Advancements and Challenges of Ammonia as a Sustainable Fuel for the Maritime Industry
Abstract
1. Introduction
2. The Shipping Industry’s Current Challenges
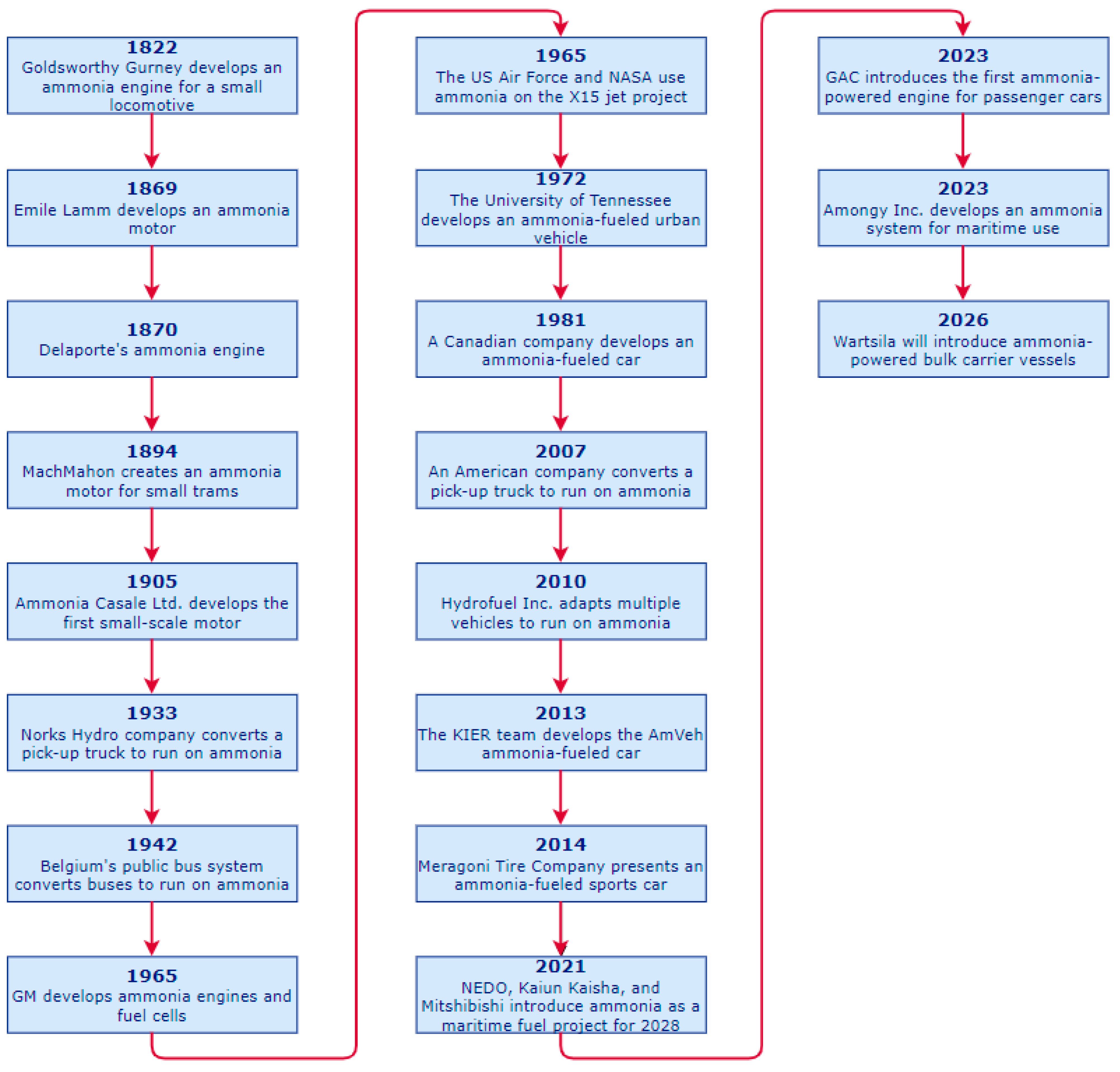
2.1. Ongoing Projects and Companies Developing Technology
2.2. Regulatory Landscape and Safety Concerns
3. NH3 Combustion
4. ICE Development
4.1. Techniques to Reduce NOx
4.1.1. Thermal DeNOx
4.1.2. Blending DeNOx
4.1.3. Homogeneous Charge Compression Ignition (HCCI)
4.1.4. Premixed Charge Compression Ignition (PCCI)
4.1.5. Lean Engine Operation
4.1.6. Scrubbing the Exhaust
4.1.7. Selective Non-Catalytic Reduction (SNCR)
4.2. Techniques to Reduce N2O
Selective Catalytic Reaction (SCR)
4.3. NH3 Blending with Other Fuels
