Abstract
This paper focuses on the selection and application of scale inhibitor by studying the problem of pipeline scaling in geothermal well development. Adding scale inhibitor can effectively reduce the treatment cost and achieve a good scale resistance effect, but the commonly used polyaspartic acid scale inhibitor has problems such as poor scale inhibition effect and large use limitations. Therefore, a new modified polyaspartic acid scale inhibitor (His-Tyr-SA-PASP) was prepared using polysuccinimide (PSI) as the raw material and histidine (His), tyrosine (Tyr), and sulfonic acid (SA) as the modification reagent. When the dosage of His-Tyr-SA-PASP was 8 mg/L, the scale inhibition rate of CaCO3 was 94.40%. In addition, the scale inhibition effect of His-Tyr-SA-PASP on CaCO3 was better than that of PASP. At the same time, under the condition of a static experiment at 75 °C, according to the ion concentration of water samples in different scale zones, this paper also identified the ratio of four composite scale inhibitors. When the dosage of compound scale inhibitor was 100 mg/L, Sodium of Polyaspartic Acid–Diethylene Triamine Penta (Methylene Phosphonic Acid)–2-Phosphonobutane-1,2,4-Tricarboxylic Acid–Amino Trimethylene Phosphonic Acid–Copolymer of Maleic and Acrylic Acid = (10:10:5:1:9), (15:10:5:2.5:2.5), (12.5:5:10:1:6.5), and (15:5:10:4:1) and the scale inhibition rate was more than 95%. Under the condition of a dynamic experiment, the optimized composite scale inhibitor still showed a scale inhibition rate of more than 90%. It provides a useful reference for the practical application of water treatment in geothermal wells and has the prospect of industrial application.
1. Introduction
The demand for oil has increased due to global economic growth, particularly in emerging economies such as China and India. To address the limited oil supply, the focus has shifted to enhancing recovery rates in oil fields using advanced technologies such as secondary water drive, CO2 drive, and polymer drive. However, these methods can lead to scale deposition in oilfield equipment [1]. Therefore, domestic and foreign researchers have conducted extensive research on the scaling problem in water treatment processes. Research has shown that the use of scale inhibitors can reduce treatment costs and improve the effectiveness of scale inhibition [2]. This is a common approach to address scaling problems. Four main types of scale inhibitors have been developed over the years: inorganic phosphorus, organophosphorus, polymer, and green composite inhibitors [3,4]. While organophosphoric acid scale inhibitors are effective at preventing scale buildup, they have several drawbacks, including high production costs, slow degradation, and environmental pollution. Examples of these inhibitors include Amino Trimethylene Phosphonic Acid (ATMP), etc. [5]. In the field of anti-scaling technology, phosphorus pollution, as a significant contributor to the eutrophication of water bodies, has become a significant challenge for water treatment. At the same time, the scarcity of freshwater resources is closely linked to energy issues, making the development of efficient water treatment technologies essential to meeting this compound challenge and solving freshwater and energy problems [6]. As a result, there is a pressing need to develop innovative green scale inhibitors [7]. Current research focuses on Polyaspartic acid (PASP), Polyepoxysuccinic acid (PESA), and natural plant extracts and their derivatives [8]. Among these, PASP is an eco-friendly scale inhibitor, with non-toxic properties and excellent biodegradability, that effectively prevents the formation of CaCO3 and CaSO4 scales [9]. However, the scale inhibition effect of PASP is limited because it only contains carboxyl groups on its side chain [10]. To enhance the scale inhibition performance of PASP, it is essential to modify it by introducing other functional groups [11]. Current research suggests that copolymers containing carboxyl and hydroxyl groups have significant advantages in scale inhibition performance [12].
