PEMFC Electrochemical Degradation Analysis of a Fuel Cell Range-Extender (FCREx) Heavy Goods Vehicle after a Break-In Period
Abstract
:1. Introduction
2. Methodology
2.1. Vehicle Dynamic Parameters
2.2. PEMFC Preparation, Specifications, and Operating Conditions
2.3. Drive Cycle Endurance Testing for PEMFCs
2.4. Power Cycle Division for a Range-Extender Vehicle
2.5. Downscaling Stack-Level Power Cycles to Cell-Level Power Cycles
2.6. ECSA Calculation
2.7. EIS Equivalent Circuit Model Fitting
3. Results and Discussion
3.1. MPML
3.2. MPNL
3.3. NPML
3.4. NPNL
3.5. MPML vs. MPNL vs. NPML vs. NPNL
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clifford, J. Running-In a New Car. Toyota UK Magazine, 1 March 2018. Available online: https://mag.toyota.co.uk/running-in-new-car/(accessed on 17 April 2024).
- Desantes, J.M.; Novella, R.; Pla, B.; Lopez-Juarez, M. Effect of dynamic and operational restrictions in the energy management strategy on fuel cell range extender electric vehicle performance and durability in driving conditions. Energy Convers. Manag. 2022, 266, 115821. [Google Scholar] [CrossRef]
- Molina, S.; Novella, R.; Pla, B.; Lopez-Juarez, M. Optimization and sizing of a fuel cell range extender vehicle for passenger car applications in driving cycle conditions. Appl. Energy 2021, 285, 116469. [Google Scholar] [CrossRef]
- Advanced Propulsion Centre UK. Going the Distance: The Tevva Hydrogen Range Extender; Advanced Propulsion Centre UK: Warwick, UK, 2024; Available online: https://www.apcuk.co.uk/impact/case-studies/tevva-hydrogen-range-extender/ (accessed on 16 April 2024).
- Chandran, M.; Palaniswamy, K.; Karthik Babu, N.B.; Das, O. A study of the influence of current ramp rate on the performance of polymer electrolyte membrane fuel cell. Sci. Rep. 2022, 12, 21888. [Google Scholar] [CrossRef]
- Qin, C.; Wang, J.; Yang, D.; Li, B.; Zhang, C. Proton exchange membrane fuel cell reversal: A review. Catalysts 2016, 6, 197. [Google Scholar] [CrossRef]
- Meng, K.; Zhou, H.; Chen, B.; Tu, Z. Dynamic current cycles effect on the degradation characteristic of a H2/O2 proton exchange membrane fuel cell. Energy 2021, 224, 120168. [Google Scholar] [CrossRef]
- Di Yang, J.; Millichamp, J.; Suter, T.; Shearing, P.R.; Brett, D.J.L.; Robinson, J.B. A Review of Drive Cycles for Electrochemical Propulsion. Energies 2023, 16, 6552. [Google Scholar] [CrossRef]
- Di Yang, J.; Shearing, P.R.; Millichamp, J.; Suter, T.; Brett, D.J.L.; Robinson, J.B. An adaptive fuel cell hybrid vehicle propulsion sizing model. iEnergy 2024, 3, 59–72. [Google Scholar] [CrossRef]
- Lion Electric. LION8 Tractor Technical Specifications; Lion Electric: Quebec, QC, Canada, 2023; Available online: https://thelionelectric.com/documents/en/LionTruck-SpecSheet-202305-SCREEN-ENUS.pdf (accessed on 17 April 2024).
- Interreg. TRANSPOWER: Electric Drayage Truck with Fuel Cell Range Extender. H2-Share. 2017. Available online: https://fuelcelltrucks.eu/project/transpower-electric-drayage-truck-with-fuel-cell-range-extender/ (accessed on 17 April 2024).
