000 | n.r. | n.r. | ||
| CO2 | n.r. | 44.000 | n.r. | n.r. | |||
| C1-C4 | n.r. | 22.000 | n.r. | n.r. | |||
| 550 | CO | n.r. | 37.000 | n.r. | n.r. | ||
| CO2 | n.r. | 39.000 | n.r. | n.r. | |||
| C1–C4 | n.r. | 24.000 | n.r. | n.r. | |||
| Rice straw | 500 | H | 4.000 | 0.000 | n.r. | n.r. | [17] |
| O2 | 0.000 | 3.000 | n.r. | n.r. | |||
| N2 | 4.000 | 93.000 | n.r. | n.r. | |||
| CO | 25.000 | 2.000 | n.r. | n.r. | |||
| CO2 | 55.000 | 5.000 | n.r. | n.r. | |||
| CH4 | 9.000 | 0.000 | n.r. | n.r. | |||
| Ethyne | 0.000 | 0.000 | n.r. | n.r. | |||
| Ethene | 1.500 | 0.000 | n.r. | n.r. | |||
| Ethane | 3.500 | 0.000 | n.r. | n.r. | |||
| Propene | 1.500 | 0.000 | n.r. | n.r. | |||
| Propane | 1.500 | 0.000 | n.r. | n.r. | |||
| Corncob | 500 | H2 | n.r. | 0.020 | n.r. | 0.020 | [26] |
| CO + H2 | n.r. | 4.030 | n.r. | 2.710 | |||
| CO2 | n.r. | 10.820 | n.r. | 5.180 | |||
| CH4 | n.r. | 0.410 | n.r. | 0.260 | |||
| Corn stalk | 500 | H2 | n.r. | 0.020 | n.r. | 0.010 | |
| CO + H2 | n.r. | 3.920 | n.r. | 4.190 | |||
| CO2 | n.r. | 9.240 | n.r. | 9.630 | |||
| CH4 | n.r. | 0.340 | n.r. | 0.390 | |||
| Sunflower residues | 500 | H2 | n.r. | 0.010 | n.r. | 0.060 | |
| CO + H2 | n.r. | 0.820 | n.r. | 1.860 | |||
| CO2 | n.r. | 8.090 | n.r. | 10.860 | |||
| CH4 | n.r. | 0.130 | n.r. | 0.390 | |||
| Olive pruning | 500 | H2 | n.r. | 0.010 | n.r. | 0.010 | |
| CO + H2 | n.r. | 1.150 | n.r. | 2.160 | |||
| CO2 | n.r. | 7.370 | n.r. | 9.150 | |||
| CH4 | n.r. | 0.090 | n.r. | 0.300 | |||
| Olive kernels | 500 | H2 | n.r. | 0.030 | n.r. | 0.020 | |
| CO + H2 | n.r. | 2.680 | n.r. | 1.500 | |||
| CO2 | n.r. | 7.500 | n.r. | 4.530 | |||
| CH4 | n.r. | 0.610 | n.r. | 0.300 | |||
| Rice straw | 445 | CO | n.r. | 24.000 | n.r. | n.r. | [78] |
| CO2 | n.r. | 66.000 | n.r. | n.r. | |||
| CH4 | n.r. | 3.500 | n.r. | n.r. | |||
| C1–C4 | n.r. | 6.500 | n.r. | n.r. | |||
| Bamboo | 405 | CO | n.r. | 32.000 | n.r. | n.r. | |
| CO2 | n.r. | 61.000 | n.r. | n.r. | |||
| CH4 | n.r. | 4.200 | n.r. | n.r. | |||
| C1–C4 | n.r. | 2.800 | n.r. | n.r. |
| Feedstock | T (°C) | Chemical Selectivity (%, w/w) | BP | CP | CBP | CCP | References |
|---|---|---|---|---|---|---|---|
| LLDPE | 500–550 | Mono-aromatics | 9.60 | 0.00 | 65.80 | 72.30 | [39] |
| Benzene | 1.30 | 0.00 | 0.00 | 0.00 | |||
| Toluene | 3.90 | 0.00 | 16.60 | 11.60 | |||
| Xylene | 1.60 | 0.00 | 26.30 | 23.20 | |||
| Poly-aromatics | 6.10 | 0.00 | 8.10 | 2.30 | |||
| C5–C12 alkenes | 23.30 | 15.40 | 9.40 | 17.10 | |||
| C5–C12 alkanes | 6.90 | 5.30 | 4.80 | 7.30 | |||
| C12+ alkenes | 38.30 | 58.40 | 3.40 | 0.00 | |||