Several studies have reported on the methods for modifying PASP. For instance, Shaopeng Zhang et al. [13] prepared modified polyaspartic acid scale inhibitors (Tyr-SA-PASP and Trp-SA-PASP), by graft copolymerization of amino/amino acids onto PASP, introducing carboxyl and sulfonic groups simultaneously. These inhibitors were used to prevent calcium sulfate buildup in cooling water. The maximum inhibition efficiency of CaSO4 was close to 98% under the test conditions of GB/T 16632-2019 [14]. Jinhui Yang et al. [15] prepared a modifier using inexpensive raw materials (maleic anhydride, ethylenediamine, NaOH) to modify polysuccinimide, obtaining a modified PASP (M-PASP) with excellent scale inhibition performance and low cost. Ying Zhang et al. [16] reacted with threonine (Thr), urea and PSI as raw materials to produce polyaspartic acid derivatives containing hydroxyl and acylamino groups. The chelating ability of the modified PASP was significantly enhanced. Huang et al. [17] introduced -CO-NH- into the side chain of PSAP with lysine to obtain the polyaspartic acid derivative (Lys-PASP). This derivative has an excellent scale inhibition effect, with a scale inhibition rate of 94% for CaCO3. In addition, researchers introduced fluorescent groups while modifying and improving the scale inhibition performance of PASP to facilitate real-time monitoring of the concentration of scale inhibitor in the water system. Among them, Yu Rong et al. [18] developed a new fluorescent tracer scale inhibitor, Tyr-Glu-PSAP, by modifying the environmentally friendly scale inhibitor polyaspartic acid (PSAP) with tyrosine (Tyr) and glutamic acid (Glu). This modification improved the CaSO4 inhibition performance of PSAP and added a fluorescent tracer effect. Currently, efforts to improve the scale inhibition performance of polyaspartic acid (PASP) not only involve direct modification but also the incorporation of substances such as oxidized starch [19], functionalized silica [20], and graphene oxide [21] into PASP. These additions increase the number of polar groups like -COOH and -NH2 in the PASP molecule, enhancing its adsorption and chelation capabilities towards Ca2+ and further bolstering its lattice distortion abilities.
To enhance the performance of polyaspartic acid, this paper employs a ring-opening grafting modification strategy to introduce sulfonic acid groups and hydroxyl-containing amino acids into the inhibitors. The negative electronegativity of the sulfonic acid group allows it to interact with the surface of scale crystals, forming a double electron layer. This interaction effectively inhibits the deposition and growth of scale crystals, such as calcium carbonate and calcium sulfate. Additionally, hydroxyl-containing amino acids are simple with regard to regulating calcium carbonate and have high biodegradability. The use of a single scale inhibitor has certain limitations and cannot fully meet the application requirements [22]. Therefore, this paper explores the synergistic effect of compounding the modified PASP with other scale inhibitor monomers to improve the scale inhibition effect. Different ratios can be adopted based on the water sample to address the scaling problem caused by various metal ions. Meanwhile, in order to determine the optimal use concentration of compounded scale inhibitors suitable for a wide range of water samples, this study conducted compounding experiments of five preferred scale inhibitors (modified PASP, DTPMPA, PBTCA, ATMP, and MA/AA) for different water samples at a constant temperature of 75 °C and 12 MPa. The experimental results assessed the influence of the concentration of the compounded scale inhibitors on the scale inhibition effect. Furthermore, this study analyzed the dynamic scale inhibition performance at different temperatures to comprehensively evaluate the practical application efficiency and scale inhibition ability of the scale inhibitors.
2. Materials and Methods
2.1. Materials
The materials used were polysuccinimide (PSI), histidine (His), tyrosine (Tyr), amino sulfonic acid (SA), sodium hydroxide, anhydrous ethanol, hydrochloric acid, Amino Trimethylene Phosphonic Acid (ATMP), Diethylenetriamine pentamethylene phosphonic acid (DTPMPA), 2-Phosphonobutane-1, 2, 4-tricarboxylic acid (PBTCA), and Copolymer of Maleic and Acrylic Acid (MA/AA), and all of which were AR products of Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China; ultra pure water was made by the laboratory in an ultra pure water machine (CM−RO−C2, Junqi Instrument Equipment Co., Ltd., Ningbo, China).
2.2. Synthesis of Polyaspartate (PASP)
A total of 0.5047 g of polysuccinimide (5 mmol) was weighed and deionized water was added to obtain a suspension. The suspension was mixed with 5 mL of 10 wt% NaOH solution and this reacted at 40 °C for 2 h. After the reaction, the solution was neutralized with diluted hydrochloric acid and 400 mL of anhydrous ethanol was added. This was stirred thoroughly to obtain a floc. The solid was filtered using a suction filter and dried in an oven at 40 °C to obtain PASP. The preparation process is shown in Figure 1.
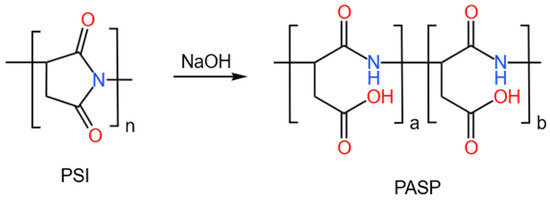
Figure 1.
Synthesis of PASP.