- Fotouhi, A.; Shateri, N.; Shona Laila, D.; Auger, D.J. Electric vehicle energy consumption estimation for a fleet management system. Int. J. Sustain. Transp. 2020, 15, 40–54. [Google Scholar] [CrossRef]
- Hack, J.; García-Salaberri, P.A.; Kok, M.D.R.; Jervis, R.; Shearing, P.R.; Brandon, N.; Brett, D.J. X-ray Micro-Computed Tomography of Polymer Electrolyte Fuel Cells: What is the Representative Elementary Area? J. Electrochem. Soc. 2020, 167, 013545. [Google Scholar] [CrossRef]
- Tsotridis, G.; Pilenga, A.; De Marco, G.; Malkow, T.; European Commission; Joint Research Centre. EU Harmonised Test Protocols for PEMFC MEA Testing in Single Cell Configuration for Automotive Applications; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Pielecha, I. Modeling of Fuel Cells Characteristics in Relation to Real Driving Conditions of FCHEV Vehicles. Energies 2022, 15, 6753. [Google Scholar] [CrossRef]
- Wang, Y.; Moura, S.J.; Advani, S.G.; Prasad, A.K. Power management system for a fuel cell/battery hybrid vehicle incorporating fuel cell and battery degradation. Int. J. Hydrogen Energy 2019, 44, 8479–8492. [Google Scholar] [CrossRef]
- Suter, T.A.M.; Smith, K.; Hack, J.; Rasha, L.; Rana, Z.; Angel, G.M.A.; Shearing, P.R.; Miller, T.S.; Brett, D.J. Engineering Catalyst Layers for Next-Generation Polymer Electrolyte Fuel Cells: A Review of Design, Materials, and Methods. Adv. Energy Mater. 2021, 11, 2101025. [Google Scholar] [CrossRef]
