Enhancing Electrode Efficiency in Proton Exchange Membrane Fuel Cells with PGM-Free Catalysts: A Mini Review
Abstract
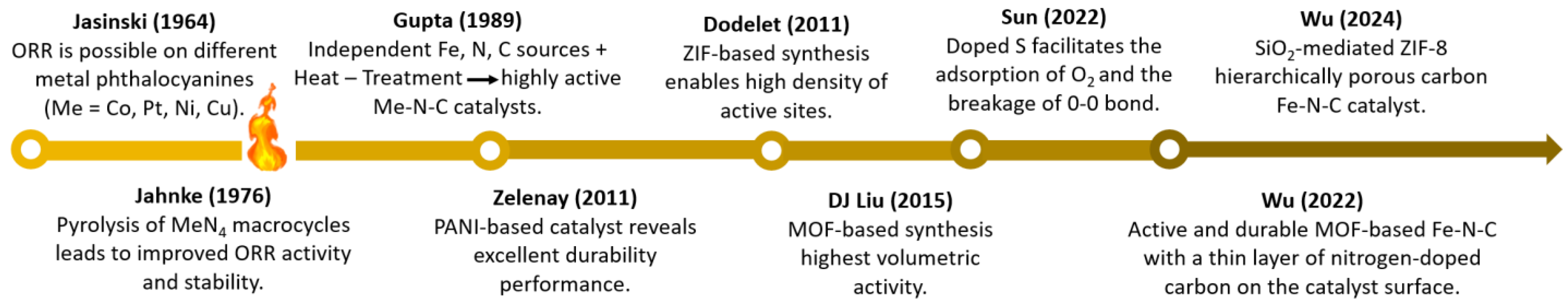
:1. Introduction
2. Platinum Group Metal (PGM)–Free Electrocatalysts
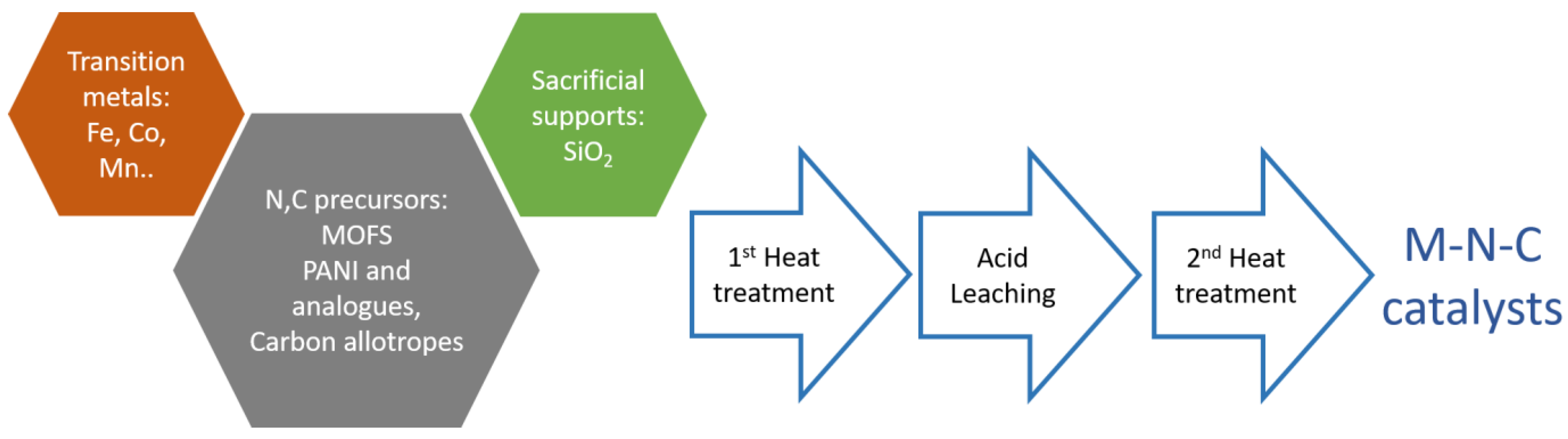
2.1. Progress on Metal-Nitrogen-Carbon (M–N–C) Catalysts
2.1.1. Polyaniline and Analogues
2.1.2. High Surface-Area Supports or Sacrificial Template Method
2.1.3. Metal–Organic Frameworks (MOFs)
2.2. Current Perspectives on Active Site Structures
2.2.1. CNx-Sites
2.2.2. Iron Carbide Sites
2.2.3. FeN4 Sites
2.3. Durability/Stability Issues in PEMFC
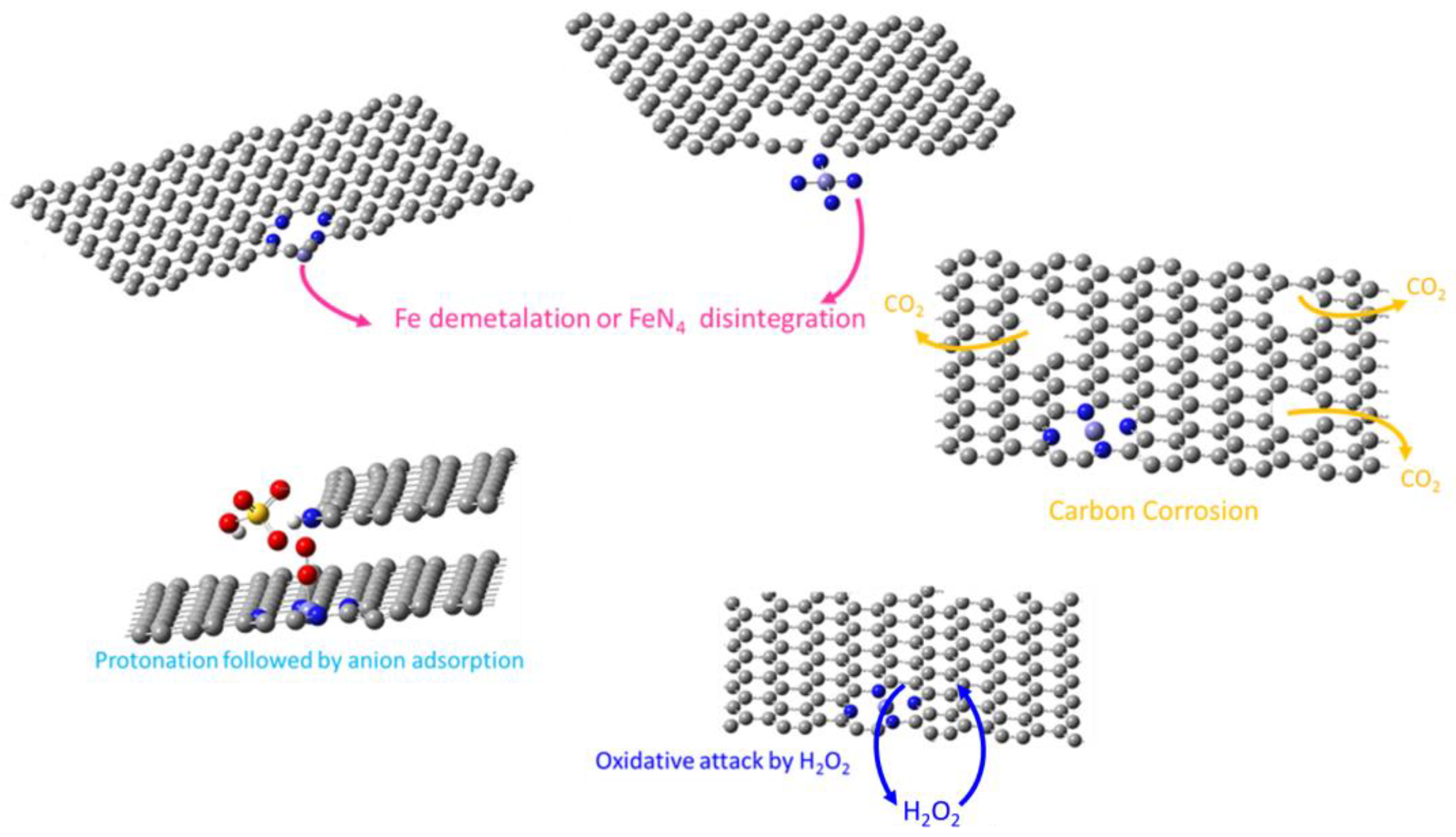
2.4. Degradation Mechanisms
2.4.1. Fe Dissolution or FeN4 Disintegration
2.4.2. Carbon Oxidation
2.4.3. Oxidative Attack of Carbon Structure by H2O2
2.4.4. Anion Adsorption of the Active Site
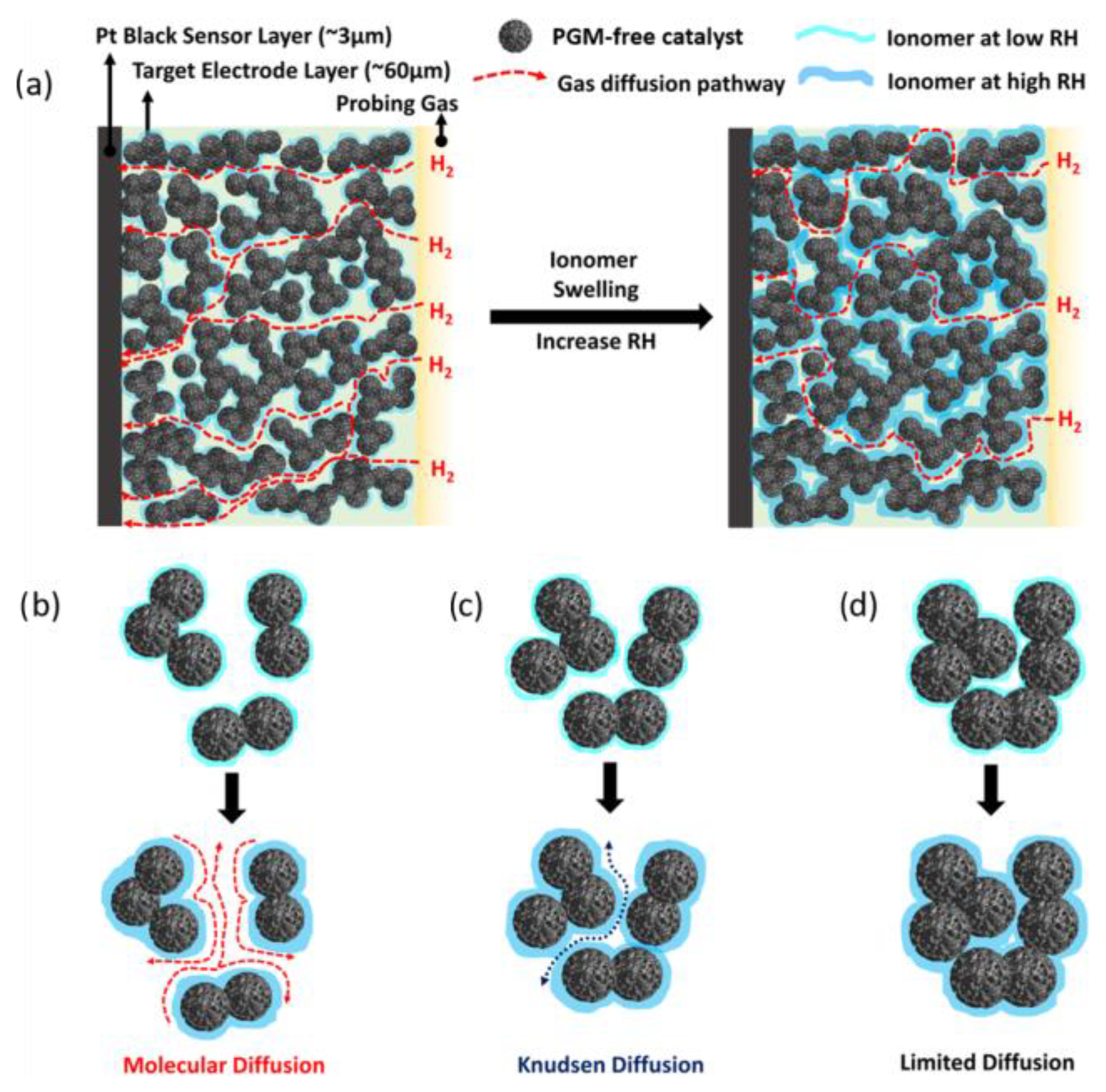
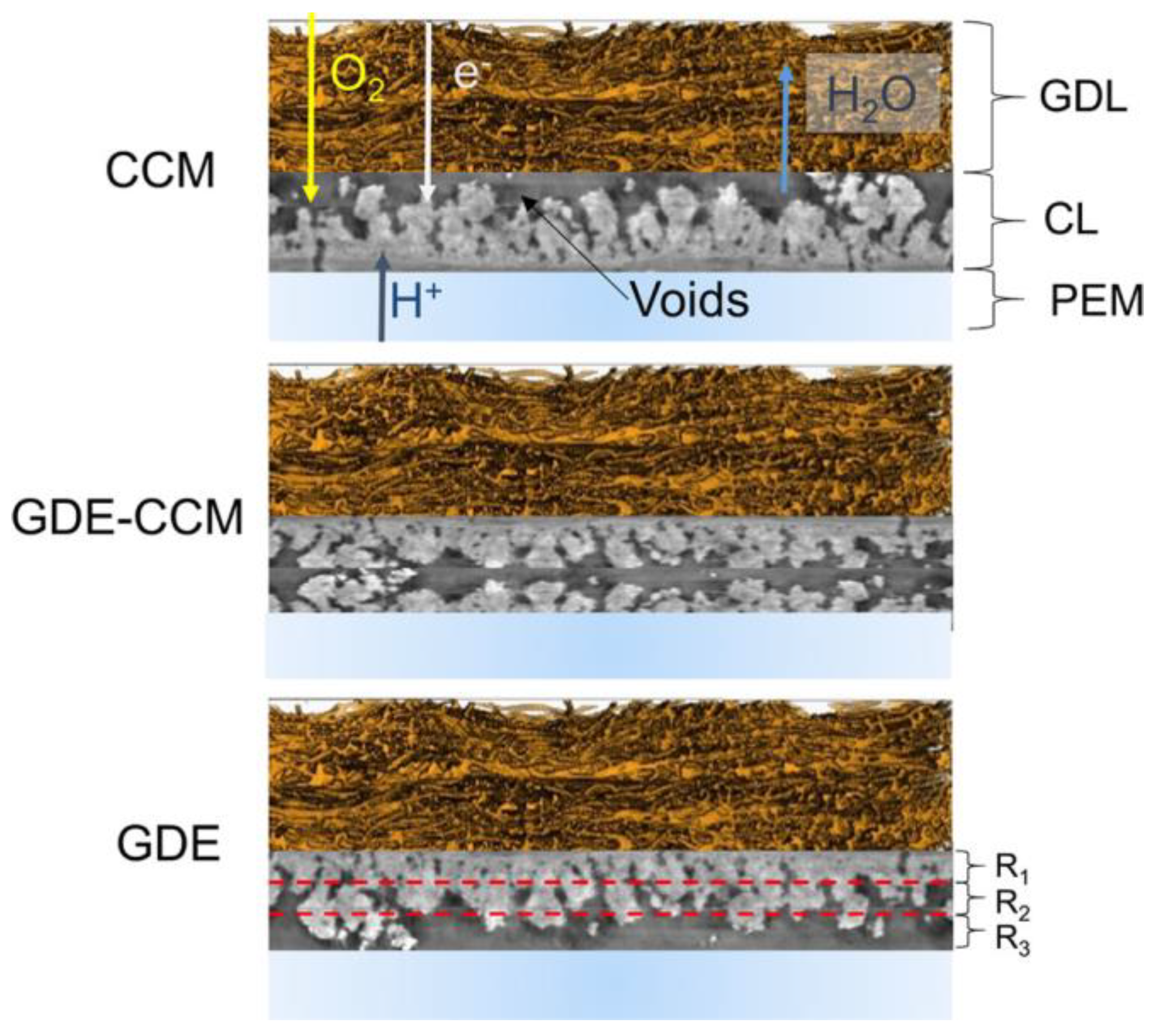
3. Electrode Structure and Mass Transport Properties
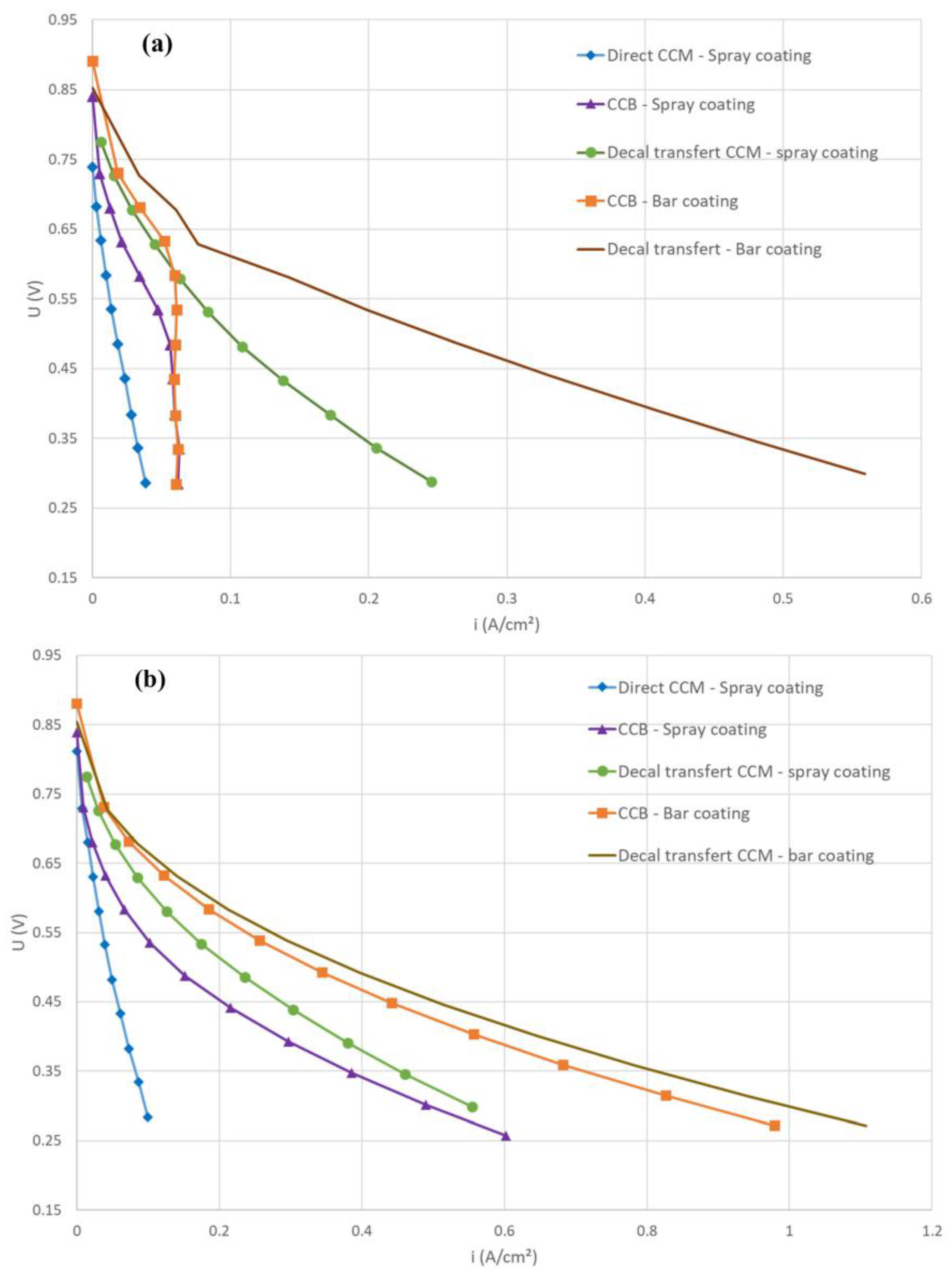
3.1. Membrane-Electrode Assembly (MEA) Fabrication
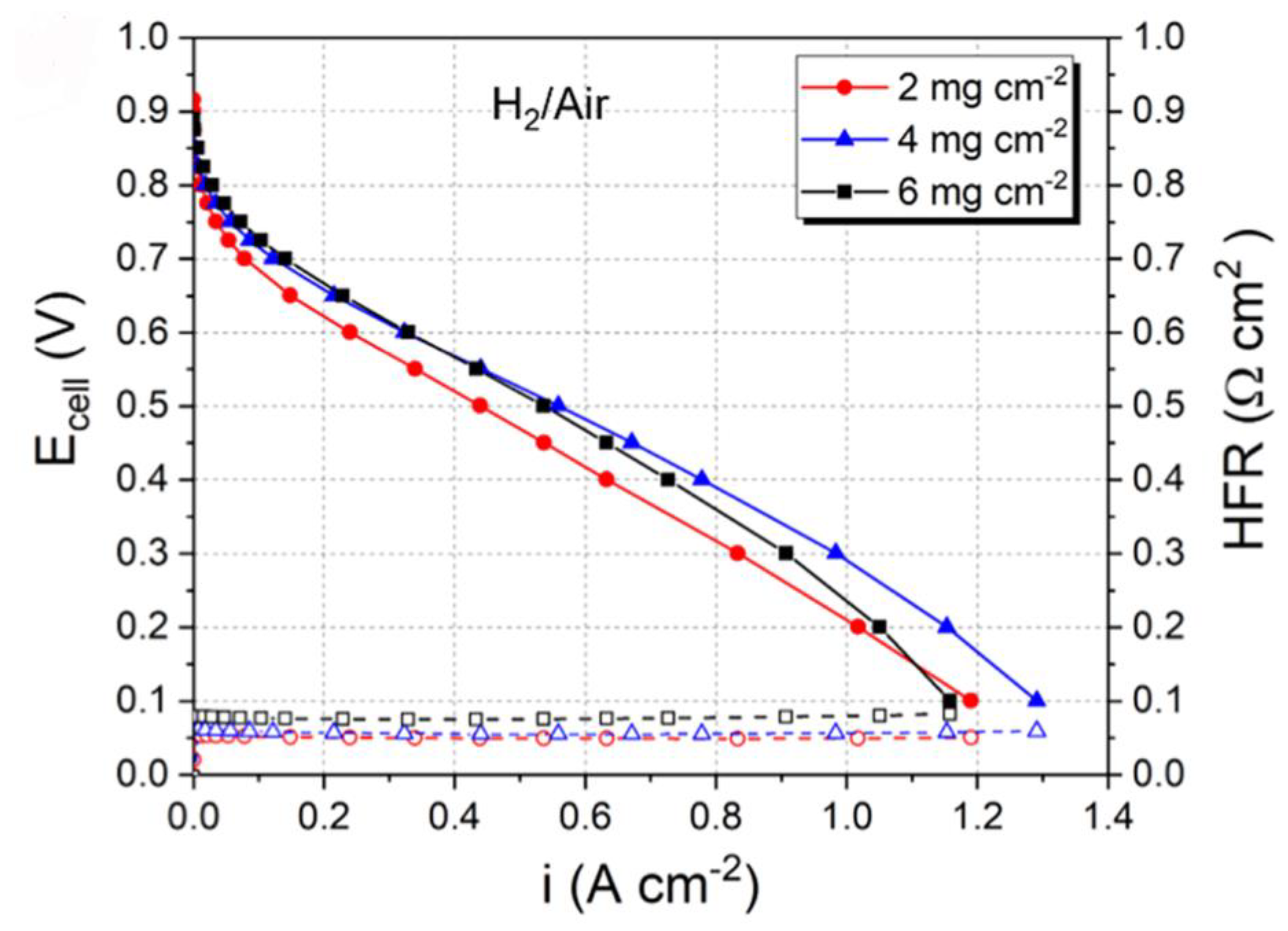
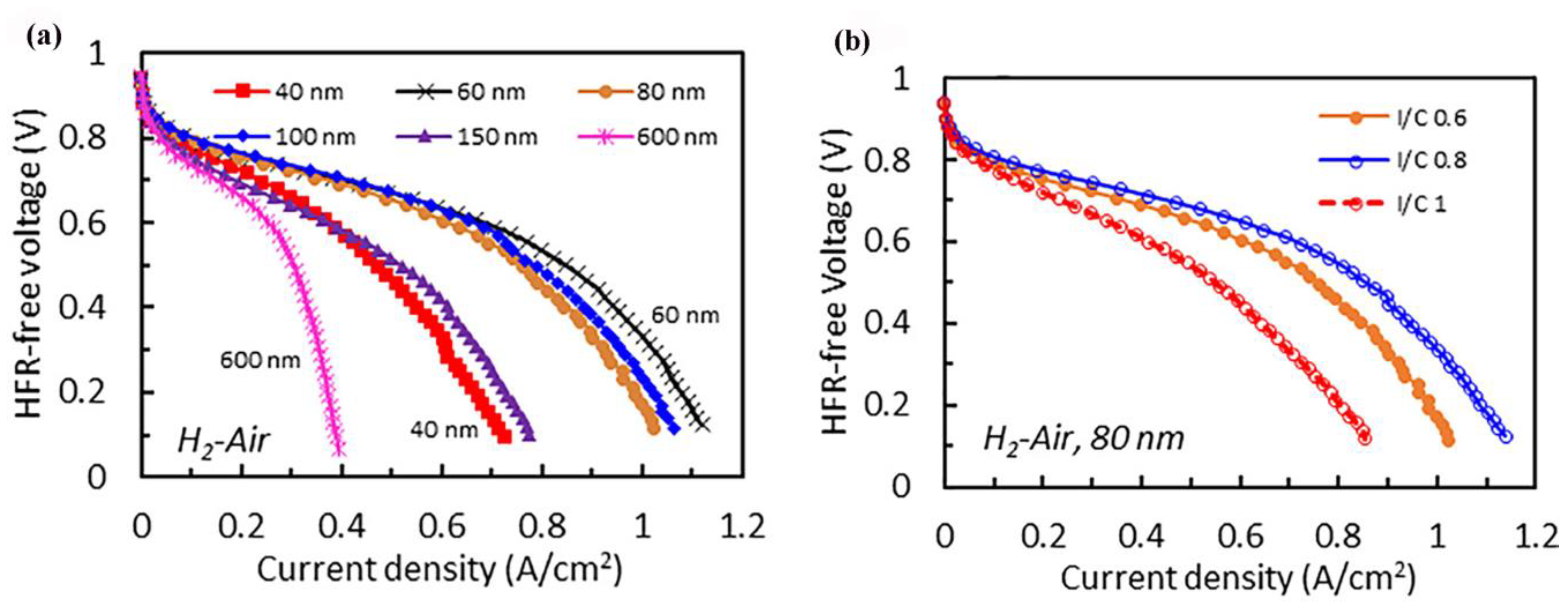
3.2. PGM-Free Catalyst Loading
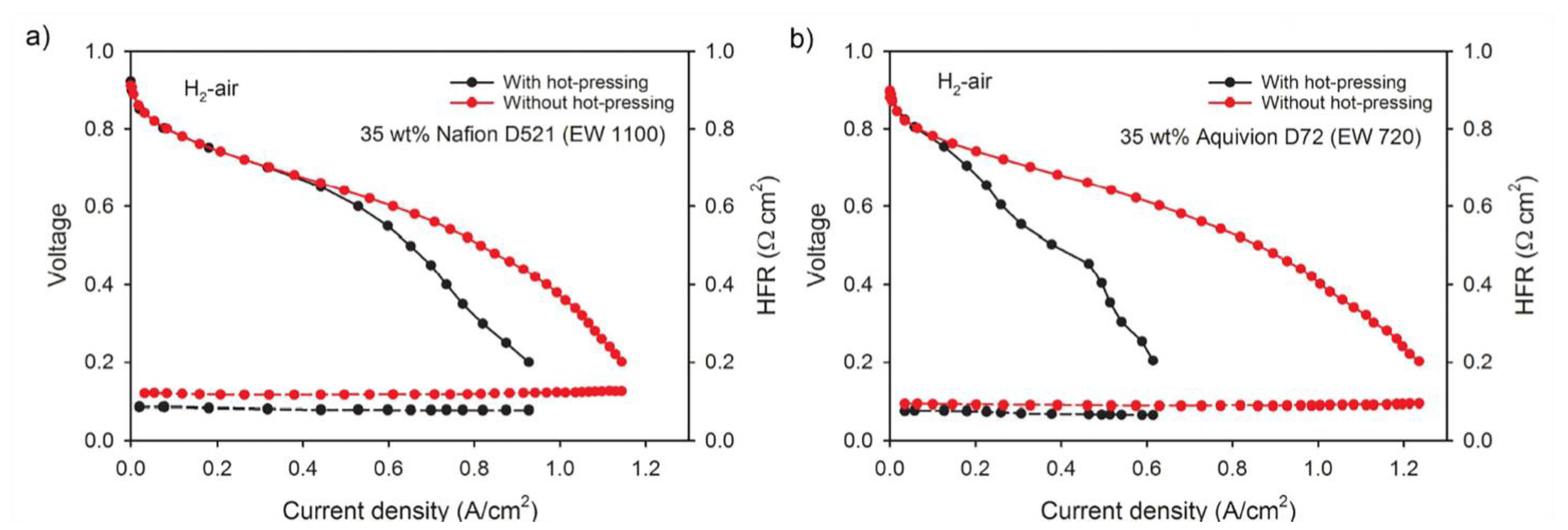
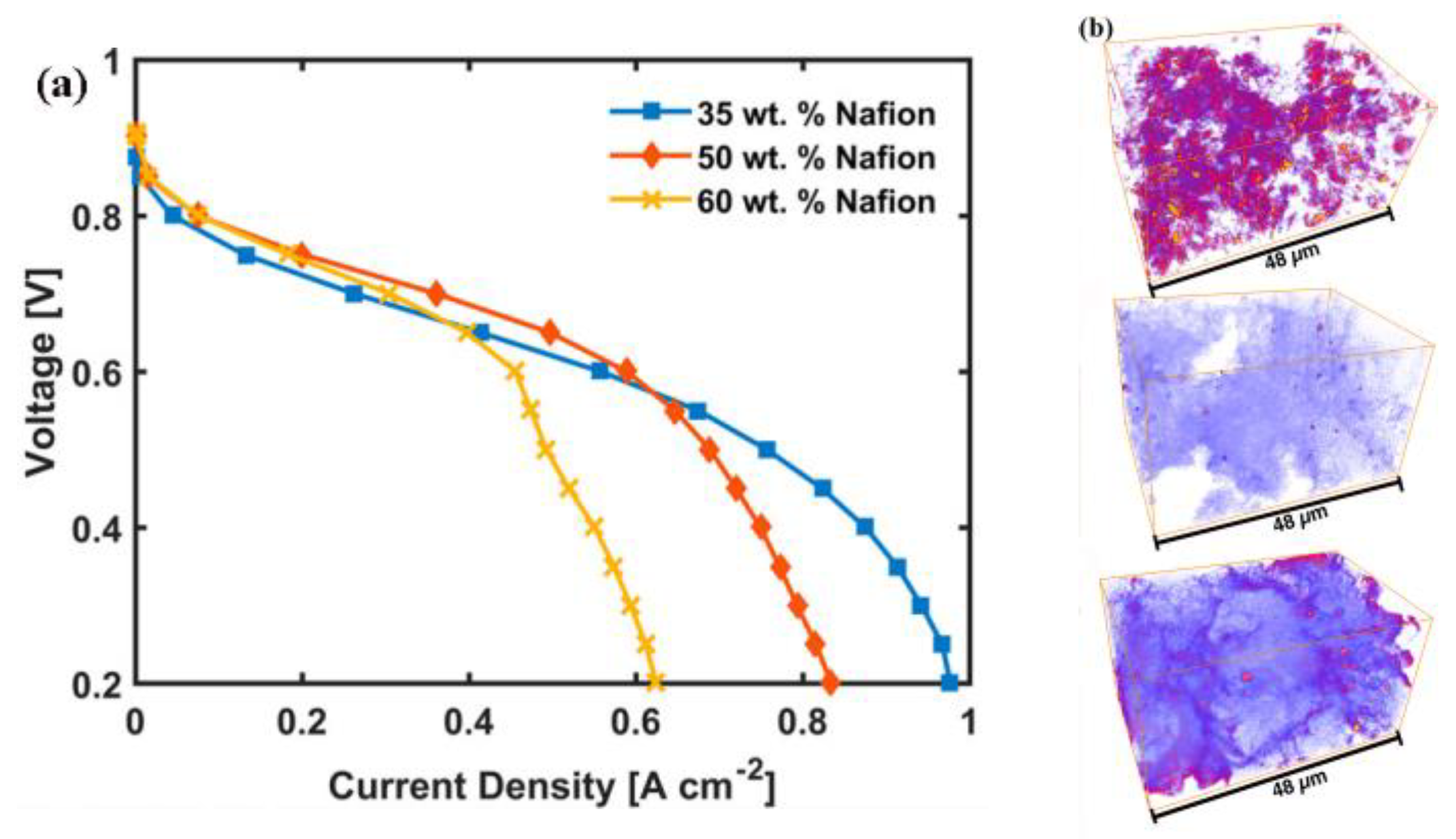
3.3. The Role of the Ionomer and Ink Composition
3.4. Operating Fuel Cell Conditions
4. Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel Cell and Electrolysis Cell Technologies and Hydrogen Infrastructure Development—A Review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Zhong, L.; Li, S. Unconventional Oxygen Reduction Reaction Mechanism and Scaling Relation on Single-Atom Catalysts. ACS Catal. 2020, 10, 4313–4318. [Google Scholar] [CrossRef]
- Mo, S.; Du, L.; Huang, Z.; Chen, J.; Zhou, Y.; Wu, P.; Meng, L.; Wang, N.; Xing, L.; Zhao, M.; et al. Recent Advances on PEM Fuel Cells: From Key Materials to Membrane Electrode Assembly. Electrochem. Energy Rev. 2023, 6, 28. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C Electrocatalyst with Dense Active Sites and Efficient Mass Transport for High-Performance Proton Exchange Membrane Fuel Cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D.; et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Samarakoon, W.; Shan, W.; Cullen, D.A.; Karakalos, S.; Chen, M.; Gu, D.; More, K.L.; Wang, G.; et al. Thermally Driven Structure and Performance Evolution of Atomically Dispersed FeN 4 Sites for Oxygen Reduction. Angew. Chem. 2019, 131, 19147–19156. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, M.; Li, X.; Ding, R.; Duan, X.; Li, J.; Wang, Y.; Li, X.; Zhang, Y.; Liu, J. Assistance of Rearrangement of Active Sites in Fe/N/C Catalyst for Harvesting Ultra-High Power Density PEMFCs. Appl. Catal. B 2022, 312, 121365. [Google Scholar] [CrossRef]
- Wang, W.; Jia, Q.; Mukerjee, S.; Chen, S. Recent Insights into the Oxygen-Reduction Electrocatalysis of Fe/N/C Materials. ACS Catal. 2019, 9, 10126–10141. [Google Scholar] [CrossRef]
- He, Y.; Wu, G. PGM-Free Oxygen-Reduction Catalyst Development for Proton-Exchange Membrane Fuel Cells: Challenges, Solutions, and Promises. Acc. Mater. Res. 2022, 3, 224–236. [Google Scholar] [CrossRef]
