CO2 Removal in Hydrogen Production Plants
Abstract
:1. Introduction
1.1. Hydrogen Production
- Heat source or as raw material for the chemical industry;
- Alternative source of fossil fuels for space heating or for power generation in thermal power plants;
- Energy storage medium to provide flexibility to the grid;
- Means of transport by the use of fuel cells or after conversion to synthetic fuels.
- To purify the gaseous stream rich in hydrogen;
- To meet environmental regulations or, generally, to reduce the greenhouse gas impact of the plant due to the emissions of CO2 to the atmosphere.
1.2. CO2 Removal
Objectives of the Study
2. The PSA Tail Gas
3. The Considered Schemes
3.1. Scheme (a)
3.2. Scheme (b)
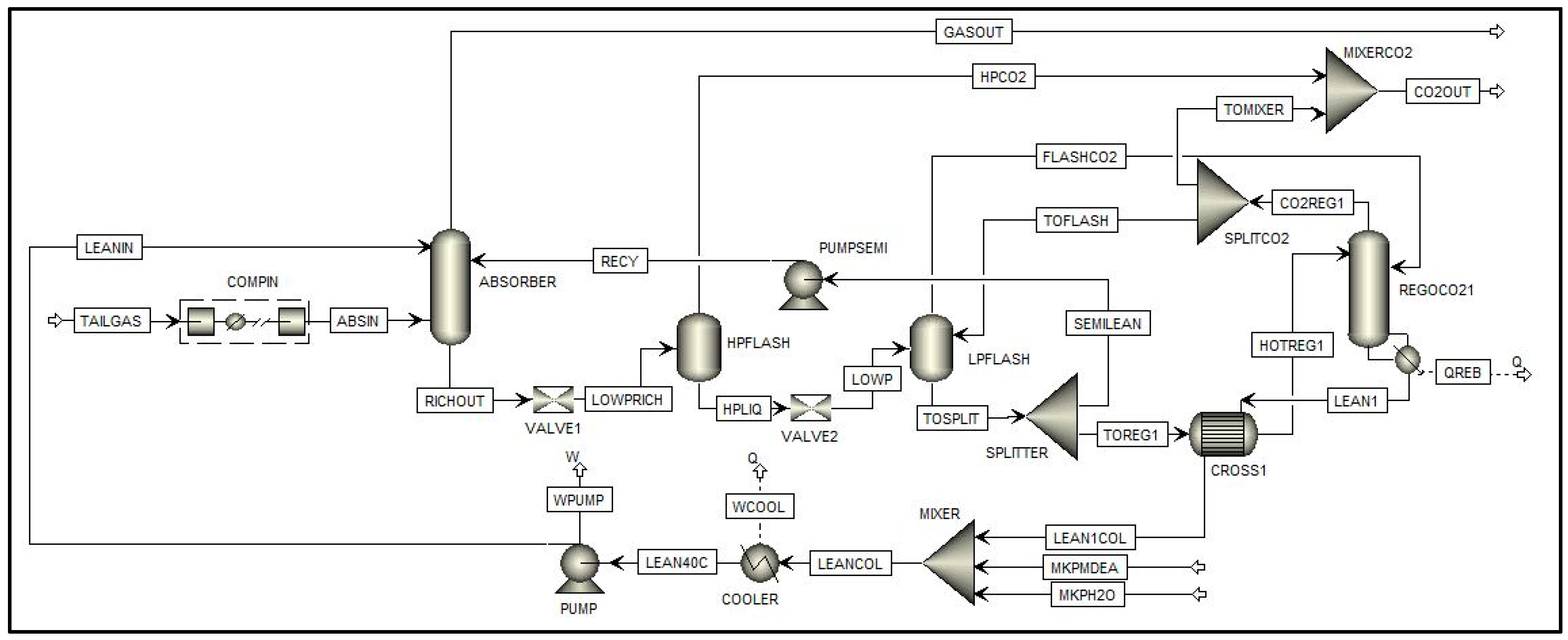
3.3. Scheme (c)
4. Methodology
4.1. Tool for Simulation and Economic Evaluation
4.2. Procedure Employed in this Study
4.2.1. Methodology Employed for the Analysis of Scheme (a)
4.2.2. Methodology Employed for the Analysis of Scheme b)
4.2.3. Methodology Employed for the Analysis of Scheme (c)
