Innovation in Methods for Incorporating Magnetite into Biocellulose for Electromagnetic Interference Shielding Effectiveness Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maintenance of Microorganism
2.2. Preparation of Pre-Inoculum
2.3. Biocellulose Production by Fermentation
2.4. Magnetite Synthesis and Incorporation Methods
2.4.1. In Situ Coprecipitation
2.4.2. Ex Situ Coprecipitation
2.5. Analysis of Results
2.5.1. Incorporation Rate
2.5.2. Physicochemical and Structural Characterization
2.5.3. Vibrating Sample Magnetometry (VSM)
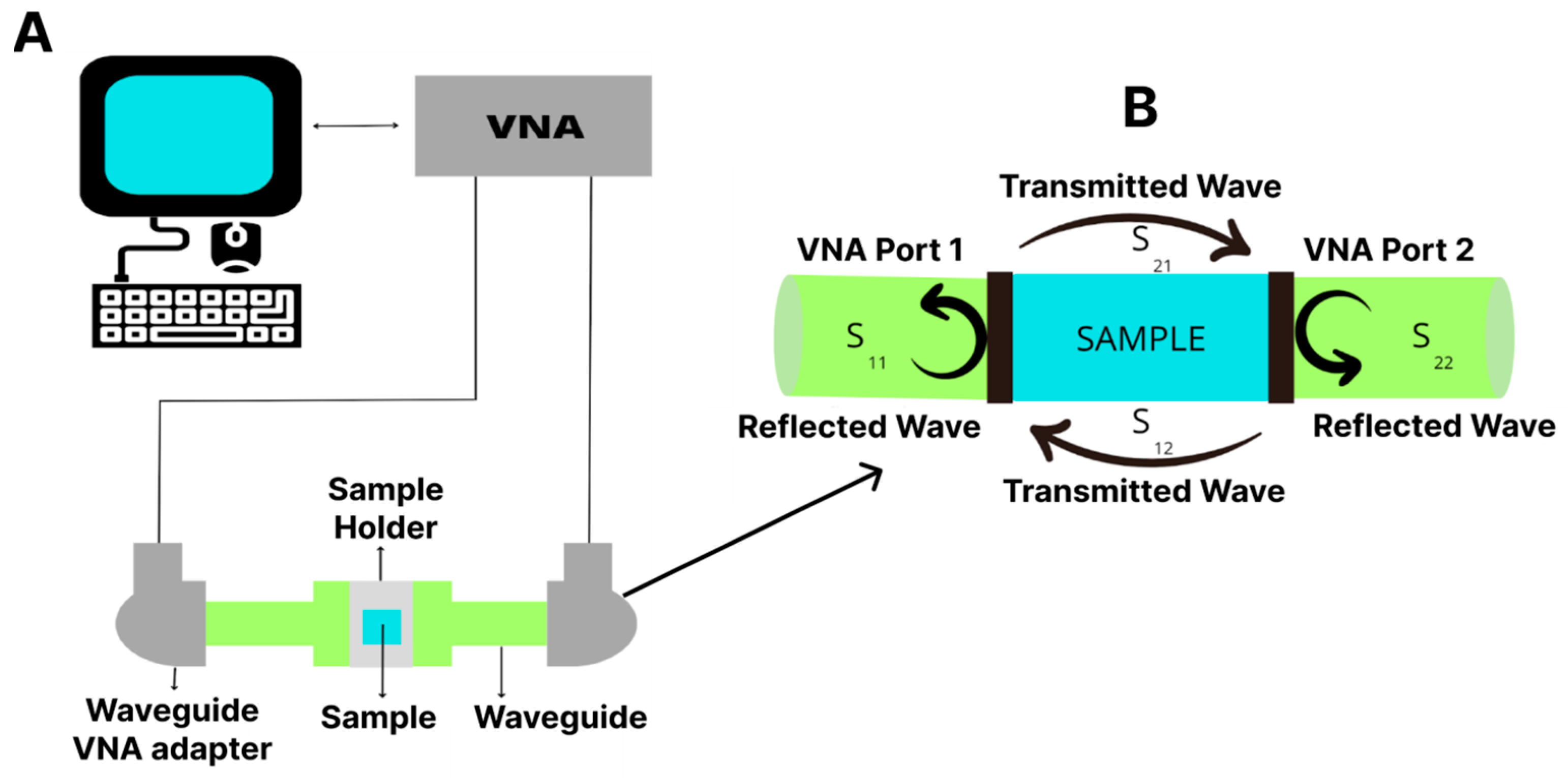
2.5.4. Magnetic Shielding
3. Results and Discussion
3.1. Incorporation Rate
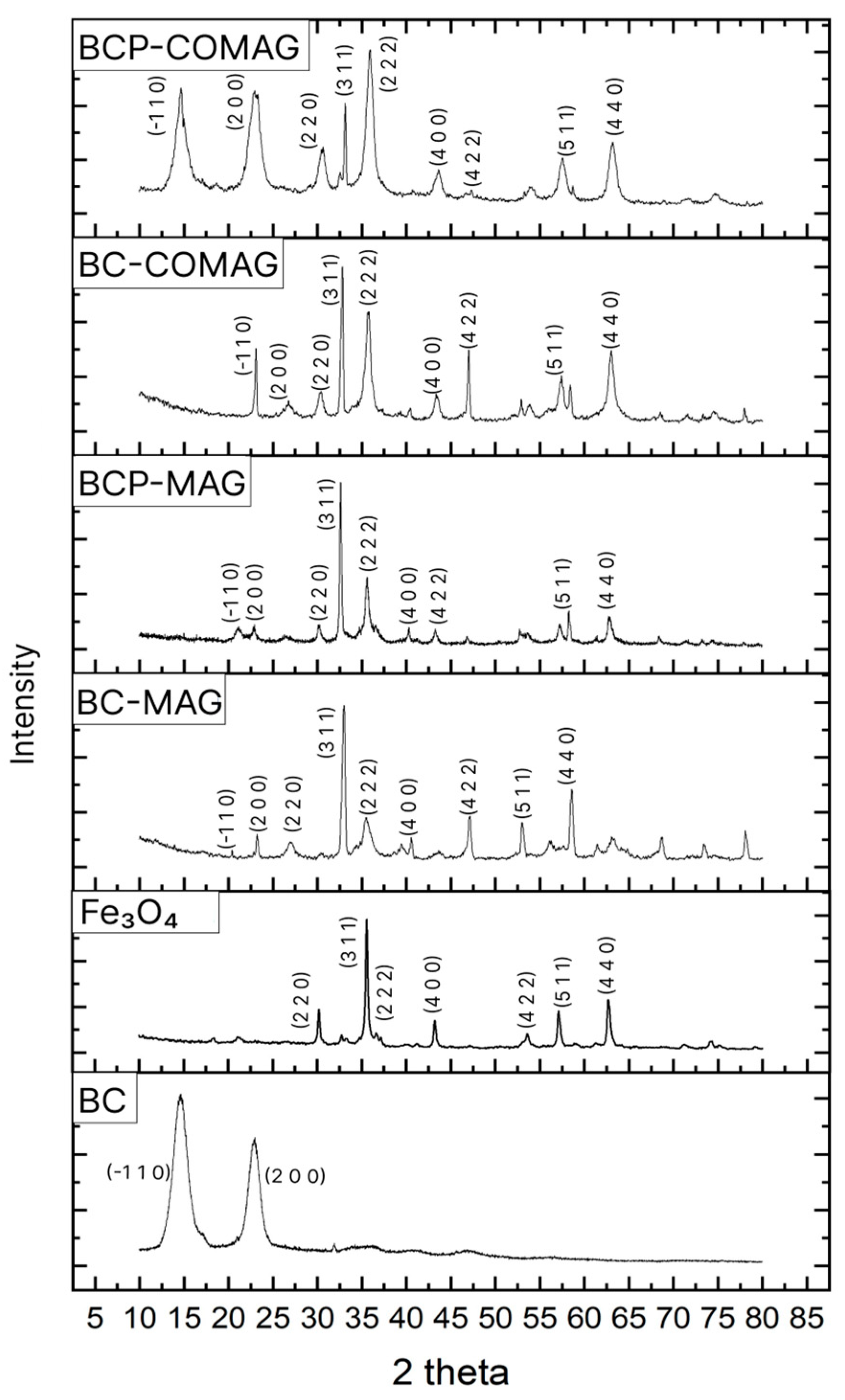
3.2. X-ray Diffraction (XRD)
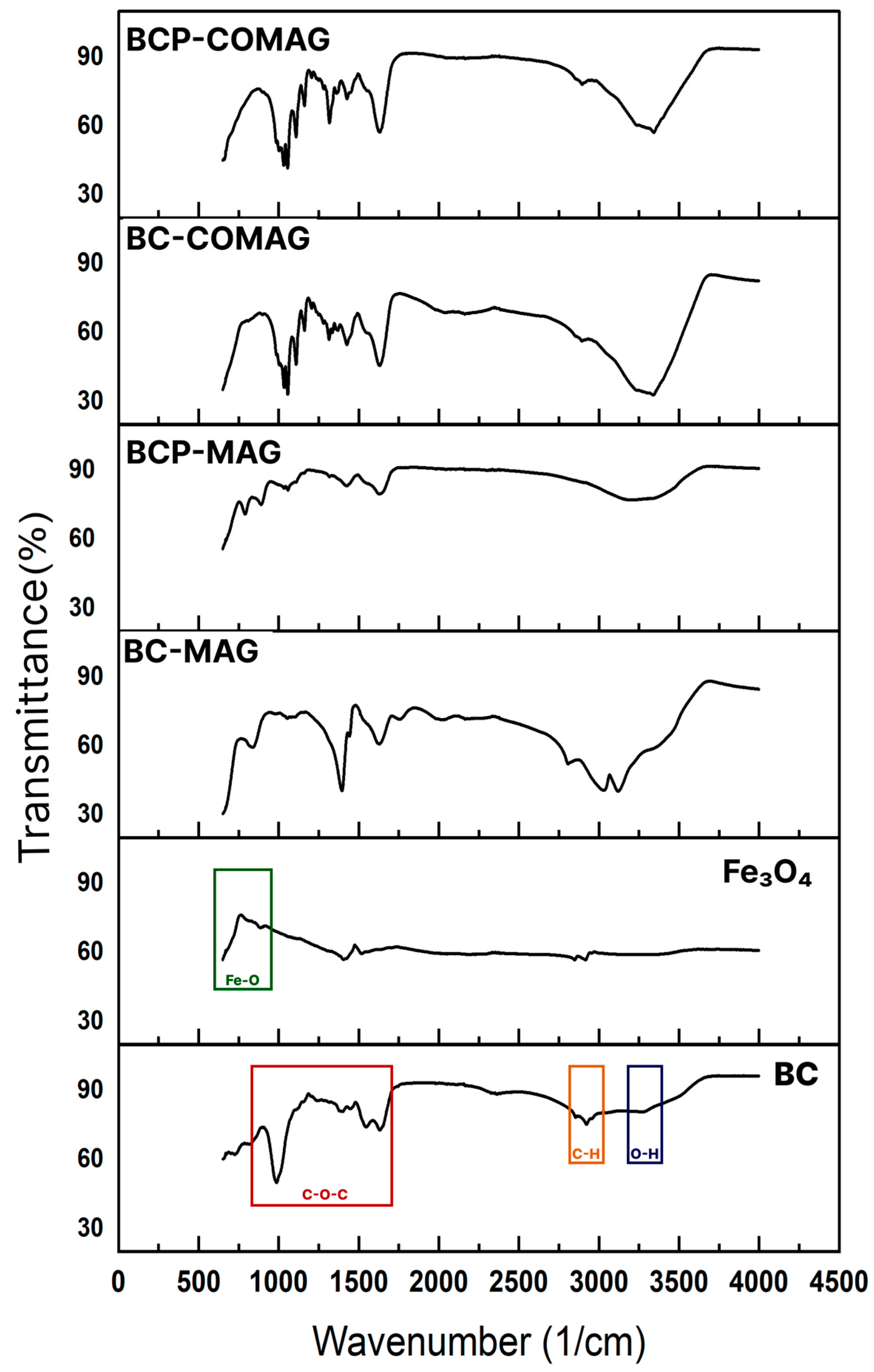
3.3. Fourier Transform Infrared (FTIR) Spectrometry
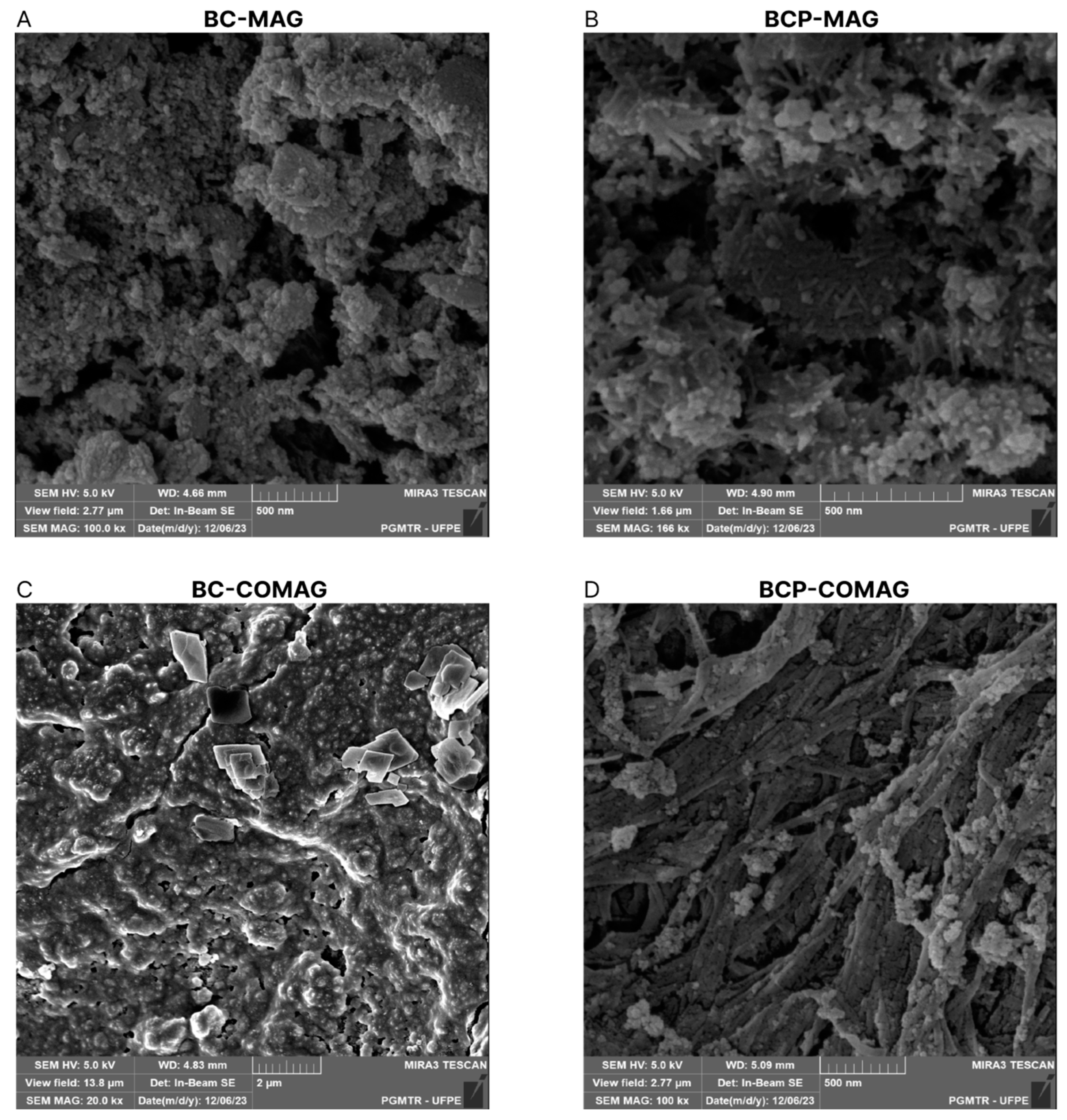
3.4. Scanning Electron Microscopy (SEM)
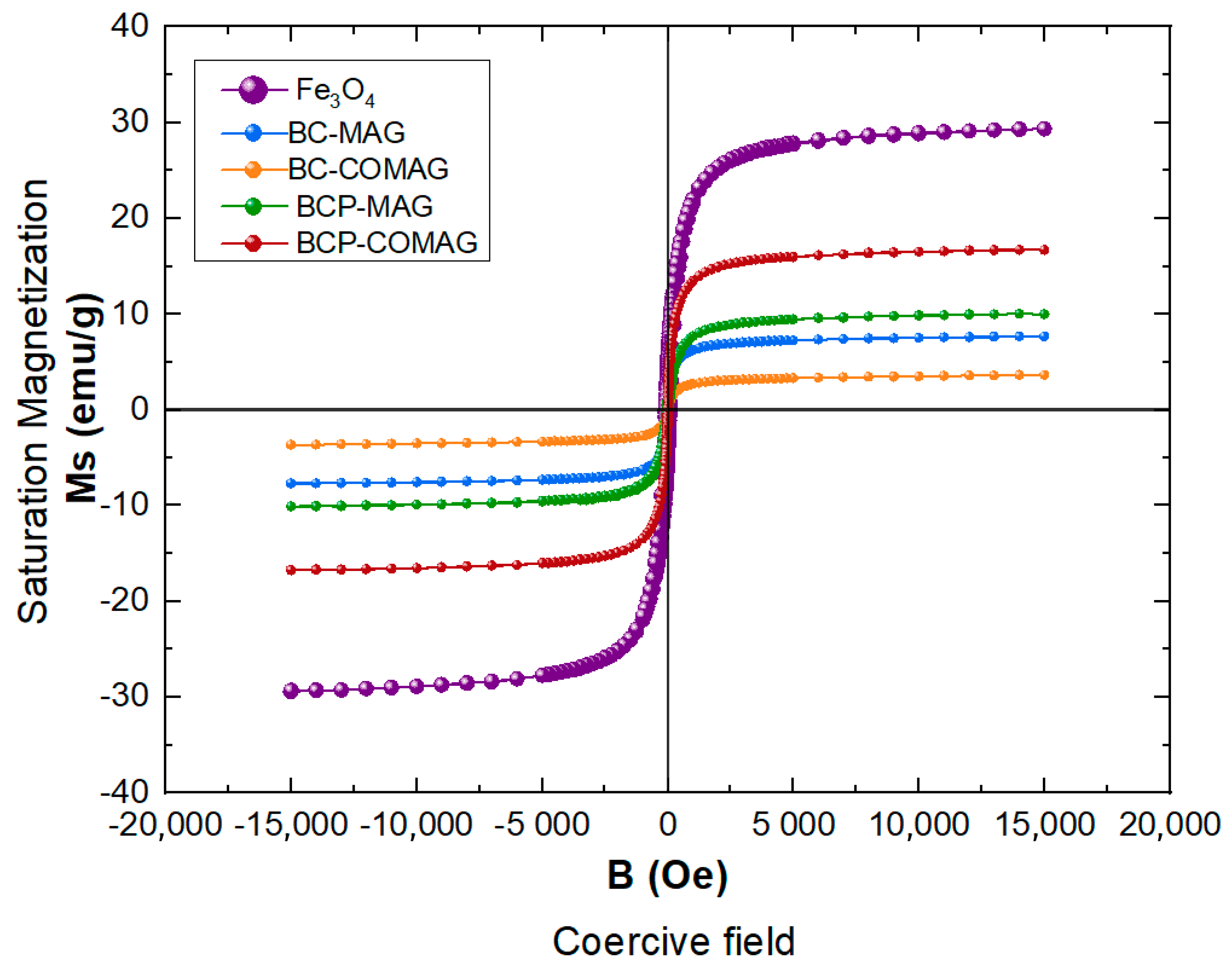
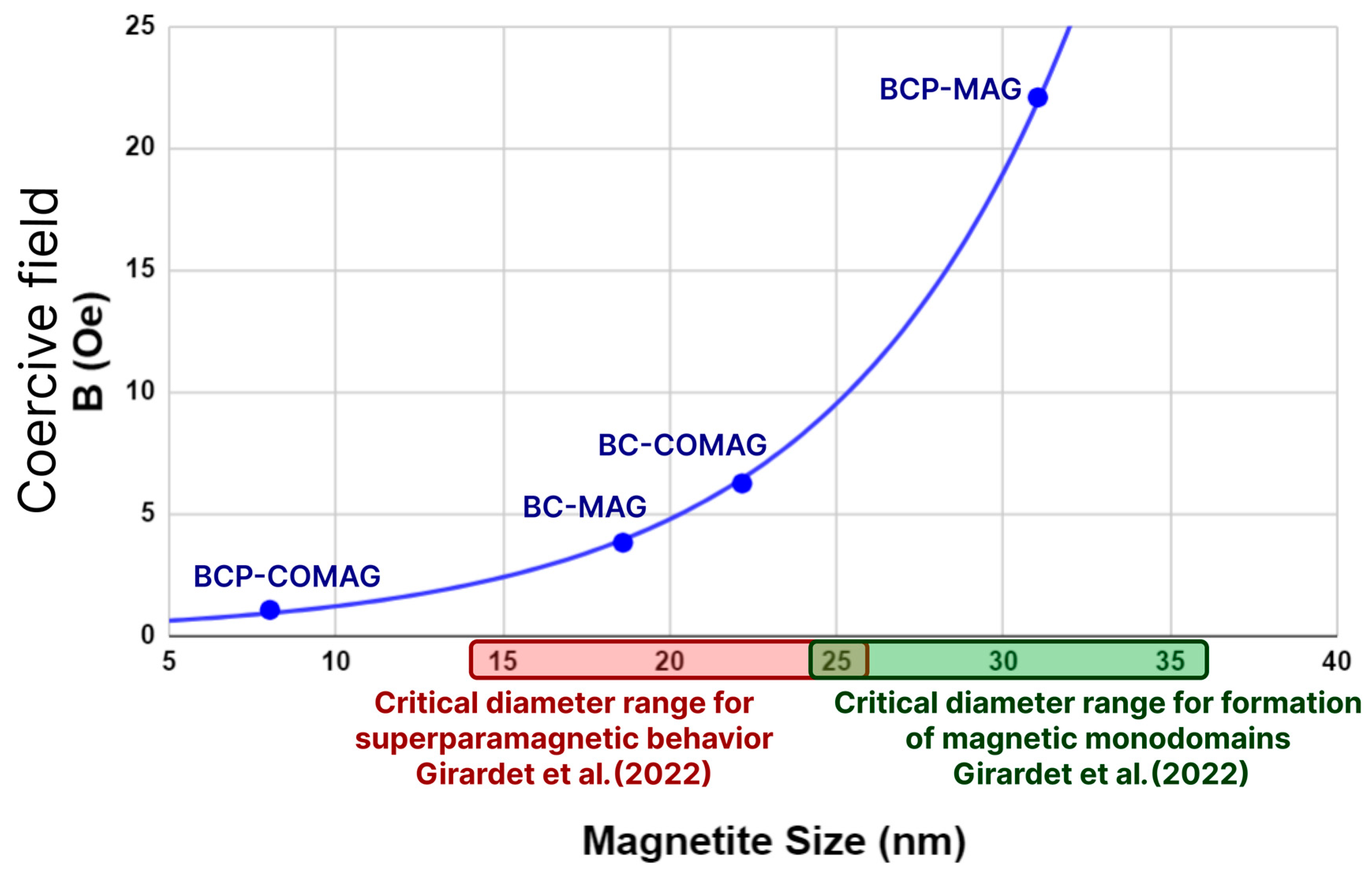
3.5. Vibrating Sample Magnetometry (VSM)
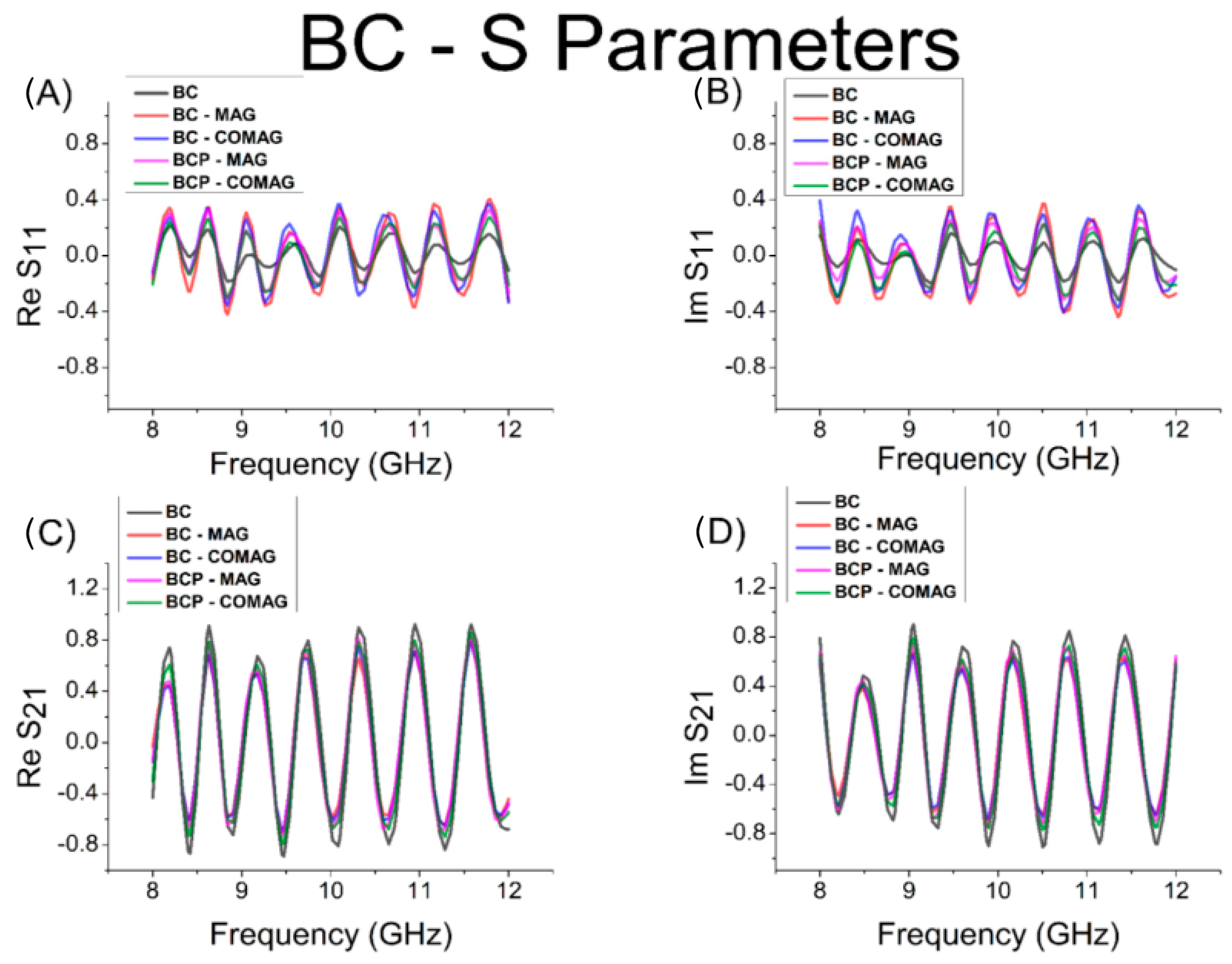
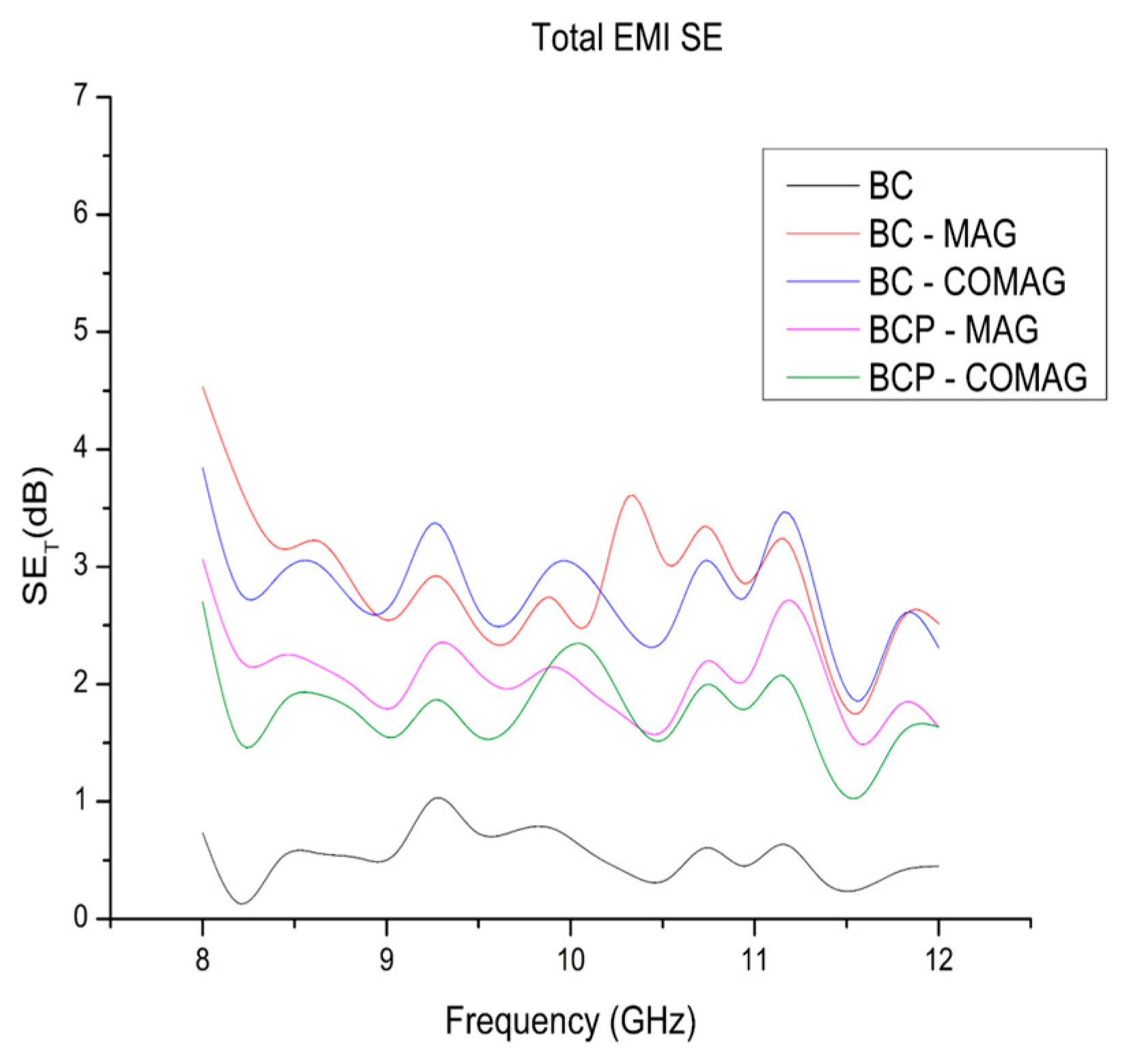
3.6. Electromagnetic Interference Shielding Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zachariah, S.M.; Grohens, Y.; Kalarikkal, N.; Thomas, S. Hybrid materials for electromagnetic shielding: A review. Polym. Compos. 2022, 43, 2507–2544. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Ray, S.S. Functional and structural facts of effective electromagnetic interference shielding materials: A review. ACS Omega 2023, 8, 8134–8158. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Ray, S.S. Electromagnetic interference cognizance and potential of advanced polymer composites toward electromagnetic interference shielding: A review. Polym. Eng. Sci. 2022, 62, 591–621. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: A review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Sriplai, N.; Pinitsoontorn, S. Bacterial cellulose-based magnetic nanocomposites: A review. Carbohydr. Polym. 2020, 254, 117228. [Google Scholar] [CrossRef] [PubMed]
- Khurd, A.S.; Kandasubramanian, B. A systematic review of cellulosic material for green electronics devices. Carbohydr. Polym. Technol. Appl. 2022, 4, 100234. [Google Scholar] [CrossRef]
- Pan, X.; Lia, J.; Ma, N.; Ma, X.; Gao, M. Bacterial cellulose hydrogel for sensors. Chem. Eng. J. 2023, 461, 142062. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Souza, K.C.; Duarte, C.R.; Duarte, I.S.; Ribeiro, F.A.S.; Silva, G.S.; Farias, P.M.A.; Sting, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Betlej, I.; Zakaria, S.; Krajewski, K.J.; BoruszewskIi, P. Bacterial cellulose–properties and its potential application. Sains Malays. 2021, 50, 493–505. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Xu, W.; Xiong, R.; Huang, C. Cellulose-based fibrous materials for self-powered wearable pressure sensor: A mini review. Cellulose 2023, 30, 1981–1998. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Pedro, L. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2019, 76, 333–335. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Potential of magnetic nano cellulose in biomedical applications: Recent advances. Biomater. Polym. Horiz. 2022, 1, 32–47. [Google Scholar] [CrossRef]
