Qualities and Quantities of Poultry Litter Biochar Characterization and Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Fuel Property Analysis
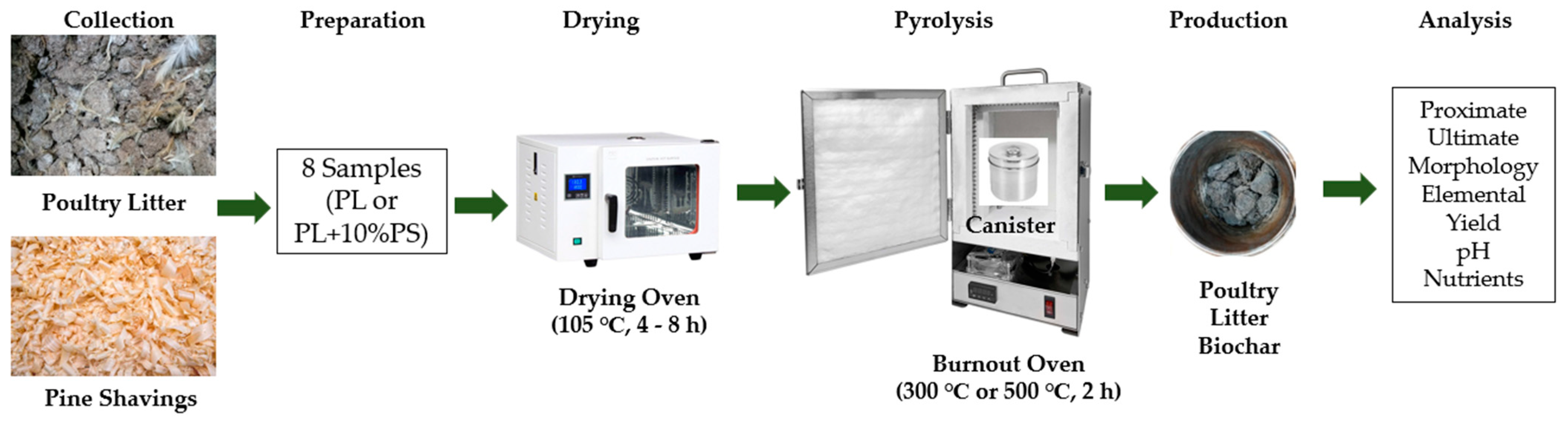
2.2. Poultry Litter Biochar Preparation and Framework
2.3. Mophology and Elemental Analysis