- Miao, Z.; Li, S.; Priest, C.; Wang, T.; Wu, G.; Li, Q. Effective Approaches for Designing Stable M–N x /C Oxygen-Reduction Catalysts for Proton-Exchange-Membrane Fuel Cells. Adv. Mater. 2022, 34, e2200595. [Google Scholar] [CrossRef]
- Kumar, K.; Dubau, L.; Jaouen, F.; Maillard, F. Review on the Degradation Mechanisms of Metal-N-C Catalysts for the Oxygen Reduction Reaction in Acid Electrolyte: Current Understanding and Mitigation Approaches. Chem. Rev. 2023, 123, 9265–9326. [Google Scholar] [CrossRef]
- Zagal, J.H.; Bedioui, F.; Dodelet, J.-P. (Eds.) N4-Macrocyclic Metal Complexes; Springer New York: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Jasinski, R. A New Fuel Cell Cathode Catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Jahnke, H.; Schönborn, M.; Zimmermann, G. Organic Dyestuffs as Catalysts for Fuel Cells. In Physical and Chemical Applications of Dyestuffs; Springer: Berlin/Heidelberg, Germany, 1976; pp. 133–181. [Google Scholar] [CrossRef]
- Gupta, S.; Tryk, D.; Bae, I.; Aldred, W.; Yeager, E. Heat-Treated Polyacrylonitrile-Based Catalysts for Oxygen Electroreduction. J. Appl. Electrochem. 1989, 19, 19–27. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-Based Cathode Catalyst with Enhanced Power Density in Polymer Electrolyte Membrane Fuel Cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Shui, J.; Chen, C.; Grabstanowicz, L.; Zhao, D.; Liu, D.-J. Highly Efficient Nonprecious Metal Catalyst Prepared with Metal–Organic Framework in a Continuous Carbon Nanofibrous Network. Proc. Natl. Acad. Sci. USA 2015, 112, 10629–10634. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Wan, L.; Zheng, Y.; Qu, X.; Zhang, H.; Wang, Y.; Zaghib, K.; Yuan, J.; Sun, S.; et al. A General Carboxylate-Assisted Approach to Boost the ORR Performance of ZIF-Derived Fe/N/C Catalysts for Proton Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2021, 31, 202009645. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Zachman, M.J.; Zeng, Y.; Yu, H.; Li, B.; Wang, M.; Braaten, J.; Liu, J.; Meyer, H.M.; et al. Atomically Dispersed Iron Sites with a Nitrogen–Carbon Coating as Highly Active and Durable Oxygen Reduction Catalysts for Fuel Cells. Nat. Energy 2022, 7, 652–663. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Yang, P.; Wan, Y.; Wang, D.; Xu, H.; Liu, L.; Xiao, L.; Li, R.; Wang, G.; et al. Atomically Dispersed Fe-N-C Catalyst with Densely Exposed Fe-N4 Active Sites for Enhanced Oxygen Reduction Reaction. Chem. Eng. J. 2024, 485, 149529. [Google Scholar] [CrossRef]
- Cui, J.; Chen, Q.; Li, X.; Zhang, S. Recent Advances in Non-Precious Metal Electrocatalysts for Oxygen Reduction in Acidic Media and PEMFCs: An Activity, Stability and Mechanism Study. Green. Chem. 2021, 23, 6898–6925. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent Advances in Polyaniline Research: Polymerization Mechanisms, Structural Aspects, Properties and Applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A Class of Non-Precious Metal Composite Catalysts for Fuel Cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Z.; Artyushkova, K.; Garzon, F.H.; Zelenay, P. Polyaniline-Derived Non-Precious Catalyst for the Polymer Electrolyte Fuel Cell Cathode. ECS Trans. 2008, 16, 159–170. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, T.; Chen, M.; Feng, H.; Yuan, R.; Zhong, C.; Yan, W.; Tian, Y.; Wu, X.; Chu, W.; et al. High-Purity Pyrrole-Type FeN 4 Sites as a Superior Oxygen Reduction Electrocatalyst. Energy Environ. Sci. 2020, 13, 111–118. [Google Scholar] [CrossRef]