- Jin, K.; Jin, C.; Wu, Y. Synthetic biology-powered microbial co-culture strategy and application of bacterial cellulose-based composite materials. Carbohydr. Polym. 2022, 283, 119171. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.C.; Amorim, J.D.P.d.; Silva Junior, C.J.G.d.; de Medeiros, A.D.M.; Santana Costa, A.F.d.; Vinhas, G.M.; Sarubbo, L.A. Magnetic bacterial cellulose biopolymers: Production and potential applications in the electronics sector. Polymers 2023, 15, 853. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.C.; Costa, A.F.d.S.; Vinhas, G.M.; Sarubbo, L.A. Synthesis of iron oxides and influence on final sizes and distribution in bacterial cellulose applications. Polymers 2023, 15, 3284. [Google Scholar] [CrossRef] [PubMed]
- Yingkamhaeng, N.; Intapan, I.; Sukyai, P. Fabrication and characterisation of functionalised superparamagnetic bacterial nanocellulose using ultrasonic-assisted in situ synthesis. Fibers Polym. 2018, 19, 489–497. [Google Scholar] [CrossRef]
- Chanthiwong, M.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Controlling the processing of co-precipitated magnetic bacterial cellulose/iron oxide nanocomposites. Mater. Des. 2020, 196, 109148. [Google Scholar] [CrossRef]
- Mira-Cuenca, C.; Meslier, T.; Roig-Sanchez, S.; Laromaine, A.; Roig, A. Patterning bacterial cellulose films with iron oxide nanoparticles and magnetic resonance imaging monitoring. ACS Appl. Polym. Mater. 2021, 3, 4959–4965. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, Z.; Qian, Y.; Xu, L.; Guo, H. Bacterial laccase immobilized on a magnetic dialdehyde cellulose without cross-linking agents for decolorization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 632, 127818. [Google Scholar] [CrossRef]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetically responsive and flexible bacterial cellulose membranes. Carbohydr. Polym. 2018, 192, 251–262. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, S.; Adstedt, K.; Kim, M.; Xiong, R.; Yu, J.; Chen, X.; Zhao, X.; Ye, C.; Tsukruk, V.V. Uniformly aligned flexible magnetic films from bacterial nanocelluloses for fast actuating optical materials. Nat. Commun. 2022, 13, 5804. [Google Scholar] [CrossRef] [PubMed]
- Salles, T.R.; da Silva Bruckamann, F.; Viana, A.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. J. Polym. Environ. 2022, 30, 2695–2713. [Google Scholar] [CrossRef]
- Salidkul, N.; Mongkolthanaruk, W.; Faungnawakij, K.; Pinitsoontorn, S. Hard magnetic membrane based on bacterial cellulose–barium ferrite nanocomposites. Carbohydr. Polym. 2021, 264, 118016. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.; Wei, W.; Lin, Z.; Chang, J.; Hao, Y. Flexible nanocomposite conductors for electromagnetic interference shielding. Nano-Micro Lett. 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, H.; Liu, T.; Wang, Z.; Wang, L.; Fan, P.; Chen, F.; Zhong, M.; Wu, B.; Liu, S.; et al. EMI performance within the conductive composite foams engineered with bimodal and uniform cell structures. J. Supercrit. Fluids 2024, 204, 106093. [Google Scholar] [CrossRef]
