Compatibility of Methanol-Hydrotreated Vegetable Oil Blends with Chosen Steels and Aluminum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Trace Metals
2.2.2. Analysis of Fuel Properties
3. Results
3.1. Visual Evaluation of Metal Corrosion
3.2. Concentration of Trace Metals in Exposed Fuel Samples
3.3. Influence of Trace Elements on Fuel Properties
3.3.1. Density and Kinematic Viscosity
3.3.2. Distillation Curve Analysis
4. Discussion
4.1. Fuel and Metal Compatibility
4.2. Effects of Trace Metals on Fuel Performance and Engines
5. Conclusions
- A blend of methanol and HVO/HVOr displays relatively poor stability due to the occurrence of phase separation between methanol and HVO/HVOr. The co-solvents 1-octanol and 1-dodecanol can be used to form stable, single-phase methanol–HVOr fuel blends.
- Visual assessment showed that methanol, HVO, HVOr, MeOH50, MeOH50-1-octanol, and the MeOH50-1-dodecanol fuel blends did not induce observable corrosion on the metallic surfaces.
- The metals were corrosion-resistant when immersed in HVO and MeOH 50 fuel samples in the studied conditions. Methanol had a slight dissolving effect on aluminum (dissolving Al) and carbon steel (dissolving Zn).
- The trace metal concentrations in MeOH50-O, MeOH50-D, and HVOr fuels did not change after metal immersion. These three fuels did not dissolve metal in the studied conditions.
- Methanol, HVO, and HVOr exhibited relatively stable characteristics when in contact with metals. The immersion of selected metals into the fuels had no impact on the density of methanol, HVO, HVOr, MeOH50, MeOH50-1-octanol, and MeOH50-1-dodecanol fuels. Moreover, the immersion did not influence kinematic viscosity and distillation behavior of methanol, HVO, and HVOr fuels.
- An experimental method needs to be developed further to study the corrosive effect of methanol in particular, and to prove and secure different metals’ resistance to corrosion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a Fuel for Internal Combustion Engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Anand, K.; Ra, Y.; Reitz, R.D.; Bunting, B. Surrogate Model Development for Fuels for Advanced Combustion Engines. Energy Fuels 2011, 25, 1474–1484. [Google Scholar] [CrossRef]
- Liu, J.; Wu, P.; Ji, Q.; Sun, P.; Wang, P.; Meng, Z.; Ma, H. Experimental Study on Effects of Pilot Injection Strategy on Combustion and Emission Characteristics of Diesel/Methanol Dual-Fuel Engine under Low Load. Energy 2022, 247, 123464. [Google Scholar] [CrossRef]
- No, S.Y. Application of Biobutanol in Advanced CI Engines—A Review. Fuel 2016, 183, 641–658. [Google Scholar] [CrossRef]
- Szeto, W.; Leung, D.Y.C. Is Hydrotreated Vegetable Oil a Superior Substitute for Fossil Diesel? A Comprehensive Review on Physicochemical Properties, Engine Performance and Emissions. Fuel 2022, 327, 125065. [Google Scholar] [CrossRef]
- Halada Nandhakrishnan, M.; Prashant Thakare, S. Alcoholate Corrosion of Ferrous Metals in Methanol-Gasoline Fuel Blends. J. Chem. Technol. Metall. 2020, 55, 2187. [Google Scholar]
- Domínguez, V.M.; Hernández, J.J.; Ramos, Á.; Giménez, B.; Rodríguez-Fernández, J. Exploring the Effect of Methanol and Ethanol on the Overall Performance and Substitution Window of a Dual-Fuel Compression-Ignition Engine Fueled with HVO. Fuel 2024, 359, 130529. [Google Scholar] [CrossRef]
- Liu, M. Methanol as a Marine Fuel-Availability and Pre-Trial Considerations; Nanyang Technological University: Singapore, 2020; Available online: https://www.ntu.edu.sg/docs/librariesprovider79/publication/mesd-webinar-2020-methanol-as-a-marine-fuel.pdf (accessed on 12 January 2024).