2.4. Yield, pH, and Extractable Nutrient Analysis
3. Results and Discussion
3.1. Fuel Property Analysis (Proximate and Ultimate Analysis) Results
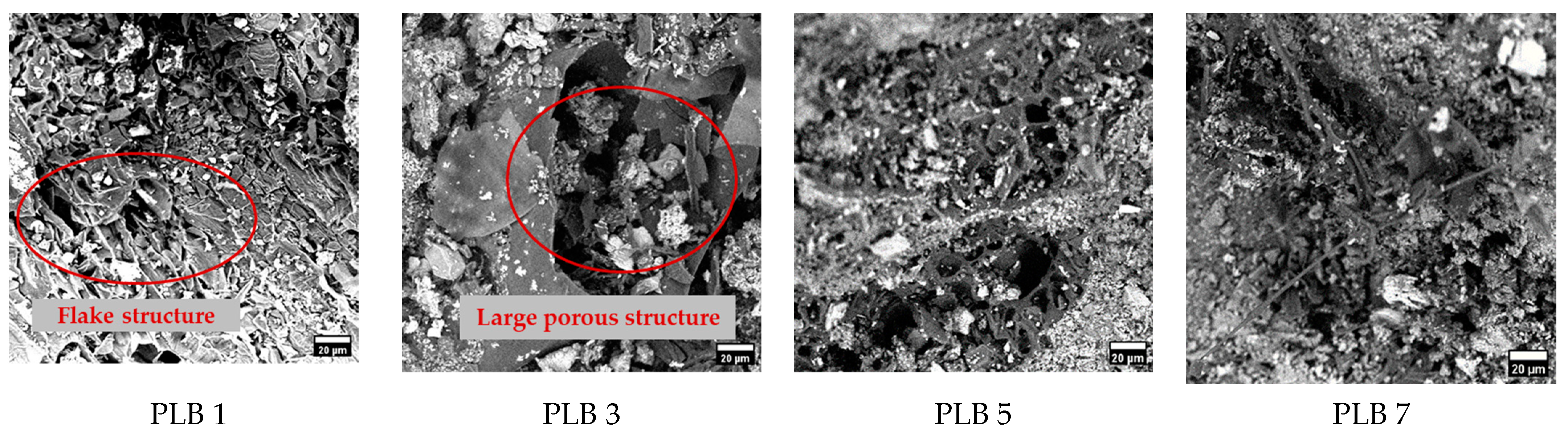
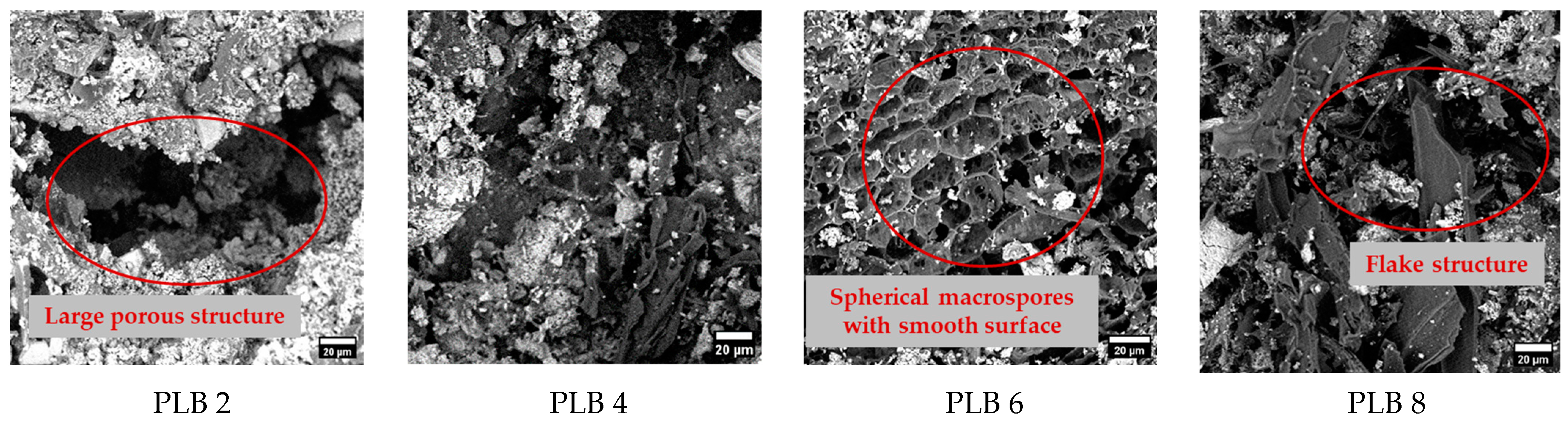
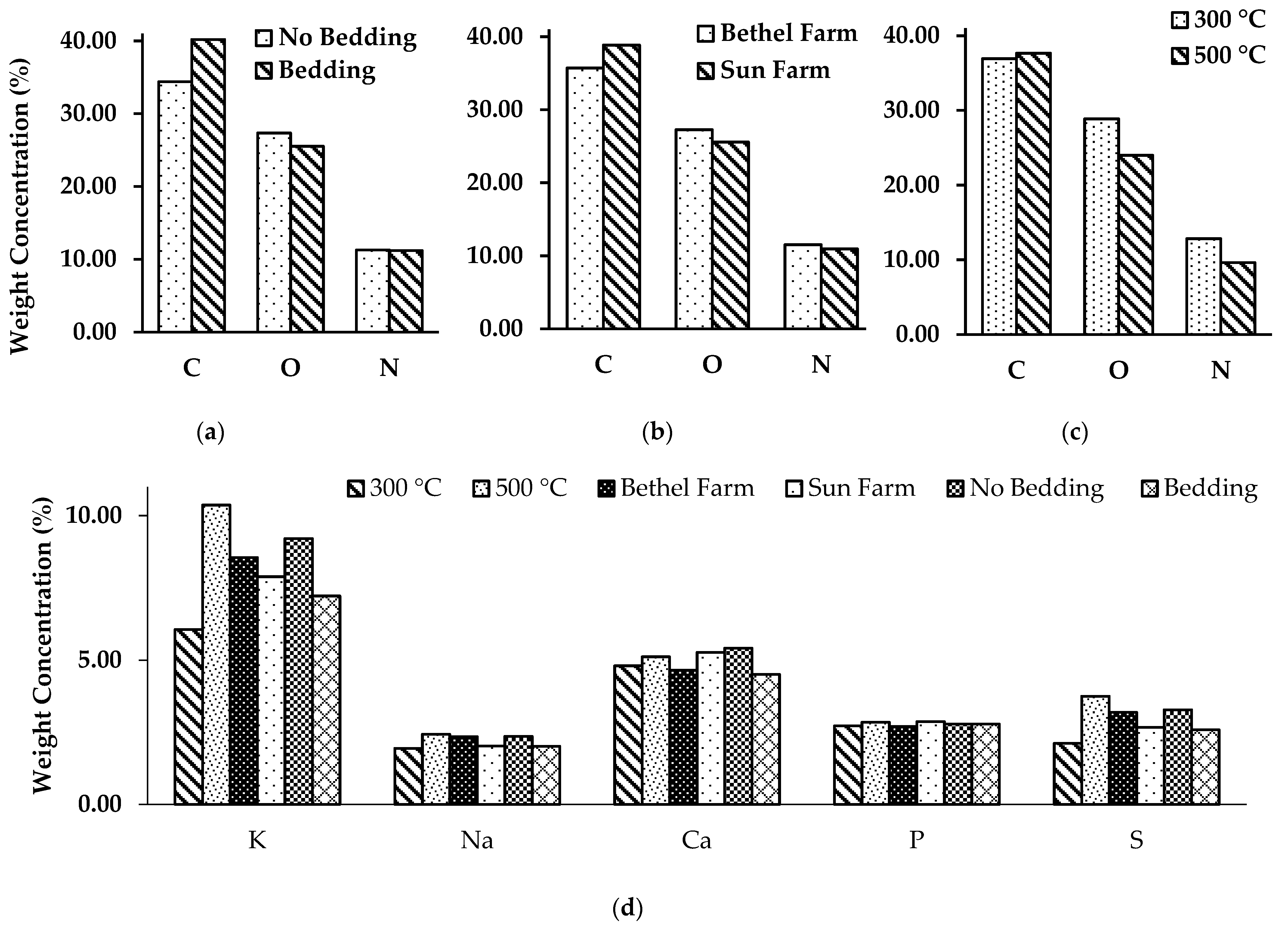
3.2. Biochar Surface & Chemical Composition Analysis