- Rezaei Niya, S.M.; Hoorfar, M. Study of proton exchange membrane fuel cells using electrochemical impedance spectroscopy technique—A review. J. Power Sources 2013, 240, 281–293. [Google Scholar] [CrossRef]
- Santana, J.; Espinoza-Andaluz, M.; Li, T.; Andersson, M. A Detailed Analysis of Internal Resistance of a PEFC Comparing High and Low Humidification of the Reactant Gases. Front. Energy Res. 2020, 8, 572333. [Google Scholar] [CrossRef]
- Kang, R.J.; Chen, Y.S. Experimental study on the effect of hydrogen sulfide on high-temperature proton exchange membrane fuel cells by using electrochemical impedance spectroscopy. Catalysts 2018, 8, 441. [Google Scholar] [CrossRef]
- Kurtz, J.; Dinh, H.; Saur, G.; Ainscough, C. Fuel Cell Technology Status: Degradation; NREL: Washington, DC, USA, 2017. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/review17/fc081_kurtz_2017_o.pdf (accessed on 17 April 2024).
- Rui, Z.; Wang, J.; Li, J.; Yao, Y.; Huo, Y.; Liu, J.; Zou, Z. A Highly Durable Quercetin-Based Proton Exchange Membrane for Fuel Cells. J. Electrochem. Soc. 2019, 166, F3052–F3057. [Google Scholar] [CrossRef]