| C12+ alkanes | 15.70 | 20.90 | 8.50 | 1.00 | |||
| Gasoline-range hydrocarbons | 45.10 | 20.70 | 84.90 | 98.00 | |||
| PS | 500 | Toluene | 6.50 | n.r. | 7.00 | n.r. | [63] |
| Ethylbenzene | 2.50 | n.r. | 7.20 | n.r. | |||
| Styrene | 63.59 | n.r. | 63.55 | n.r. | |||
| α-Methylstyrene | 6.60 | n.r. | 7.00 | n.r. | |||
| 1,3-Diphenylpropane | 6.00 | n.r. | 8.50 | n.r. | |||
| LDPE | 550 | n-Paraffins | 33.40 | n.r. | 4.04 | n.r. | [37] |
| Is-Paraffins | 0.00 | n.r. | 4.10 | n.r. | |||
| 1-Olefins | 51.00 | n.r. | 8.60 | n.r. | |||
| Olefins | 15.60 | n.r. | 30.80 | n.r. | |||
| Aromatics | 0.00 | n.r. | 52.10 | n.r. | |||
| HDPE | 550 | n-Paraffins | 37.00 | n.r. | 3.80 | n.r. | |
| Is-Paraffins | 0.00 | n.r. | 2.90 | n.r. | |||
| 1-Olefins | 44.00 | n.r. | 18.70 | n.r. | |||
| Olefins | 19.00 | n.r. | 24.00 | n.r. | |||
| Aromatics | 0.00 | n.r. | 50.60 | n.r. | |||
| Plastic mixture | 500 | Toluene | 8.10 | n.r. | n.r. | n.r. | [57] |
| Dimethyl-heptene | 5.90 | n.r. | n.r. | n.r. | |||
| Ethylbenzene | 5.00 | n.r. | n.r. | n.r. | |||
| Xylenes | <3.00 | n.r. | n.r. | n.r. | |||
| Styrene | 48.40 | n.r. | n.r. | n.r. | |||
| α-Methylstyrene | 4.20 | n.r. | n.r. | n.r. | |||
| Naphthalene | <3.00 | n.r. | n.r. | n.r. | |||
| Municipal solid waste | 550 | N-paraffin | 14.60 | n.r. | 13.60 | n.r. | [45] |
| N-olefin | 18.60 | n.r. | 14.10 | n.r. | |||
| Phenol and derivatives | 10.30 | n.r. | 8.50 | n.r. | |||
| Aldehyde | 5.30 | n.r. | 3.80 | n.r. | |||
| Keton | 2.60 | n.r. | 2.50 | n.r. | |||
| Alcohols | 7.90 | n.r. | 1.50 | n.r. | |||
| Carboxylic acid | 9.00 | n.r. | 8.90 | n.r. | |||
| Other | 31.70 | n.r. | 47.20 | n.r. | |||
| Construction waste | 500 | Acids | 19.00 | 18.00 | 16.50 | n.r. | [48] |
| Oxygenates | 36.00 | 32.50 | 28.50 | n.r. | |||
| Aromatics | 1.0 | 2.00 | 2.00 | n.r. | |||
| Phenolics | 27.0 | 43.00 | 37.50 | n.r. | |||
| N-compounds | 2.7 | 3.00 | 0.80 | n.r. | |||
| Hydrocarbons | 0.0 | 0.00 | 0.40 | n.r. | |||
| Rice straw | 500 | Alkanes | 11.33 | 4.27 | n.r. | n.r. | [17] |
| Alkenes | 10.18 | 0.50 | n.r. | n.r. | |||
| Alcohols | 3.89 | 6.91 | n.r. | n.r. | |||
| Ketones | 19.03 | 17.72 | n.r. | n.r. | |||
| Phenols | 27.72 | 33.04 | n.r. | n.r. | |||
| Aromatics | 25.27 | 2.01 | n.r. | n.r. | |||
| Nitriles | 1.56 | n.r. | n.r. | n.r. | |||
| Others | n.r. | 29.31 | n.r. | n.r. | |||
| Waste furniture sawdust | 500 | Acetic acid | n.r. | 13.70 | n.r. | n.r. | [32] |
| Pyrazine | n.r. | 0.50 | n.r. | n.r. | |||
| Cyclopentanone | n.r. | 0.30 | n.r. | n.r. | |||
| 2-Furanmethanol | n.r. | 1.50 | n.r. | n.r. | |||