5. Results and Discussion
5.1. Scheme (a)
5.2. Scheme (b)
5.3. Scheme (c)
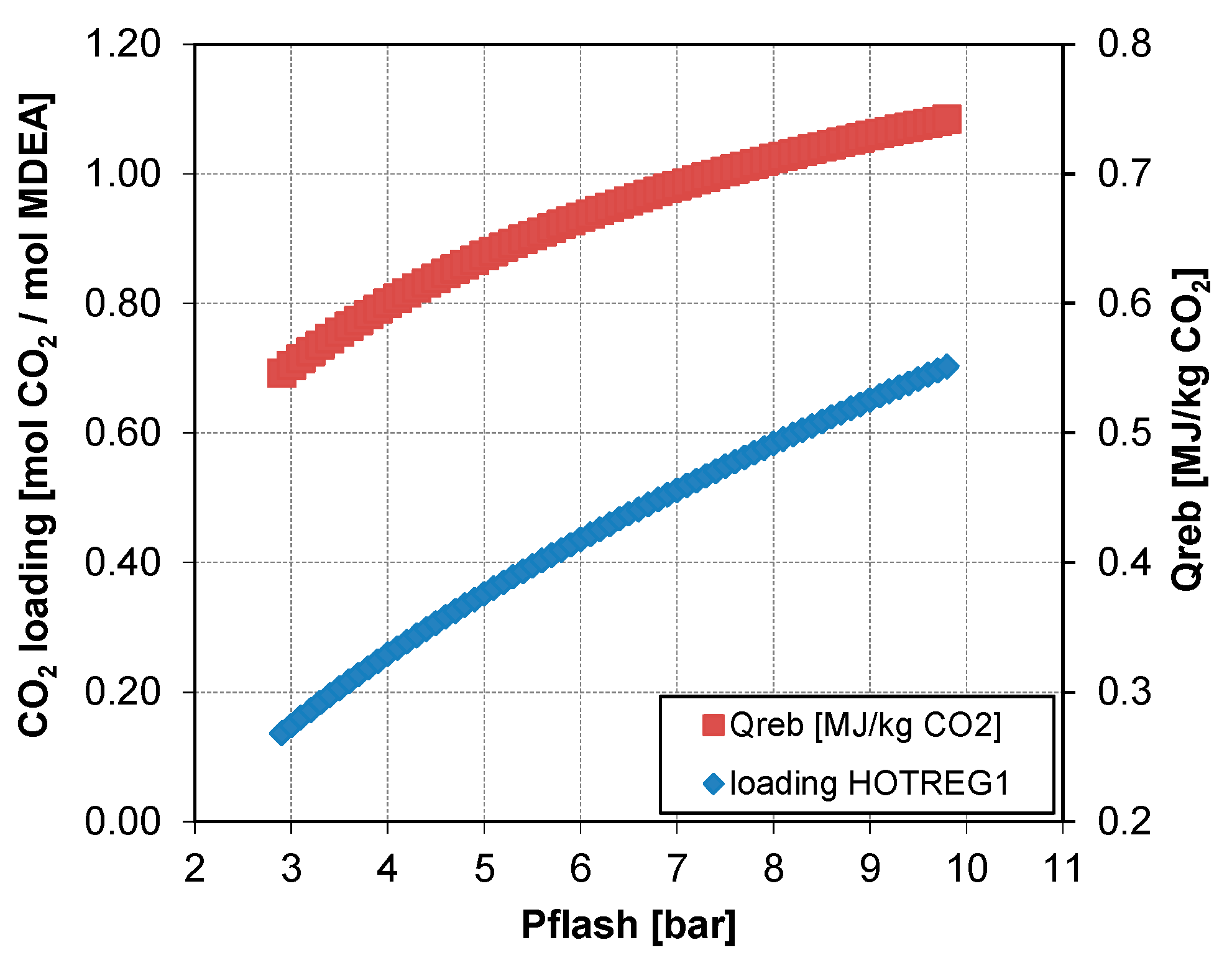
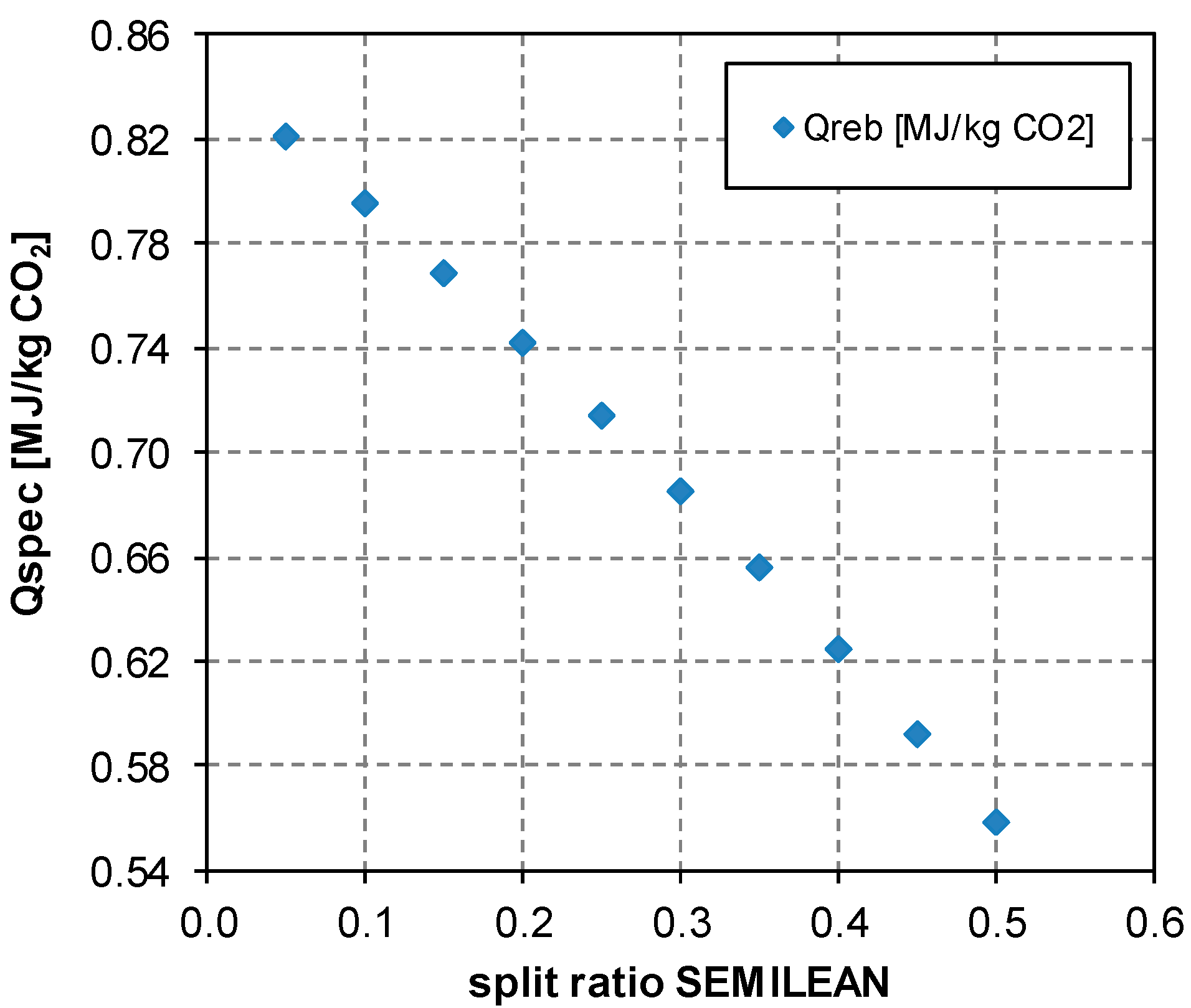
5.3.1. Analysis of the Split Fraction of the CO2-Rich Vapor Stream
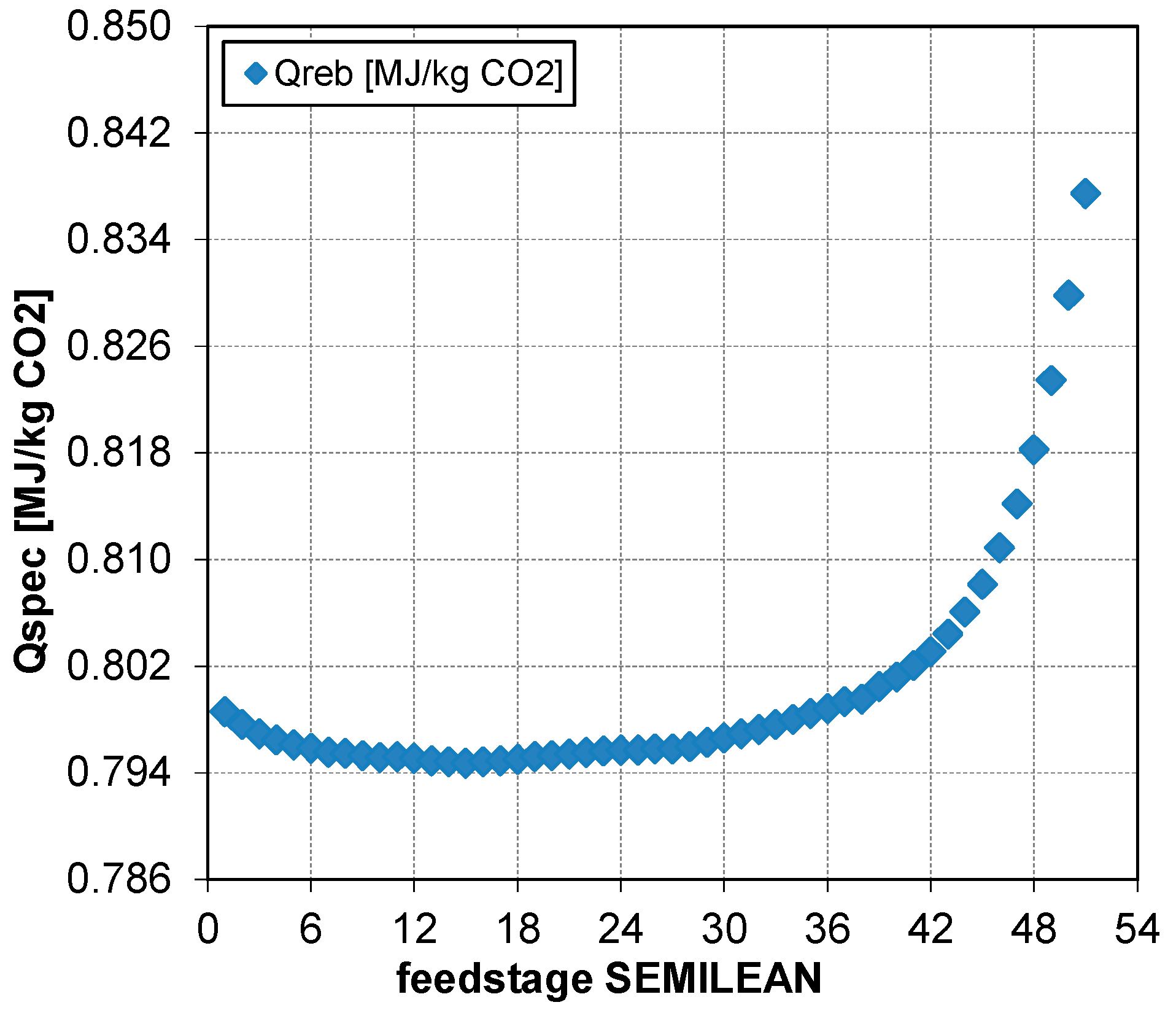
5.3.2. Effect of the Semi-Lean Feed Stage
5.3.3. Effect of the Split Fraction
6. Comparison among the Schemes
6.1. Technical Comparison
6.2. Economic Comparison
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronyms | |
| CCS | CO2 Capture and Storage |
| CCSU | CO2 Capture, Storage and Utilization |
| CCU | CO2 Capture and Utilization |
| IEAGHG | International Energy Agency Greenhouse Gas R&D Programme |
| MDEA | MethylDiEthanolAmine |
| NPV | Net Present Value |
| PSA | Pressure Swing Adsorption |
| SMR | Steam Methane Reforming |
References
- Voss, C. CO2 removal by PSA: An industrial view on opportunities and challenges. Adsorption 2014, 20, 295–299. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 15 April 2024).