- Wissner, N.; Healy, S.; Cames, M.; Sutter, J. Methanol as a Marine Fuel; Naturschutzbund Deutschland: Stuttgart, Germany, 2023. [Google Scholar]
- Hunicz, J.; Mikulski, M.; Shukla, P.C.; Gęca, M.S. Partially Premixed Combustion of Hydrotreated Vegetable Oil in a Diesel Engine: Sensitivity to Boost and Exhaust Gas Recirculation. Fuel 2022, 307, 121910. [Google Scholar] [CrossRef]
- Niemi, S.; Vauhkonen, V.; Mannonen, S.; Ovaska, T.; Nilsson, O.; Sirviö, K.; Heikkilä, S.; Kiijärvi, J. Effects of Wood-Based Renewable Diesel Fuel Blends on the Performance and Emissions of a Non-Road Diesel Engine. Fuel 2016, 186, 1–10. [Google Scholar] [CrossRef]
- Spoof-Tuomi, K.; Vauhkonen, V.; Niemi, S.; Ovaska, T.; Lehtonen, V.; Heikkilä, S.; Nilsson, O. Effects of Crude Tall Oil Based Renewable Diesel on the Performance and Emissions of a Non-Road Diesel Engine; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2021. [Google Scholar]
- Ciniviz, M.; Köse, H.; Canli, E.; Solmaz, Ö. An Experimental Investigation on Effects of Methanol Blended Diesel Fuels to Engine Performance and Emissions of a Diesel Engine. Sci. Res. Essays 2011, 6, 3189–3199. [Google Scholar] [CrossRef]
- Duan, Q.; Yin, X.; Wang, X.; Kou, H.; Zeng, K. Experimental Study of Knock Combustion and Direct Injection on Knock Suppression in a High Compression Ratio Methanol Engine. Fuel 2022, 311, 122505. [Google Scholar] [CrossRef]
- Hunicz, J.; Matijošius, J.; Rimkus, A.; Kilikevičius, A.; Kordos, P.; Mikulski, M. Efficient Hydrotreated Vegetable Oil Combustion under Partially Premixed Conditions with Heavy Exhaust Gas Recirculation. Fuel 2020, 268, 117350. [Google Scholar] [CrossRef]
- Wang-Alho, H.; Sirviö, K.; Balogun, F.; Kaivosoja, J.; Nuortila, C.; Mikulski, M.; Niemi, S. Properties of Chemically Stabilized Methanol-HVO Blends. Agron. Res. 2024, 22. [Google Scholar] [CrossRef]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M. A Review of the Effect of Biodiesel on the Corrosion Behavior of Metals/Alloys in Diesel Engines. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2923–2943. [Google Scholar] [CrossRef]
- Wang-Alho, H.; Sirviö, K.; Hissa, M.; Mikulski, M.; Niemi, S. Methanol-HVO Blends for Efficient Low-Temperature Combustion: Analytical Research on Fuel Properties. Agron. Res. 2023, 21, 994–1005. [Google Scholar] [CrossRef]
- Sathish Kumar, T.; Ashok, B. Material Compatibility of SI Engine Components towards Corrosive Effects on Methanol-Gasoline Blends for Flex Fuel Applications. Mater. Chem. Phys. 2023, 296, 127344. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Alcohols as Alternative Fuels: An Overview. Appl. Catal. A Gen. 2011, 404, 1–11. [Google Scholar] [CrossRef]
- Martin, A.; O’malley, J. Compatibility of Methanol Fuel Blends with Gasoline Vehicles and Engines in Indonesia; The International Council on Clean Transportation: Washington, DC, USA, 2021. [Google Scholar]
- Muthuraman, V.S.; Patel, A.; Shreya, V.; Vaidyanathan, A.; Reshwanth, K.N.G.L.; Karthick, C.; Jacqueline, P.J.; Jan Gęca, M.; Ashok, B.; Sivagami, K.; et al. Progress on Compatibility Issues of Alcohols on Automotive Materials: Kinetics, Challenges and Future Prospects—A Comprehensive Review. Process Saf. Environ. Prot. 2022, 162, 463–493. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, S.; Luo, J.; Zhang, B.; Zhang, H.; Xiao, R. Optimize the Co-Solvent for Methanol in Diesel with Group of Oxygen-Containing Reagents: Molecular Structure and Intermolecular Forces Analysis. Fuel Process. Technol. 2021, 222, 106980. [Google Scholar] [CrossRef]
- Yilmaz, N.; Atmanli, A.; Vigil, F.M. Quaternary Blends of Diesel, Biodiesel, Higher Alcohols and Vegetable Oil in a Compression Ignition Engine. Fuel 2018, 212, 462–469. [Google Scholar] [CrossRef]