- Walsh, P.P.; Murphy, E.; Horan, D. The role of science, technology and innovation in the UN 2030 agenda. Technol. Forecast. Soc. Chang. 2020, 154, 119957. [Google Scholar] [CrossRef]
- Tahalyani, J.; Akhtar, M.J.; Kar, K.K. Flexible, stretchable and lightweight polyurethane and graphene nanoplatelets nanocomposite for high-performance EMI shielding application. Mater. Today Commun. 2022, 33, 104586. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zheng, Q.; Zheng, Y.-J.; Cao, M.-S. Green EMI shielding: Dielectric/magnetic “genes” and design philosophy. Carbon 2023, 206, 124–141. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Luo, J.; Tusiime, R.; Lu, C.; Xue, Y.; Zhou, J.; Liu, Y.; Zhang, H.; Yu, J. Fe3O4 uniformly decorated reduced graphene oxide aerogel for epoxy nanocomposites with high EMI shielding performance. Compos. Commun. 2022, 36, 101391. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, M.; Ren, Y.; Shen, B.; Chen, J.; Wang, E.; Zhao, J.; Zheng, W. Carbon fabric composites with NiCo compounds: Structure evolution and EMI shielding performance. Appl. Surf. Sci. 2023, 627, 157275. [Google Scholar] [CrossRef]
- Rayar, A.; Naveen, C.S.; Onkarappa, H.S.; Betageri, V.S.; Prasanna, G.D. EMI shielding applications of PANI-Ferrite nanocomposite materials: A review. Synth. Met. 2023, 295, 117338. [Google Scholar] [CrossRef]
- Yang, X.; Xuan, L.; Men, W.W.; Wu, X.; Lan, D.; Shi, Y.; Jia, H.; Duan, Y. Carbonyl iron/glass fiber cloth composites: Achieving multi-spectrum stealth in a wide temperature range. Chem. Eng. J. 2024, 491, 151862. [Google Scholar] [CrossRef]
- He, D.; Su, Q.; Liu, D.; Xia, L.; Huang, X.; Lan, D.; Liu, Y.; Huang, Y.; Zhong, B. Surface engineering strategy for MXene to tailor electromagnetic wave absorption performance. Chem. Eng. J. 2024, 491, 152041. [Google Scholar] [CrossRef]
- Radasky, W.A.; Baum, C.E.; Wik, M.W. Introduction to the special issue on high-power electromagnetics (HPEM) and intentional electromagnetic interference (IEMI). IEEE Trans. Electromagn. Compat. 2004, 46, 314–321. [Google Scholar] [CrossRef]
- Jie, H.; Zhao, Z.; Zeng, Y.; Chang, Y.; Fan, F.; Wang, C.; See, K.Y. A review of intentional electromagnetic interference in power electronics: Conducted and radiated susceptibility. IET Power Electron. 2024, in press. [CrossRef]
- Deeraj, B.D.S.; Jayan, J.S.; Raman, A.; Saritha, A.; Joseph, K. Recent prospects and trends on zeolitic imidazolate frameworks for microwave absorption and EMI shielding applications. Synth. Met. 2023, 296, 117354. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Zhang, P.; Wan, C. Outstanding EMI shielding properties of Al2O3 reinforced with sandwiched graphene/CNTs. J. Eur. Ceram. Soc. 2023, 43, 4082–4087. [Google Scholar] [CrossRef]
- Fan, B.; Xing, L.; Yang, K.; Yang, Y.; Zhou, F.; Tong, G.; Wu, W. Salt-templated graphene nanosheet foams filled in silicon rubber toward prominent EMI shielding effectiveness and high thermal conductivity. Carbon 2023, 207, 317–327. [Google Scholar] [CrossRef]
- Mondal, D.; Bhattacharya, D.; Mondal, T.; Kundu, M.; Sarkar, S.; Mandal, T.K.; Paul, B.K.; Das, S. Rare earth ion-infused α-MnO2 nano-rods for excellent EMI shielding efficiency: Experimental and theoretical insights. Sustain. Mater. Technol. 2023, 38, e00772. [Google Scholar] [CrossRef]