- Chapman, A.; Itaoka, K.; Hirose, K.; Davidson, F.T.; Nagasawa, K.; Lloyd, A.C.; Webber, M.E.; Kurban, Z.; Managi, S.; Tamaki, T.; et al. A review of four case studies assessing the potential for hydrogen penetration of the future energy system. Int. J. Hydrogen Energy 2019, 44, 6371–6382. [Google Scholar] [CrossRef]
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 4569–4574. [Google Scholar] [CrossRef]
- Ball, M.; Wietschel, M. The future of hydrogen—Opportunities and challenges. Int. J. Hydrogen Energy 2009, 34, 615–627. [Google Scholar] [CrossRef]
- Papadias, D.D.; Ahmed, S.; Kumar, R.; Joseck, F. Hydrogen quality for fuel cell vehicles—A modeling study of the sensitivity of impurity content in hydrogen to the process variables in the SMR–PSA pathway. Int. J. Hydrogen Energy 2009, 34, 6021–6035. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sust. Energ Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Mofarahi, M. Reduction of CO2 capture plant energy requirement by selecting a suitable solvent and analyzing the operating parameters. Int. J. Energy Res. 2013, 37, 973–981. [Google Scholar] [CrossRef]
- Spatolisano, E.; Restelli, F.; Matichecchia, A.; Pellegrini, L.A.; de Angelis, A.R.; Cattaneo, S.; Roccaro, E. Assessing opportunities and weaknesses of green hydrogen transport via LOHC through a detailed techno-economic analysis. Int. J. Hydrogen Energy 2024, 52, 703–717. [Google Scholar] [CrossRef]
- Herrmann, A.; Mädlow, A.; Krause, H. Key performance indicators evaluation of a domestic hydrogen fuel cell CHP. Int. J. Hydrogen Energy 2019, 44, 19061–19066. [Google Scholar] [CrossRef]
- Akal, D.; Öztuna, S.; Büyükakın, M.K. A review of hydrogen usage in internal combustion engines (gasoline-Lpg-diesel) from combustion performance aspect. Int. J. Hydrogen Energy 2020, 45, 35257–35268. [Google Scholar] [CrossRef]
- Pakpour, F.; Najafpour, G.; Tabatabaei, M.; Tohidfar, M.; Younesi, H. Biohydrogen production from CO-rich syngas via a locally isolated Rhodopseudomonas palustris PT. Bioprocess Biosyst. Eng. 2014, 37, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Acharya, B. 16—Production of bio-syngas and biohydrogen via gasification. In Handbook of Biofuels Production; Luque, R., Campelo, J., Clark, J., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 420–459. [Google Scholar]
- Kumar, A.; Sarkar, S. Chapter 21—Biohydrogen Production from Bio-oil. In Biofuels; Pandey, A., Larroche, C., Ricke, S.C., Dussap, C.-G., Gnansounou, E., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 481–497. [Google Scholar]
- Bermudez, J.M.; Fidalgo, B. 15—Production of bio-syngas and bio-hydrogen via gasification. In Handbook of Biofuels Production, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 431–494. [Google Scholar]
- Muradov, N. Low to near-zero CO2 production of hydrogen from fossil fuels: Status and perspectives. Int. J. Hydrogen Energy 2017, 42, 14058–14088. [Google Scholar] [CrossRef]
- Riis, T.; Hagen, E.F.; Vie, P.J.; Ulleberg, Ø. Hydrogen Production and Storage-R&D Priorities and Gaps; International Energy Agency (IEA): Paris, France, 2005. [Google Scholar]
- Rostrup-Nielsen, J.R.; Rostrup-Nielsen, T. Large-Scale Hydrogen Production. Cattech 2002, 6, 150–159. [Google Scholar] [CrossRef]
- Spatolisano, E.; De Guido, G.; Pellegrini, L.A.; Calemma, V.; de Angelis, A.R.; Nali, M. Hydrogen sulphide to hydrogen via H2S methane reformation: Thermodynamics and process scheme assessment. Int. J. Hydrogen Energy 2022, 47, 15612–15623. [Google Scholar] [CrossRef]
- Spatolisano, E.; De Guido, G.; Pellegrini, L.A.; Calemma, V.; de Angelis, A.R.; Nali, M. Process sensitivity analysis and techno-economic assessment of hydrogen sulphide to hydrogen via H2S methane reformation. J. Clean Prod. 2022, 330, 129889. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Ozcan, H.; Dincer, I. Comparative cost evaluation of nuclear hydrogen production methods with the Hydrogen Economy Evaluation Program (HEEP). Int. J. Hydrogen Energy 2015, 40, 11168–11177. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A. Haber-Bosch process intensification: A first step towards small-scale distributed ammonia production. Chem. Eng. Res. Des. 2023, 195, 651–661. [Google Scholar] [CrossRef]