| 2-Methyl-2-cyclopenten-1-one | n.r. | 0.60 | n.r. | n.r. | |||
| 2(5H)-Furanone | n.r. | 2.20 | n.r. | n.r. | |||
| 5-Methyl-2(5H)-furanone | n.r. | 0.30 | n.r. | n.r. | |||
| 3-Methyl-2-cyclopenten-1-one | n.r. | 0.60 | n.r. | n.r. | |||
| Phenol | n.r. | 0.70 | n.r. | n.r. | |||
| 2-Hydroxy-3-methyl-2-cyclopenten-1-one | n.r. | 2.40 | n.r. | n.r. | |||
| 2-Methyl-phenol | n.r. | 0.30 | n.r. | n.r. | |||
| Benzoic acid | n.r. | 2.20 | n.r. | n.r. | |||
| 2-Methoxy- phenol | n.r. | 1.10 | n.r. | n.r. | |||
| 3-Hydroxy-2-methyl-4H-pyran-4-one | n.r. | 0.40 | n.r. | n.r. | |||
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | n.r. | 0.30 | n.r. | n.r. | |||
| 2-Methoxy-4-methyl- phenol | n.r. | 0.70 | n.r. | n.r. | |||
| 2-Methoxy-4-vinylphenol | n.r. | 1.30 | n.r. | n.r. | |||
| 2,6-Dimethoxy-phenol | n.r. | 0.50 | n.r. | n.r. | |||
| 2-Methoxy-4-(1-propenyl)-phenol | n.r. | 2.40 | n.r. | n.r. | |||
| 2-Methyl-1H-Isoindole-1,3(2H)-dione | n.r. | 1.10 | n.r. | n.r. | |||
| 1H-Isoindole-1,3(2H)-dione | n.r. | 3.20 | n.r. | n.r. | |||
| 1-(4-Hydroxy-3-methoxyphenyl)-ethanone | n.r. | 1.60 | n.r. | n.r. | |||
| 4-Vinyl-2-methoxy-phenol | n.r. | 0.20 | n.r. | n.r. | |||
| Bis(2-ethylhexyl) phthalate | n.r. | 1.70 | n.r. | n.r. | |||
| Rice straw | 445 | 2(5H)-Furanone | n.r. | 2.35 | n.r. | n.r. | [78] |
| Furfural | n.r. | 2.63 | n.r. | n.r. | |||
| 3-Methyl-2-cyclopenten-1-one | n.r. | 0.62 | n.r. | n.r. | |||
| 2-Hydroxy-2-cyclopenten-1-one | n.r. | 1.17 | n.r. | n.r. | |||
| Phenol | n.r. | 1.09 | n.r. | n.r. | |||
| o-Cresol | n.r. | 0.66 | n.r. | n.r. | |||
| p-Cresol | n.r. | 1.33 | n.r. | n.r. | |||
| o-Guaiacol | n.r. | 2.31 | n.r. | n.r. | |||
| m-Xylenol | n.r. | 0.74 | n.r. | n.r. | |||
| o-Xylenol | n.r. | 0.09 | n.r. | n.r. | |||
| 4-Ethyl-phenol | n.r. | 2.53 | n.r. | n.r. | |||
| Pyrocatechol | n.r. | 3.08 | n.r. | n.r. | |||
| 4-Methyl-benzaldehyde | n.r. | 4.17 | n.r. | n.r. | |||
| Hydroquinone | n.r. | 0.83 | n.r. | n.r. | |||
| 3-Methyl-pyrocatechol | n.r. | 1.29 | n.r. | n.r. | |||
| 3-Methoxy-pyrocatecho | n.r. | 0.66 | n.r. | n.r. | |||
| 4-Methyl-catechol | n.r. | 1.34 | n.r. | n.r. | |||
| Levoglucosan | n.r. | 10.64 | n.r. | n.r. | |||
| p-Ethylguaiacol | n.r. | 1.48 | n.r. | n.r. | |||
| p-Vinylguaiacol | n.r. | 5.10 | n.r. | n.r. | |||
| Syringol | n.r. | 2.54 | n.r. | n.r. | |||
| Eugenol | n.r. | 1.25 | n.r. | n.r. | |||
| Vanillin | n.r. | 0.99 | n.r. | n.r. | |||
| 4-Hydroxy-benzaldehyde | n.r. | 0.42 | n.r. | n.r. | |||
| (E)-Isoeugenol | n.r. | 1.74 | n.r. | n.r. | |||
| Bamboo | 405 | 2-Furancarboxaldehyd | n.r. | 1.74 | n.r. | n.r. | |