N2O and NH3 Slipping
4.4. Waste Heat Recovery Strategies
5. Numerical Models Applied to ICEs Using NH3
6. Regulatory Framework and Safety Conditions
6.1. ISO Standards
6.2. American Regulations
6.3. IGC Code
6.4. IGF Code
7. Environmental Impact
8. Future Outlook
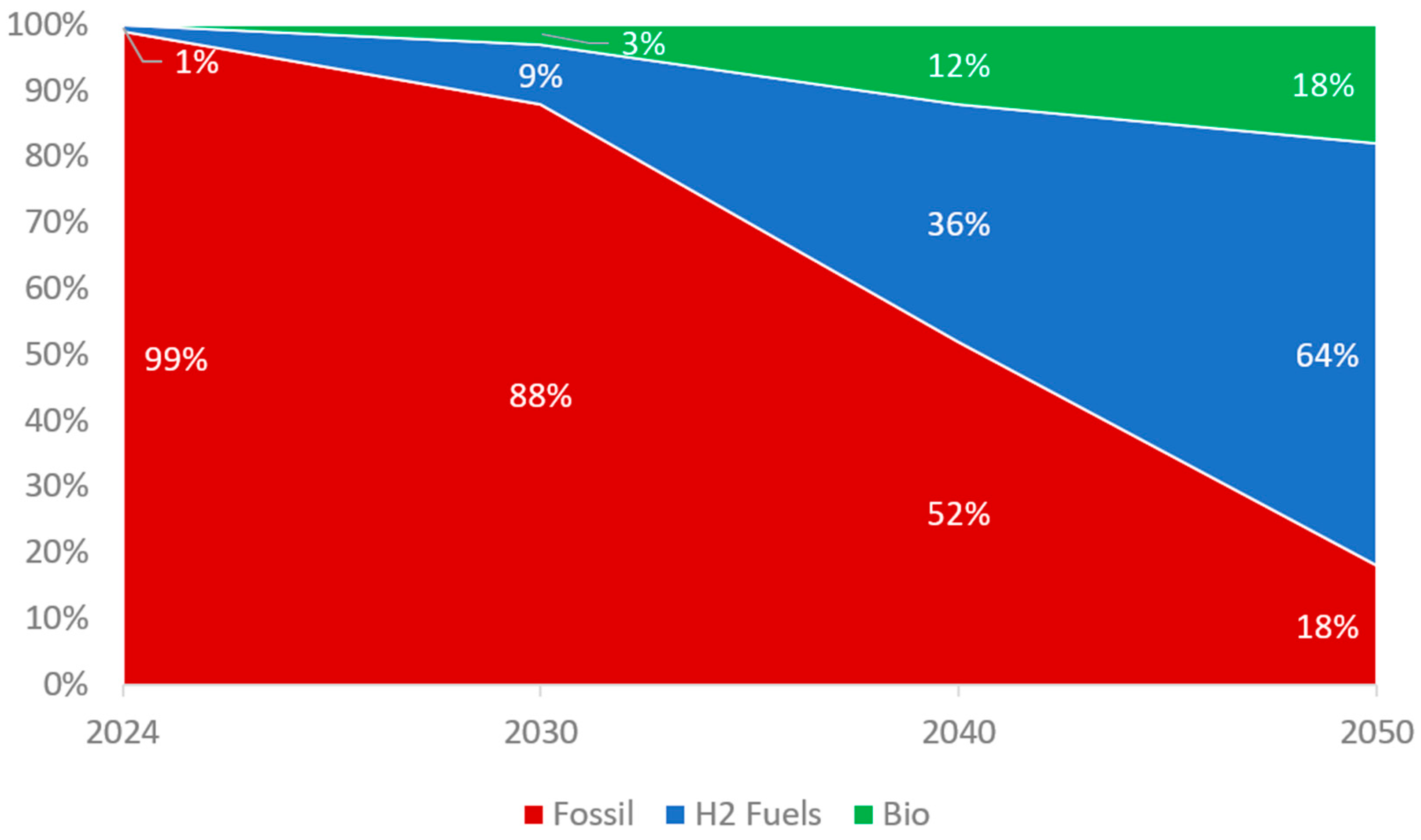
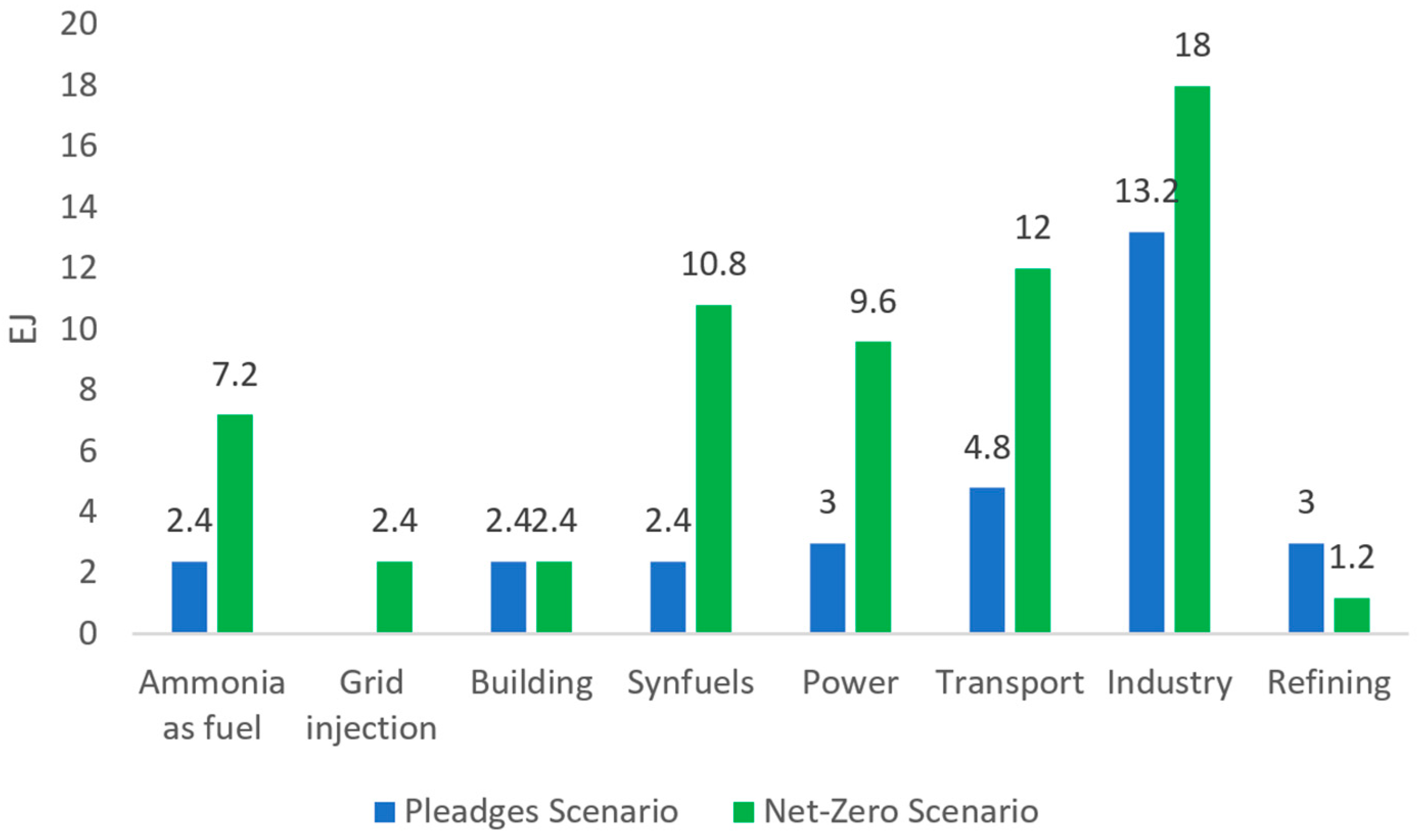
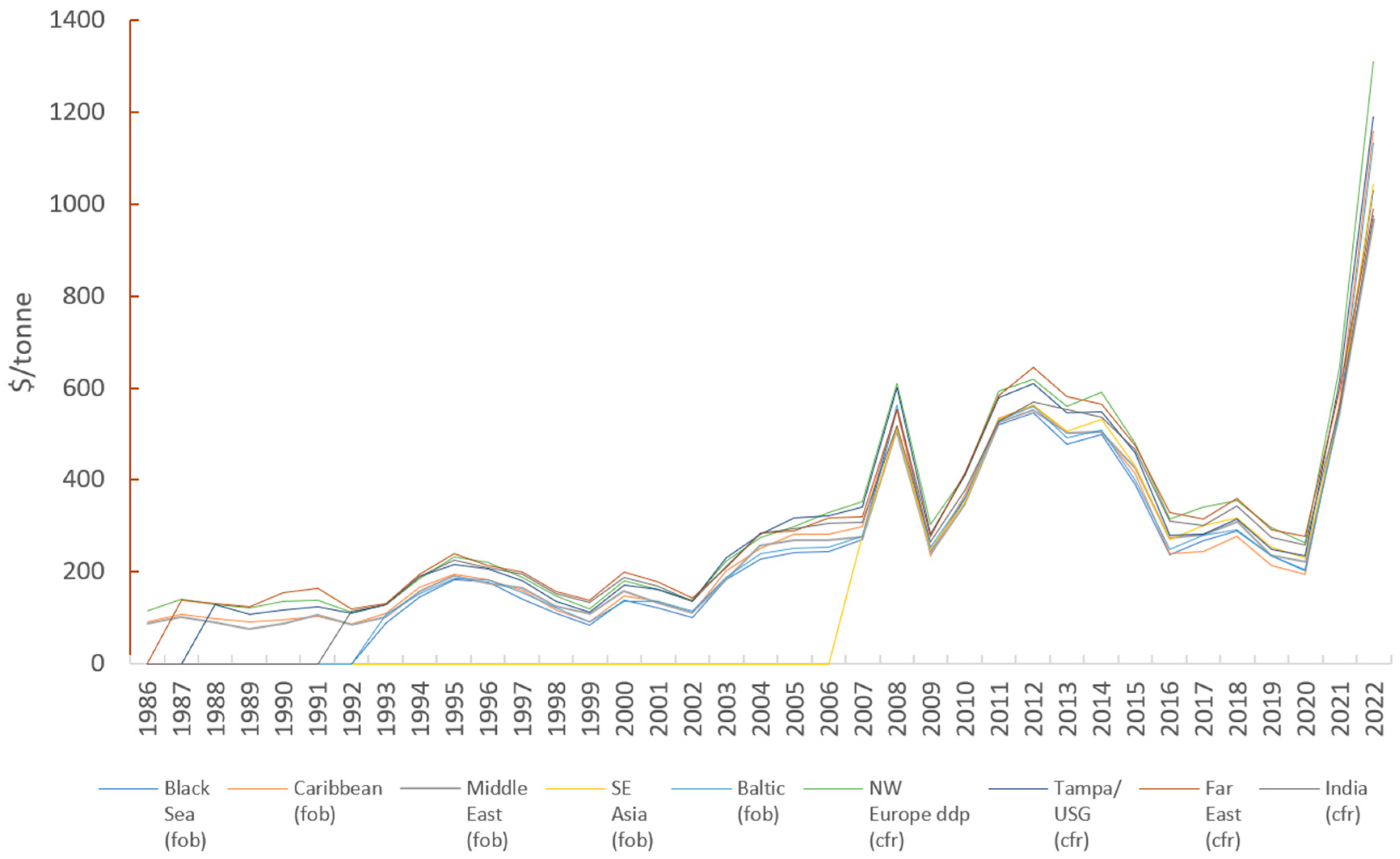
8.1. Market Trends
8.2. NH3 Challenges
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- ICS. The Role of Maritime Trade in the Post COVID Recovery—Europe Focus. Available online: https://www.ics-shipping.org/event/the-role-of-maritime-trade-in-the-post-covid-recovery-europe-focus/ (accessed on 17 September 2023).
