Combined Effect of Pressure and Temperature on Nitrogen Reduction Reaction in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrodes
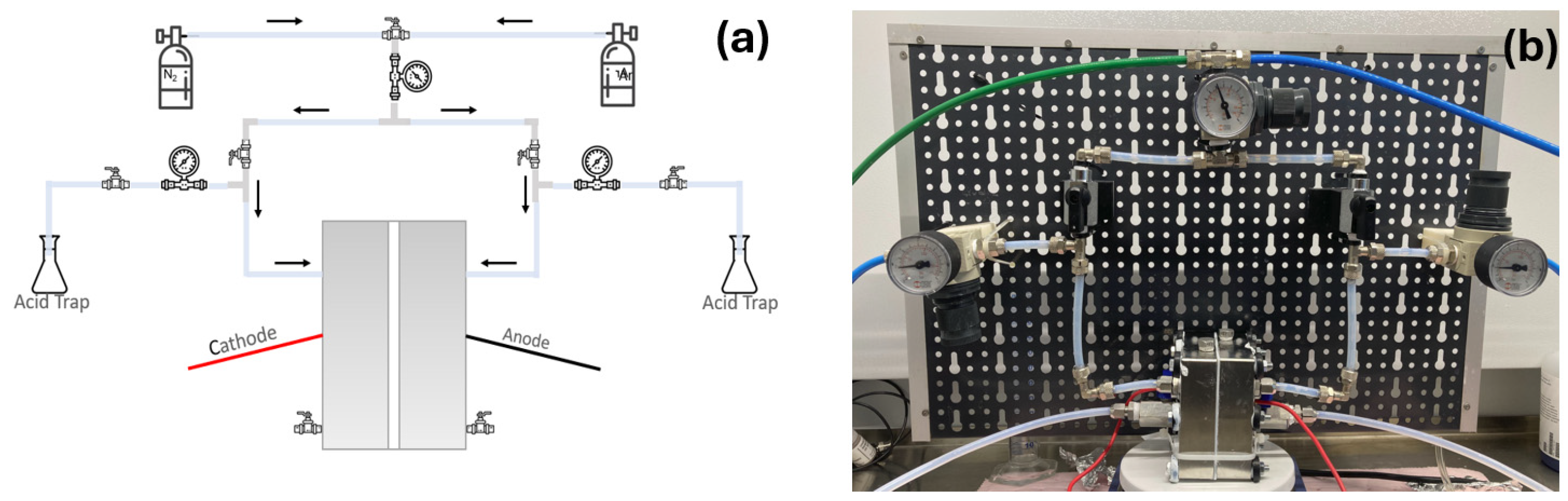
2.2. Pressurized Electrochemical H-Cell
2.3. Electrochemical Ammonia Synthesis
2.4. Ammonia Detection
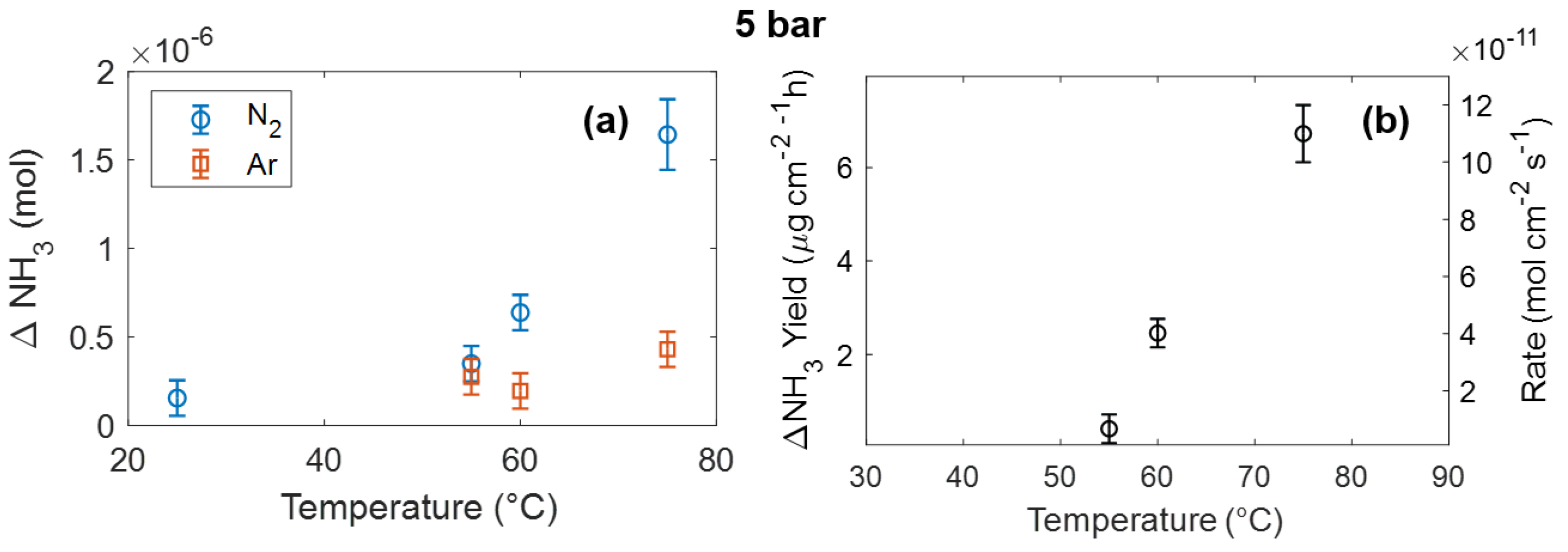
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thamdrup, B. New Pathways and Processes in the Global Nitrogen Cycle. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 407–428. [Google Scholar] [CrossRef]
- Liu, H. Ammonia synthesis catalyst: 100 years: Practice, enlightenment and challenge. Chin. J. Catal. 2014, 35, 1619–1640. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vasileiou, E.; Vourros, A.; Stoukides, M. Progress in the Electrochemical Synthesis of Ammonia. Catal. Today 2017, 286, 2–13. [Google Scholar] [CrossRef]
- Singh, A.R.; Rohr, B.A.; Schwalbe, J.A.; Cargnello, M.; Chan, K.; Jaramillo, T.F.; Chorkendorff, I.; Nørskov, J.K. Electrochemical Ammonia Synthesis—The Selectivity Challenge. ACS Catal. 2017, 7, 706–709. [Google Scholar] [CrossRef]
- Ouyang, L.; Liang, J.; Luo, Y.; Zheng, D.; Sun, S.; Liu, Q.; Hamdy, M.S.; Sun, X.; Ying, B. Recent Advances in Electrocatalytic Ammonia Synthesis. Chin. J. Catal. 2023, 50, 6–44. [Google Scholar] [CrossRef]
- Shafiq, F.; Yang, L.; Zhu, W. Recent progress in the advanced strategies, rational design, and engineering of electrocatalysts for nitrate reduction toward ammonia. Phys. Chem. Chem. Phys. 2024, 26, 11208–11216. [Google Scholar] [CrossRef]
- Wonsang, J.; Hwang, Y.J. Material strategies in the electrochemical nitrate reduction reaction to ammonia production. Mater. Chem. Front. 2021, 5, 6803–6823. [Google Scholar]
- Rao, X.; Liu, M.; Chien, M.; Inoue, C.; Zhang, J.; Liu, Y. Recent progress in noble metal electrocatalysts for nitrogen-to-ammonia conversion. Renew. Sustain. Energy Rev. 2022, 168, 112845. [Google Scholar] [CrossRef]
- Santhosh, C.R.; Sankannavar, R. A comprehensive review on electrochemical green ammonia synthesis: From conventional to distinctive strategies for efficient nitrogen fixation. Appl. Energy 2023, 352, 121960. [Google Scholar] [CrossRef]