- Denholm, P.; Hand, M.; Jackson, M.; Ong, S. Land-Use Requirements of Modern Wind Power Plants in the United States; NREL/TP-6A2-45834; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2009. [Google Scholar]
- Palmer-Wilson, K.; Donald, J.; Robertson, B.; Lyseng, B.; Keller, V.; Fowler, M.; Wade, C.; Scholtysik, S.; Wild, P.; Rowe, A. Impact of land requirements on electricity system decarbonisation pathways. Energy Policy 2019, 129, 193–205. [Google Scholar] [CrossRef]
- Roque, L.A.C.; Paiva, L.T.; Fernandes, M.C.R.M.; Fontes, D.B.M.M.; Fontes, F.A.C.C. Layout optimization of an airborne wind energy farm for maximum power generation. Energy Rep. 2020, 6, 165–171. [Google Scholar] [CrossRef]
- Ghazvini, M.; Sadeghzadeh, M.; Ahmadi, M.H.; Moosavi, S.; Pourfayaz, F. Geothermal energy use in hydrogen production: A review. Int. J. Energy Res. 2019, 43, 7823–7851. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Innovation in hydrogen production. Int. J. Hydrogen Energy 2017, 42, 14843–14864. [Google Scholar] [CrossRef]
- Simbeck, D.; Chang, E. Hydrogen Supply: Cost Estimate for Hydrogen Pathways—Scoping Analysis; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2002. [Google Scholar]
- Bouvart, F.; Prieur, A. Comparison of life cycle GHG emissions and energy consumption of combined electricity and H2 production pathways with CCS: Selection of technologies with natural gas, coal and lignite as fuel for the European HYPOGEN Programme. Energy Procedia 2009, 1, 3779–3786. [Google Scholar] [CrossRef]
- Del Ben, L. Study of Energy Saving Configurations in the CO2 Removal Section of a SMR-Based H2 Plant. Master’s Thesis, Politecnico di Milano, Milano, Italy, 2018. [Google Scholar]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tan, C.-S. A Review: CO2 Utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Alper, E.; Yuksel Orhan, O. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Olah, G.A.; Mathew, T.; Goeppert, A.; Surya Prakash, G.K. Difference and Significance of Regenerative Versus Renewable Carbon Fuels and Products. Top. Catal. 2018, 61, 522–529. [Google Scholar] [CrossRef]
- Armstrong, K.; Styring, P. Assessing the Potential of Utilization and Storage Strategies for Post-Combustion CO2 Emissions Reduction. Front. Energy Res. 2015, 3, 8. [Google Scholar] [CrossRef]
- Hunt, A.J.; Sin, E.H.K.; Marriott, R.; Clark, J.H. Generation, Capture, and Utilization of Industrial Carbon Dioxide. ChemSusChem 2010, 3, 306–322. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Nagatoshi, Y.; Fujita, Y. Accelerating Soybean Breeding in a CO2-Supplemented Growth Chamber. Plant Cell Physiol. 2018, 60, 77–84. [Google Scholar] [CrossRef]
- Thorne, R.J.; Sundseth, K.; Bouman, E.; Czarnowska, L.; Mathisen, A.; Skagestad, R.; Stanek, W.; Pacyna, J.M.; Pacyna, E.G. Technical and environmental viability of a European CO2 EOR system. Int. J. Greenh. Gas Control 2020, 92, 102857. [Google Scholar] [CrossRef]
- Melzer, L.S. CO2 Transport—Building on the current framework to meet the demands of widely deployed, commercial scale CCS systems. In Proceedings of the 6th annual Conference on Carbon Capture and Sequestration, Pittsburgh, PA, USA, 7–10 May 2007. [Google Scholar]
- Tarun, C.B.; Croiset, E.; Douglas, P.L.; Gupta, M.; Chowdhury, M.H.M. Techno-economic study of CO2 capture from natural gas based hydrogen plants. Int. J. Greenh. Gas Control 2007, 1, 55–61. [Google Scholar] [CrossRef]
- Reddy, S.; Vyas, S. Recovery of Carbon Dioxide and Hydrogen from PSA Tail Gas. Energy Procedia 2009, 1, 149–154. [Google Scholar] [CrossRef]
- Shi, W.; Yang, H.; Shen, Y.; Fu, Q.; Zhang, D.; Fu, B. Two-stage PSA/VSA to produce H2 with CO2 capture via steam methane reforming (SMR). Int. J. Hydrogen Energy 2018, 43, 19057–19074. [Google Scholar] [CrossRef]
- Chen, Y.; Ahn, H. Feasibility Study of Vacuum Pressure Swing Adsorption for CO2 Capture From an SMR Hydrogen Plant: Comparison Between Synthesis Gas Capture and Tail Gas Capture. Front. Chem. Eng. 2021, 3, 742963. [Google Scholar] [CrossRef]
- Shahid, M.Z.; Kim, J.-K. Design and economic evaluation of a novel amine-based CO2 capture process for SMR-based hydrogen production plants. J. Clean Prod. 2023, 402, 136704. [Google Scholar] [CrossRef]
- United Nation. The Paris Agreement. Available online: http://unfccc.int/paris_agreement/items/9485.php (accessed on 15 April 2024).