- Wu, G.; Nie, Y. N, S Codoped Iron-Carbon-Based Electrocatalyst for Oxygen Reduction Reaction via Salt Recrystallization Strategy. Chem. Lett. 2021, 50, 124–127. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herrmann-Geppert, I.; Behrends, J.; Lips, K.; Fiechter, S.; Bogdanoff, P. On an Easy Way to Prepare Metal–Nitrogen Doped Carbon with Exclusive Presence of MeN 4-Type Sites Active for the ORR. J. Am. Chem. Soc. 2016, 138, 635–640. [Google Scholar] [CrossRef]
- Ding, W.; Li, L.; Xiong, K.; Wang, Y.; Li, W.; Nie, Y.; Chen, S.; Qi, X.; Wei, Z. Shape Fixing via Salt Recrystallization: A Morphology-Controlled Approach to Convert Nanostructured Polymer to Carbon Nanomaterial as a Highly Active Catalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 5414–5420. [Google Scholar] [CrossRef]
- Zamani, P.; Higgins, D.; Hassan, F.; Jiang, G.; Wu, J.; Abureden, S.; Chen, Z. Electrospun Iron–Polyaniline–Polyacrylonitrile Derived Nanofibers as Non–Precious Oxygen Reduction Reaction Catalysts for PEM Fuel Cells. Electrochim. Acta 2014, 139, 111–116. [Google Scholar] [CrossRef]
- Fu, X.; Zamani, P.; Choi, J.; Hassan, F.M.; Jiang, G.; Higgins, D.C.; Zhang, Y.; Hoque, M.A.; Chen, Z. In Situ Polymer Graphenization Ingrained with Nanoporosity in a Nitrogenous Electrocatalyst Boosting the Performance of Polymer-Electrolyte-Membrane Fuel Cells. Adv. Mater. 2017, 29, 1604456. [Google Scholar] [CrossRef]
- Serov, A.; Workman, M.J.; Artyushkova, K.; Atanassov, P.; McCool, G.; McKinney, S.; Romero, H.; Halevi, B.; Stephenson, T. Highly Stable Precious Metal-Free Cathode Catalyst for Fuel Cell Application. J. Power Sources 2016, 327, 557–564. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Atanassov, P. Fe-N-C Oxygen Reduction Fuel Cell Catalyst Derived from Carbendazim: Synthesis, Structure, and Reactivity. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Wu, R.; Song, Y.; Huang, X.; Chen, S.; Ibraheem, S.; Deng, J.; Li, J.; Qi, X.; Wei, Z. High-density active sites porous Fe/N/C electrocatalyst boosting the performance of proton exchange membrane fuel cells. J. Power Sources 2018, 401, 287–295. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Kim, K.; Kim, S.; Lee, S.; Han, J.W.; Lee, J. Versatile Strategy for Tuning ORR Activity of a Single Fe-N 4 Site by Controlling Electron-Withdrawing/Donating Properties of a Carbon Plane. J. Am. Chem. Soc. 2019, 141, 6254–6262. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically Dispersed Metal–Nitrogen–Carbon Catalysts for Fuel Cells: Advances in Catalyst Design, Electrode Performance, and Durability Improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Mao, X.; Liu, D.; He, W.; Li, J.; Liu, J.; Yan, X.; Chen, J.; Song, L.; et al. Edge-Rich Fe−N 4 Active Sites in Defective Carbon for Oxygen Reduction Catalysis. Adv. Mater. 2020, 32, 202000966. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Lin, H.; Gupta, S.; Wang, C.; Tao, Z.; Fu, H.; Wang, T.; Zheng, J.; Wu, G.; et al. Directly Converting Fe-Doped Metal–Organic Frameworks into Highly Active and Stable Fe-N-C Catalysts for Oxygen Reduction in Acid. Nano Energy 2016, 25, 110–119. [Google Scholar] [CrossRef]