2.3. Ring-Opening Graft Modification of PASP
A total of 0.5047 g of polysuccinimide (5 mmol) was weighed and deionized water was added to obtain suspension B. Suspension B was placed into a 40 °C water bath, heated, and stirred. A total of 0.3875 g of histidine (2.5 mmol), 0.4530 g of tyrosine (2.5 mmol), and 0.25 g of sulfamic acid (2.5 mmol) were weighed, and these were dissolved in 5 mL of 10% NaOH solution to obtain solution C. Solution C was slowly added into suspension B using a constant-pressure dropping funnel; the dropping was completed within 1 h and then it reacted for two hours under alkaline conditions. After completing the reaction, the solution was neutralized with diluted hydrochloric acid and 400 mL of ethanol absolute was added. This was stirred thoroughly to obtain floccules. The solid was filtered using a suction filter and dried in an oven at 40 °C until it reached a constant weight to obtain the final product; the preparation process is shown in Figure 2.
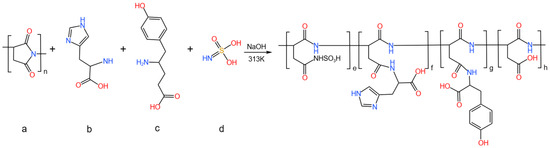
Figure 2.
Synthesis of modified PASP (His-Tyr-SA-PASP).
2.4. Fourier-Transform Infrared Spectra
The test samples were placed in a mortar with potassium bromide in a ratio of 1:100 for thorough grinding, and after which the mixtures were placed in a test mold for pressing; the test was performed in a transmission mode (TR) of 4000–500 cm−1.
2.5. Evaluation Method of Scale Inhibition Effect
According to the method outlined in GB/T 16632-2019 entitled “Test method for scale inhibitor performance of water treatment agents using calcium carbonate deposition combined with EDTA titration method”, the scale inhibitor rate of His-Tyr-SA-PASP against CaCO3 was determined. The calculation formula for the anti-scaling performance of the copolymer is as follows:
In the equation, η is the scale inhibition rate; A1 is the calcium ion concentration of the blank control group without scale inhibitor after the experiment, mg/mL; A2 is the calcium ion concentration of the sample added with scale inhibitor after the experiment, mg/mL; and A is the concentration of calcium ion in the sample before the experiment, mg/mL.
2.6. Dynamic Scale Inhibition Experimental Device and Experimental Method
The dynamic scale inhibition experimental device mainly includes a liquid storage bottle, a circulating pipeline, a peristaltic pump, and an oven, as shown in Figure 3.
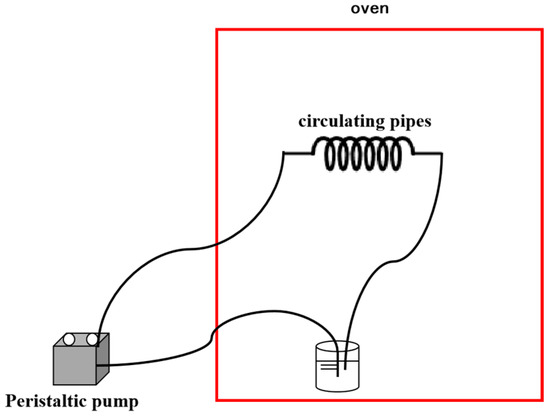
Figure 3.
Dynamic scale inhibition experimental setup.
The dynamic scale inhibition experiment was performed using a peristaltic pump (BT100-2J) for flow control, with a flow rate of 15 mL/min maintained throughout the experiment, controlling temperature using an oven, setting the oven temperature (50 °C, 60 °C, 70 °C and 80 °C), and placing a 500 mL water sample into a reservoir bottle. Then, the peristaltic pump was turned on to circulate the experimental water sample for 12 h.
3. Results
3.1. FTIR and1H NMR Analysis of His-Tyr-SA-PASP
The FTIR spectrum is shown in Figure 4a. The band at 1068 cm−1 corresponds to the vibration of the sulfonic acid group, indicating that the sulfonic acid group was successfully grafted onto the side chain of PASP. The band at 1400 cm−1 represents the bending vibration of N-H and the tensile vibration of C-N in -CONH, the tensile vibration at 1470 cm−1 corresponds to the tensile vibration of C=C in the imidazole ring, 1598 cm−1 shows the stretching vibration peak of the C=O bond in the amide, and 3203 cm−1 corresponds to the stretching vibration peak of the -OH in the benzene ring. The above evidence shows that aminosulfonic acid, L-histidine, and L-tyrosine successfully modified PASP.