- ICS. Evolving EU Sanctions Keep Ship Owners on Alert|International Chamber of Shipping. Available online: https://www.ics-shipping.org/news-item/evolving-eu-sanctions-keep-ship-owners-on-alert/ (accessed on 17 September 2023).
- Fajgelbaum, P.D.; Khandelwal, A.K. The Economic Impacts of the US–China Trade War. Annu. Rev. Econ. 2022, 14, 205–228. [Google Scholar] [CrossRef]
- IEA. International Shipping. Available online: https://www.iea.org/reports/tracking-clean-energy-progress-2023 (accessed on 17 September 2023).
- ICS. Annual Overview on Maritime Trade Policy by the International Chamber of Shipping. Available online: https://www.ics-shipping.org/wp-content/uploads/2022/09/ICS-Trade-Policy-Review-2022.pdf (accessed on 9 June 2024).
- IMO. Revised GHG Reduction Strategy for Global Shipping Adopted. Available online: https://www.imo.org/en/MediaCentre/PressBriefings/pages/Revised-GHG-reduction-strategy-for-global-shipping-adopted-.aspx (accessed on 11 June 2024).
- Machaj, K.; Kupecki, J.; Malecha, Z.; Morawski, A.W.; Skrzypkiewicz, M.; Stanclik, M.; Chorowski, M. Ammonia as a potential marine fuel: A review. Energy Strategy Rev. 2022, 44, 100926. [Google Scholar] [CrossRef]
- Wu, S.; Salmon, N.; Li, M.M.; Bañares-Alcántara, R.; Tsang, S.C. Energy Decarbonization via Green H2 or NH3? ACS Energy Lett. 2022, 7, 1021–1033. [Google Scholar] [CrossRef]
- Manigandan, S.; Ryu, J.I.; Kumar, T.R.P.; Elgendi, M. Hydrogen and ammonia as a primary fuel—A critical review of production technologies, diesel engine applications, and challenges. Fuel 2023, 352, 129100. [Google Scholar] [CrossRef]
- Li, J.; Lai, S.; Chen, D.; Wu, R.; Kobayashi, N.; Deng, L.; Huang, H. A Review on Combustion Characteristics of Ammonia as a Carbon-Free Fuel. Front. Energy Res. 2021, 9, 760356. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M. New recipes for producing a high-octane gasoline based on naphtha from natural gas condensate. Fuel 2020, 276, 118075. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R.; Nayak-Luke, R. Optimization of green ammonia distribution systems for intercontinental energy transport. iScience 2021, 24, 102903. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Fan, Y.; Li, J.; Wu, K.; Gao, Z.; Li, A.; Zhu, L.; Huang, Z. Fuel reactivity stratification assisted jet ignition for low-speed two-stroke ammonia marine engine. Int. J. Hydrogen Energy 2023, 49, 470–585. [Google Scholar] [CrossRef]
- Wardana, M.K.; Lim, O. Investigation of ammonia homogenization and NOx reduction quantity by remodeling urea injector shapes in heavy-duty diesel engines. Appl. Energy 2022, 323, 119586. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, B. Analysis of explosion and laminar combustion characteristics of premixed ammonia-air/oxygen mixtures. Fuel 2023, 351, 128860. [Google Scholar] [CrossRef]
- Kang, L.; Pan, W.; Zhang, J.; Wang, W.; Tang, C. A review on ammonia blends combustion for industrial applications. Fuel 2023, 332, 126150. [Google Scholar] [CrossRef]
- McKinsey & Company. Maritime Decarbonization in the Shipping Industry. Available online: https://www.mckinsey.com/industries/travel-logistics-and-infrastructure/our-insights/charting-fuel-choices-as-the-shipping-industry-sails-toward-net-zero#/ (accessed on 29 January 2024).
- SEA\LNG Ltd. Comparison of Alternative Marine Fuels. Available online: https://sea-lng.org/wp-content/uploads/2020/04/Alternative-Marine-Fuels-Study_final_report_25.09.19.pdf (accessed on 9 June 2024).
- Cardiff University. New Funding to Unlock the Power of Ammonia. Available online: https://www.cardiff.ac.uk/news/view/2426218-new-funding-to-unlock-the-power-of-ammonia (accessed on 17 September 2023).
- European Commission. Advanced Materials and Reactors for Energy Storage through Ammonia. Available online: https://cordis.europa.eu/project/id/862482 (accessed on 17 September 2023).
- MAN Energy Solutions. Groundbreaking First Ammonia Engine Test Completed. Available online: https://www.man-es.com/docs/default-source/press-releases-new/pr-first-ammonia-test_en.pdf?sfvrsn=8da490cf_2 (accessed on 9 June 2024).
- MAN Energy Solutions. MAN Energy Solutions Upgrading Four-Stroke Engines for Green Future-Fuels. Available online: https://www.man-es.com/company/press-releases/press-details/2021/11/29/man-energy-solutions-upgrading-four-stroke-engines-for-green-future-fuels (accessed on 9 June 2024).
- Fuels and Lubes. Wärtsilä Launches New 4-Stroke Engine that Can Run on Ammonia. Available online: https://www.fuelsandlubes.com/wartsila-launches-new-4-stroke-engine-that-can-run-on-ammonia/ (accessed on 17 September 2023).
- Splash247. Plans Revealed for Zero-Emission Ammonia Bunker Vessels. Available online: https://splash247.com/plans-revealed-for-zero-emission-ammonia-bunker-vessels/ (accessed on 6 January 2024).
- Amogy. Amogy’s Ammonia-to-Power System Issued Successful Completion of Technology Verification from Lloyd’s Register. Available online: https://amogy.co/amogys-ammonia-to-power-system-issued-successful-completion-of-technology-verification-from-lloyds-register/ (accessed on 6 January 2024).
- GAC Motor. Gac Unveils Industry-First Innovations at Gac Tech Day 2023. Available online: https://www.gac-motor.com/en/media/newsdetail/id/296.html (accessed on 6 January 2024).
- Nathan, S. The Global Shipping Industry—Demands, Challenges, and the Future Outlook. Available online: https://www.goodfirms.co/resources/global-shipping-industry-demands-challenges-future (accessed on 20 September 2023).
- DNV. Energy Transition Outlook 2023—Maritime Forecast to 2050. Available online: https://www.dnv.com/publications/energy-transition-outlook-2023-247935/ (accessed on 9 June 2024).
- IEA. International Shipping. Available online: https://www.iea.org/energy-system/transport/international-shipping#tracking (accessed on 26 September 2023).
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- DVN. Alternative Fuels Insights for the Shipping Industry. Available online: https://www.dnv.com/services/alternative-fuels-insight-128171 (accessed on 26 September 2023).
- DNV. DNV Unites with 22 Industry Partners on a Landmark Ammonia Study. Available online: https://www.dnv.com/news/dnv-unites-with-22-industry-partners-on-a-landmark-ammonia-study-202318 (accessed on 7 October 2023).
- Amogy. Amogy Presents World’s First Ammonia-Powered, Zero-Emission Semi Truck. Available online: https://fuelcellsworks.com/news/amogy-presents-worlds-first-ammonia-powered-zero-emission-semi-truck/ (accessed on 6 January 2024).
- ABS. Technology Trends—Exploring the Future of Maritime Innovation. Available online: https://ww2.eagle.org/en/publication-flip/technology-trends.html (accessed on 27 October 2023).
- Navigator Gas. Navigator Gas Awarded Approval in Principle for Ammonia Fuelled Gas Carrier by Classification Society DNV. Available online: https://navigatorgas.com/navigator-gas-awarded-approval-in-principle-for-ammonia-fuelled-gas-carrier-by-classification-society-dnv/ (accessed on 7 October 2023).
- Anglo American. Anglo American Completes First Maritime Biofuel Trial towards Decarbonising Ocean Freight. Available online: https://www.angloamerican.com/media/press-releases/2021/29-06-2021 (accessed on 7 October 2023).
- ClassNK. Available online: https://www.classnk.or.jp/hp/en/index.html (accessed on 7 October 2023).
- DNV. Maritime Cyber Priority 2023: Staying Secure in an Era of Connectivity. Available online: https://www.dnv.com/cybersecurity/cyber-insights/maritime-cyber-priority-2023.html (accessed on 7 October 2023).