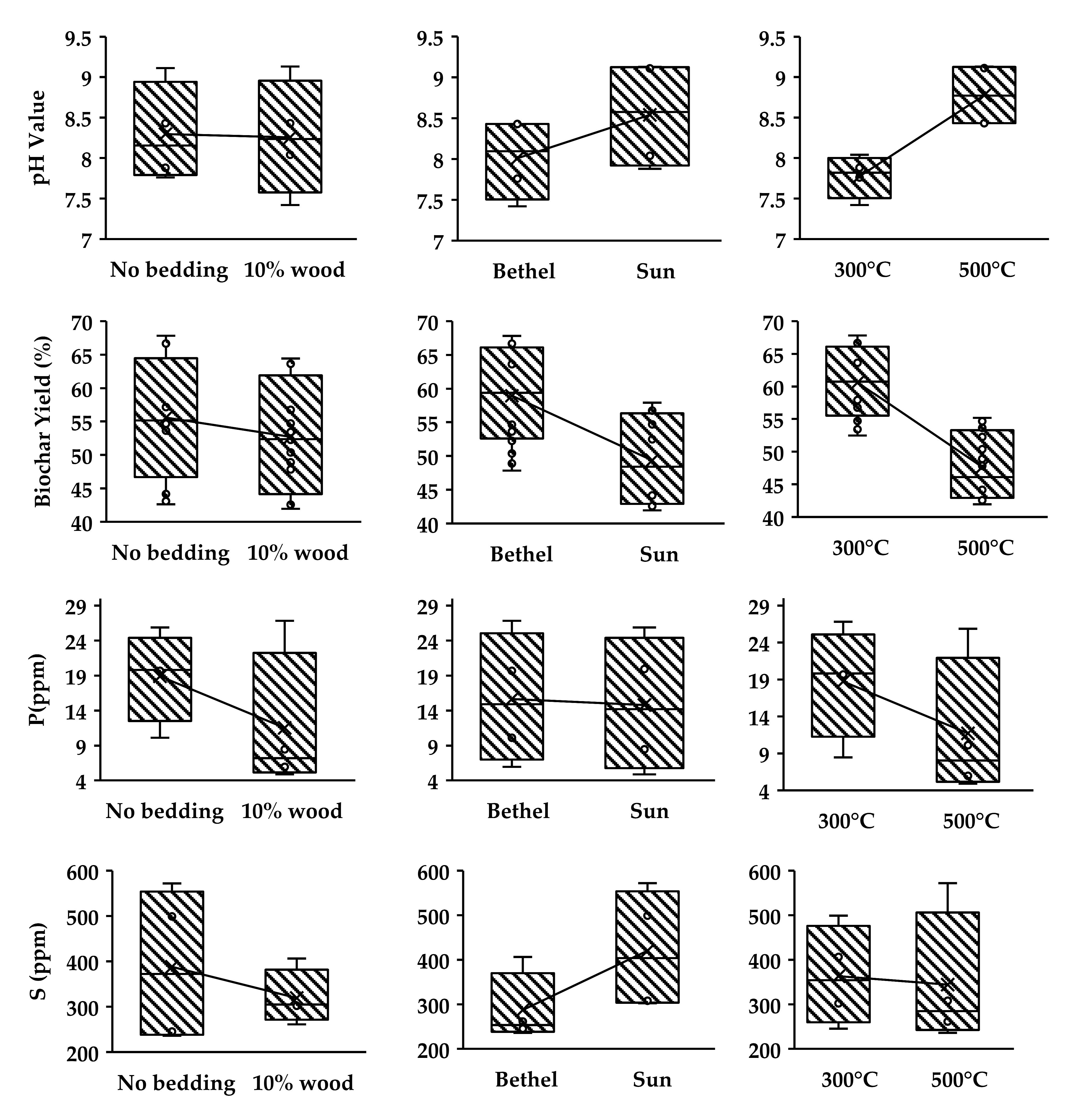
3.3. pH and BIOCHAR Yield Calculations & Extractable Nutrient Analysis by Ion Exchange Resin Method
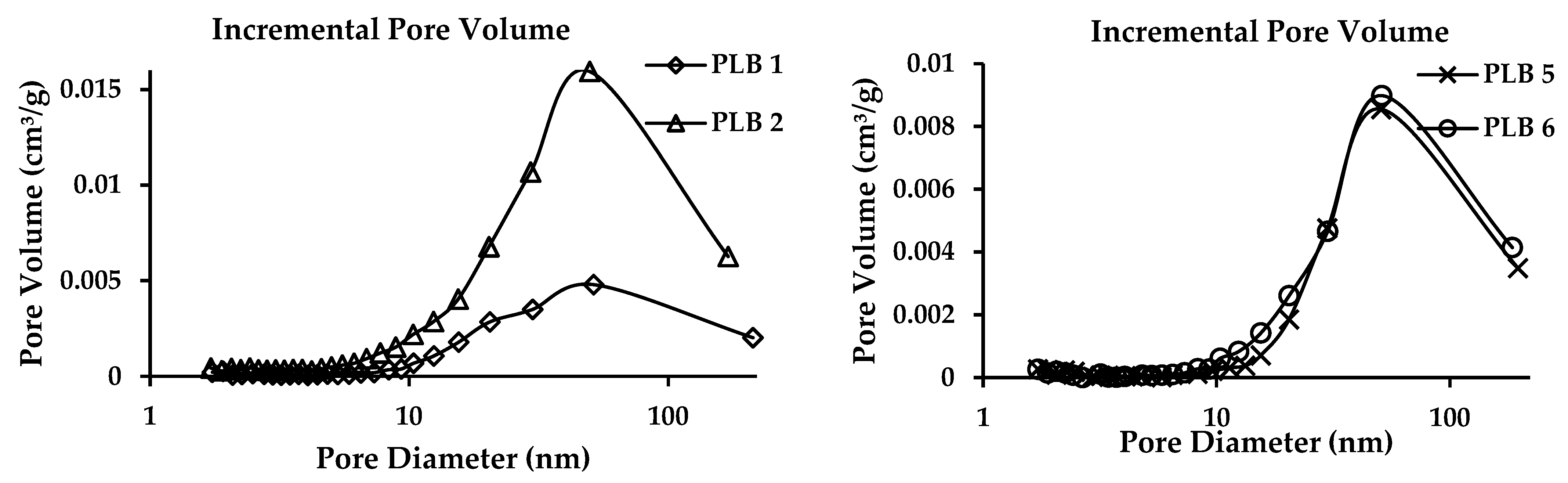
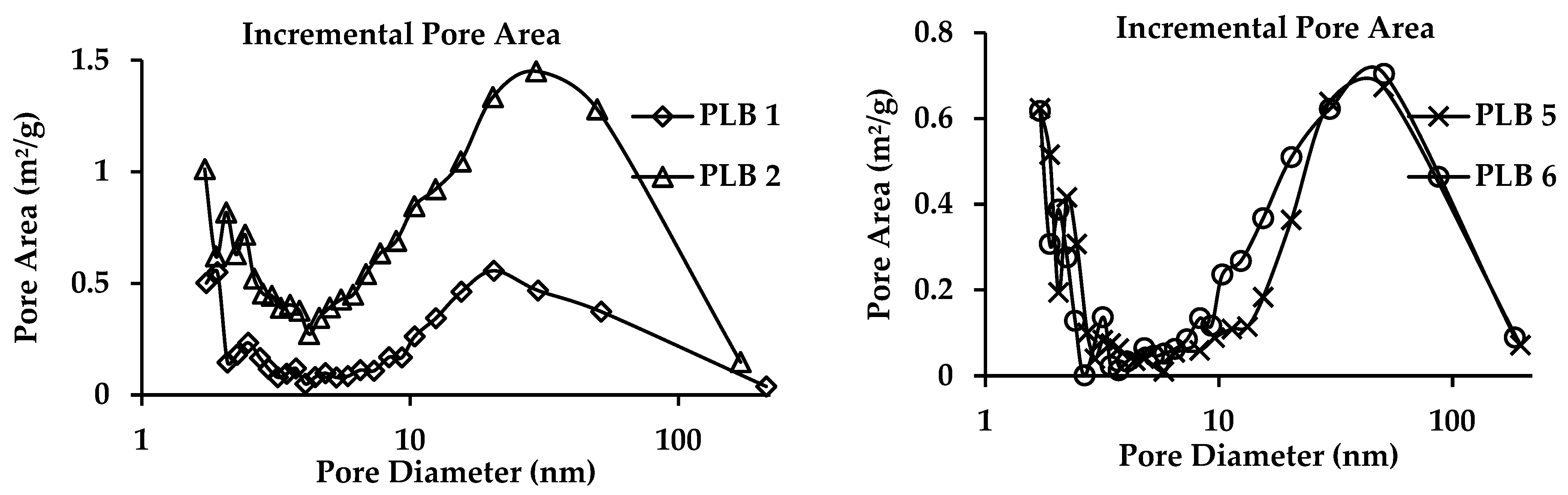
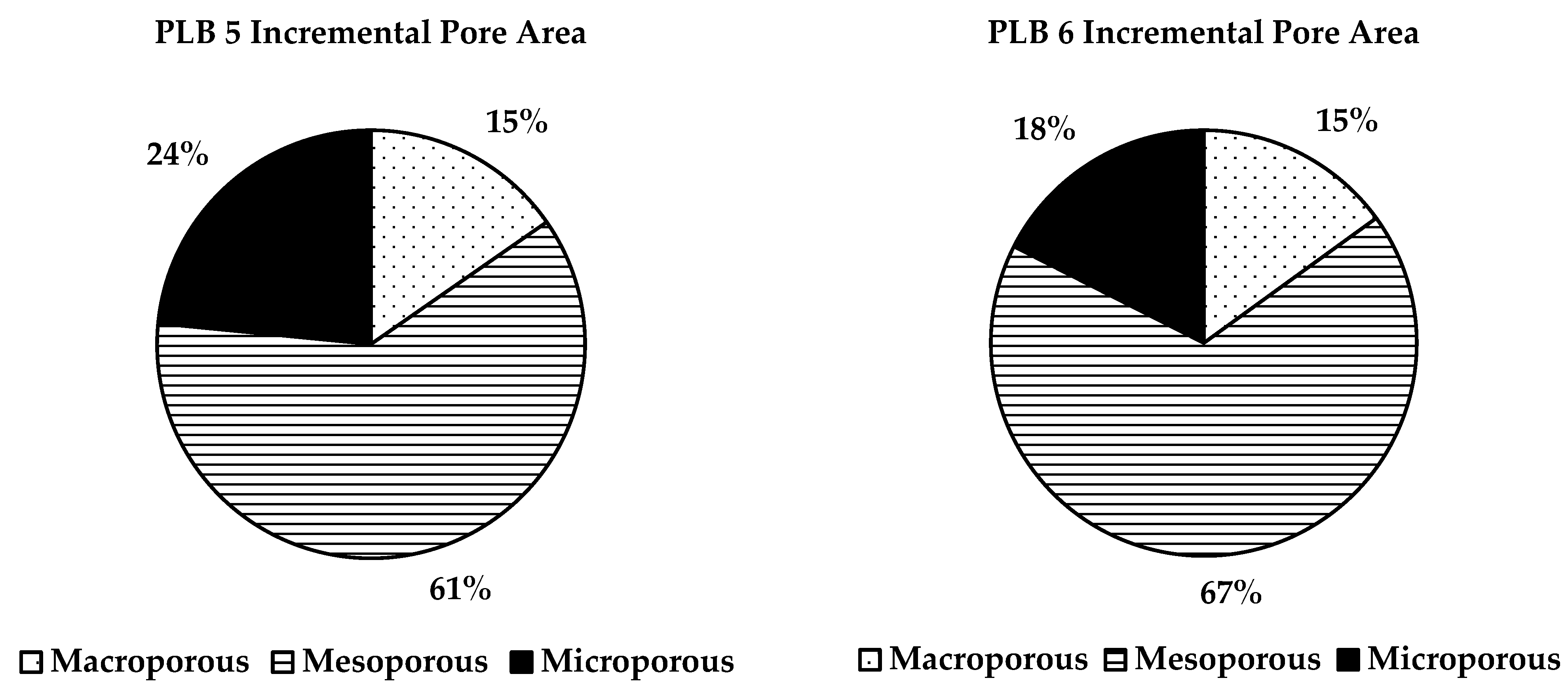
3.4. BET Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity evaluation and emission characteristics of poultry litter co-combustion process. Appl. Sci. 2019, 9, 4116. [Google Scholar] [CrossRef]
- Parker, D.D.; Lichtenberg, E. Impacts of agricultural nutrient regulation in a heterogeneous region. In Proceedings of the American Agricultural Economics Association Annual Meeting, Denver, CO, USA, 1–4 July 2004. [Google Scholar]
- Tabeling, K. Delaware’s Chicken Industry Breaks $5B Sales. Delaware Business Times. 9 March 2023. Available online: https://delawarebusinesstimes.com/news/delawares-chicken-5b-sales/ (accessed on 6 January 2024).