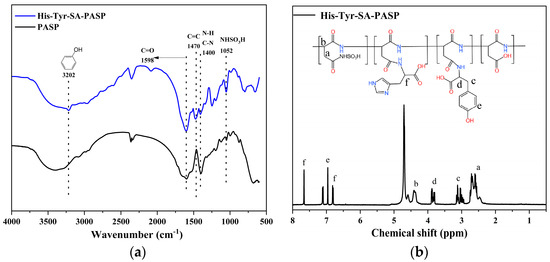
Figure 4.
(a) FTIR spectra of His-Tyr-SA-PASP; (b) 1H NMR spectrum of His-Tyr-SA-PASP.
The 1H NMR spectrum is shown in Figure 4b. The peak of the D2O solvent appears at 4.75 ppm, the methylene resonance peak in PASP occurs in the range of 2.4–2.8 ppm, and the methylene resonance peak in PASP occurs at 4.4 ppm. At the same time, the resonance peak of methylene in the side chain tyrosine can be observed in the range of 2.8 ppm to 3.2 ppm, and the resonance peak of methylene in tyrosine can be observed at 3.9 ppm. The resonance peak of the methylene of the benzene ring in tyrosine can be seen at 7.0 ppm, while the resonance peak of the methylene of the imidazole ring in histidine can be seen at 6.9 ppm and 7.7 ppm. Based on these observations, it can be concluded that His-Tyr-SA-PASP was successfully synthesized.
3.2. Scale Inhibition Performance of His-Tyr-SA-PASP and Its Composite Scale Inhibitor
3.2.1. Scale Inhibition Performance of Scale Inhibitor Monomer
According to GB/T 16632-2019 entitled “Test method for scale inhibitor performance of water treatment agents using calcium carbonate deposition”, the scale inhibitor properties of scale inhibitors and their composite products were determined. The experimental conditions for preventing CaCO3 scaling were as follows: the mass concentrations of Ca2+ and HCO3− were both 250 mg/L (expressed as CaCO3) and the test temperature was 80 °C, with a constant temperature for 10 h and a solution pH of 9.0. The scale inhibitor rate was measured when the scale inhibitor was added at a dose of 100 mg/L, and the scaling inhibition rates of the various scale inhibitors used individually were analyzed. The results are shown in Table 1.

Table 1.
Scale inhibition efficiency of different scale inhibitors.
- After modification, the scale inhibition rate of PASP significantly improved, but the scale inhibition rates of modified PASP, DTPMPA, PBTCA, ATMP, and MA/AA were less than 90%, which did not meet the scale inhibition requirements. Therefore, this work developed and optimized suitable scale inhibitors for different water types, pH values, and scale ion concentrations of the water samples. Experiments were conducted on the synergistic effect of various scale inhibitors and the optimal scale inhibition rate was explored.
3.2.2. Scale Inhibition Performance of Compound SCALE Inhibitor
Static Scale Inhibition Experiment of Composite Scale Inhibitor
- In the field of water treatment, regional differences in ion concentrations in water samples and variations in pH lead to a wide variety of scaling phenomena. As a result of these differences, different types of scales are formed. In addition, different scale inhibitors have different inhibiting effects on various scaling conditions. Therefore, in order to solve the scaling problem of a specific water body more effectively, it is necessary to compound different scale inhibitors and optimize their ratios to achieve a better anti-scaling effect. Among the existing scale inhibitors, organophosphorus scale inhibitors have significant scale inhibition effects and require small dosages. However, their high phosphorus content can easily lead to environmental pollution. Conversely, copolymer scale inhibitors demonstrate high scale inhibition efficiency and low or non-toxicity. However, high phosphorus content in these inhibitors can easily lead to environmental pollution. In light of this, it is preferable to use the composite application of multiple scale inhibitors and optimize the ratio of these inhibitors to achieve the optimal anti-scaling effectiveness. In this context, modified polyphosphates (PASPs) show considerable potential to act in synergy with other scale inhibitors and exhibit highly efficient scale inhibition properties. Consequently, compounding them with organophosphate and copolymer scale inhibitors is expected to be an effective strategy to enhance the anti-scaling effect.