- Wang, G.; Deng, J.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Turning on Electrocatalytic Oxygen Reduction by Creating Robust Fe–N x Species in Hollow Carbon Frameworks via in Situ Growth of Fe Doped ZIFs on g-C 3 N 4. Nanoscale 2020, 12, 5601–5611. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zheng, L.; Shang, J.; Wan, X.; Hu, R.; Guo, X.; Hong, S.; Shui, J. Preparation of Fe–N–C Catalysts with FeN x (x = 1, 3, 4) Active Sites and Comparison of Their Activities for the Oxygen Reduction Reaction and Performances in Proton Exchange Membrane Fuel Cells. J. Mater. Chem. A Mater. 2019, 7, 26147–26153. [Google Scholar] [CrossRef]
- Mehmood, A.; Gong, M.; Jaouen, F.; Roy, A.; Zitolo, A.; Khan, A.; Sougrati, M.-T.; Primbs, M.; Bonastre, A.M.; Fongalland, D.; et al. High Loading of Single Atomic Iron Sites in Fe–NC Oxygen Reduction Catalysts for Proton Exchange Membrane Fuel Cells. Nat. Catal. 2022, 5, 311–323. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Sun, F.; Chen, G.; Wang, J.; Peng, L.; Chen, W.; Shang, L.; Zhao, J.; Sun-Waterhouse, D.; et al. Molten NaCl-Assisted Synthesis of Porous Fe-N-C Electrocatalysts with a High Density of Catalytically Accessible FeN 4 Active Sites and Outstanding Oxygen Reduction Reaction Performance. Adv. Energy Mater. 2021, 11. [Google Scholar] [CrossRef]
- Wiesener, K. N4-Chelates as Electrocatalyst for Cathodic Oxygen Reduction. Electrochim. Acta 1986, 31, 1073–1078. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, D.; Liu, W.; Li, X.; Chen, Q. Fluorine Enhanced Pyridinic-N Configuration as an Ultra-Active Site for Oxygen Reduction Reaction in Both Alkaline and Acidic Electrolytes. Carbon N. Y. 2022, 187, 67–77. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wei, D.; Li, M. A “MOF-Protective-Pyrolysis” Strategy for the Preparation of Fe–N–C Catalysts and the Role of Fe, N, and C in the Oxygen Reduction Reaction in Acidic Medium. ACS Appl. Mater. Interfaces 2019, 11, 35755–35763. [Google Scholar] [CrossRef]
- Tran, T.-N.; Shin, C.-H.; Lee, B.-J.; Samdani, J.S.; Park, J.-D.; Kang, T.-H.; Yu, J.-S. Fe–N-Functionalized Carbon Electrocatalyst Derived from a Zeolitic Imidazolate Framework for Oxygen Reduction: Fe and NH3 Treatment Effects. Catal. Sci. Technol. 2018, 8, 5368–5381. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, X.; Pan, C.; Xiang, X.; Hao, C.; Meng, Q.; Tian, Z.Q.; Shen, P.K.; Jiang, S.P. N Species Tuning Strategy in N, S Co-Doped Graphene Nanosheets for Electrocatalytic Activity and Selectivity of Oxygen Redox Reactions. Chem. Eng. J. 2022, 431, 133216. [Google Scholar] [CrossRef]
- Wei, J.; Liang, Y.; Hu, Y.; Kong, B.; Simon, G.P.; Zhang, J.; Jiang, S.P.; Wang, H. A Versatile Iron–Tannin-Framework Ink Coating Strategy to Fabricate Biomass-Derived Iron Carbide/Fe-N-Carbon Catalysts for Efficient Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Wu, Y.; Liang, Z.; Guo, W.; Zhi, C.; Qu, C.; Gao, S.; Zhu, B.; Zhang, H.; Zou, R. Fabricating Hierarchically Porous and Fe3C-Embeded Nitrogen-Rich Carbon Nanofibers as Exceptional Electocatalysts for Oxygen Reduction. Carbon. N. Y 2019, 142, 115–122. [Google Scholar] [CrossRef]