| 2(5H)-Furanone | n.r. | 0.98 | n.r. | n.r. | |||
| 5-Methyl-2(3H)-furanone | n.r. | 1.68 | n.r. | n.r. | |||
| Acetic acid | n.r. | 1.07 | n.r. | n.r. | |||
| 3-Methyl-2-cyclopenten-1-one | n.r. | 0.17 | n.r. | n.r. | |||
| 2-Hydroxy-3-methyl-2-cyclopenten-1-one | n.r. | 1.10 | n.r. | n.r. | |||
| Phenol | n.r. | 0.64 | n.r. | n.r. | |||
| o-Cresol | n.r. | 0.55 | n.r. | n.r. | |||
| p-Cresol | n.r. | 0.60 | n.r. | n.r. | |||
| o-Guaiacol | n.r. | 2.25 | n.r. | n.r. | |||
| m-Xylenol | n.r. | 0.14 | n.r. | n.r. | |||
| o-Xylenol | n.r. | 0.08 | n.r. | n.r. | |||
| 4-Ethyl-phenol | n.r. | 1.18 | n.r. | n.r. | |||
| Pyrocatechol | n.r. | 2.27 | n.r. | n.r. | |||
| 4-Methyl-benzaldehyde | n.r. | 8.02 | n.r. | n.r. | |||
| 3-Methyl-pyrocatechol | n.r. | 0.71 | n.r. | n.r. | |||
| Levoglucosan | n.r. | 2.14 | n.r. | n.r. | |||
| p-Ethylguaiacol | n.r. | 1.55 | n.r. | n.r. | |||
| 4-Methyl-pyrocatechol | n.r. | 0.70 | n.r. | n.r. | |||
| p-Vinylguaiacol | n.r. | 4.01 | n.r. | n.r. | |||
| Syringol | n.r. | 4.61 | n.r. | n.r. | |||
| 4-Hydroxy-benzaldehyde | n.r. | 0.94 | n.r. | n.r. | |||
| 2-Methoxy-4-(1-propenyl)-phenol | n.r. | 5.74 | n.r. | n.r. | |||
| 2,6-Dimethoxy-4-(2-propenyl)-phenol | n.r. | 6.98 | n.r. | n.r. |
| Feedstock | T (°C) | Elemental Contents (%, w/w) | BP | CP | CBP | CCP | References |
|---|---|---|---|---|---|---|---|
| LLDPE | 500–550 | C | 84.97 | 83.92 | 88.76 | 86.82 | [39] |
| H | 13.82 | 15.10 | 10.63 | 12.65 | |||
| N | 0.33 | 0.28 | 0.14 | 0.15 | |||
| O * | 0.79 | 0.58 | 0.47 | 0.32 | |||
| S | 0.09 | 0.12 | 0.00 | 0.06 | |||
| HHV (MJ/kg) | 46.03 | 47.29 | 43.35 | 45.23 | |||
| Plastic mixture | 500 | C | 86.50 | n.r. | n.r. | n.r. | [57] |
| H | 11.30 | n.r. | n.r. | n.r. | |||
| Cl | 0.50 | n.r. | n.r. | n.r. | |||
| Other | 1.50 | n.r. | n.r. | n.r. | |||
| HHV (MJ/kg) | 43.30 | n.r. | n.r. | n.r. | |||
| Waste tires | 550 | C | n.r. | 86.20 | n.r. | n.r. | [74] |
| H | n.r. | 10.30 | n.r. | n.r. | |||
| N | n.r. | 0.80 | n.r. | n.r. | |||
| S | n.r. | 0.80 | n.r. | n.r. | |||
| HHV (MJ/kg) | n.r. | 42.45 | n.r. | n.r. | |||
| Packages (Tetra Pak) | 650 | C | n.r. | 3.57 | n.r. | n.r. | [73] |
| H | n.r. | 10.80 | n.r. | n.r. | |||
| N | n.r. | 0.43 | n.r. | n.r. | |||
| O * | n.r. | 85.19 | n.r. | n.r. | |||
| S | n.r. | 0.01 | n.r. | n.r. | |||
| 750 | C | n.r. | 1.69 | n.r. | 1.42 | ||
| H | n.r. | 9.36 | n.r. | 12.80 | |||
| N | n.r. | 0.13 | n.r. | 0.31 | |||
| O * | n.r. | 88.81 | n.r. | 85.45 | |||
| S | n.r. | 0.01 | n.r. | 0.02 | |||
| 850 | C | n.r. | 3.03 | n.r. | 1.11 | ||
| H | n.r. | 11.60 | n.r. | 12.18 | |||
| N | n.r. | 0.28 | n.r. | 0.14 | |||