- Rajesh Kumar, B.; Saravanan, S. Use of Higher Alcohol Biofuels in Diesel Engines: A Review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Mofijur, M.; Shuvho, M.B.A.; Chowdhury, M.A.K.; Kalam, M.A.; Masjuki, H.H.; Chowdhury, M.A. A Study on the Corrosion Characteristics of Internal Combustion Engine Materials in Second-Generation Jatropha Curcas Biodiesel. Energies 2021, 14, 4352. [Google Scholar] [CrossRef]
- Nam Cao Ho Chi, D.; Dao Nam, C.; Anh Tuan Ho Chi, H. The 11th Seatuc Symposium Impact of Coconut Oil Acid Component on Fuel System Materials Durability and Corrosion in Diesel Engines. In Proceedings of the SEATUC, Nakhon Ratchasima, Thailand, 23–24 February 2022. [Google Scholar]
- Liu, Z.; Guo, Z.; Rao, X.; Xu, Y.; Sheng, C.; Yuan, C. A Comprehensive Review on the Material Performance Affected by Gaseous Alternative Fuels in Internal Combustion Engines. Eng. Fail. Anal. 2022, 139, 106507. [Google Scholar] [CrossRef]
- Worldwide Fuel Charter Committee. Biodiesel Guidelines. 2009. Available online: https://www.acea.auto/uploads/publications/20090423_B100_Guideline.pdf (accessed on 12 January 2024).
- Mdluli, N.S.; Nomngongo, P.N.; Mketo, N. A Critical Review on Application of Extraction Methods Prior to Spectrometric Determination of Trace-Metals in Oily Matrices. Crit. Rev. Anal. Chem. 2022, 52, 1–18. [Google Scholar] [CrossRef]
- Neste Corporation. Neste Renewable Diesel Handbook. 2020. Available online: https://www.sustainable-ships.org/stories/2023/neste-renewable-diesel-handbook (accessed on 12 January 2024).
- Lampinen, A. Uusiutuvan Liikenne-Energian Tiekartta; Karelia University of Applied Sciences: Joensuu, Finland, 2009; ISBN 9789516041004. Available online: https://www.theseus.fi/handle/10024/127014 (accessed on 12 January 2024). (In Finnish)
- Metallinjalostajat, R. Teräskirja, 9th ed.; Bookwell Oy: Helsinki, Finland, 2014. (In Finnish) [Google Scholar]
- EN 14538; Fat and Oil Derivatives—Fatty Acid Methyl Ester (FAME)—Determination of Ca, K, Mg and Na Content by Optical Emission Spectral Analysis with Inductively Coupled Plasma (ICP OES). European Committee for Standardization: Brussels, Belgium, 2006.
- EN 14107; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Phosphorus Content by Inductively Coupled Plasma (ICP) Emission Spectrometry. European Committee for Standardization: Brussels, Belgium, 2003.
- ASTM D7042-21a; Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- Novotny-Farkas, F.; Böhme, W.; Stabinger, H.; Belitsch, W. Customer Portrait. In The Stabinger Viscometer: A New and Unique Instrument for Oil Service Laboratories; Anton Paar: Vienna, Austria, 2010; p. 4. [Google Scholar]
- ASTM D7345-17; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure (Micro Distillation Method). ASTM International: West Conshohocken, PA, USA, 2024.
- Kalghatgi, G.; Kalghatgi, G. FuelEngine Interactions; SAE International: Warrendale, PA, USA, 2014; p. 255. [Google Scholar]
- Joo, S.; Suh, D. Facile and Precise Quantitative Determination of Silicon in Naphtha by Inductively Coupled Plasma-Optical Emission Spectroscopy. J. Anal. Sci. Technol. 2022, 13, 30. [Google Scholar] [CrossRef]
- Yahya, S.I.; Aghel, B. Estimation of Kinematic Viscosity of Biodiesel-Diesel Blends: Comparison among Accuracy of Intelligent and Empirical Paradigms. Renew. Energy 2021, 177, 318–326. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Roberts, W.L.; Dibble, R.W. Feasibility of Using Less Viscous and Lower Cetane (LVLC) Fuels in a Diesel Engine: A Review. Renew. Sustain. Energy Rev. 2015, 51, 1166–1190. [Google Scholar] [CrossRef]
- ASTM G31-72(2004); Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2012.