- Jiang, R.; Li, L.; Sheng, T.; Hu, G.; Chen, Y.; Wang, L. Edge-Site Engineering of Atomically Dispersed Fe–N 4 by Selective C–N Bond Cleavage for Enhanced Oxygen Reduction Reaction Activities. J. Am. Chem. Soc. 2018, 140, 11594–11598. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow Spheres of Iron Carbide Nanoparticles Encased in Graphitic Layers as Oxygen Reduction Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.-T.; Jaouen, F.; Mukerjee, S. Highly Active Oxygen Reduction Non-Platinum Group Metal Electrocatalyst without Direct Metal–Nitrogen Coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herranz, J.; Larouche, N.; Arruda, T.M.; Lefèvre, M.; Jaouen, F.; Bogdanoff, P.; Fiechter, S.; Abs-Wurmbach, I.; Mukerjee, S.; et al. Structure of the Catalytic Sites in Fe/N/C-Catalysts for O2-Reduction in PEM Fuel Cells. Phys. Chem. Chem. Phys. 2012, 14, 11673. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Auerbach, H.; Tait, C.E.; Martinaiou, I.; Kumar, S.C.N.; Kübel, C.; Sergeev, I.; Wille, H.; Behrends, J.; Wolny, J.A.; et al. Elucidating the Structural Composition of an Fe–N–C Catalyst by Nuclear- and Electron-Resonance Techniques. Angew. Chem. Int. Ed. 2019, 58, 10486–10492. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, Z. Identifying the Impact of the Covalent-Bonded Carbon Matrix to FeN4 Sites for Acidic Oxygen Reduction. Nat. Commun. 2022, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Seo, B.; Wang, B.; Zamel, N.; Jiao, K.; Adroher, X.C. Fundamentals, Materials, and Machine Learning of Polymer Electrolyte Membrane Fuel Cell Technology. Energy AI 2020, 1, 100014. [Google Scholar] [CrossRef]
- Osmieri, L.; Meyer, Q. Recent Advances in Integrating Platinum Group Metal-Free Catalysts in Proton Exchange Membrane Fuel Cells. Curr. Opin. Electrochem. 2022, 31, 100847. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.; Kishimoto, T.; Imashiro, Y. A Review of the Stability and Durability of Non-Precious Metal Catalysts for the Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J.J. Degradation Mechanisms of Pt/C Fuel Cell Catalysts under Simulated Start–Stop Conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J.J. Oxygen Electrochemistry as a Cornerstone for Sustainable Energy Conversion. Angew. Chem. Int. Ed. 2014, 53, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Martinaiou, I. Degradation Studies of Me-N-C Catalysts for the Oxygen Reduction Reaction in Fuel Cells. Ph.D. Thesis, Technische Universität Darmstadt, Darmstadt, Germany, 2018. [Google Scholar]
- Calle-Vallejo, F.; Martínez, J.I.; Rossmeisl, J. Density Functional Studies of Functionalized Graphitic Materials with Late Transition Metals for Oxygen Reduction Reactions. Phys. Chem. Chem. Phys. 2011, 13, 15639. [Google Scholar] [CrossRef]
- Dy, E.S.; Roman, T.A.; Kubota, Y.; Miyamoto, K.; Kasai, H. Exploring Haem-Based Alternatives for Oxygen Reduction Catalysis in Fuel Cells—A Status Report of Our First Principles Calculations. J. Phys. Condens. Matter 2007, 19, 445010. [Google Scholar] [CrossRef]
- Chenitz, R.; Kramm, U.I.; Lefèvre, M.; Glibin, V.; Zhang, G.; Sun, S.; Dodelet, J.-P. A Specific Demetalation of Fe–N 4 Catalytic Sites in the Micropores of NC_Ar + NH 3 Is at the Origin of the Initial Activity Loss of the Highly Active Fe/N/C Catalyst Used for the Reduction of Oxygen in PEM Fuel Cells. Energy Environ. Sci. 2018, 11, 365–382. [Google Scholar] [CrossRef]