- DTPMPA in organic phosphates has a significant chelating effect on metal ions, especially in the treatment of various types of scale, but the agent has poor tolerance to calcium ions, and its effectiveness decreases in strong alkali or high-temperature environments. PBTCA is suitable for alkaline water and can effectively inhibit scaling. By adjusting the ratio of DTPMPA and PBTCA, effective scale inhibition can be carried out for water bodies with different pH values. The dissociation characteristics of ATMP in water enable it to form stable multi-ring chelates with calcium, magnesium, and other metal ions, effectively destroying the formation of scale. Therefore, for the Mg2+ present in the water sample, it is appropriate to add ATMP for synergistic effect in the scale inhibitor.
- Copolymer scale inhibitor MA/AA, as a low-molecular-weight polyelectrolyte, is copolymerized by maleic acid and acrylic acid in a certain proportion. It has a significant dispersion effect on carbonates and has good thermal stability. It can maintain its effectiveness even in a high temperature environment of 300 °C. In addition, MA/AA shows good compatibility and synergistic effect with other water treatment agents. To enhance the high-temperature applicability of the scale inhibitor, it is necessary to choose to add a suitable amount of MA/AA for synergistic effect in the scale inhibitor.
- We conducted research on the prices of scale inhibitors in the market. The price of PASP is 6.5–12 CNY/kg, DTPMPA is 5.23–12.8 CNY/kg, PBTCA is 4.6–18 CNY/kg, ATMP is 6–7.8 CNY/kg, and MA/AA is 8.50 CNY/kg. However, when it comes to modified PASP scale inhibitors, compared to unmodified PASP with other raw materials added, their prices will be higher than ordinary PASP scale inhibitors. Despite this, by compounding them with other scale inhibitors, their scale inhibition performance can be improved, costs can be reduced, and better economic benefits may be obtained.
- Under the conditions of 75 °C temperature and 12 MPa pressure, to achieve the optimal concentration of mixed scale inhibitor for different water samples in different regions, the five selected scale inhibitors (modified PASP, DTPMPA, PBTCA, ATMP, and MA/AA) were used for mixing according to different water sample types. The experimental evaluation looked at the effect of mixed scale inhibitor concentration on the scale inhibition of the scaling water samples. Table 2 provides the formula of the mixed scale inhibitor; Table 3 shows the ion concentrations of the four water samples.
 Table 2. The ratios of compound scale inhibitor.
Table 2. The ratios of compound scale inhibitor. Table 3. Ion concentrations of water samples.
Table 3. Ion concentrations of water samples.
Figure 5a shows the influence curve of the scale inhibitor on the scale inhibition effect of water sample A under different composite ratios. For the non-alkaline property of water sample A, the ratio of DTPMPA and PBTCA is 10:5. As the salinity of the water sample is not high, the amount of PASP is set at 10%. In addition, in view of the low concentration of Mg2+ in the water sample, a certain amount of ATMP was added to the formula to improve the scale inhibition efficiency, and a certain amount of MA/AA was added to strengthen the synergistic effect between scale inhibitors and enhance their thermal stability. In the experimental testing of water sample A, the mixed scale inhibitor formula-6 (modified PASP:DTPMPA:PBTCA:ATMP:MA/AA = 10:10:5:1:9) achieved a scale inhibition rate of 95.1%.
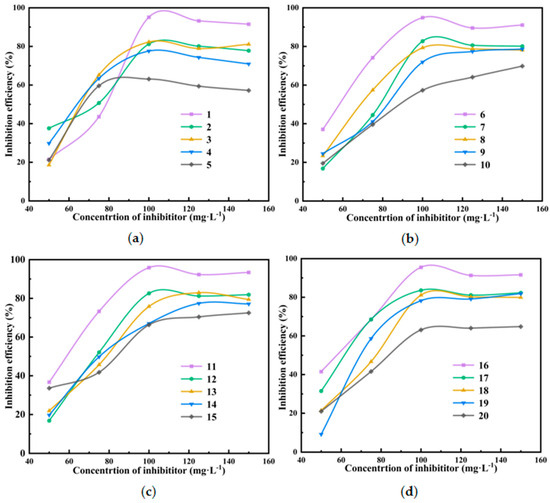
Figure 5.
Scale inhibition effect of different compound scale inhibitors on water sample A (a), water sample B (b), water sample C (c), and water sample D (d).
Figure 5b shows the influence curve of the scale inhibitor on the scale inhibition effect of water sample B under different composite ratios. The pH value of water sample B was also non-alkaline, so the ratio of DTPMPA and PBTCA was the same as that of water sample A. However, the mineralization of water sample B was higher, so the amount of PASP was increased to 15%, and the proportion of ATMP and MA/AA was adjusted according to the actual situation. In the experimental testing of water sample B, the mixed scale inhibitor formula-6 (modified PASP:DTPMPA:PBTCA:ATMP:MA/AA = 15:10:5:2.5:2.5) achieved a scale inhibition rate of 96.4%.