- DNV. Maritime Forecast to 2050. Available online: https://www.dnv.com/maritime/publications/maritime-forecast-2023/download-the-report.html (accessed on 7 October 2023).
- DNV. Transport in Transition. Available online: https://verit.dnv.com/Publications/transport-in-transition-242808 (accessed on 7 October 2023).
- Equinor. Shipping in Equinor. Available online: https://www.equinor.com/energy/shipping (accessed on 7 October 2023).
- Global Maritime Forum. What Now: From Ambition to Action. Available online: https://www.globalmaritimeforum.org/annual-summit-2023/ (accessed on 7 October 2023).
- ITOCHU. ITOCHU’s ‘Integrated Project’ of Ammonia Fueled Ships with Supply Chain Is Adopted to the ‘Green Innovation Fund Project. Available online: https://www.itochu.co.jp/en/news/press/2021/211026.html (accessed on 7 October 2023).
- Itochu. Execution of Memorandum of Understanding for Ammonia Bunkering Safety for Ammonia-Fueled Container Carrier. Available online: https://www.itochu.co.jp/en/news/press/2023/230922_2.html (accessed on 7 October 2023).
- Ship Technology. K Line, Itochu and Others Collaborate on Ammonia-Powered Ship Project. Available online: https://www.ship-technology.com/news/k-line-ammonia-powered/ (accessed on 7 October 2023).
- MAN Energy Solutions. Making the Maritime Energy Transition. Available online: https://www.man-es.com/discover/making-the-maritime-energy-transition (accessed on 7 October 2023).
- Nihon Shipyard. Maritime Consortium Successfully Completes Ammonia Co-Firing Test Using Cutting-Edge Ammonia-Fueled Engine. Available online: https://www.nsyc.co.jp/wp-content/uploads/2023/05/230516_news_en.pdf (accessed on 7 October 2023).
- Nordic Innovation. NoGAPS: Nordic Green Ammonia Powered Ship. Available online: https://www.nordicinnovation.org/2021/nogaps-nordic-green-ammonia-powered-ship (accessed on 7 October 2023).
- Itochu. ITOCHU Announces Supply of Marine Ammonia Fuel in Japan and Joint Development of Supply Sites. Available online: https://www.itochu.co.jp/en/news/press/2021/210312.html (accessed on 7 October 2023).
- Maritime-executive. Korean Design for Ammonia Bunker Vessel Awarded AiP. Available online: https://maritime-executive.com/article/korean-design-for-ammonia-bunker-vessel-awarded-aip (accessed on 7 October 2023).
- Vopak. Vopak Singapore Explores Expanding Its Ammonia Infrastructure for Low Carbon Power Generation and Bunker Fuel. Available online: https://www.vopak.com/newsroom/news/news-vopak-singapore-explores-expanding-its-ammonia-infrastructure-low-carbon-power?language_content_entity=en (accessed on 7 October 2023).
- Wärtsilä. Wärtsilä and Eidesvik Offshore to Cooperate in World’s First Ammonia Conversion Project. Available online: https://www.wartsila.com/media/news/08-10-2021-wartsila-and-eidesvik-offshore-to-cooperate-in-world-s-first-ammonia-conversion-project-2987432 (accessed on 7 October 2023).
- Wärtsilä. Wärtsilä Coordinates EU Funded Project to Accelerate Ammonia Engine Development. Available online: https://www.wartsila.com/media/news/05-04-2022-wartsila-coordinates-eu-funded-project-to-accelerate-ammonia-engine-development-3079950 (accessed on 7 October 2023).
- Yara. Yara Clean Ammonia and Bunker Holding Sign an MOU to Develop the Market for Ammonia as A shipping Fuel. Available online: https://www.yara.com/corporate-releases/yara-clean-ammonia-and-bunker-holding-sign-an-mou-to-develop-the-market-for-ammonia-as-a-shipping-fuel/ (accessed on 7 October 2023).
- RINA. Overcoming Ammonia’s Toxicity Challenge: A Technical Perspective. Available online: https://rina.org.uk/industry-news/research-and-education/overcoming-ammonias-toxicity-challenge-a-technical-perspective/ (accessed on 29 January 2024).
- Rötzer, J. Regulations and their Scope of Applicability. In Design and Construction of LNG Storage Tanks; Ernst &Sohn Verlag GmbH & Co. KG: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, A.E. Recommendations for the Safe and Reliable Inspection of Atmospheric, Refrigerated Ammonia Storage Tanks-EFMA European Fertilizer Manufacturers’ Association. Available online: https://ureaknowhow.com/pdflib/659_2012_EFMA_Recommendations_refrigerated_ammonia_storage_tanks.pdf (accessed on 9 June 2024).
- Herbinet, O.; Bartocci, P.; Dana, A.G. On the use of ammonia as a fuel—A perspective. Fuel Commun. 2022, 11, 100064. [Google Scholar] [CrossRef]
- Chavando, J.A.M.; Silva, V.B.; da Cruz Tarelho, L.A.; Cardoso, J.S.; Hall, M.J.; Eusébio, D. Ammonia as an alternative. In Combustion Chemistry and the Carbon Neutral Future: What will the Next 25 Years of Research Require? Elsevier: Amsterdam, The Netherlands, 2023; pp. 179–208. [Google Scholar] [CrossRef]
- Lammel, G.; Graßl, H. Greenhouse effect of NOX. Environ. Sci. Pollut. Res. Int. 1995, 2, 40–45. [Google Scholar] [CrossRef]
- Our World in Data. Nitrous Oxide Emissions. 2022. Available online: https://ourworldindata.org/grapher/nitrous-oxide-emissions (accessed on 17 June 2024).
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- US EPA. Acid Rain Program. Available online: https://www.epa.gov/acidrain/acid-rain-program (accessed on 15 July 2023).
- Ozgen, S.; Cernuschi, S.; Caserini, S. An overview of nitrogen oxides emissions from biomass combustion for domestic heat production. Renew. Sustain. Energy Rev. 2021, 135, 110113. [Google Scholar] [CrossRef]
- Ruksana Moreea-Taha. NOx Modelling and Prediction. Available online: https://www.sustainable-carbon.org/wp-content/uploads/dlm_uploads/reports/emmisionscontrol/NOx-modelling-prediction-CCC-31.pdf (accessed on 9 June 2024).
- ur Rahman, Z.; Wang, X.; Zhang, J.; Yang, Z.; Dai, G.; Verma, P.; Mikulcic, H.; Vujanovic, M.; Tan, H.; Axelbaum, R.L. Nitrogen evolution, NOX formation and reduction in pressurized oxy coal combustion. Renew. Sustain. Energy Rev. 2022, 157, 112020. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. Detailed Kinetic Modelling of Chemistry and Temperature Effects on Ammonia Oxidation. Combust. Sci. Technol. 2007, 99, 253–276. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia oxidation at high pressure and intermediate temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical kinetic modeling of ammonia oxidation with improved reaction mechanism for ammonia/air and ammonia/hydrogen/air combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Hayakawa, A.; Hayashi, M.; Kovaleva, M.; Gotama, G.J.; Okafor, E.C.; Colson, S.; Mashruk, S.; Valera-Medina, A.; Kudo, T.; Kobayashi, H. Experimental and numerical study of product gas and N2O emission characteristics of ammonia/hydrogen/air premixed laminar flames stabilized in a stagnation flow. Proc. Combust. Inst. 2023, 39, 1625–1633. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; He, X.; Chen, W.; Xu, H. Data-driven enabling technologies in soft sensors of modern internal combustion engines: Perspectives. Energy 2023, 272, 127067. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Teoh, Y.H.; How, H.G.; Le, T.D.; Nguyen, H.T.; Loo, D.L.; Rashid, T.; Sher, F. A review on production and implementation of hydrogen as a green fuel in internal combustion engines. Fuel 2023, 333, 126525. [Google Scholar] [CrossRef]
- Werrell, K.P. The strategic bombing of germany in world war ii: Costs and accomplishments. J. Am. Hist. 1986, 73, 702–713. [Google Scholar] [CrossRef]
- Koch, E. Ammonia–A fuel for motor buses. J. Inst. Pet. 1945, 31, 213. [Google Scholar]
- Lyon, R.K.; Fanwood, N.J. Method for the Reduction of the Concentration of NO in Combustion Effluents Using Ammonia. U.S. Patent No. 3,900,554, 19 August 1975. [Google Scholar]
- Lyon, R.K. Thermal DeNOx Controlling nitrogen oxides emissions by a noncatalytic process. Environ. Sci. Technol. 1987, 21, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.K.; Cole, J.A. A reexamination of the RapreNOx process. Combust Flame 1990, 82, 435–443. [Google Scholar] [CrossRef]
- ACharalambides, G.; Charalambides, A.G. Homogenous Charge Compression Ignition (HCCI) Engines. In Advances in Internal Combustion Engines and Fuel Technologies; Intechopen: London, UK, 2013. [Google Scholar] [CrossRef]
- Sharma, T.K.; Rao, G.A.; Murthy, K.M. Effective reduction of NOx emissions of a HCCI (Homogeneous charge compression ignition) engine by enhanced rate of heat transfer under varying conditions of operation. Energy 2015, 93, 2102–2115. [Google Scholar] [CrossRef]
- Wang, C.; Deng, J.; Ding, W.; Huang, Y.; Tang, Y.; Li, L. Thermodynamic analysis of employing argon as the diluent and adding hydrogen in an HCCI ammonia engine: Ignition characteristics and performances of combustion and NO emissions. Int. J. Hydrogen Energy 2024, 49, 293–300. [Google Scholar] [CrossRef]
- Lee, D.; Song, H.H. Development of combustion strategy for the internal combustion engine fueled by ammonia and its operating characteristics. J. Mech. Sci. Technol. 2018, 32, 1905–1925. [Google Scholar] [CrossRef]
- Masoumi, S.; Houshfar, E.; Ashjaee, M. Experimental and numerical analysis of ammonia/hydrogen combustion under artificial exhaust gas recirculation. Fuel 2024, 357, 130081. [Google Scholar] [CrossRef]
- USP Technologies. Nitrogen Oxides Pollutant Removal—NOx Abatement. Available online: https://www.h2o2.com/industrial/applications.aspx?pid=101 (accessed on 15 July 2023).