- Martinaiou, I.; Monteverde Videla, A.H.A.; Weidler, N.; Kübler, M.; Wallace, W.D.Z.; Paul, S.; Wagner, S.; Shahraei, A.; Stark, R.W.; Specchia, S.; et al. Activity and Degradation Study of an Fe-N-C Catalyst for ORR in Direct Methanol Fuel Cell (DMFC). Appl. Catal. B 2020, 262, 118217. [Google Scholar] [CrossRef]
- Kocha, S.S. Electrochemical Degradation. In Polymer Electrolyte Fuel Cell Degradation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 89–214. [Google Scholar] [CrossRef]
- Martinaiou, I.; Shahraei, A.; Grimm, F.; Zhang, H.; Wittich, C.; Klemenz, S.; Dolique, S.J.; Kleebe, H.-J.; Stark, R.W.; Kramm, U.I. Effect of Metal Species on the Stability of Me-N-C Catalysts during Accelerated Stress Tests Mimicking the Start-up and Shut-down Conditions. Electrochim. Acta 2017, 243, 183–196. [Google Scholar] [CrossRef]
- Meyer, Q.; Liu, S.; Li, Y.; Zhao, C. Operando Detection of Oxygen Reduction Reaction Kinetics of Fe–N–C Catalysts in Proton Exchange Membrane Fuel Cells. J. Power Sources 2022, 533, 231058. [Google Scholar] [CrossRef]
- Liu, S.; Meyer, Q.; Jia, C.; Wang, S.; Rong, C.; Nie, Y.; Zhao, C. Operando Deconvolution of the Degradation Mechanisms of Iron–Nitrogen–Carbon Catalysts in Proton Exchange Membrane Fuel Cells. Energy Environ. Sci. 2023, 16, 3792–3802. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Jiang, Q.-Z.; Sun, L.; Yuan, X.; Shao, Z.; Ma, Z.-F. 3D Non-Precious Metal-Based Electrocatalysts for the Oxygen Reduction Reaction in Acid Media. Int. J. Hydrogen Energy 2010, 35, 8295–8302. [Google Scholar] [CrossRef]
- Kramm, U.I.; Abs-Wurmbach, I.; Herrmann-Geppert, I.; Radnik, J.; Fiechter, S.; Bogdanoff, P. Influence of the Electron-Density of FeN[Sub 4]-Centers Towards the Catalytic Activity of Pyrolyzed FeTMPPCl-Based ORR-Electrocatalysts. J Electrochem Soc 2011, 158, B69. [Google Scholar] [CrossRef]
- Herranz, J.; Jaouen, F.; Lefèvre, M.; Kramm, U.I.; Proietti, E.; Dodelet, J.-P.; Bogdanoff, P.; Fiechter, S.; Abs-Wurmbach, I.; Bertrand, P.; et al. Unveiling N-Protonation and Anion-Binding Effects on Fe/N/C Catalysts for O 2 Reduction in Proton-Exchange-Membrane Fuel Cells. J. Phys. Chem. C 2011, 115, 16087–16097. [Google Scholar] [CrossRef]
- Jaouen, F.; Jones, D.; Coutard, N.; Artero, V.; Strasser, P.; Kucernak, A. Toward Platinum Group Metal-Free Catalysts for Hydrogen/Air Proton-Exchange Membrane Fuel Cells. Johns. Matthey Technol. Rev. 2018, 62, 231–255. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Sui, S.; Sun, T.; Yan, Y.; Du, S. Cathode Design for Proton Exchange Membrane Fuel Cells in Automotive Applications. Automot. Innov. 2021, 4, 144–164. [Google Scholar] [CrossRef]
- Lopez-Haro, M.; Guétaz, L.; Printemps, T.; Morin, A.; Escribano, S.; Jouneau, P.-H.; Bayle-Guillemaud, P.; Chandezon, F.; Gebel, G. Three-Dimensional Analysis of Nafion Layers in Fuel Cell Electrodes. Nat. Commun. 2014, 5, 5229. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Osmieri, L.; Star, A.G.; Pfeilsticker, J.; Neyerlin, K.C. Elucidating the Role of Ionomer in the Performance of Platinum Group Metal-Free Catalyst Layer via in Situ Electrochemical Diagnostics. J. Electrochem. Soc. 2020, 167, 044519. [Google Scholar] [CrossRef]