- Tang, X.; Yang, M.; Shi, L.; Hou, Z.; Xu, S.; Sun, C. Adaptive state-of-health temperature sensitivity characteristics for durability improvement of PEM fuel cells. Chem. Eng. J. 2024, 491, 151951. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X. Hollow Micro- and Nanomaterials: Synthesis and Applications. In Advanced Nanomaterials for Pollutant Sensing and Environmental Catalysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–38. [Google Scholar] [CrossRef]

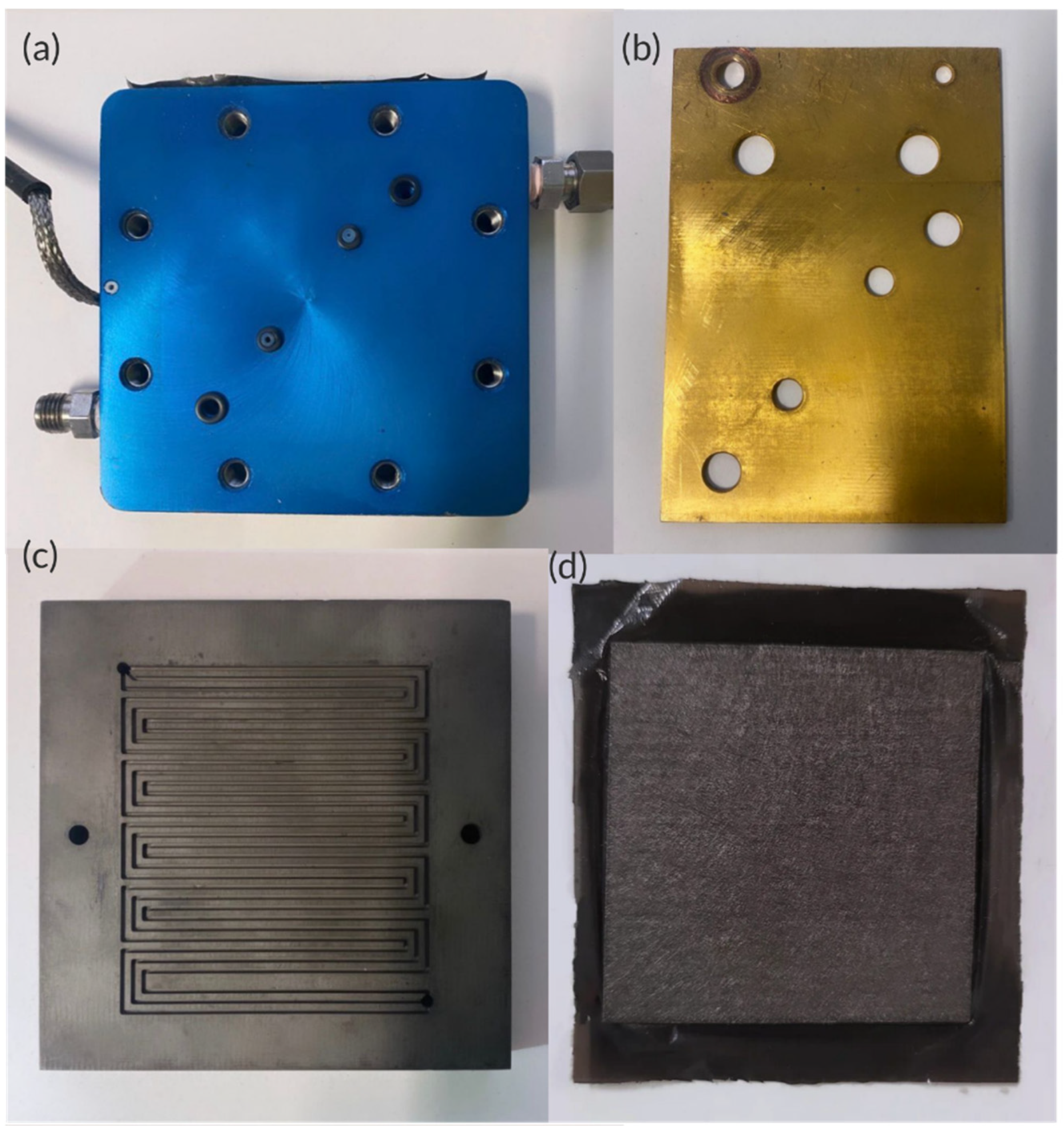












| Parameters | Description | Value |
|---|---|---|
| Cd | Drag coefficient | 0.36 |
| A | Frontal area (m2) | 9 |
| GVM | Gross vehicle mass (kg) | 37,195 |
| Air density (kg m−1) | 1.225 | |
| Static rolling coefficient | 0.008 |
| Parameter | Unit | Value |
|---|---|---|
| Cell temperature | °C | 80 |
| Anode gas inlet dew point temperature (DPT) | °C | 64 |
| Anode gas inlet absolute pressure | kPa | 250 |
| Anode stoichiometry | - | 1.3 |
| Cathode gas inlet dew point temperature (DPT) | °C | 53 |
| Cathode gas inlet absolute pressure | kPa | 230 |
| Cathode stoichiometry | - | 1.5 |
| Technique | Conditions |
|---|---|
| Polarisation curve | OCV to 0.3 V to OCV; 30 s pt−1; 0.025 V pt−1 |
| CV | 0.06 V to 1 V to 0.06 V; 20 mV/s; 0.1 s pt−1 |
| LSV | 0.06 V to 0.6 V; 5 mV s−1; 0.1 pt s−1 |
| EIS | AC 10%; 10,000 to 0.1 Hz |
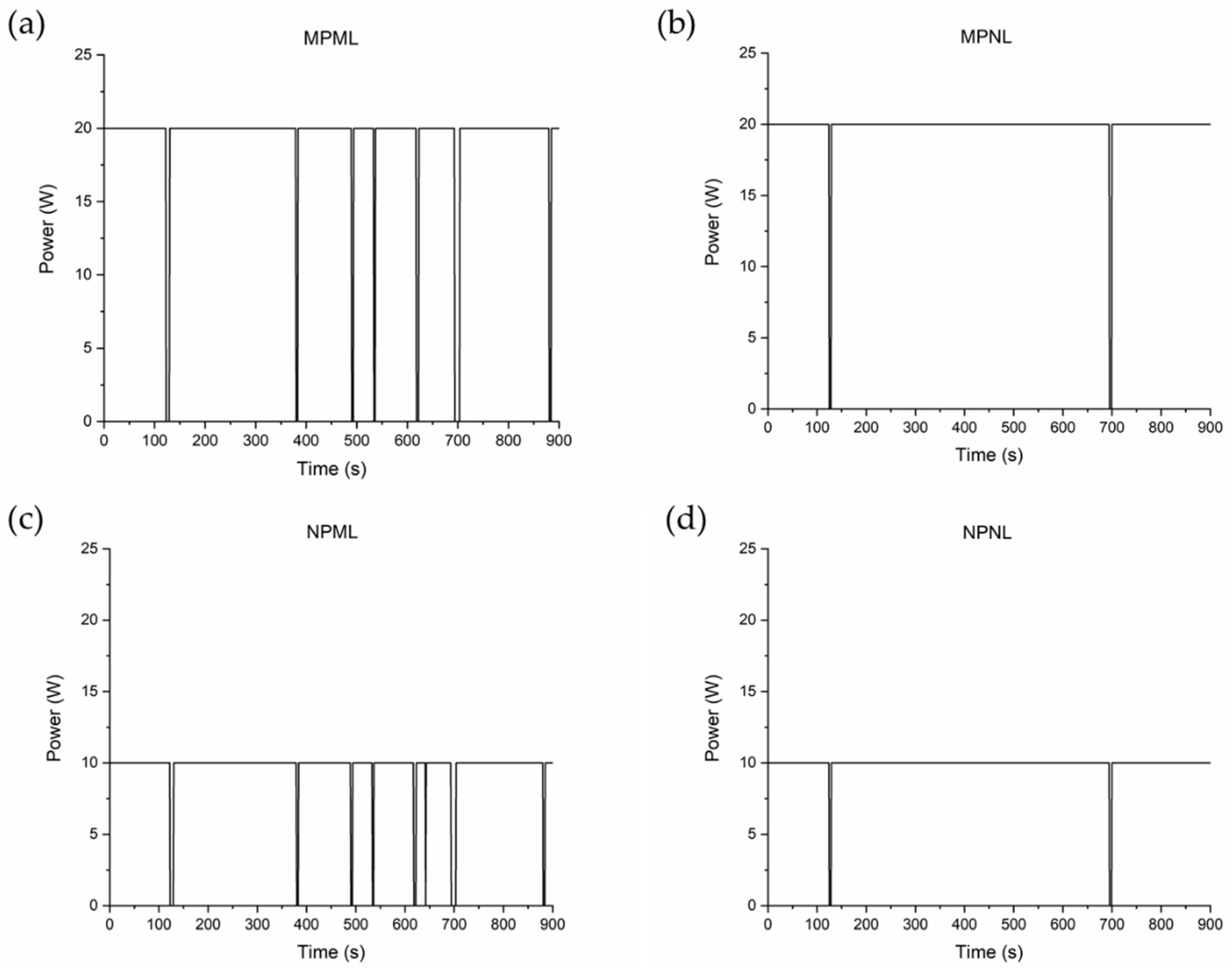
| Operation Terminology | PEMFC Maximum Power (W) | LiB Maximum Power (W) |
|---|---|---|
| MPML | 20 | 20 |