- Sorrels, J.L.; Randall, D.D.; Fry, C.R.; Schaffner, K.S. Chapter 1—Selective Noncatalytic Reduction. Available online: https://www.epa.gov/sites/default/files/2017-12/documents/sncrcostmanualchapter7thedition20162017revisions.pdf (accessed on 9 June 2024).
- Locci, C.; Vervisch, L.; Farcy, B.; Domingo, P.; Perret, N. Selective Non-catalytic Reduction (SNCR) of Nitrogen Oxide Emissions: A Perspective from Numerical Modeling. Flow Turbul. Combust. 2017, 100, 301–340. [Google Scholar] [CrossRef]
- Kulish, O.N.; Zaporozhskii, K.I.; Orlova, M.N. Two-Stage Selective Non-Catalytic Process for Nitrogen Oxide Removal from Thermal Generating Unit Flue Gases. Dokl. Chem. 2023, 511, 197–201. [Google Scholar] [CrossRef]
- Horton, J.; Linero, A.; Miller, F.M.G. Use of SNCR to control emissions of oxides of nitrogen from cement plants. In Proceedings of the IEEE Cement Industry Technical Conference, 2006. Conference Record, Phoenix, AZ, USA, 9–14 April 2006; Volume 2006, pp. 316–344. [Google Scholar] [CrossRef]
- Institute of Clean Air Companies (ICAC). Selective Non-Catalytic Reduction (SNCR) for Controlling NOx Emissions. Available online: https://cdn.ymaws.com/www.icac.com/resource/resmgr/Standards_WhitePapers/SNCR_Whitepaper_Final.pdf (accessed on 9 June 2024).
- The Cadmus Group, Inc. Bechtel Power Corporation, and Science Applications International Corporation. Selective Noncatalytic Reduction for NOx Control on Coal-fired Boilers, Draft Report. Prepared for the U.S. Environmental Protection Agency. 1998. Available online: https://downloads.regulations.gov/EPA-R08-OAR-2011-0851-0037/attachment_32.pdf (accessed on 9 June 2024).
- EPRI (Electric Power Research Institute). SNCR Guidelines Update. Report Number 1004727; EPRI: Palo Alto, CA, USA, 2004. [Google Scholar]
- Zhang, X.; Shen, Q.; He, C.; Ma, C.; Cheng, J.; Li, L.; Hao, Z. Investigation of selective catalytic reduction of N2O by NH3 over an Fe-mordenite catalyst: Reaction mechanism and O2 effect. ACS Catal. 2012, 2, 512–520. [Google Scholar] [CrossRef]
- Pedersen, K.A.; Lewandowski, M.T.; Schulze-Netzer, C.; Pasternak, M.; Løvås, T. Ammonia in Dual-Fueled Internal Combustion Engines: Impact on NOx, N2O, and Soot Formation. Energy Fuels 2023, 37, 17585–17604. [Google Scholar] [CrossRef]
- Azure. What Is SCR System? Available online: https://azurechemical.com/blog/what-is-scr-system/ (accessed on 16 June 2024).
- Liang, W.; Law, C.K. Enhancing ammonia combustion using reactivity stratification with hydrogen addition. Proc. Combust. Inst. 2023, 39, 4419–4426. [Google Scholar] [CrossRef]
- SI, K.; Lim, M.; Lee, Y.; Lee, J.; Yang, W. Evaluation of effects of ammonia co-firing on the thermal performances of supercritical pulverized coal and circulating fluidized bed boilers. Energy Convers Manag. 2023, 276, 116528. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Eusébio, D.; Tarelho, L.A.C.; Hall, M.J.; Dana, A.G. Numerical modelling of ammonia-coal co-firing in a pilot-scale fluidized bed reactor: Influence of ammonia addition for emissions control. Energy Convers. Manag. 2022, 254, 115226. [Google Scholar] [CrossRef]
- Hong, D.; Yuan, L.; Wang, C. Competition between NH3-O2 reaction and char-O2 reaction and its influence on NO generation and reduction during char/NH3 co-combustion: Reactive molecular dynamic simulations. Fuel 2022, 324, 124666. [Google Scholar] [CrossRef]
- Xia, Y.; Hashimoto, N.; Fujita, O. Turbulent flame propagation limits of polymethylmethacrylate particle cloud–ammonia–air co-combustion. Proc. Combust. Inst. 2023, 39, 3519–3528. [Google Scholar] [CrossRef]
- Alfazazi, A.; Es-sebbar, E.T.; Zhang, X.; Dally, B.; Abdullah, M.; Younes, M.; Sarathy, S.M. Counterflow flame extinction of ammonia and its blends with hydrogen and C1–C3 hydrocarbons. Appl. Energy Combust. Sci. 2022, 12, 100099. [Google Scholar] [CrossRef]
- Huang, F.; Guo, S.; Wang, L.; Yang, Z.; Kong, W. Experimental and numerical study on the performances of a free-piston engine generator using ammonia and methane fuel mixtures. Fuel 2023, 341, 127654. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Elbaz, A.M.; Han, X.; He, Y.; Costa, M.; Konnov, A.A.; Roberts, W.L. Experimental study and kinetic analysis of the laminar burning velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air premixed flames at elevated pressures. Combust. Flame 2020, 221, 270–287. [Google Scholar] [CrossRef]
- Wu, Z.; Lv, J.; Liu, X.; Wu, W.; Zhou, S.; Yan, B.; Chen, G. Adiabatic laminar burning velocities and NO generation paths of NH3/H2 premixed flames. J. Energy Inst. 2023, 108, 101225. [Google Scholar] [CrossRef]
- Bayramoğlu, K.; Bahlekeh, A.; Masera, K. Numerical investigation of the hydrogen, ammonia and methane fuel blends on the combustion emissions and performance. Int. J. Hydrogen Energy 2023, 48, 39586–39598. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, B.; Gu, M.; Luo, K.; Fan, J.; Wang, Y. Studying the mechanism and impact of H2O-rich atmosphere on N oxidation during ammonia combustion and ammonia-coal co-combustion. Fuel 2023, 352, 129092. [Google Scholar] [CrossRef]
- Uddeen, K.; Tang, Q.; Shi, H.; Magnotti, G.; Turner, J. A novel multiple spark ignition strategy to achieve pure ammonia combustion in an optical spark-ignition engine. Fuel 2023, 349, 128741. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, C.; Zhang, B.; Li, J. Effects of swirl intensity on flame stability and NO emission in swirl-stabilized ammonia/methane combustion. Appl. Energy Combust. Sci. 2023, 14, 100138. [Google Scholar] [CrossRef]
- DNV. Smells Like Sustainability: Harnessing Ammonia as Ship Fuel. Available online: https://www.dnv.com/expert-story/maritime-impact/Harnessing-ammonia-as-ship-fuel.html (accessed on 11 July 2023).