- Arroyo-Caire, J.; Diaz-Perez, M.A.; Lara-Angulo, M.A.; Serrano-Ruiz, J.C. A Conceptual Approach for the Design of New Catalysts for Ammonia Synthesis: A Metal—Support Interactions Review. Nanomaterials 2023, 13, 2914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xie, H.; Chang, L.; Zhang, X.; Zhu, X.; Tong, X.; Wang, T.; Luo, Y.; Wei, P.; Wang, Z.; et al. Recent progress in the electrochemical ammonia synthesis under ambient conditions. EnergyChem 2019, 1, 100011. [Google Scholar] [CrossRef]
- Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J.K. A Theoretical Evaluation of Possible Transition Metal Electro-Catalysts for N2 Reduction. Phys. Chem. Chem. Phys. 2011, 14, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Gruber, N.; Galloway, J.N. An Earth-System Perspective of the Global Nitrogen Cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Rong, W.; Wei, S.; Duan, L. Regulating kinetics and thermodynamics of electrochemical nitrogen reduction with metal single atom catalysts in a pressurized electrolyzer. Proc. Natl. Acad. Sci. USA 2020, 117, 29462–29468. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Shaigan, N.; Malek, A.; Fatih, K.; Gyenge, E.; Wilkinson, D.P. Strategies in cell design and operation for the electrosynthesis of ammonia: Status and prospects. Energy Environ. Sci. 2022, 15, 2259–2287. [Google Scholar] [CrossRef]
- Kolen, M.; Ripepi, D.; Smith, W.A.; Burdyny, T.; Mulder, F.M. Overcoming Nitrogen Reduction to Ammonia Detection Challenges: The Case for Leapfrogging to Gas Diffusion Electrode Platforms. ACS Catal. 2022, 12, 5726–5735. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, G.; Milazzo, R.G.; Leonardi, M.; Scalese, S.; Farina, R.A.; Lombardo, S.; Privitera, S.M.S. Ultra-Low Loading of Gold on Nickel Foam for Nitrogen Electrochemistry. Nanomaterials 2023, 13, 2850. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, R.G.; Privitera, S.M.S.; D’Angelo, D.; Scalese, S.; Di Franco, S.; Maita, F.; Lombardo, S. Spontaneous galvanic displacement of Pt nanostructures on nickel foam: Synthesis, characterization and use for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 7903–7910. [Google Scholar] [CrossRef]