Figure 5c shows the influence curve of the scale inhibitor on the scale inhibition effect of water sample C under different composite ratios. The pH value of water sample C was alkaline and the proportion of PBTCA needed to be increased, so the proportion of DTPMPA and PBTCA was set to 5:10. The mineralization of the water sample was medium, the amount of PASP was adjusted to 12.5%, and the proportion of ATMP and MA/AA was adjusted as required. In the experimental testing of water sample C, the mixed scale inhibitor formula-11 (modified PASP:DTPMPA:PBTCA:ATMP:MA/AA = 12.5:5:10:1:6.5) had the best scale resistance effect, and the scale resistance rate reached 95.9%.
Figure 5d shows the influence curve of the scale inhibitor on the scale inhibition effect of water sample D under different composite ratios. The pH value of water sample D was similar to that of water sample C, so the ratio settings of DTPMPA and PBTCA were the same. The mineralization of the water sample was high, so the scale inhibition effect was better when the content of PASP was 15%. By adjusting the compounding ratio, the mixed scale inhibitor formula-16 (modified PASP:DTPMPA:PBTCA:ATMP:MA/AA = 15:5:10:4:1) achieved a scale inhibition rate of 96.3%.
A study was conducted to investigate the effect of pH on the scale inhibition effect of composite water samples. It was observed that the compounding ratio of scale inhibitors had a significant impact on the anti-scaling effect under different pH conditions. In non-alkaline water samples, the optimum scale inhibitor compounding ratio of 10:5 DTPMPA to PBTCA was found to be effective in inhibiting the scaling process. In alkaline water samples, the same scale inhibitor combination in a ratio of 5:15 demonstrated superior anti-scaling performance. This indicates that for water samples with varying pH values, the ratio of scale inhibitor compounding should be adjusted in a timely manner to ensure that the optimal anti-scaling effect is achieved. For instance, in the case of water sample D, the alkalinity of the sample was diminished by the addition of acid, rendering the original formulation ratio inapplicable and necessitating its adjustment to maintain the actual scale inhibition performance. In addition, the proportion of PASP and ATMP could be properly adjusted to adapt to the difference in the salinity of water samples and magnesium ion and sulfate content. Through the study of four different water samples, the proportion of four composite scale inhibitors with a high scale inhibition effect was selected, providing a useful reference for the practical application of water treatment in different regions.
Dynamic Scale Inhibition Experiment of Composite Scale Inhibitor
According to the dynamic scale inhibition experimental device and method, the prepared water samples (A, B, C, and D) were put into the reservoir bottle, and 100 ppm antiscalant (composite scale inhibitor-1, composite scale inhibitor-6, composite scale inhibitor-11, and composite scale inhibitor-16) was added at different temperatures (50 °C, 60 °C, 70 °C, and 80 °C) for 24 h of dynamic scaling test experiments; the relationship between the scale inhibition rate and temperature of the composite scale inhibitor was obtained, as shown in Figure 6.
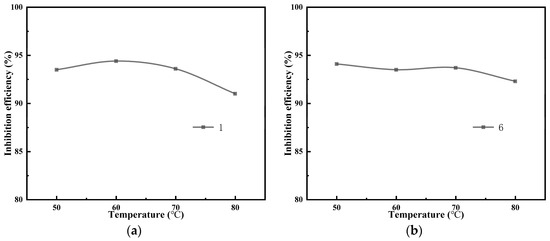
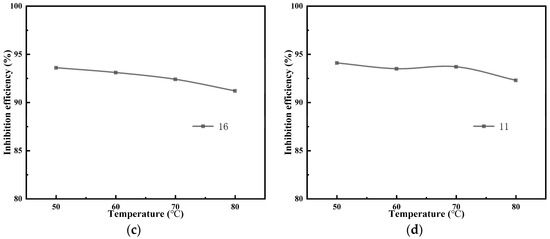
Figure 6.
Scale inhibition effect of the composite scale inhibitor at different temperatures on water sample A (a), water sample B (b), water sample C (c), and water sample D (d).