- Anand, S.; Muniyappan, S.; Racik, K.M.; Manikandan, A.; Mani, D.; Nandhini, S.; Karuppasamy, P.; Pandian, M.S.; Ramasamy, P.; Chandar, N.K. Fabrication of binary to quaternary PVDF based flexible composite films and ultrathin sandwich structured quaternary PVDF/CB/g-C3N4/BaFe11.5Al0.5O19 composite films for efficient EMI shielding performance. Synth. Met. 2022, 291, 117199. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Q.; Li, M.; Song, L.; Zhang, F.; Min, Z.; Wang, H.; Zhu, Y.; Zhang, R.; Lan, D.; et al. Magnetic media synergistic carbon fiber@ Ni/NiO composites for high-efficiency electromagnetic wave absorption. Chem. Eng. J. 2024, 492, 152245. [Google Scholar] [CrossRef]
- Jia, F.; Lu, Z.; Li, S.; Zhang, J.; Liu, Y.; Wang, H.; Xu, X.; Du, A.; Guo, D.; Yan, N. Asymmetric c-MWCNT/AgNWs/PANFs hybrid film constructed by tailoring conductive-blocks strategy for efficient EMI shielding. Carbon 2024, 217, 118600. [Google Scholar] [CrossRef]
- Pu, X.; Feng, Z.; Sun, S.; Bi, K.; Hao, Y. Absorption-dominant EMI shielding performance of multiple folded Bi2Se3/PVDF nanocomposite films. J. Mater. 2023, 10, 27–36. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacterxylinum: II. Preparation of freeze—Dried cells capable of polymerized glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hungund, B.; Gupta, S.G. Improved production of bacterial cellulose from Gluconacetobacter persimmonis GH-2. J. Microb. Biochem. Technol. 2010, 2, 5. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef] [PubMed]
- Galdino, C.J.S., Jr.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Advancement in conductive cotton fabrics through in situ polymerization of polypyrrole-nanocellulose composites. Carbohydr. Polym. 2016, 151, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.H.; Alonso, J.; Khurshid, H.; Lampen-Kelley, P.; Chandra, S.; Repa, K.S.; Nemati, Z.; Das, R.; Iglesias, O.; Srikanth1, H. Exchange bias effects in iron oxide-based nanoparticle systems. Nanomaterials 2016, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Li, Y.; Yi, D.; Zhai, W.; Wei, X.; Zheng, W. Microcellular graphene foam for improved broadband electromagnetic interference shielding. Carbon 2016, 102, 154–160. [Google Scholar] [CrossRef]
- Yan, A.; Liu, Y.; Wu, Z.; Gan, X.; Li, F.; Tao, J.; Li, C.; Yi, J. RGO reinforced Cu foam with enhanced mechanical and electromagnetic shielding properties. J. Mater. Res. Technol. 2022, 21, 2965–2975. [Google Scholar] [CrossRef]
- Yazdani, F.; Seddigh, M. Magnetite nanoparticles synthesized by co-precipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Chaabane, L.; Chahdoura, H.; Mehdaoui, R.; Snoussi, M.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. Functionalization of developed bacterial cellulose with magnetite nanoparticles for nanobiotechnology and nanomedicine applications. Carbohydr. Polym. 2020, 247, 116707. [Google Scholar] [CrossRef] [PubMed]
- David, I.; Welch, A.J.E. The oxidation of magnetite and related spinels. Constitution of gamma ferric oxide. Trans. Faraday Soc. 1956, 52, 1642–1650. [Google Scholar] [CrossRef]