- Matějovský, L.; Macák, J.; Pospíšil, M.; Baroš, P.; Staš, M.; Krausová, A. Study of Corrosion of Metallic Materials in Ethanol-Gasoline Blends: Application of Electrochemical Methods. Energy Fuels 2017, 31, 10880–10889. [Google Scholar] [CrossRef]
- Patel, C.; Chandra, K.; Hwang, J.; Agarwal, R.A.; Gupta, N.; Bae, C.; Gupta, T.; Agarwal, A.K. Comparative Compression Ignition Engine Performance, Combustion, and Emission Characteristics, and Trace Metals in Particulates from Waste Cooking Oil, Jatropha and Karanja Oil Derived Biodiesels. Fuel 2019, 236, 1366–1376. [Google Scholar] [CrossRef]
- Stepien, Z.; Krasodomski, W. Effect of Trace Zinc Amounts Introduced in Various Chemical Structures in Diesel Fuel on Coke Deposits of Fuel Injectors of a CI Engine. Int. J. Eng. Res. 2020, 21, 755–765. [Google Scholar] [CrossRef]
- Wen, J.; Wang, X.; Zhang, Y.; Zhu, H.; Chen, Q.; Tian, Y.; Shi, X.; Shi, G.; Feng, Y. PM2.5 Source Profiles and Relative Heavy Metal Risk of Ship Emissions: Source Samples from Diverse Ships, Engines, and Navigation Processes. Atmos. Environ. 2018, 191, 55–63. [Google Scholar] [CrossRef]
- Fang, W.; Yang, Y.; Xu, Z. PM10 and PM2.5 and Health Risk Assessment for Heavy Metals in a Typical Factory for Cathode Ray Tube Television Recycling. Environ. Sci. Technol. 2013, 47, 12469–12476. [Google Scholar] [CrossRef] [PubMed]
- Morajkar, P.P.; Abdrabou, M.K.; Raj, A.; Elkadi, M.; Stephen, S.; Ibrahim Ali, M. Transmission of Trace Metals from Fuels to Soot Particles: An ICP-MS and Soot Nanostructural Disorder Study Using Diesel and Diesel/Karanja Biodiesel Blend. Fuel 2020, 280, 118631. [Google Scholar] [CrossRef]


| Carbon Steel | Stainless Steel | MoC210M/25CrMo4+SH | Aluminum EN AW-6082 Alloy | |
|---|---|---|---|---|
| Cu (%) | 0.20 | 0.96 | 0.20 | 0.01 |
| Fe (%) | >70 | >70 | >70 | 0.20 |
| Mn (%) | 1.13 | 1.66 | 0.81 | 0.41 |
| Si (%) | 0.24 | 0.27 | 0.27 | 1.07 |
| V (%) | 0.06 | 0.01 | ||
| Pb (%) | 0.0003 | 0.0007 | <0.05 | |
| C (%) | 0.13 | 0.023 | 0.27 | |
| Cr (%) | 0.20 | 18.10 | 0.99 | 0.13 |
| Ni (%) | 0.15 | 8.01 | 0.19 | |
| Mo (%) | 0.03 | 0.29 | 0.22 | |
| Mg (%) | 0.70 | |||
| Zn (%) | 0.01 | |||
| Ti (%) | 0.02 |
| Sample Matrix (Fuel/Metal) | Carbon Steel | Stainless Steel | MoC210M/25CrMo4+SH | Aluminum |