These four kinds of compound scale inhibitor optimized by static scale inhibition experiments all showed a good scale inhibition effect under the conditions of dynamic experiments, and the scale inhibition rate of the four kinds of composite scale inhibitors was greater than 90%. Concurrently, the scale inhibition rate of the composite scale inhibitor exhibited a decline over time, yet remained above 90% throughout the 12 h experimental period. In addition, the scale inhibition rates of the composite scale inhibitors all showed a decreasing trend with an increase in temperature; the main reasons for this are as follows [23,24,25]: (1) the temperature increase led to supersaturation of the solution and accelerated the generation of calcium carbonate precipitation; (2) a higher temperature reduced the chelating ability of scale inhibitors and metal ions, increased the possibility of collision between scale crystallites, and was beneficial to the formation of a large number of precipitates; and (3) a higher temperature led to partial hydrolysis of the scale inhibitor, thus reducing the scale inhibition effect.
4. Conclusions
The modified polyaspartic acid scale inhibitor His-Tyr-SA-PASP was successfully synthesized by graft modification of PASP with Tyr, His, and SA. The synthesized product was characterized by the infrared spectrum, which showed that the modified scale inhibitor had introduced various scale-inhibiting groups such as sulfonic acid groups, carboxyl groups, phenolic hydroxyl groups, and amide groups. The nuclear magnetic resonance hydrogen spectrum showed that the scale inhibitor was introduced into L-histidine and L-tyrosine, and His-Tyr-SA-PASP was successfully synthesized.
The scale inhibition rate of the modified PASP significantly improved, with the scale inhibition rate reaching 82.6%. However, the use of a single scale inhibitor has certain limitations, which cannot fully meet the application needs. It should be compounded with other scale inhibitor monomers to improve the scale inhibition effect.
Among the scale inhibitors, DTPMPA has a good scale inhibition effect on a variety of scales, but has poor tolerance to calcium ions. PBTCA is suitable for alkaline water and can effectively inhibit scaling. By adjusting the ratio of DTPMPA and PBTCA, scale inhibition can be carried out for water bodies with different pH values. For Mg2+ in the water sample, an appropriate amount of ATMP can be added to compound the scale inhibitor to enhance its high-temperature applicability, while MA/AA can coordinate and enhance the resistance of the compound scale inhibitor.
According to the scaling type of water samples in different regions, four kinds of composite scale inhibitor proportions were selected, and the general rule of the composite proportion was obtained: in non-alkaline water samples, the ratio of DTPMPA:PBTCA is 10:5, which can provide a good scale inhibition effect, while in alkaline water samples, the ratio of DTPMPA:PBTCA is 5:15, which is more effective. In addition, the proportion of PASP and ATMP can be properly adjusted to adapt to the difference in the salinity of water samples and magnesium ion and sulfate content. The preferred composite scale inhibitor achieved a scale inhibition rate of more than 90% under both static and dynamic scaling tests, and had a very good scale inhibition effect.
Author Contributions
Conceptualization, methodology, validation, writing—original draft preparation, writing—review and editing, W.G. and J.A.; validation, investigation, writing—review and editing, L.S. and K.W.; methodology, validation, visualization, supervision, X.Y. and Q.G.; data curation, investigation, supervision, project administration, D.K.; project administration, validation, formal analysis, M.L.; review and editing, resources, funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the scientific research and technology development project of China Petroleum Group Oilfield Technology Service Co., Ltd.—grant No. 2024T-001-004.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
Thanks to the State Key Laboratory of Heavy Oil Processing of the China University of Petroleum for its support in analysis and testing. Thanks to the Research Institute of Unconventional Oil and Gas Science and Technology, China University of Petroleum, for its support in analysis and testing.