- Zheng, H.; Daghagheleh, O.; Ma, Y.; Taferner, B.; Schenk, J.; Kapelyushin, Y. Phase transition of magnetite ore fines during oxidation probed by in situ high-temperature X-ray diffraction. Met. Mater. Trans. B 2023, 54BI, 1195. [Google Scholar] [CrossRef]
- Wroblewski, C.; Volford, T.; Martos, B.; Samoluk, J.; Martos, P. High yield synthesis and application of magnetite nanoparticles (Fe3O4). Magnetochemistry 2020, 6, 22. [Google Scholar] [CrossRef]
- Sabur, A.; Gafur, A. Crystallographic, morphological, magnetic, and thermalcharacterization of superparamagnetic magnetite nanoparticles (Fe3O4) synthesized by chemical coprecipitation method and calcined at 250 °C for 4 hr. J. Nanomater. 2024, 2024, 577778. [Google Scholar] [CrossRef]
- Aphesteguy, J.C.; Kurlyandskaya, G.V.; De Celis, J.P.; Safronov, A.P.; Schegoleva, N.N. Magnetite nanoparticles prepared by co-precipitation method in different conditions. Mater. Chem. Phys. 2015, 161, 243–249. [Google Scholar] [CrossRef]
- Suppiah, D.D.; Abd Hamid, S.B. One step facile synthesis of ferromagnetic magnetite nanoparticles. J. Magn. Magn. Mater. 2016, 414, 204–208. [Google Scholar] [CrossRef]
- Wen, X.; Yang, J.; He, B.; Gu, Z. Preparation of monodisperse magnetite nanoparticles under mild conditions. Curr. Appl. Phys. 2008, 8, 535–541. [Google Scholar] [CrossRef]
- Riaz, S.; Bashir, M.; Naseem, S. Iron oxide nanoparticles prepared by modified co-precipitation method. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Girardet, T.; Venturini, P.; Martinez, H.; Dupin, J.-C.; Cleymand, F.; Fleutot, S. Spinel. Magnetic iron oxide nanoparticles: Properties, synthesis and washing methods. Appl. Sci. 2022, 12, 8127. [Google Scholar] [CrossRef]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of nanocomposite film comprising of polyvinyl alcohol (PVA) incorporated with bacterial cellulose nanocrystals and magnetite nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef] [PubMed]
- Niranjana, J.S.; Koyakutty, H.; Thomas, A.A.; Bushiri, M.J. Novel synthesis of multi-layered rGO/Fe3O4 nanocomposite in a single step and its efficient electrochemical sensing of vitamin C. Mater. Sci. Eng. B 2023, 290, 116283. [Google Scholar] [CrossRef]
- Zhou, J.; Li, R.; Liu, S.; Li, Q.; Zhang, L.; Zhang, L.; Guan, J. Structure and magnetic properties of regenerated cellulose/Fe3O4 nanocomposite films. J. Appl. Polym. Sci. 2009, 111, 2477–2484. [Google Scholar] [CrossRef]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetic bacterial cellulose and carbon nanofiber aerogel by simple immersion and pyrolysis. J. Mater. Sci. 2019, 55, 9. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Spaldin, N.A. Magnetic Materials: Fundamentals and Applications, 2nd ed.; Cambridge University Press: Santa Barbara, CA, USA, 2012. [Google Scholar]
- Zeng, M.; Laromaine, A.; Feng, W.; Levkin, P.A.; Roig, A. Origami magnetic cellulose: Controlled magnetic fraction and patterning of flexible bacterial cellulose. J. Mater. Chem. C 2014, 2, 6312–6318. [Google Scholar] [CrossRef]
- IEEE Std 299-2006; IEEE Standard Method for Measuring the Effectiveness of Electromagnetic Shielding Enclosures. IEEE: Piscataway, NJ, USA, 2007; pp. 1–52. [CrossRef]