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef]
- Dalólio, F.S.; da Silva, J.N.; de Oliveira, A.C.C.; Tinôco, I.D.F.F.; Barbosa, R.C.; de Oliveira Resende, M.; Albino, L.F.T.; Coelho, S.T. Poultry litter as biomass energy: A review and future perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in poultry litter disposal technology–a review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.; Bandara, W. Potential of Using Poultry Litter as a Feedstock for Energy Production; Louisiana Forest Product Development Center: Baton Rouge, LA, USA, 2010. [Google Scholar]
- Lamm, M.; Markow, L.; Bernhardt, C.; Pelton, T. Blind Eye to Big Chicken. The Environmental Integrity Project, 28 October 2021. [Google Scholar]
- Kim, S.S.; Agblevor, F.A.; Lim, J. Fast pyrolysis of chicken litter and turkey litter in a fluidized bed reactor. J. Ind. Eng. Chem. 2009, 15, 247–252. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Van der Drift, A.; Boerrigter, H. Synthesis Gas from Biomass for Fuels and Chemicals; ECN Biomass, Coal and Environmental Research: Petten, The Netherlands, 2006; pp. 1–31. [Google Scholar]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Zolfi Bavariani, M.; Ronaghi, A.; Ghasemi, R. Influence of pyrolysis temperatures on FTIR analysis, nutrient bioavailability, and agricultural use of poultry manure biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for soil amendment. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. [Google Scholar]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application research of biochar for the remediation of soil heavy metals contamination: A review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef]
- Lima, I.M.; Ro, K.S.; Reddy, G.B.; Boykin, D.L.; Klasson, K.T. Efficacy of chicken litter and wood biochars and their activated counterparts in heavy metal clean up from wastewater. Agriculture 2015, 5, 806–825. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.; Lee, S.J.; Bolan, N.; Chung, J.W.; Edraki, M. Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water Air Soil Pollut. 2013, 224, 1711. [Google Scholar] [CrossRef]
- Sri Shalini, S.; Palanivelu, K.; Ramachandran, A.; Raghavan, V. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Convers. Biorefinery 2021, 11, 2247–2267. [Google Scholar] [CrossRef]
- Man, K.Y.; Chow, K.L.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Use of biochar as feed supplements for animal farming. Crit. Rev. Environ. Sci. Technol. 2021, 51, 187–217. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, S.; Su, C. Impact of biochar on the bioremediation and phytoremediation of heavy metal (loid)s in soil. In Advances in Bioremediation and Phytoremediation; IntechOpen: London, UK, 2018; Volume 149. [Google Scholar]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 139–163. [Google Scholar]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Joardar, J.C.; Mondal, B.; Sikder, S. Comparative study of poultry litter and poultry litter biochar application in the soil for plant growth. SN Appl. Sci. 2020, 2, 1770. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chang, Y.F. Quality evaluation of poultry litter biochar produced at different pyrolysis temperatures as a sustainable management approach and its impact on soil carbon mineralization. Agronomy 2021, 11, 1692. [Google Scholar] [CrossRef]
- Pereira, M.E.; Varanda, L.D.; de Carvalho, N.R.; Sette, C.R., Jr.; de Padua, F.A.; De Conti, A.C.; Yamaji, F.M. Biochar produced from poultry litter waste. Res. Soc. Dev. 2021, 10, e351101119704. [Google Scholar] [CrossRef]
- Clarke, J.; Olea, M. The Effect of Temperature and Treatment Regime on the Physical, Chemical, and Biological Properties of Poultry Litter Biochar. Preprints 2024, 2024030998. [Google Scholar] [CrossRef]
- ASTM D5373; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D3302; Standard Test Method for Total Moisture in Coal. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D3173; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D3174; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D3175; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D5865; Standard Test Method for Gross Calorific Value of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D5864; Standard Test Method for Determining Aerobic Aquatic Biodegradation of Lubricants or Their Components. ASTM International: West Conshohocken, PA, USA, 2023.
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- ISO 9277; Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. International Standard Organization: Geneva, Switzerland, 2022.
- Singh, S.V.; Chaturvedi, S.; Dhyani, V.C.; Kasivelu, G. Pyrolysis temperature influences the characteristics of rice straw and husk biochar and sorption/desorption behaviour of their biourea composite. Bioresour. Technol. 2020, 314, 123674. [Google Scholar]
- ASTM D4972; Standard Test Method for pH of Soils. ASTM International: West Conshohocken, PA, USA, 2019.
- Skogley, E.O. The universal bioavailability environment/soil test unibest. Commun. Soil Sci. Plant Anal. 1992, 23, 2225–2246. [Google Scholar] [CrossRef]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.M.; Abdulaha-Al Baquy, M.; Akhter, S.; Sen, R.; Barman, A.; Khatun, M.R. Liming effects of poultry litter derived biochar on soil acidity amelioration and maize growth. Ecotoxicol. Environ. Saf. 2020, 202, 110865. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; Melo, I.C.N.D.; Melo, L.C.; Magriotis, Z.M.; Sanchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- IBI-STD-2.1; Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. International Biochar Initiative: Canandaigua, NY, USA, 2015; p. 23.