- Marine Log. Why MAN Sees Engine Retrofits as Key to Shipping Decarbonization. Available online: https://www.marinelog.com/technology/why-man-sees-engine-retrofits-as-key-to-shipping-decarbonization/ (accessed on 12 January 2024).
- Te Maritime Executive. Wärtsilä Introduces First Commercial 4-Stroke Ammonia Dual-Fuel Engine. Available online: https://maritime-executive.com/article/waertsilae-introduces-first-commercial-4-stroke-ammonia-dual-fuel-engine (accessed on 12 January 2024).
- James, G.W.; Giovanni, C.; Christine, L.K. The Influence of Ammonia Slip Catalysts on Ammonia, N2O and NOx Emissions for Diesel Engines. Available online: https://www.jstor.org/stable/44650854 (accessed on 29 January 2024).
- Singh, D.V.; Pedersen, E. A review of waste heat recovery technologies for maritime applications. Energy Convers. Manag. 2016, 111, 315–328. [Google Scholar] [CrossRef]
- Konur, O.; Colpan, C.O.; Saatcioglu, O.Y. A comprehensive review on organic Rankine cycle systems used as waste heat recovery technologies for marine applications. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 4083–4122. [Google Scholar] [CrossRef]
- Ng, C.W.; Tam, I.C.K.; Wu, D. Thermo-Economic Performance of an Organic Rankine Cycle System Recovering Waste Heat Onboard an Offshore Service Vessel. J. Mar. Sci. Eng. 2020, 8, 351. [Google Scholar] [CrossRef]
- de la Fuente, S.S.; Cao, T.; Pujol, A.G.; Romagnoli, A. Waste heat recovery on ships. In Sustainable Energy Systems on Ships: Novel Technologies for Low Carbon Shipping; Elsevier: Amsterdam, The Netherlands, 2022; pp. 123–195. [Google Scholar] [CrossRef]
- Gao, H.; Chen, F. Thermo-Economic Analysis of a Bottoming Kalina Cycle for Internal Combustion Engine Exhaust Heat Recovery. Energies 2018, 11, 3044. [Google Scholar] [CrossRef]
- Larsen, U.; Van Nguyen, T.; Knudsen, T.; Haglind, F. System analysis and optimisation of a Kalina split-cycle for waste heat recovery on large marine diesel engines. Energy 2014, 64, 484–494. [Google Scholar] [CrossRef]
- Yustiarza, F. Analysis of Waste Heat to Power Technologies in a Maritime Engine System. Master’s Thesis, Master’s Degree in Environomical Pathways for Sustainable Energy Systems. Aalto University, Espoo, Finland, 2023. [Google Scholar]
- Berni, F.; Pessina, V.; Teodosio, L.; d’Adamo, A.; Borghi, M.; Fontanesi, S. An integrated 0D/1D/3D numerical framework to predict performance, emissions, knock and heat transfer in ICEs fueled with NH3–H2 mixtures: The conversion of a marine Diesel engine as case study. Int. J. Hydrogen Energy 2023, 50, 908–938. [Google Scholar] [CrossRef]
- Wu, X.; Feng, Y.; Gao, Y.; Xia, C.; Zhu, Y.; Shreka, M.; Ming, P. Numerical simulation of lean premixed combustion characteristics and emissions of natural gas-ammonia dual-fuel marine engine with the pre-chamber ignition system. Fuel 2023, 343, 127990. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, B.; Wang, J.; Zhu, L.; Xiao, J.; Sun, Z.; Huang, Z. Numerical analysis of ammonia HCCI combustion in a free piston engine through trajectory-based combustion control. Fuel 2023, 341, 127634. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, D.; Yang, W.; Qian, Y.; Mao, Y.; Lu, X. Investigation on the potential of using carbon-free ammonia in large two-stroke marine engines by dual-fuel combustion strategy. Energy 2023, 263, 125748. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Q.; Zhao, N.; Chen, M.; Zheng, H. Numerically study of CH4/NH3 combustion characteristics in an industrial gas turbine combustor based on a reduced mechanism. Fuel 2022, 327, 124897. [Google Scholar] [CrossRef]
- Wu, R.; Zeng, Z.; Liu, H.; Guo, K. Numerical Investigation on NH3/H2/Air Combustion Flow in a Multiple Direct-Injection Combustor. Energy Fuels 2023, 37, 17499–17515. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Dai, L.; Zhang, M.; Yu, C. Numerical Study on the Combustion Properties of Ammonia/DME and Ammonia/DMM Mixtures. Energies 2023, 16, 6929. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Chavando, J.A.M.; Eusébio, D.; Hall, M.J. Numerical modelling of the coal phase-out through ammonia and biomass co-firing in a pilot-scale fluidized bed reactor. Fuel Commun. 2022, 10, 100055. [Google Scholar] [CrossRef]
- Zanobetti, F.; Pio, G.; Jafarzadeh, S.; Ortiz, M.M.; Cozzani, V. Inherent safety of clean fuels for maritime transport. Process Saf. Environ. Prot. 2023, 174, 1044–1055. [Google Scholar] [CrossRef]
- ABS, CE-DELFT, and ARCSILEA. Potential of Ammonia as Fuel in Shipping. 2022. Available online: www.emsa.europa.eu (accessed on 26 June 2024).
- ISO 8217:2017; Petroleum Products—Fuels (Class F)—Specifications of Marine Fuels. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/64247.html (accessed on 9 June 2023).
- ISO 5771:2008; Rubber Hoses and Hose Assemblies for Transferring Anhydrous Ammonia—Specification. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/43694.html (accessed on 9 June 2023).
- ISO 7103:1982; Liquefied Anhydrous Ammonia for Industrial Use—Sampling—Taking a Laboratory Sample. ISO: Geneva, Switzerland, 1982. Available online: https://www.iso.org/standard/13688.html (accessed on 9 June 2023).
- ISO 7105:1985; Liquefied Anhydrous Ammonia for Industrial Use—Determination of Water Content—Karl Fischer Method. ISO: Geneva, Switzerland, 1985. Available online: https://www.iso.org/standard/13690.html (accessed on 9 June 2023).
- ISO 7106:1985; Liquefied Anhydrous Ammonia for Industrial Use—Determination of Oil Content—Gravimetric and Infra-Red Spectrometric Methods. ISO: Geneva, Switzerland, 1985. Available online: https://www.iso.org/standard/13691.html (accessed on 9 June 2023).
- ISO 6957:1988; Copper Alloys—Ammonia Test for Stress Corrosion Resistance. ISO: Geneva, Switzerland, 1988. Available online: https://www.iso.org/standard/13507.html (accessed on 9 June 2023).
- ISO 17179:2016; Stationary Source Emissions—Determination of the Mass Concentration of Ammonia in Flue Gas—Performance Characteristics of Automated Measuring Systems. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/59257.html (accessed on 9 June 2023).
- ISO 21593:2019; Ships and Marine Technology—Technical Requirements for Dry-Disconnect/Connect Couplings for Bunkering Liquefied Natural Gas. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/71167.html (accessed on 9 June 2023).
- ISO 20519:2021; Ships and Marine Technology—Specification for Bunkering of Liquefied Natural Gas Fuelled Vessels. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/80842.html (accessed on 9 June 2023).
- ISO/TS 18683:2021; Guidelines for Safety and Risk Assessment of LNG Fuel Bunkering Operations. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/76811.html (accessed on 9 June 2023).
- Derek Matthiessen, J.D. Regulatory Framework for NH3 Fuel. Available online: https://www.ammoniaenergy.org/paper/regulatory-framework-for-nh3-fuel/ (accessed on 9 June 2023).
- ANSI. CGA G-2.1—American National Standard Safety Requirements for the Storage and Handling of Anhydrous Ammonia (ANSI K61.1). Available online: https://webstore.ansi.org/standards/cga/cga-1272198?_ga=2.244520965.127570802.1686422280-1231710072.1686422280 (accessed on 9 June 2023).