- Li, J.; Zhang, W.; Yang, L.; Yin, S. Conductive fabrics based on carbon nanotube/Ti3C2Tx MXene/polyaniline/liquid metal quaternary composites with improved performance of EMI shielding and joule heating. Compos. Commun. 2023, 38, 101476. [Google Scholar] [CrossRef]
- Lyu, S.; Zhao, T.; Wang, Y.; Han, H.; Li, T.; Zhang, C.; Li, D.; Wang, J.-K.; Huang, J.; Yu, P.; et al. Ti3C2Tx-coated diatom frustules-derived porous SiO2 composites with high EMI shielding and mechanical properties. Ceram. Int. 2022, 48, 22845–22853. [Google Scholar] [CrossRef]
- Erfanian, E.; Moaref, R.; Ajdary, R.; Tam, K.C.; Rojas, O.J.; Kamkar, M.; Sundararaj, U. Electrochemically synthesized graphene/TEMPO-oxidized cellulose nanofibrils hydrogels: Highly conductive green inks for 3D printing of robust structured EMI shielding aerogels. Carbon 2023, 210, 118037. [Google Scholar] [CrossRef]
- Yang, J.; Liao, X.; Wang, G.; Chen, J.; Song, P.; Tang, W.; Guo, F.; Liu, F.; Li, G. Heterogeneous silicon rubber composite foam with gradient porous structure for highly absorbed ultra-efficient electromagnetic interference shielding. Compos. Sci. Technol. 2021, 206, 108663. [Google Scholar] [CrossRef]
- Li, J.; Cui, M.; Wen, J.; Chen, Y.; Shi, B.; Fan, H.; Xiang, J. Leather-like hierarchical porous composites with outstanding electromagnetic interference shielding effectiveness and durability. Compos. B Eng. 2021, 225, 109272. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.; Gao, C.; Liu, Z.; Wang, X.; Fatehi, P.; Wang, S.; Kong, F. Carbonyl iron/glass fiber/bacterial cellulose/Fe3O4/methyltrimethoxylsilane flexible film with hydrophobic for effective electromagnetic shielding. Int. J. Biol. Macromol. 2023, 243, 125195. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Li, Q.; Xue, Y.; Zhou, B.; Feng, Y.; Liu, C. Reinforcing and toughening bacterial cellulose/MXene films assisted by interfacial multiple cross-linking for electromagnetic interference shielding and photothermal response. J. Colloid Interface Sci. 2023, 652 Pt B, 1645–1652. [Google Scholar] [CrossRef]
- Xing, W.; Xi, J.; Cai, W.; Zhang, W.; Wang, B.; Chen, L.; Hu, Y. Preparation and properties of multifunctional polyurethane synthetic leather nanocomposites. Compos.—A Appl. Sci. Manuf. 2023, 169, 107534. [Google Scholar] [CrossRef]
- Fan, M.; Xia, X.; Li, S.; Zhang, R.; Wu, L.; Qu, M.; Tang, P.; Bin, Y. Sustainable bacterial cellulose reinforced carbon nanotube buckypaper and its multifunctionality for electromagnetic interference shielding, Joule heating and humidity sensing. Chem. Eng. J. 2022, 441, 136103. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, Y.; Jiang, D.; Bai, L.; Li, Z.; Huo, P.; Liu, C.; Liu, Y. Electromagnetic interference shielding of flexible carboxymethyl cellulose/MWCNT@Fe3O4 composite film with ultralow reflection loss. Int. J. Biol. Macromol. 2024, 257 Pt 1, 128604. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Micropor. Mesopor. Mat. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room temperature coprecipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.; Ashraf, M.; Javid, A.; Abid, H.A.; Ahmad, S.; Nawab, Y.; Rasheed, A.; Xue, Z.; Nosheen, A. Recent advances in electromagnetic interference (EMI) shielding textiles: A comprehensive review. Synth. Met. 2023, 294, 117305. [Google Scholar] [CrossRef]