| MPNL | 20 | 10 |
| NPML | 10 | 20 |
| NPNL | 10 | 10 |
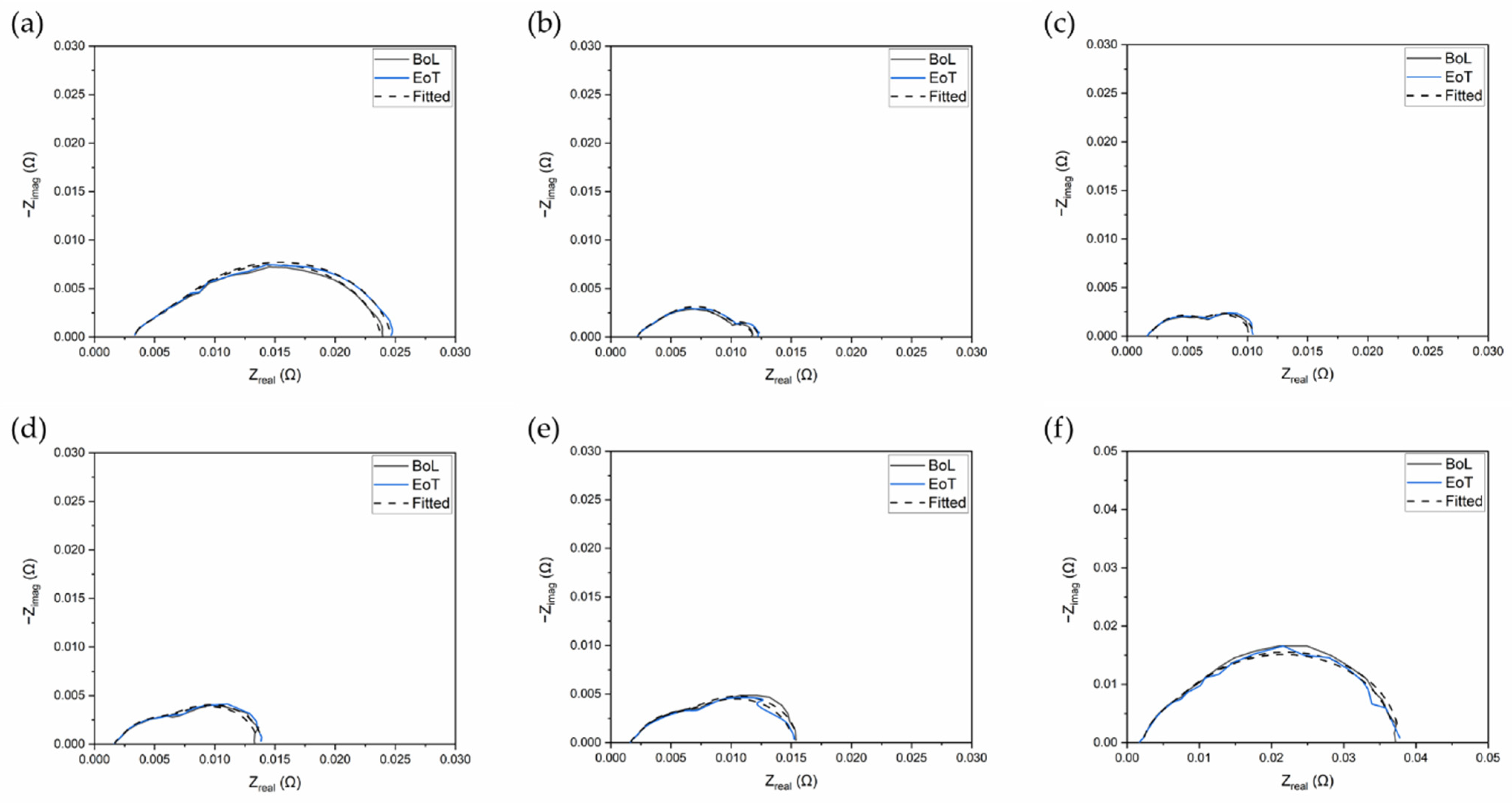
| Current Density or Voltage (mA cm−2 or V) | RΩ BoL (mΩ cm2) | RΩ EoT (mΩ cm2) | Ran BoL (mΩ cm2) | Ran EoT (mΩ cm2) | Rca BoL (mΩ cm2) | Rca EoT (mΩ cm2) | Rm BoL (mΩ cm2) | Rm EoT (mΩ cm2) |
|---|---|---|---|---|---|---|---|---|
| 100 mA cm−2 | 108 | 84.2 | 45.8 | 32.0 | 475 | 551 | 66.9 | 24.7 |
| 300 mA cm−2 | 72.6 | 64.5 | 77.4 | 79.2 | 74.4 | 94.8 | 123 | 148 |
| 800 mA cm−2 | 53.6 | 50.7 | 120 | 15.3 | 116 | 147 | 18.3 | 159 |
| 1200 mA cm−2 | 51.4 | 49.1 | 137 | 19.2 | 241 | 176 | 17.9 | 440 |
| 0.65 V | 51.5 | 49.6 | 136 | 14.6 | 195 | 152 | 16.5 | 229 |
| 0.5 V | 51.7 | 49.0 | 156 | 206 | 693 | 855 | 19.4 | 16.8 |
| Current Density or Voltage (mA cm−2 or V) | RΩ BoL (mΩ cm2) | RΩ EoT (mΩ cm2) | Ran BoL (mΩ cm2) | Ran EoT (mΩ cm2) | Rca BoL (mΩ cm2) | Rca EoT (mΩ cm2) | Rm BoL (mΩ cm2) | Rm EoT (mΩ cm2) |
|---|---|---|---|---|---|---|---|---|
| 100 mA cm−2 | 78.4 | 82.2 | 27.0 | 27.2 | 435 | 483 | 39.7 | 30.2 |
| 300 mA cm−2 | 60.0 | 63.9 | 170 | 186 | 46.2 | 50.7 | 23.0 | 24.0 |
| 800 mA cm−2 | 45.3 | 46.1 | 30.3 | 29.7 | 113 | 134 | 83.6 | 93.3 |
| 1200 mA cm−2 | 44.1 | 44.9 | 28.9 | 25.7 | 213 | 273 | 102 | 115 |
| 0.65 V | 44.1 | 45.0 | 21.9 | 11.6 | 253 | 145 | 112 | 261 |
| 0.5 V | 45.7 | 45.6 | 126 | 16.7 | 819 | 128 | 14.6 | 863 |