- Kim, P.; Johnson, A.; Edmunds, C.W.; Radosevich, M.; Vogt, F.; Rials, T.G.; Labbé, N. Surface functionality and carbon structures in lignocellulosic-derived biochars produced by fast pyrolysis. Energy Fuels 2011, 25, 4693–4703. [Google Scholar] [CrossRef]
- Tang, M.M.; Bacon, R. Carbonization of cellulose fibers—I. Low temperature pyrolysis. Carbon 1964, 2, 211–220. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xinag, J.; Sun, L.; Yang, T.; Zhang, A.; Wang, Y.; Chen, G. Effects of pyrolysis temperature on characteristics of porosity in biomass chars. In Proceedings of the 2009 International Conference on Energy and Environment Technology, Guilin, China, 16–18 October 2009; Volume 1, pp. 109–112. [Google Scholar]
- Huang, H.; Reddy, N.G.; Huang, X.; Chen, P.; Wang, P.; Zhang, Y.; Huang, Y.; Lin, P.; Garg, A. Effects of pyrolysis temperature, feedstock type and compaction on water retention of biochar amended soil. Sci. Rep. 2021, 11, 7419. [Google Scholar] [CrossRef]
- Keskinen, R.; Hyväluoma, J.; Sohlo, L.; Help, H.; Rasa, K. Fertilizer and soil conditioner value of broiler manure biochars. Biochar 2019, 1, 259–270. [Google Scholar] [CrossRef]
- Deinert, L.; Hossen, S.; Ikoyi, I.; Kwapinksi, W.; Noll, M.; Schmalenberger, A. Poultry litter biochar soil amendment affects microbial community structures, promotes phosphorus cycling and growth of barley (Hordeum vulgare). Eur. J. Soil Biol. 2024, 120, 103591. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Harris, K.; Gaskin, J.; Das, K.C. The nitrogen contained in carbonized poultry litter is not plant available. Open Agric. 2018, 3, 284–290. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Lu, S. Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Effect of one-step and two-step H3PO4 activation on activated carbon characteristics. Bioresour. Technol. Rep. 2019, 8, 100307. [Google Scholar] [CrossRef]


| Experimental Condition | PL Source | Bedding | Reaction Temperature | Serial No. |
|---|---|---|---|---|
| 1 | Bethel | No bedding | 300 °C | PLB 1 |
| 2 | Bethel | No bedding | 500 °C | PLB 2 |
| 3 | Bethel | 10% wood | 300 °C | PLB 3 |
| 4 | Bethel | 10% wood | 500 °C | PLB 4 |
| 5 | Sun | No bedding | 300 °C | PLB 5 |
| 6 | Sun | No bedding | 500 °C | PLB 6 |
| 7 | Sun | 10% wood | 300 °C | PLB 7 |
| 8 | Sun | 10% wood | 500 °C | PLB 8 |
| Source | PL | PL | PS 1 | PLB | |
|---|---|---|---|---|---|
| Sun Farm | Bethel Farm | Bedding | Bethel Farm | ||
| Ultimate analysis (dry-based, wt.%) | C | 27.86 | 28.01 | 43.42 | 34.48 |
| H | 6.28 | 5.97 | 8.39 | 1.77 | |
| O | 33.49 | 27.06 | <25.54 | 4.99 | |
| N | 4.02 | 4.54 | <0.2 | 4.28 | |
| S | 1.72 | 1.42 | 0.06 | 2.06 | |
| Proximate analysis (dry-based, wt.%) | VM 2 | 65.31 | 57.94 | 69.6 | 23.36 |