- Feng, X.; Wang, C.; Shang, S.; Liu, H.; Huang, X.; Jiang, J.; Song, Z.; Zhang, H. Self-healing, EMI shielding, and antibacterial properties of recyclable cellulose liquid metal hydrogel sensor. Carbohydr. Polym. 2023, 311, 120786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Luo, H.; Gao, Q.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. Sandwich-like high-efficient EMI shielding materials based on 3D conductive network and porous microfiber skeleton. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130163. [Google Scholar] [CrossRef]
- Souto, L.F.C.; Soares, B.G. Electromagnetic wave absorption, EMI shielding effectiveness and electrical properties of ethylene–vinyl acetate (EVA)/polyaniline (PAni) blends prepared by in situ polymerization. Synth. Met. 2023, 298, 117441. [Google Scholar] [CrossRef]
- Jia, X.; Li, Y.; Shen, B.; Zheng, W. Evaluation, fabrication, and dynamic performance regulation of green EMI-shielding materials with low reflectivity: A review. Compos. B Eng. 2022, 233, 109652. [Google Scholar] [CrossRef]
- Hou, T.; Wang, B.; Ma, M.; Feng, A.; Huang, Z.; Zhang, Y.; Jia, Z.; Tan, G.; Cao, H.; Wu, G. Preparation of two-dimensional titanium carbide (Ti3C2Tx) and NiCo2O4 composites to achieve excellent microwave absorption properties. Compos. B Eng. 2020, 180, 107577. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Kyosev, Y.; Kremenakova, D.; Militky, J. The novel approach of EMI shielding simulation for metal coated nonwoven textiles with optimized textile module. Polym. Test. 2022, 114, 107706. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Zhao, J.; Li, X.-Z.; Jiang, H.; Cai, Y.-J.; Yang, X.; Liu, Y.; Wei, N.-J.; Li, Y.-B.; Wu, Y.-G.; et al. Metal-grade laminated nanofiber films with outstanding EMI shielding performances and high-temperature resistance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132701. [Google Scholar] [CrossRef]
- Erdogan, N.; Erden, F.; Astarlioglu, A.T.; Ozdemir, M.; Ozbay, S.; Aygun, G.; Ozyuzer, L. ITO/Au/ITO multilayer thin films on transparent polycarbonate with enhanced EMI shielding properties. Curr. Appl. Phys. 2020, 20, 489–497. [Google Scholar] [CrossRef]
- Sahoo, R.; Sundara, R.; Subramanian, V. Influence of molecular weight of PVP on the structure of silver nanowires for EMI shielding application. Mater. Today Proc. 2023, 94, 29–34. [Google Scholar] [CrossRef]
- He, D.; Qian, L.; Chen, X.; He, B.; Li, J. Durable cellulose paper by grafting thiol groups and controlling silver deposition for ultrahigh electromagnetic interference shielding. Int. J. Biol. Macromol. 2023, 248, 125972. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, J.; Ren, H.; Xue, Y.; Yang, C.; Yuan, T.; Yang, Z.; Liu, Y.; Zhang, H.; Yu, J. Simultaneously improving the EMI shielding performances and mechanical properties of CF/PEKK composites via MXene interfacial modification. J. Mater. Sci. Technol. 2023, 154, 202–209. [Google Scholar] [CrossRef]
- Lee, S.; Park, D.; Cho, Y.; Lee, J.; Kim, J. Highly thermally conductive and EMI shielding composite reinforced with aligned carbon fibers and MXene. Synth. Met. 2022, 291, 117183. [Google Scholar] [CrossRef]