| Current Density or Voltage (mA cm−2 or V) | RΩ BoL (mΩ cm2) | RΩ EoT (mΩ cm2) | Ran BoL (mΩ cm2) | Ran EoT (mΩ cm2) | Rca BoL (mΩ cm2) | Rca EoT (mΩ cm2) | Rm BoL (mΩ cm2) | Rm EoT (mΩ cm2) |
|---|---|---|---|---|---|---|---|---|
| 100 mA cm−2 | 84.0 | 83.8 | 52.7 | 32.6 | 89.8 | 455 | 350 | 45.1 |
| 300 mA cm−2 | 56.9 | 57.9 | 49.6 | 183 | 51.1 | 46.3 | 133 | 22.2 |
| 800 mA cm−2 | 44.2 | 44.4 | 108 | 20.9 | 91.8 | 107 | 10.1 | 90.2 |
| 1200 mA cm−2 | 43.5 | 43.8 | 120 | 113 | 168 | 176 | 8.80 | 10.7 |
| 0.65 V | 42.3 | 43.8 | 26.2 | 116 | 224 | 193 | 96.8 | 10.9 |
| 0.5 V | 45.1 | 45.4 | 18.1 | 15.2 | 769 | 121 | 129 | 705 |
| Current Density or Voltage (mA cm−2 or V) | RΩ BoL (mΩ cm2) | RΩ EoT (mΩ cm2) | Ran BoL (mΩ cm2) | Ran EoT (mΩ cm2) | Rca BoL (mΩ cm2) | Rca EoT (mΩ cm2) | Rm BoL (mΩ cm2) | Rm EoT (mΩ cm2) |
|---|---|---|---|---|---|---|---|---|
| 100 mA cm−2 | 85.0 | 84.3 | 31.5 | 32.9 | 425 | 450 | 54.6 | 49.7 |
| 300 mA cm−2 | 57.6 | 54.7 | 175 | 68.7 | 40.5 | 55.3 | 22.0 | 128 |
| 800 mA cm−2 | 43.4 | 43.0 | 110 | 112 | 91.8 | 97.9 | 9.80 | 10.6 |
| 1200 mA cm−2 | 42.5 | 42.1 | 125 | 123 | 163 | 173 | 8.90 | 9.40 |
| 0.65 V | 42.5 | 41.7 | 124 | 141 | 209 | 187 | 12.0 | 8.20 |
| 0.5 V | 43.9 | 43.8 | 153 | 166 | 732 | 703 | 14.2 | 12.4 |