| O * | n.r. | 85.07 | n.r. | 86.57 | |||
| S | n.r. | 0.02 | n.r. | 0.01 | |||
| Pinewood sawdust | 483 | C | n.r. | 50.90 | n.r. | n.r. | [69] |
| H | n.r. | 6.80 | n.r. | n.r. | |||
| N | n.r. | 0.20 | n.r. | n.r. | |||
| O | n.r. | 42.00 | n.r. | n.r. | |||
| HHV (MJ/kg) | n.r. | 23.30 | n.r. | n.r. | |||
| Wood pellets | 450 | C | n.r. | 55.69 | n.r. | n.r. | [76] |
| H | n.r. | 7.93 | n.r. | n.r. | |||
| N | n.r. | 0.36 | n.r. | n.r. | |||
| O * | n.r. | 36.02 | n.r. | n.r. | |||
| HHV (MJ/kg) | n.r. | 24.2 | n.r. | n.r. | |||
| Barley straw | 450 | C | n.r. | 62.57 | n.r. | n.r. | |
| H | n.r. | 8.12 | n.r. | n.r. | |||
| N | n.r. | 1.41 | n.r. | n.r. | |||
| O * | n.r. | 25.79 | n.r. | n.r. | |||
| HHV (MJ/kg) | n.r. | 28.90 | n.r. | n.r. | |||
| Rice straw | 500 | C | 74.0 | 50.00 | n.r. | n.r. | [17] |
| H | 7.00 | 6.00 | n.r. | n.r. | |||
| N | 3.00 | 2.00 | n.r. | n.r. | |||
| O | 15.00 | 42.00 | n.r. | n.r. | |||
| S | 1.00 | 0.00 | n.r. | n.r. | |||
| Rice straw | 445 | C | n.r. | 49.19 | n.r. | n.r. | [78] |
| H | n.r. | 5.55 | n.r. | n.r. | |||
| N | n.r. | 1.83 | n.r. | n.r. | |||
| S | n.r. | 0.33 | n.r. | n.r. | |||
| O * | n.r. | 43.10 | n.r. | n.r. | |||
| HHV (MJ/kg) | n.r. | 18.60 | n.r. | n.r. | |||
| Bamboo | 405 | C | n.r. | 41.39 | n.r. | n.r. | |
| H | n.r. | 7.03 | n.r. | n.r. | |||
| N | n.r. | 2.01 | n.r. | n.r. | |||
| S | n.r. | 0.02 | n.r. | n.r. | |||
| O * | n.r. | 49.55 | n.r. | n.r. | |||
| HHV (MJ/kg) | n.r. | 17.4 | n.r. | n.r. | |||
| Polanga | n.r. | B | 4.14 | n.r. | n.r. | n.r. | [62] |
| C | 77.43 | n.r. | n.r. | n.r. | |||
| O | 13.29 | n.r. | n.r. | n.r. | |||
| Mg | 0.10 | n.r. | n.r. | n.r. | |||
| Al | 0.97 | n.r. | n.r. | n.r. | |||
| Cl | 0.03 | n.r. | n.r. | n.r. | |||
| Mn | 0.08 | n.r. | n.r. | n.r. | |||
| Fe | 0.13 | n.r. | n.r. | n.r. | |||
| Mo | 3.30 | n.r. | n.r. | n.r. | |||
| Sb | 0.81 | n.r. | n.r. | n.r. | |||
| S | 0.02 | n.r. | n.r. | n.r. | |||
| HHV (MJ/kg) | 21.64 | n.r. | n.r. | n.r. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, A.B.; Leiva-Candia, D.E.; Dorado, M.P. An Overview of Pyrolysis as Waste Treatment to Produce Eco-Energy. Energies 2024, 17, 2852. https://doi.org/10.3390/en17122852
Cuevas AB, Leiva-Candia DE, Dorado MP. An Overview of Pyrolysis as Waste Treatment to Produce Eco-Energy. Energies. 2024; 17(12):2852. https://doi.org/10.3390/en17122852
Chicago/Turabian StyleCuevas, Ana B., David E. Leiva-Candia, and M. P. Dorado. 2024. "An Overview of Pyrolysis as Waste Treatment to Produce Eco-Energy" Energies 17, no. 12: 2852. https://doi.org/10.3390/en17122852