- Aroonwilas, A.; Veawab, A. Characterization and Comparison of the CO2 Absorption Performance into Single and Blended Alkanolamines in a Packed Column. Ind. Eng. Chem. Res. 2004, 43, 2228–2237. [Google Scholar] [CrossRef]
- Khan, S.N.; Hailegiorgis, S.M.; Man, Z.; Shariff, A.M.; Garg, S. Thermophysical properties of concentrated aqueous solution of N-methyldiethanolamine (MDEA), piperazine (PZ), and ionic liquids hybrid solvent for CO2 capture. J. Mol. Liq. 2017, 229 (Suppl. C), 221–229. [Google Scholar] [CrossRef]
- Rochelle, G.T. Thermal degradation of amines for CO2 capture. Curr. Opin. Chem. Eng. 2012, 1, 183–190. [Google Scholar] [CrossRef]
- Lepaumier, H.; Picq, D.; Carrette, P.-L. New Amines for CO2 Capture. I. Mechanisms of Amine Degradation in the Presence of CO2. Ind. Eng. Chem. Res. 2009, 48, 9061–9067. [Google Scholar] [CrossRef]
- Oyenekan, B.A.; Rochelle, G.T. Energy Performance of Stripper Configurations for CO2 Capture by Aqueous Amines. Ind. Eng. Chem. Res. 2005, 45, 2457–2464. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R. Gas Purification, 5th ed.; Gulf Publishing Company, Book Division: Houston, TX, USA, 1997. [Google Scholar]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Moioli, S.; Spatolisano, E.; Pellegrini, L.A. Techno-Economic Assessment for the Best Flexible Operation of the CO2 Removal Section by Potassium Taurate Solvent in a Coal-Fired Power Plant. Energies 2024, 17, 1736. [Google Scholar] [CrossRef]
- Chalmers, H.; Lucquiaud, M.; Gibbins, J.; Leach, M. Flexible Operation of Coal Fired Power Plants with Postcombustion Capture of Carbon Dioxide. J. Environ. Eng.-ASCE 2009, 135, 449–458. [Google Scholar] [CrossRef]
- Lucquiaud, M.; Fernandez, E.S.; Chalmers, H.; Mac Dowell, N.; Gibbins, J. Enhanced operating flexibility and optimised off-design operation of coal plants with post-combustion capture. Energy Procedia 2014, 63, 7494–7507. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A. Operating the CO2 absorption plant in a post-combustion unit in flexible mode for cost reduction. Chem. Eng. Res. Des. 2019, 147, 604–614. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A. Optimal Operation of a CO2 Absorption Plant in a Post-Combustion Unit for Cost Reduction. Chem. Eng. Trans. 2018, 69, 151–156. [Google Scholar]
- Errey, O.; Chalmers, H.; Lucquiaud, M.; Gibbins, J. Valuing Responsive Operation of Post-combustion CCS Power Plants in Low Carbon Electricity Markets. Energy Procedia 2014, 63, 7471–7484. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A. Fixed and Capture Level Reduction operating modes for carbon dioxide removal in a Natural Gas Combined Cycle power plant. J. Clean Prod. 2020, 254, 120016. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Moioli, S. Design of the CO2 removal section for PSA tail gas treatment in hydrogen production plant. Front. Energy Res. 2020, 8, 77. [Google Scholar] [CrossRef]
- Cohen, S.M.; Rochelle, G.T.; Webber, M.E. Optimal operation of flexible post-combustion CO2 capture in response to volatile electricity prices. Energy Procedia 2011, 4, 2604–2611. [Google Scholar] [CrossRef]
- Alie, C.; Backham, L.; Croiset, E.; Douglas, P.L. Simulation of CO2 capture using MEA scrubbing: A flowsheet decomposition method. Energy Convers. Manag. 2005, 46, 475–487. [Google Scholar] [CrossRef]
- Davison, J. Performance and costs of power plants with capture and storage of CO2. Energy 2007, 32, 1163–1176. [Google Scholar] [CrossRef]