Conflicts of Interest
Author WenLong Gao, LiWei Sun, Miao Li, XiAn Ye, QingChun Gao and DongLiang Kong are employed by the company GWDC Drilling Engineering and Technology Research Institute of China National Petroleum Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from the scientific research and technology development project of China Petroleum Group Oilfield Technology Service Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Cui, K.; Zhao, L.; Sun, G. Study on scale inhibition performance and molecular dynamics simulation of a pipeline scale inhibitor in an oil field. J. Yan’an Univ. (Nat. Sci. Ed.) 2023, 42, 9–15. [Google Scholar]
- Zhang, T.; Zhang, D.; Liu, D. Polyaspartic acid modified by fluorescent carbon quantum dots as an environmentally friendly scale inhibitor for calcium sulphate. Desalination 2024, 584, 117740. [Google Scholar] [CrossRef]
- Hai, X.; Liu, Z. Study on synergistic effect of scale inhibitor combination. Petrochem. Appl. 2012, 31, 95–97. [Google Scholar]
- Yang, Y.; Xie, B.; Han, W. Study on the synergistic effect and influencing factors of the composite scale inhibitor PASP-ATMP. Appl. Chem. Ind. 2012, 41, 834–836. [Google Scholar]
- Ye, P.; Zhu, D.; Wang, W. Experimental study on factors influencing the scale inhibition performance of organic phosphoric acid and polycarboxylic acid. Ind. Water Wastewater 2007, 5, 93–96. [Google Scholar]
- Elhenawy, Y.; Fouad, K.; Bassyouni, M. Design and performance a novel hybrid membrane distillation/humidification–dehumidification system. Energy Convers. Manag. 2023, 286, 117039. [Google Scholar] [CrossRef]
- Macedo, R.; Marques, N.; Paulucci, L. Water-soluble carboxymethylchitosan as green scale inhibitor in oil wells. Carbohydr. Polym. 2019, 215, 137–142. [Google Scholar] [CrossRef]
- Xu, J.; Jing, G.; Liu, T. Research progress of green scale inhibitors: A mini review. Pet. Sci. Technol. 2022, 40, 59–72. [Google Scholar] [CrossRef]
- Hasson, D.; Shemer, H.; Sher, A. State of the art of friendly “green” scale control inhibitors: A review article. Ind. Eng. Chem. Res. 2011, 50, 7601–7607. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Fang, Y. Green and high effective scale inhibitor based on ring-opening graft modification of polyaspartic acid. Catalysts 2021, 11, 802. [Google Scholar] [CrossRef]
- Migahed, M.; Rashwan, S.; Kamel, M. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants. J. Mol. Liq. 2016, 224, 849–858. [Google Scholar] [CrossRef]
- Ling, G.; Li, Z.; Li, N. Research progress of green scale inhibitors for oilfield development. Oilfield Chem. 2022, 39, 564–570. [Google Scholar]
- Zhang, S.; Qu, H.; Yang, Z. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 2017, 419, 152–159. [Google Scholar] [CrossRef]
- GB/T 16632–2019; Determination of Scale Inhibition Properties of Water Treatment Agents, Calcium Carbonate Deposition Method. China National Standard: Shanghai, China, 2019.
- Yang, J.; Hu, Z.; Wang, Z.; Wu, C.; Dong, L.; Meng, X.; Lin, X.; Zhao, J.; Chen, Y. Preparation and scale inhibition performance of modified polyaspartic acid (M-PASP). J. Mol. Liq. 2024, 401, 124712. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Zhang, Q.; Li, Y.; Yao, P.; Huo, H. A novel polyaspartic acid derivative with multifunctional groups for scale inhibition application. Environ. Technol. 2018, 39, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, X.; Han, J. Synthesis of multi branched polyaspartic acid derivatives and their scale inhibition performance. Ind. Water Treat. 2017, 37, 51–54. [Google Scholar]
- Yu, R.; Cheng, K.; Fu, S. Synthesis and properties of amino acid modified PASP. Funct. Mater. 2022, 53, 12184–12188. [Google Scholar]
- Chen, Y.; Chen, X.; Liang, Y. Synthesis of polyaspartic acid-oxidized starch copolymer and evaluation of its inhibition performance and dispersion capacity. Dispers. Sci. Technol. 2021, 42, 1926–1935. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, X.; Zhao, X. Nanosilica modified with polyaspartic acid as an industrial circulating water scale inhibitor. NPJ Clean. Water 2021, 4, 46. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Liang, Y. Synthesis of polyaspartic acid/graphene oxide grafted copolymer and evaluation of scale inhibition and dispersion performance. Diam. Relat. Mater. 2020, 108, 107949. [Google Scholar] [CrossRef]
- Song, S.; Jia, W.; Wang, B. Study on scaling mechanism and scale resistance in pre-oxidation process of heavy oil hot mining sewage. Appl. Chem. Ind. 2020, 49, 2206–2210. [Google Scholar]
- Zhou, G.; Luo, J.; Liu, C. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. [Google Scholar] [CrossRef]
- Elgendy, A.; Elkholy, A.; El Basiony, N. Monte Carlo simulation for the antiscaling performance of Gemini ionic liquids. J. Mol. Liq. 2019, 285, 408–415. [Google Scholar] [CrossRef]
- Issabayev, Y.; Boiko, G.; Lyubchenko, N. Synthesis of unexplored aminophosphonic acid and evaluation as scale inhibitor for industrial water applications. J. Water Process Eng. 2018, 22, 198–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).