- EPA. The Public Health and Welfare Chapter 85—Air Pollution Prevention and Control—Sec. 7412—Hazardous Air Pollutants. Available online: https://www.govinfo.gov/content/pkg/USCODE-2013-title42/html/USCODE-2013-title42-chap85-subchapI-partA-sec7412.htm (accessed on 9 June 2023).
- EPA. List of Regulated Substances under the Risk Management Program Program. Available online: https://www.epa.gov/rmp/list-regulated-substances-under-risk-management-program-program (accessed on 9 June 2023).
- EPA. CFR Part 355—Emergency Planning and Notification. 2008. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-J/part-355#subpart-C (accessed on 9 June 2023).
- DHS. Chemical Facility Anti-Terrorism Standards (CFATS) Ammonia Flyer. Available online: https://www.cisa.gov/resources-tools/resources/chemical-facility-anti-terrorism-standards-cfats-ammonia-flyer (accessed on 9 June 2023).
- OSHA, 1910.111—Storage and Handling of Anhydrous Ammonia. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.111 (accessed on 9 June 2023).
- OSHA, 1910.119—Process Safety Management of Highly Hazardous Chemicals. | Occupational Safety and Health Administration. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.119 (accessed on 9 June 2023).
- OSHA. Hazard Communication. Available online: https://www.osha.gov/laws-regs/federalregister/2012-03-26 (accessed on 9 June 2023).
- DOT and ECFR. Title 49 Transportation. Available online: https://www.ecfr.gov/current/title-49/subtitle-B/chapter-I (accessed on 9 June 2023).
- IMO. IGC Code. Available online: https://www.imo.org/en/ourwork/safety/pages/igc-code.aspx (accessed on 9 June 2023).
- OMI. International Code of Safety for Ship Using Gases or Other Low-Flashpoint Fuels (IGF Code). Available online: https://www.imo.org/es/OurWork/Safety/Paginas/IGF-Code.aspx (accessed on 9 June 2023).
- EU. ‘Fit for 55’: Council Adopts Key Pieces of Legislation Delivering on 2030 Climate Targets. Available online: https://www.consilium.europa.eu/en/press/press-releases/2023/04/25/fit-for-55-council-adopts-key-pieces-of-legislation-delivering-on-2030-climate-targets/ (accessed on 9 June 2023).
- IMO. Guidelines on Life Cycle GHG Intensity of Marine Fuels (LCA Guidelines). Available online: https://www.imo.org/en/OurWork/Environment/Pages/Lifecycle-GHG---carbon-intensity-guidelines.aspx (accessed on 9 June 2023).
- Parkar, S. AEA Webinar: Ammonia at Sea The Maritime Decarbonisation Hub. Available online: https://www.ammoniaenergy.org/wp-content/uploads/2023/02/Maritime-Insights-speaker-slides-Feb-2023.pdf (accessed on 9 June 2024).
- Lloyd’s Register Maritime Decarbonisation Hub. The Future of Maritime Fuels The Future of Maritime Fuels; Lloyd’s Register Maritime Decarbonisation Hub: London, UK, 2023. [Google Scholar]
- Mordor Intelligence. Maritime Freight Transport Market—Trends, Share & Overview. Available online: https://www.mordorintelligence.com/industry-reports/global-maritime-freight-transport-market (accessed on 31 January 2024).
- International Chamber of Shipping. Shipping and World Trade: World Seaborne Trade. Available online: https://www.ics-shipping.org/shipping-fact/shipping-and-world-trade-world-seaborne-trade/ (accessed on 31 January 2024).
- Unctad. Review of Maritime Transport. 2023-Chapter 1: International Maritime Trade. Available online: https://unctad.org/publication/review-maritime-transport-2023 (accessed on 9 June 2024).
- Nayak-Luke, R.M.; Bañares-Alcántara, R. Techno-economic viability of islanded green ammonia as a carbon-free energy vector and as a substitute for conventional production. Energy Environ. Sci. 2020, 13, 2957–2966. [Google Scholar] [CrossRef]
- IHS Markit. Ammonia Outlook—Quarterly Outlook for the International Ammonia Market Prepared by Fertecon Analysts. 2022. Available online: https://commodityinsights.spglobal.com/rs/325-KYL-599/images/T4-Ammonia-Outlook-Report-Aug22.pdf (accessed on 17 February 2024).

| Energy Source | Fossil (without CCs) | Bio | Renewable (C) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fuel | HFO + Scrubber | Low Sulfur Fuels | LNG | Methanol | LPG | HVO (Advanced Biodiesel) | NH3 | H2 | Fully Electric |
| High-priority parameters | |||||||||
| Energy density | 5 | 5 | 4 | 4 | 4 | 5 | 3 | 2 | 1 |
| Technological maturity | 4 | 4 | 4 | 3 | 3 | 5 | 2 | 2 | 1 |
| Local emissions | 2 | 2 | 4 | 4 | 4 | 2 | 3 | 5 | 5 |
| GHG emissions | 1 | 1 | (B) | 2 | 2 | 4 | 5 | 5 | 5 |
| Energy cost | 5 | 4 | 5 | 3 | 4 | 2 | 1 | 1 | (D) |
| Capital cost | 4 | 5 | 4 | 4 | 4 | 5 | 4 | 1 | 5 |
| Converter storage | 5 | 5 | 3 | 4 | 4 | 5 | 4 | 1 | 1 |
| Bunkering availability | 5 | 5 | 4 | 3 | 3 | 2 | 2 | 1 | 2 |
| Commercial readiness (A) | 5 | 5 | 5 | 4 | 4 | 3 | 2 | 1 | (E) |
| Other key parameters | |||||||||
| Flammability | 5 | 5 | 5 | 3 | 5 | 5 | 4 | 1 | 5 |
| Toxicity | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 5 | 5 |
| Regulations and guidelines | 5 | 5 | 5 | 4 | 3 | 5 | 3 | 1 | 4 |
| Global production capacity and locations | 5 | 5 | 5 | 4 | 4 | 2 | 3 | 3 | 1 |
| LNG | LPG | CH3OH | H2 | Ships with Scrubbers | |||||
|---|---|---|---|---|---|---|---|---|---|
| Year | In Operation | On Order | In Operation | On Order | In Operation | On Order | In Operation | On Order | |
| 2015 | 63 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 243 |
| 2016 | 82 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 312 |
| 2017 | 99 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 387 |
| 2018 | 124 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 740 |
| 2019 | 157 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 3178 |
| 2020 | 186 | 0 | 3 | 0 | 12 | 0 | 0 | 0 | 4362 |
| 2021 | 247 | 0 | 21 | 0 | 15 | 0 | 0 | 0 | 4581 |
| 2022 | 355 | 0 | 52 | 0 | 24 | 0 | 0 | 0 | 4807 |
| 2023 | 431 | 110 | 74 | 40 | 28 | 2 | 2 | 5 | 5095 |
| 2024 | 431 | 290 | 74 | 55 | 28 | 33 | TBD | TBD | 5230 |
| 2025 | 431 | 431 | 74 | 76 | 28 | 76 | TBD | TBD | 5246 |
| 2026 | 431 | 504 | 74 | 95 | 28 | 137 | TBD | TBD | TBD |
| 2027 | 431 | 534 | 74 | 99 | 28 | 172 | TBD | TBD | TBD |
| 2028 | 431 | 539 | TBD | TBD | 28 | 176 | TBD | TBD | TBD |
| Players | Project | Description | Year | Ref. |
|---|---|---|---|---|
| Azane | Zero-emission NH3 bunker vessels | It has successfully created an innovative bunker vessel design that produces zero emissions. | 2023 | [24] |
| Amogy Inc. | Successful completion of technology verification | NH3-to-power system for maritime uses. | 2023 | [25] |
| NH3-powered semi-truck | Amogy Inc. effectively conducted trials on its inaugural NH3-powered semi-truck, which produced zero emissions, by integrating its proprietary technology into John Deere tractors and drones. | 2023 | [33] | |
| GAC | Trumpchi E9 NH3 | The first passenger vehicle in its class to utilize NH3. | 2023 | [26] |
| ABS | Technology trends | ABS, a major technology company, has built a base for the marine industry to adapt to the digital world and switch to sustainable operations, leading to a net-zero carbon path and a sustainable future. | 2022 | [34] |
| Navigator Gas | AiP | DNV, a classification society, has given Navigator Holdings Ltd. preliminary approval for its company, Navigator Gas LLC, to build a gas carrier that runs on NH3. DNV and the Norwegian Maritime Authority approved the design based on the unique features code (GF NH3). | 2021 | [35] |
| Anglo American | Biodiesel as a maritime fuel | Anglo American tested sustainable biodiesel on a hired capesize ship from Singapore to South Africa. Biodiesel from Singapore’s food and beverage waste cooking oil reduces CO2 emissions by 5% compared to 100% traditional marine fuel. | 2021 | [36] |