- Husebye, J.; Anantharaman, R.; Fleten, S.-E. Techno-economic assessment of flexible solvent regeneration & storage for base load coal-fired power generation with post combustion CO2 capture. Energy Procedia 2011, 4, 2612–2619. [Google Scholar]
- Meissner, R.E.; Wagner, U. Low-energy process recovers CO2. Technol 1983, 7, 55–58. [Google Scholar]
- Moioli, S.; Pellegrini, L.A.; Picutti, B.; Vergani, P. Improved rate-based modeling of H2S and CO2 removal by MDEA scrubbing. Ind. Eng. Chem. Res. 2013, 52, 2056–2065. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; Langé, S.; Moioli, S.; Picutti, B.; Vergani, P. Influence of Gas Impurities on Thermodynamics of Amine Solutions. 1. Aromatics. Ind. Eng. Chem. Res. 2013, 52, 2018–2024. [Google Scholar] [CrossRef]
- Langé, S.; Pellegrini, L.A.; Moioli, S.; Picutti, B.; Vergani, P. Influence of Gas Impurities on Thermodynamics of Amine Solutions. 2. Mercaptans. Ind. Eng. Chem. Res. 2013, 52, 2025–2031. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A.; Bhattacharyya, D. Analysys, Synthesis & Design of Chemical Processes, 4th ed.; Prentice Hall International Series in the Physical and Chemical Engineering Sciences; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineers; McGraw Hill Book Co.: Singapore, 1991. [Google Scholar]
- Moioli, S.; De Guido, G.; Pellegrini, L.A.; Fasola, E.; Riva, E.R.; Alberti, D.; Carrara, A. Techno-economic assessment of the CO2 value chain with CCUS applied to a waste-to-energy Italian plant. Chem. Eng. Sci. 2024, 287, 119717. [Google Scholar] [CrossRef]
- Dasgupta, S.; Nanoti, A.; Gupta, P.; Jena, D.; Goswami, A.N.; Garg, M.O. Carbon Di-Oxide Removal with Mesoporous Adsorbents in a Single Column Pressure Swing Adsorber. Sep. Sci. Technol. 2009, 44, 3973–3983. [Google Scholar] [CrossRef]






| Parameter | Unit | Value |
|---|---|---|
| Temperature | [°C] | 28 |
| Pressure | [MPa] | 1.1 |
| Molar Flow | [kmol/h] | 2106.3 |
| Mass Flow | [kg/h] | 60,658 |
| Composition | ||
| CO2 | [mol/mol] | 0.5095 |
| CO | [mol/mol] | 0.1454 |
| Hydrogen | [mol/mol] | 0.2369 |
| Nitrogen | [mol/mol] | 0.0062 |
| Methane | [mol/mol] | 0.0945 |
| H2O | [mol/mol] | 0.0076 |
| Scheme (a) | Scheme (a) | Scheme (c) | ||
|---|---|---|---|---|
| Variable | Unit | Scheme (a)—4.5 bar | Optimized Scheme (a)—3 bar | Optimized Scheme (c) |
| Number of flash units | [-] | 1 | 1 | 2 |
| Pressure of the first flash | [MPa] | 0.45 | 0.3 | 0.98 |
| Pressure of the second flash | [MPa] | NA | NA | 0.3 |
| % split TOSPLIT to SEMILEAN | [%] | NA | NA | 50 |
| Regenerator type | [-] | distillation | distillation | reboiled stripping |
| Condenser duty | [MW] | 0.67 | 0.67 | NA |
| Reboiler duty | [MW] | 10.63 | 8.94 | 7.07 |
| COOLER duty | [MW] | 22.2 | 22.2 | 12.3 |
| Cooler of CO2-rich stream duty | [MW] | NA | NA | 1.62 |
| PUMP duty | [MWel] | 0.126 | 0.126 | 0.085 |
| PUMPSEMI duty | [MWel] | NA | NA | 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moioli, S.; Pellegrini, L.A. CO2 Removal in Hydrogen Production Plants. Energies 2024, 17, 3089. https://doi.org/10.3390/en17133089
Moioli S, Pellegrini LA. CO2 Removal in Hydrogen Production Plants. Energies. 2024; 17(13):3089. https://doi.org/10.3390/en17133089
Chicago/Turabian StyleMoioli, Stefania, and Laura A. Pellegrini. 2024. "CO2 Removal in Hydrogen Production Plants" Energies 17, no. 13: 3089. https://doi.org/10.3390/en17133089