- Verma, R.; Thakur, P.; Chauhan, A.; Jasrotia, R.; Thakur, A. A review on MXene and its composites for electromagnetic interference (EMI) shielding applications. Carbon 2023, 208, 170–190. [Google Scholar] [CrossRef]
- Mathur, P.; Raman, S. Electromagnetic interference (EMI): Measurement and reduction techniques. J. Electroni. Mater. 2020, 49, 2975–2998. [Google Scholar] [CrossRef]

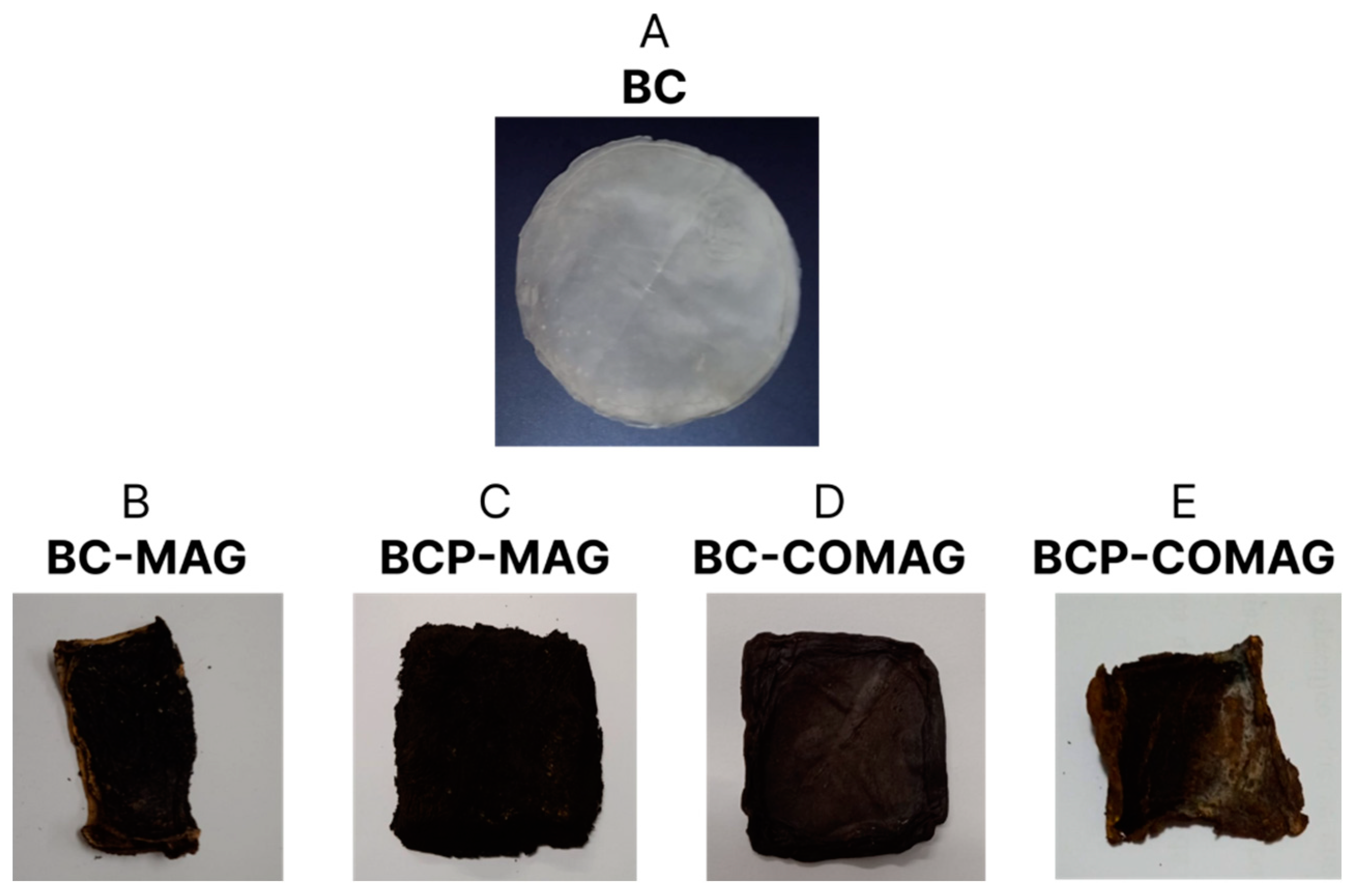




| Sample | State of BC Used | Type of Fe3O4 Synthesis |
|---|---|---|
| BC-MAG | Not processed | in situ coprecipitation |
| BCP-MAG | Processed | in situ coprecipitation |
| BC-COMAG | Not processed | ex situ coprecipitation |
| BCP-COMAG | Processed | ex situ coprecipitation |
| Sample | Percentage of Magnetite in Sample (%) |
|---|---|
| BC-MAG | 78.57 |
| BCP-MAG | 82.92 |
| BC-COMAG | 80.39 |
| BCP-COMAG | 83.62 |
| Sample | Mean Crystallite Size (nm) |
|---|---|
| Free magnetite | 20.20 |
| BC-MAG | 18.60 |
| BCP-MAG | 31.03 |
| BC-COMAG | 22.17 |
| BCP-COMAG | 8.02 |
| Sample | Saturation Magnetization (emu/g) | Coercive Field (Oe) |
|---|---|---|
| Fe3O4 | 29 | 87.57 |
| BC-MAG | 8 | 3.80 |
| BCP-MAG | 10 | 22.07 |
| BC-COMAG | 3 | 6.23 |
| BCP-COMAG | 17 | 1.04 |
| Material | MXene/BC | MXene/ Leather | CNT/ BC-BP | MWCNT@Fe3O4/CMC | BC-MAG | BC-COMAG |
|---|---|---|---|---|---|---|
| Mean EMI SE (dB) | 41.0 | 30.1 | 24.0 | 0.23 | 2.9 | 2.8 |
| Thickness (mm) | 0.015 | 19 | 0.036 | 0.028 | 1.1 | 0.3 |
| EMI SE/Thickness (dB/mm) | 2733 | 1.6 | 667 | 8.2 | 2.6 | 9.3 |
| Frequency (GHz) | 8–12 | 8.2–12.4 | 5.85–8.2 | 8–12 | 8–12 | 8–12 |
| Reference | [79] | [80] | [81] | [82] | This work | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, T.C.; dos Santos, A.R.; Chacon, J.L.d.S.P.; Durval, Í.J.B.; Costa, A.F.d.S.; Padrón Hernández, E.; Converti, A.; Vinhas, G.M.; Sarubbo, L.A. Innovation in Methods for Incorporating Magnetite into Biocellulose for Electromagnetic Interference Shielding Effectiveness Applications. Energies 2024, 17, 3202. https://doi.org/10.3390/en17133202
de Souza TC, dos Santos AR, Chacon JLdSP, Durval ÍJB, Costa AFdS, Padrón Hernández E, Converti A, Vinhas GM, Sarubbo LA. Innovation in Methods for Incorporating Magnetite into Biocellulose for Electromagnetic Interference Shielding Effectiveness Applications. Energies. 2024; 17(13):3202. https://doi.org/10.3390/en17133202
Chicago/Turabian Stylede Souza, Thaís Cavalcante, Alexsandro Ramos dos Santos, João Luiz da Silva Pereira Chacon, Ítalo José Batista Durval, Andréa Fernanda de Santana Costa, Eduardo Padrón Hernández, Attilio Converti, Glória Maria Vinhas, and Leonie Asfora Sarubbo. 2024. "Innovation in Methods for Incorporating Magnetite into Biocellulose for Electromagnetic Interference Shielding Effectiveness Applications" Energies 17, no. 13: 3202. https://doi.org/10.3390/en17133202
APA Stylede Souza, T. C., dos Santos, A. R., Chacon, J. L. d. S. P., Durval, Í. J. B., Costa, A. F. d. S., Padrón Hernández, E., Converti, A., Vinhas, G. M., & Sarubbo, L. A. (2024). Innovation in Methods for Incorporating Magnetite into Biocellulose for Electromagnetic Interference Shielding Effectiveness Applications. Energies, 17(13), 3202. https://doi.org/10.3390/en17133202