| FC 3 | 8.06 | 9.06 | 8.01 | 24.23 | |
| A 4 | 26.63 | 33.00 | 22.39 | 52.42 | |
| HHV 5 | 13,782 | 13,698 | 19,745 | 14,596 | |
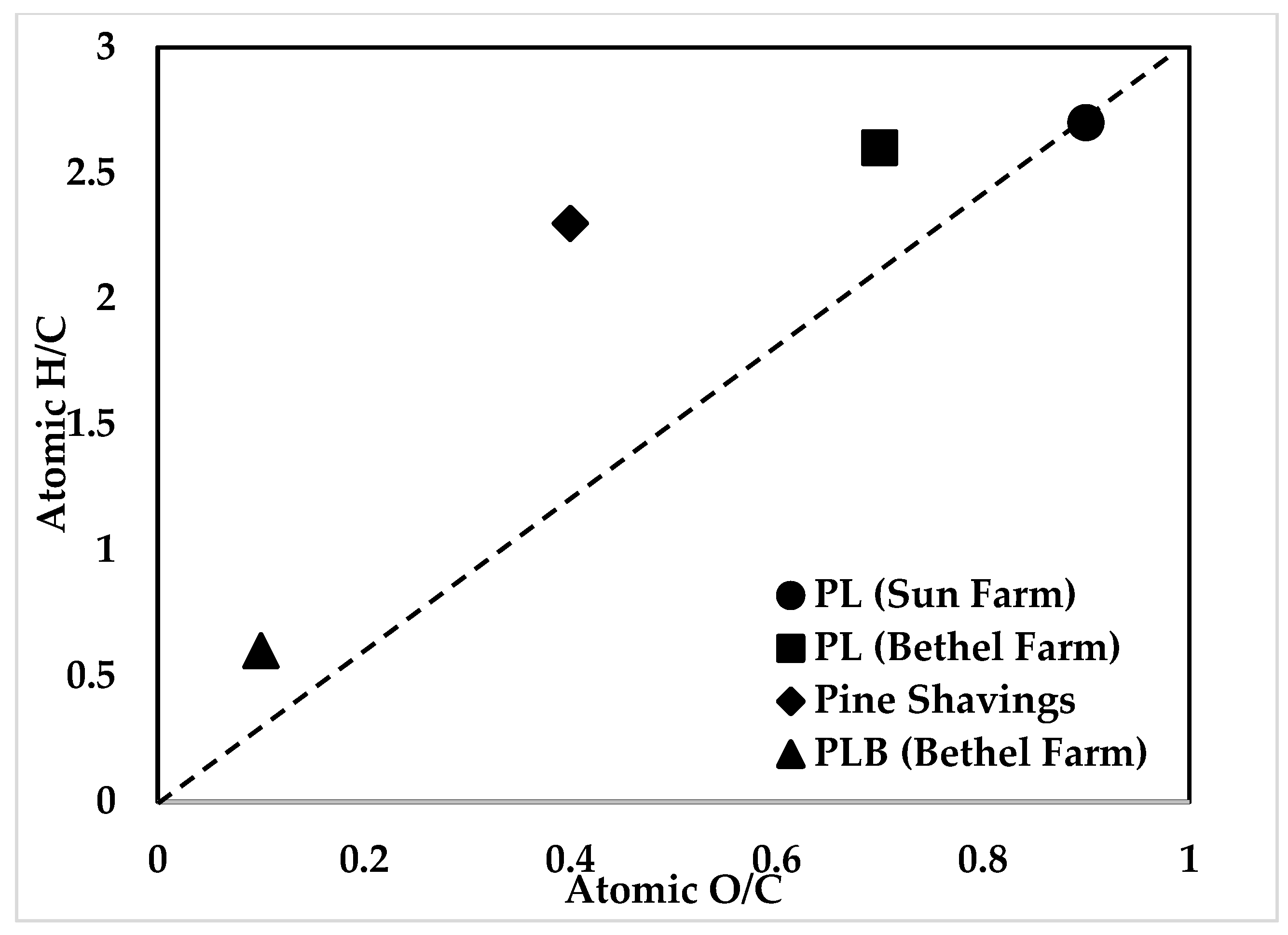
| Atomic ratio | O/C | 0.9 | 0.7 | 0.4 | 0.1 |
| H/C | 2.7 | 2.6 | 2.3 | 0.6 |
| Series No. | C | O | N | K | Ca | P | S | Minor Elements |
|---|---|---|---|---|---|---|---|---|
| PLB 1 | 37.91 | 30.21 | 12.32 | 5.61 | 3.63 | 2.51 | 1.99 | 5.81 |
| PLB 2 | 28.70 | 26.56 | 9.77 | 14.34 | 5.19 | 2.89 | 6.13 | 6.42 |
| PLB 3 | 38.52 | 27.03 | 13.63 | 5.64 | 5.25 | 1.89 | 1.71 | 6.32 |
| PLB 4 | 37.73 | 25.28 | 10.34 | 8.58 | 4.51 | 3.50 | 2.93 | 7.13 |
| PLB 5 | 34.46 | 27.64 | 13.46 | 7.33 | 6.40 | 3.32 | 2.27 | 5.12 |
| PLB 6 | 36.46 | 24.95 | 9.52 | 9.52 | 6.42 | 2.4 | 2.71 | 8.02 |
| PLB 7 | 36.78 | 30.58 | 11.91 | 5.65 | 3.92 | 3.15 | 2.48 | 5.53 |
| PLB 8 | 47.70 | 19.16 | 8.84 | 9.01 | 4.33 | 2.58 | 3.21 | 5.16 |
| Total N | P | K | S | |
|---|---|---|---|---|
| PLB 1 | 1.69 | 19.68 | 892.59 | 245.47 |
| PLB 2 | 1.61 | 10.13 | 954.78 | 235.98 |
| PLB 3 | 1.61 | 26.82 | 1110.03 | 406.25 |
| PLB 4 | 1.61 | 5.98 | 875.62 | 261.23 |
| PLB 5 | 3.97 | 19.95 | 1610.11 | 499.23 |
| PLB 6 | 13.79 | 25.87 | 1715.16 | 571.96 |
| PLB 7 | 1.61 | 8.47 | 1021.24 | 302.09 |
| PLB 8 | 1.61 | 4.9 | 1329.36 | 308.07 |
| Average: | 3.4 | 15.2 | 1188.6 | 353.8 |
| PL (Sun Farm) | 69.13 | 55.74 | 1260 | 577.86 |
| Soil (near Bethel Farm) | 9.14 | 137.32 | 19.32 | 8.37 |
| Suggested optional range | 32.0–60.0 | 8.0–20.0 | 38.0–80.0 | 7.0–22.0 |
| Sample | BET Surface Area (m2/g) | Adsorption Cumulative Volume (m3/g) | Adsorption Average Pore Diameter (nm) |
|---|---|---|---|
| PLB 1 | 5.71 | 0.0196 | 13.59 |
| PLB 2 | 18.82 | 0.0589 | 12.74 |
| PLB 5 | 5.30 | 0.0219 | 16.54 |
| PLB 6 | 7.31 | 0.0253 | 14.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Qian, X.; Alamu, S.O.; Brown, K.; Lee, S.W.; Kang, D.-H. Qualities and Quantities of Poultry Litter Biochar Characterization and Investigation. Energies 2024, 17, 2885. https://doi.org/10.3390/en17122885
Yang Y, Qian X, Alamu SO, Brown K, Lee SW, Kang D-H. Qualities and Quantities of Poultry Litter Biochar Characterization and Investigation. Energies. 2024; 17(12):2885. https://doi.org/10.3390/en17122885
Chicago/Turabian StyleYang, Yulai, Xuejun Qian, Samuel O. Alamu, Kayla Brown, Seong W. Lee, and Dong-Hee Kang. 2024. "Qualities and Quantities of Poultry Litter Biochar Characterization and Investigation" Energies 17, no. 12: 2885. https://doi.org/10.3390/en17122885
APA StyleYang, Y., Qian, X., Alamu, S. O., Brown, K., Lee, S. W., & Kang, D.-H. (2024). Qualities and Quantities of Poultry Litter Biochar Characterization and Investigation. Energies, 17(12), 2885. https://doi.org/10.3390/en17122885