| Operating Power | Voltage Decrease at 600 mA cm−2 (%) | Voltage Decrease at 600 mA cm−2 (% h−1) | Voltage Decrease at 1200 mA cm−2 (%) | Voltage Decrease at 1200 mA cm−2 (% h−1) | Maximum Power Decrease (%) | Maximum Power Decrease (% h−1) | ECSA Decrease (%) |
|---|---|---|---|---|---|---|---|
| MPML | 1.37 | 0.055 | 3.33 | 0.133 | 15 | 0.60 | 11.68 |
| MPNL | 0 | 0 | 0 | 0 | 4.55 | 0.18 | 16.62 |
| NPML | 1.32 | 0.053 | 1.52 | 0.061 | 4.17 | 0.17 | 9.54 |
| NPNL | 0 | 0 | 1.45 | 0.058 | 4 | 0.16 | 8.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-D.; Suter, T.; Millichamp, J.; Owen, R.E.; Du, W.; Shearing, P.R.; Brett, D.J.L.; Robinson, J.B. PEMFC Electrochemical Degradation Analysis of a Fuel Cell Range-Extender (FCREx) Heavy Goods Vehicle after a Break-In Period. Energies 2024, 17, 2980. https://doi.org/10.3390/en17122980
Yang J-D, Suter T, Millichamp J, Owen RE, Du W, Shearing PR, Brett DJL, Robinson JB. PEMFC Electrochemical Degradation Analysis of a Fuel Cell Range-Extender (FCREx) Heavy Goods Vehicle after a Break-In Period. Energies. 2024; 17(12):2980. https://doi.org/10.3390/en17122980
Chicago/Turabian StyleYang, Jia-Di, Theo Suter, Jason Millichamp, Rhodri E. Owen, Wenjia Du, Paul R. Shearing, Dan J. L. Brett, and James B. Robinson. 2024. "PEMFC Electrochemical Degradation Analysis of a Fuel Cell Range-Extender (FCREx) Heavy Goods Vehicle after a Break-In Period" Energies 17, no. 12: 2980. https://doi.org/10.3390/en17122980