|---|---|---|---|---|
| MeOH | MeOH CS | MeOH SS | MeOH CrMo | MeOH Al |
| HVO | HVO CS | HVO SS | HVO CrMo | HVO Al |
| MeOH50 | MeOH50 CS | MeOH50 SS | MeOH50 CrMo | MeOH50 Al |
| HVOr | HVOr CS | HVOr SS | HVOr CrMo | HVOr Al |
| MeOH50-O | MeOH50-O CS | MeOH50-O SS | MeOH50-O CrMo | MeOH50-O Al |
| MeOH50-D | MeOH50-D CS | MeOH50-D SS | MeOH50-D CrMo | MeOH50-D Al |
| Sample | Al ppm | Cu ppm | Fe ppm | Mn ppm | Pb ppm | Si ppm | V ppm | Zn ppm |
|---|---|---|---|---|---|---|---|---|
| HVO | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| HVO CS | <1 | <1 | <1 | <1 | <1 | < 2 | <1 | <1 |
| HVO SS | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| HVO CrMo | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| HVO Al | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| MeOH CS | <1 | <1 | <1 | <1 | <1 | 3 | <1 | 2 |
| MeOH SS | <1 | <1 | <1 | <1 | <1 | 3 | <1 | <1 |
| MeOH CrMo | <1 | <1 | <1 | <1 | <1 | 3 | <1 | <1 |
| MeOH Al | 2 | <1 | <1 | <1 | <1 | 3 | <1 | <1 |
| MeOH50 CS | <1 | 1 | <1 | <1 | <1 | 5 | <1 | 1 |
| MeOH50 SS | <1 | <1 | <1 | <1 | <1 | 5 | <1 | <1 |
| MeOH50 CrMo | <1 | <1 | <1 | <1 | <1 | 3 | <1 | 1 |
| MeOH50 Al | <1 | <1 | <1 | <1 | <1 | 3 | <1 | <1 |
| HVOr | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| HVOr CS | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| HVOr SS | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| HVOr CrMo | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| HVOr Al | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-O | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-O CS | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-O SS | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-O CrMo | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-O Al | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-D | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-D CS | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-D SS | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-D CrMo | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| MeOH50-D Al | <1 | <1 | <1 | <2 | <4 | <1 | <1 | <1 |
| Sample | Density (kg/m3) 15 °C (RSD < 1.0%) | Kinematic Viscosity, (mm2/s) 40 °C (RSD < 1.0%) | Initial Boiling Point, (°C) (RSD < 1.1%) | Final Boiling Point, (°C) (RSD < 1.1%) |