- Leonardi, M.; Tranchida, G.; Corso, R.; Milazzo, R.G.; Lombardo, S.A.; Privitera, S.M.S. Role of the Membrane Transport Mechanism in Electrochemical Nitrogen Reduction Experiments. Membranes 2022, 12, 969. [Google Scholar] [CrossRef]
- Cai, X.; Iriawan, H.; Yang, F.; Luo, L.; Shen, S.; Shao-Horn, Y.; Zhang, J. Interaction of Ammonia with Nafion and Electrolyte in Electrocatalytic Nitrogen Reduction Study. J. Phys. Chem. Lett. 2021, 12, 6861–6866. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, G.; Milazzo, R.G.; Leonardi, M.; Scalese, S.; Pulvirenti, L.; Condorelli, G.G.; Bongiorno, C.; Lombardo, S.; Privitera, S.M.S. Strategies to Improve the Catalytic Activity of Fe-Based Catalysts for Nitrogen Reduction Reaction. Int. J. Hydrogen Energy 2023, 48, 25328–25338. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, L.; Ruther, R.E.; Hamers, R.J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 2013, 12, 836–841. [Google Scholar] [CrossRef] [PubMed]
- NIST Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 10 January 2024).
- Vanderzee, C.; King, D.L. The enthalpies of solution and formation of ammonia. J. Chem. Thermodyn. 1972, 4, 675–683. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Wang, Z.; Li, Y.; Xu, Y.; Li, X.; Xue, H.; Wang, L. Electrochemical fabrication of porous Au film on Ni foam for nitrogen reduction to ammonia. Small 2019, 15, 1804769. [Google Scholar] [CrossRef]
- Chen, D.; Luo, M.; Ning, S.; Lan, J.; Peng, W.; Lu, Y.; Chan, T.; Tan, Y. Single-Atom Gold Isolated Onto Nanoporous MoSe2 for Boosting Electrochemical Nitrogen Reduction. Small 2022, 18, 2104043. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tranchida, G.; Milazzo, R.G.; Lombardo, S.A.; Privitera, S.M.S. Combined Effect of Pressure and Temperature on Nitrogen Reduction Reaction in Water. Energies 2024, 17, 2963. https://doi.org/10.3390/en17122963
Tranchida G, Milazzo RG, Lombardo SA, Privitera SMS. Combined Effect of Pressure and Temperature on Nitrogen Reduction Reaction in Water. Energies. 2024; 17(12):2963. https://doi.org/10.3390/en17122963
Chicago/Turabian StyleTranchida, Giuseppe, Rachela G. Milazzo, Salvatore A. Lombardo, and Stefania M. S. Privitera. 2024. "Combined Effect of Pressure and Temperature on Nitrogen Reduction Reaction in Water" Energies 17, no. 12: 2963. https://doi.org/10.3390/en17122963