- Zhao, Z.; Hossain, M.D.; Xu, C.; Lu, Z.; Liu, Y.-S.; Hsieh, S.-H.; Lee, I.; Gao, W.; Yang, J.; Merinov, B.V.; et al. Tailoring a Three-Phase Microenvironment for High-Performance Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Matter 2020, 3, 1774–1790. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef]
- Osmieri, L.; Wang, H.; Neyerlin, K.C. Impact of Fabrication and Testing Parameters on the Performance of a Polymer Electrolyte Fuel Cell with Platinum Group Metal (PGM)-Free Cathode Catalyst. J. Electrochem. Soc. 2021, 168, 014503. [Google Scholar] [CrossRef]
- Liu, J.; Talarposhti, M.R.; Asset, T.; Sabarirajan, D.C.; Parkinson, D.Y.; Atanassov, P.; Zenyuk, I.V. Understanding the Role of Interfaces for Water Management in Platinum Group Metal-Free Electrodes in Polymer Electrolyte Fuel Cells. ACS Appl. Energy Mater. 2019, 2, 3542–3553. [Google Scholar] [CrossRef]
- Jankovic, J.; Zhang, S.; Putz, A.; Saha, M.S.; Susac, D. Multiscale Imaging and Transport Modeling for Fuel Cell Electrodes. J. Mater. Res. 2019, 34, 579–591. [Google Scholar] [CrossRef]
- Pavlicek, R.; Barton, S.C.; Leonard, N.; Romero, H.; McKinney, S.; McCool, G.; Serov, A.; Abbott, D.; Atanassov, P.; Mukerjee, S. Resolving Challenges of Mass Transport in Non Pt-Group Metal Catalysts for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, F589–F596. [Google Scholar] [CrossRef]
- Stariha, S.; Artyushkova, K.; Workman, M.J.; Serov, A.; Mckinney, S.; Halevi, B.; Atanassov, P. PGM-Free Fe-N-C Catalysts for Oxygen Reduction Reaction: Catalyst Layer Design. J. Power Sources 2016, 326, 43–49. [Google Scholar] [CrossRef]
- Lopes, T.; Kucernak, A.; Malko, D.; Ticianelli, E.A. Mechanistic Insights into the Oxygen Reduction Reaction on Metal–N–C Electrocatalysts under Fuel Cell Conditions. ChemElectroChem 2016, 3, 1580–1590. [Google Scholar] [CrossRef]
- Serov, A.; Shum, A.D.; Xiao, X.; De Andrade, V.; Artyushkova, K.; Zenyuk, I.V.; Atanassov, P. Nano-Structured Platinum Group Metal-Free Catalysts and Their Integration in Fuel Cell Electrode Architectures. Appl. Catal. B 2018, 237, 1139–1147. [Google Scholar] [CrossRef]
- Normile, S.J.; Sabarirajan, D.C.; Calzada, O.; De Andrade, V.; Xiao, X.; Mandal, P.; Parkinson, D.Y.; Serov, A.; Atanassov, P.; Zenyuk, I.V. Direct Observations of Liquid Water Formation at Nano- and Micro-Scale in Platinum Group Metal-Free Electrodes by Operando X-ray Computed Tomography. Mater. Today Energy 2018, 9, 187–197. [Google Scholar] [CrossRef]
- Toudret, P.; Blachot, J.-F.; Heitzmann, M.; Jacques, P.-A. Impact of the Cathode Layer Printing Process on the Performance of MEA Integrating PGM Free Catalyst. Catalysts 2021, 11, 669. [Google Scholar] [CrossRef]
- Yin, X.; Lin, L.; Chung, H.T.; Babu, S.K.; Martinez, U.; Purdy, G.M.; Zelenay, P. Effects of MEA Fabrication and Ionomer Composition on Fuel Cell Performance of PGM-Free ORR Catalyst. ECS Trans. 2017, 77, 1273. [Google Scholar] [CrossRef]
- Baricci, A.; Bisello, A.; Serov, A.; Odgaard, M.; Atanassov, P.; Casalegno, A. Analysis of the Effect of Catalyst Layer Thickness on the Performance and Durability of Platinum Group Metal-Free Catalysts for Polymer Electrolyte Membrane Fuel Cells. Sustain. Energy Fuels 2019, 3, 3375–3386. [Google Scholar] [CrossRef]