| Class NK | ClassNK is a ship classification society. The society is expanding its ship-related activities and services to protect human life, property, and the marine environment at sea. | 2023 | [37] | |
| DVN | Maritime Cyber Priority 2023 | The new global report, Marine Cyber Priority: Staying Secure in the Era of Connectedness by DNV, examines shifting marine cyber security attitudes and practices. | 2023 | [38] |
| Maritime Forecast 2050 | It gives maritime stakeholders valuable insights into how to make decisions in their decarbonization journey. | 2023 | [39] | |
| Transport transition | DNV forecasts primary fuel, electrical, and infrastructure changes in marine, aircraft, and road transport during the next 30 years. Even though 78% of road travel is electrified, these sectors account for 25% of emissions and will climb to 30% by 2050. | 2023 | [40] | |
| Equinor | The corporation is investing in offshore wind and rebuilding its shipping fleet. Dual-fuel LPG and LNG propulsion are being introduced aboard LPG carriers, shuttle tankers, and Aframax/LR2s. | 2023 | [41] | |
| Global Maritime Forum | What now, from ambition to action | The 2023 Global Maritime Forum Annual Summit brings together leading maritime voices and others to address the possibilities and challenges that will define global seaborne trade. | 2023 | [42] |
| ITOCHU | NH3-fueled ships | Develop propulsion systems and hulls to introduce NH3-fueled ships under Japan’s leadership by 2028. | 2021 | [43] |
| Bunkering safety for NH3-fueled ships | ITOCHU promotes NH3-powered container ships. | 2023 | [44] | |
| K Line, Itochu, NS United | NH3-powered ship project | NEDO, Kaiun Kaisha, and Mitsui E&S Machinery will collaborate with the players to introduce NH3-powered vessels by 2028, using NH3 as a marine fuel for propulsion systems and hulls. | 2021 | [45] |
| MAN Energy Solutions | Dual-fuel two-stroke engines | MAN Energy Solutions is developing research engines based on highly adaptable combustion engines for LNG, LPG, ethane, methanol, and carbon-free NH3 for the marine energy transition and significant ship propulsion. | 2023 | [46] |
| Nihon Shipyard | Co-Firing test using a cutting-edge NH3-fueled engine | NYK, Japan Engine Corporation, IHI Power Systems, and Nihon Shipyard launched the project in October 2021 to create naval vessels using local NH3-fueled engines. IHI Power Systems tested a coastal vessel 280 mm bore four-stroke NH3 marine engine in April 2023. All demonstration equipment did not leak NH3 during operation or shutdown, and dinitrogen monoxide and unburnt NH3 emissions were almost nil. | 2023 | [47] |
| Nordic Innovation | NoGAPS | The study concludes that technical and regulatory permissions for an NH3-powered vessel do not prevent the launch of M/S NoGAPS. Green H2 can produce NH3, a zero-emission fuel. Creating and convincing investors and operators of a business strategy is the most complex challenge. | 2021 | [48] |
| Ube Industries | Ube Industries, Japan’s biggest NH3 manufacturer, will study maritime NH3 fuel supply and onshore facilities. | 2021 | [49] | |
| Uyeno Transtech | Korean NH3 bunker vessel design | Japanese cooperation and the Korean Register’s Approval in Principle for an NH3 bunkering vessel are helping the Korean shipping sector accept NH3 as a marine fuel. | 2020 | [50] |
| Vopak Singapore | Vopak Singapore may extend its NH3 infrastructure for low-carbon electricity and bunker fuel. | 2022 | [51] | |
| Wärtsilä | First NH3 conversion project | Wärtsilä and Norwegian ship owner Eidesvik Offshore ASA have agreed to convert an offshore supply vessel (OSV) to an NH3-fueled combustion engine. The world’s first NH3-only project seeks to operate with minimal ignition fuel. The upgrade will allow the vessel to use 70% NH3, lowering CO2 emissions. Both companies support industrial decarbonization. Since 2003, Eidesvik has supported ecological technologies by using LNG fuel in its fleet. The EU-funded ShipFC project will equip the Viking Energy platform vessel with a 2 MW green NH3 fuel cell. | 2021 | [52] |
| NH3 2–4 | Wärtsilä leads EU-funded NH3 2- and 4-stroke engines. | 2022 | [53] | |
| Jera, Mou, Yara | Yara Clean NH3 and Bunker Holding Group inked an MOU to boost the maritime fuel market for clean NH3. | 2023 | [54] |
| Description | Type | Blend | Ref |
|---|---|---|---|
| Combustion at different temperatures, O2 equivalence ratios, and NH3 co-combustion ratios. | Simulation | Char/NH3 | [99] |
| It describes the limits of turbulent flame propagation and the associated mechanism for solid particle cloud–NH3–air co-combustion. | Experimental | Solid/NH3 | [100] |
| It stimulates the combustion of NH3 with different carbon fuels. | Simulation | CH4, C2H6, C3H8, and H2 | [101] |
| It asses the performance of a free-piston engine generator fueled by NH3 and methane mixtures. | Experimental | CH4/NH3 | [102] |
| It provides experimental and kinetic data for the combustion of Syngas/NH3. | Experimental | Syngas/NH3 | [103] |
| It provides experimental data on LBV and an unsatisfactory activation energy (Ea) prediction. | Experimental | NH3/H2 | [104] |
| It assesses the adiabatic flame temperature and reactivity of different blends. | Numerical method | CH4/H2/NH3 | [105] |
| It investigates the influence of H2O on NH3 combustion. | Simulation | H2O/NH3 | [106] |
| This study examines how pure NH3 burns in an optical spark ignition engine when multiple spark ignition spots exist. | Experimental | NH3 | [107] |
| It assesses the effects of swirl strength on the features of combustion of NH3/methane. | Numerical method | CH4/NH3 | [108] |
| Description | Blend | Ref. |
|---|---|---|
| The research introduces a robust numerical framework utilizing 0D, 1D, and 3D tools for conducting CFD calculations on internal combustion engines powered by NH3-H2 mixtures. The model can be improved by incorporating predictive models for knock and emissions, turbulence, and robust chemical mechanisms. | NH3/H2 | [122] |
| This paper studies the NH3/CH4 flame structure, temperature, and species field. The model can be improved by incorporating robust chemical mechanisms. | NH3/CH4 | [124] |
| This research examines the combustion properties of NH3/H2/air in various direct-injection combustors, focusing on the impacts of equivalency ratio, H2 mixing ratio, and emission characteristics. | NH3/H2/air | [125] |
| The research uses dimethyl ether (DME), a highly reactive oxygenated fuel, in combination with NH3 to address NH3’s low reactivity. The model can be improved by incorporating a more accurate model for NH2/NOx. | NH3/DME | [126] |
| The paper suggests using a mix of NH3, coal, and biomass to lower the carbon footprint of coal-fired processes and lower the costs of biomass exploration so that the feedstock supply is not affected, all while making power plants more efficient. | NH3/coal/biomass | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavando, A.; Silva, V.; Cardoso, J.; Eusebio, D. Advancements and Challenges of Ammonia as a Sustainable Fuel for the Maritime Industry. Energies 2024, 17, 3183. https://doi.org/10.3390/en17133183
Chavando A, Silva V, Cardoso J, Eusebio D. Advancements and Challenges of Ammonia as a Sustainable Fuel for the Maritime Industry. Energies. 2024; 17(13):3183. https://doi.org/10.3390/en17133183
Chicago/Turabian StyleChavando, Antonio, Valter Silva, João Cardoso, and Daniela Eusebio. 2024. "Advancements and Challenges of Ammonia as a Sustainable Fuel for the Maritime Industry" Energies 17, no. 13: 3183. https://doi.org/10.3390/en17133183
APA StyleChavando, A., Silva, V., Cardoso, J., & Eusebio, D. (2024). Advancements and Challenges of Ammonia as a Sustainable Fuel for the Maritime Industry. Energies, 17(13), 3183. https://doi.org/10.3390/en17133183