|---|---|---|---|---|
| HVO | 781 | 3.14 | 223 | 309 |
| HVO CS | 781 | 3.12 | 223 | 300 |
| HVO SS | 781 | 3.14 | 223 | 301 |
| HVO CrMo | 781 | 3.13 | 223 | 300 |
| HVO Al | 781 | 3.14 | 223 | 307 |
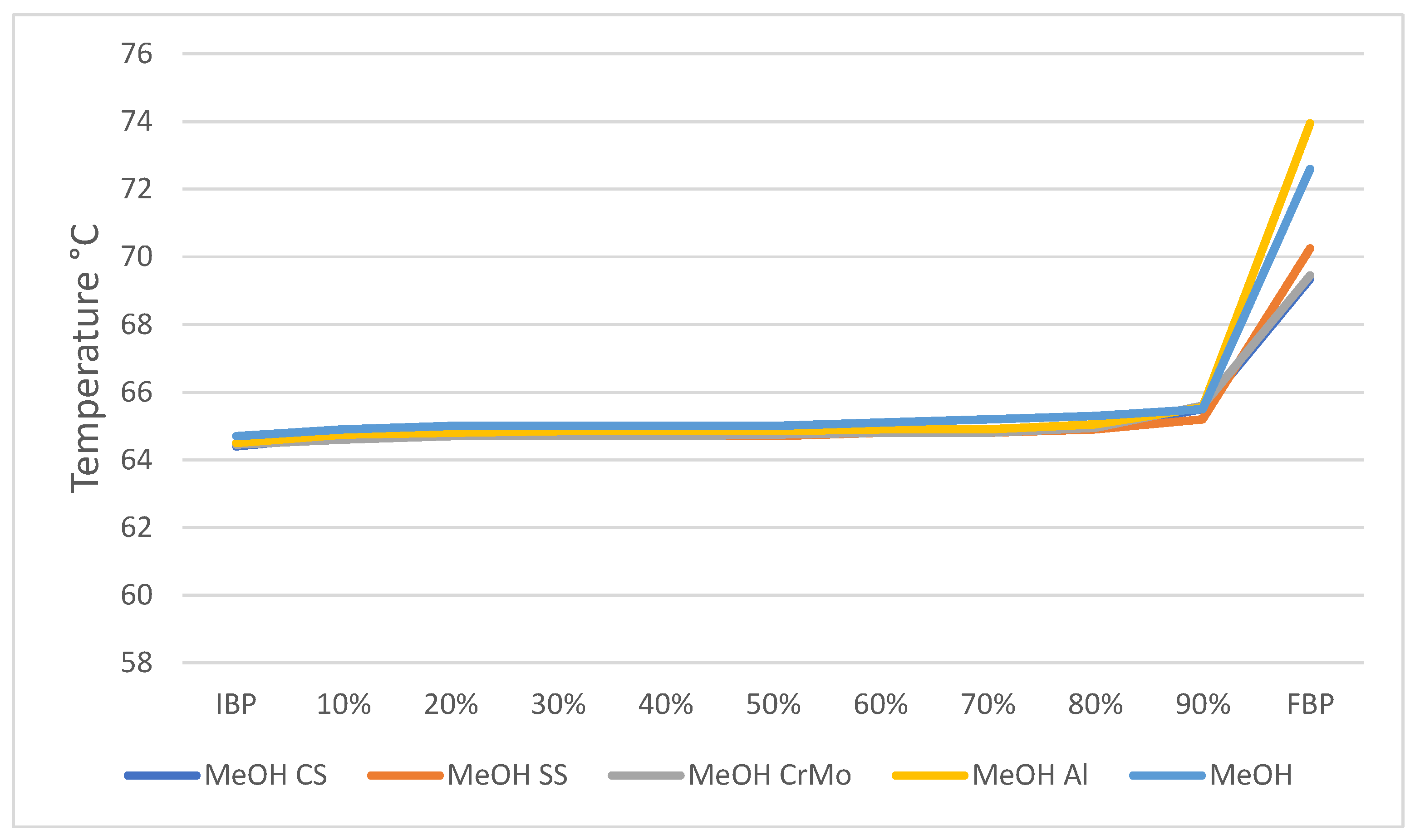
| MeOH | 795 | 0.55 | 65 | 73 |
| MeOH CS | 795 | 0.56 | 64 | 69 |
| MeOH SS | 795 | 0.58 | 64 | 70 |
| MeOH CrMo | 795 | 0.56 | 64 | 69 |
| MeOH Al | 795 | 0.57 | 65 | 74 |
| MeOH50 | 793 | 1.25 | 65 | 244 |
| MeOH50 CS | 793 | 0.85 | 66 | 260 |
| MeOH50 SS | 794 | 1.30 | 66 | 252 |
| MeOH50 CrMo | 794 | 1.14 | n/a | n/a |
| MeOH50 Al | 793 | 1.04 | 65 | 257 |
| HVOr | 781 | 3.06 | 223 | 307 |
| HVOr CS | 781 | 3.05 | 224 | 309 |
| HVOr SS | 781 | 3.05 | 222 | 308 |
| HVOr CrMo | 781 | 3.06 | 223 | 309 |
| HVOr Al | 781 | 3.06 | 222 | 309 |
| MeOH50-O | 798 | 1.33 | 68 | 259 |
| MeOH50-O CS | 797 | 1.31 | 68 | 257 |
| MeOH50-O SS | 797 | 1.33 | 65 | 256 |
| MeOH50-O CrMo | 797 | 1.35 | 68 | 257 |
| MeOH50-O Al | 797 | 1.40 | 69 | 259 |
| MeOH50-D | 797 | 1.61 | 66 | 244 |
| MeOH50-D CS | 797 | 1.49 | 66 | 246 |
| MeOH50-D SS | 797 | 1.45 | 65 | 248 |
| MeOH50-D CrMo | 797 | 1.48 | 64 | 228 |
| MeOH50-D Al | 797 | 1.58 | 66 | 252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang-Alho, H.; Sirviö, K.; Nuortila, C.; Kaivosoja, J.; Mikulski, M.; Niemi, S. Compatibility of Methanol-Hydrotreated Vegetable Oil Blends with Chosen Steels and Aluminum. Energies 2024, 17, 3423. https://doi.org/10.3390/en17143423
Wang-Alho H, Sirviö K, Nuortila C, Kaivosoja J, Mikulski M, Niemi S. Compatibility of Methanol-Hydrotreated Vegetable Oil Blends with Chosen Steels and Aluminum. Energies. 2024; 17(14):3423. https://doi.org/10.3390/en17143423
Chicago/Turabian StyleWang-Alho, Huaying, Katriina Sirviö, Carolin Nuortila, Jonna Kaivosoja, Maciej Mikulski, and Seppo Niemi. 2024. "Compatibility of Methanol-Hydrotreated Vegetable Oil Blends with Chosen Steels and Aluminum" Energies 17, no. 14: 3423. https://doi.org/10.3390/en17143423
APA StyleWang-Alho, H., Sirviö, K., Nuortila, C., Kaivosoja, J., Mikulski, M., & Niemi, S. (2024). Compatibility of Methanol-Hydrotreated Vegetable Oil Blends with Chosen Steels and Aluminum. Energies, 17(14), 3423. https://doi.org/10.3390/en17143423