- Artyushkova, K.; Habel-Rodriguez, D.; Olson, T.S.; Atanassov, P. Optimization of Ink Composition Based on a Non-Platinum Cathode for Single Membrane Electrode Assembly Proton Exchange Membrane Fuel Cells. J. Power Sources 2013, 226, 112–121. [Google Scholar] [CrossRef]
- Stariha, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Non-PGM Membrane Electrode Assemblies: Optimization for Performance. Int. J. Hydrogen Energy 2015, 40, 14676–14682. [Google Scholar] [CrossRef]
- Uddin, A.; Dunsmore, L.; Zhang, H.; Hu, L.; Wu, G.; Litster, S. High Power Density Platinum Group Metal-Free Cathodes for Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 2216–2224. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct Atomic-Level Insight into the Active Sites of a High-Performance PGM-Free ORR Catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]
- Komini Babu, S.; Chung, H.T.; Zelenay, P.; Litster, S. Resolving Electrode Morphology’s Impact on Platinum Group Metal-Free Cathode Performance Using Nano-CT of 3D Hierarchical Pore and Ionomer Distribution. ACS Appl. Mater. Interfaces 2016, 8, 32764–32777. [Google Scholar] [CrossRef]
- Malko, D.; Lopes, T.; Ticianelli, E.A.; Kucernak, A. A Catalyst Layer Optimisation Approach Using Electrochemical Impedance Spectroscopy for PEM Fuel Cells Operated with Pyrolysed Transition Metal-N-C Catalysts. J. Power Sources 2016, 323, 189–200. [Google Scholar] [CrossRef]
- Osmieri, L.; Mauger, S.; Ulsh, M.; Neyerlin, K.C.; Bender, G. Use of a Segmented Cell for the Combinatorial Development of Platinum Group Metal-Free Electrodes for Polymer Electrolyte Fuel Cells. J. Power Sources 2020, 452, 227829. [Google Scholar] [CrossRef]
- Osmieri, L.; Wang, G.; Cetinbas, F.C.; Khandavalli, S.; Park, J.; Medina, S.; Mauger, S.A.; Ulsh, M.; Pylypenko, S.; Myers, D.J.; et al. Utilizing Ink Composition to Tune Bulk-Electrode Gas Transport, Performance, and Operational Robustness for a Fe–N–C Catalyst in Polymer Electrolyte Fuel Cell. Nano Energy 2020, 75, 104943. [Google Scholar] [CrossRef]
- Khandavalli, S.; Iyer, R.; Park, J.H.; Myers, D.J.; Neyerlin, K.C.; Ulsh, M.; Mauger, S.A. Effect of Dispersion Medium Composition and Ionomer Concentration on the Microstructure and Rheology of Fe–N–C Platinum Group Metal-Free Catalyst Inks for Polymer Electrolyte Membrane Fuel Cells. Langmuir 2020, 36, 12247–12260. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Kishimoto, T.; Zhou, Y.; Sato, T.; Bai, K.; Ozaki, J.; Imashiro, Y.; Ye, S. Critical Advancements in Achieving High Power and Stable Nonprecious Metal Catalyst–Based MEAs for Real-World Proton Exchange Membrane Fuel Cell Applications. Sci. Adv. 2018, 4, eaar7180. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Kishimoto, T.; Sato, T.; Kobayashi, Y.; Narizuka, K.; Ozaki, J.; Zhou, Y.; Marquez, E.; Bai, K.; Ye, S. New Insights into Non-Precious Metal Catalyst Layer Designs for Proton Exchange Membrane Fuel Cells: Improving Performance and Stability. J. Power Sources 2017, 344, 39–45. [Google Scholar] [CrossRef]
- Toudret, P.; Wolanin, J.; Gebel, G.; Morin, A. Investigation of Ionomer Hydration and Local Relative Humidity in Platinum and Non-Noble Based Catalyst Layers in Proton Exchange Membrane Fuel Cells Using SAXS. J. Power Sources 2023, 586, 233671. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Mao, Z.; Gao, X.; Marquez, E.; Choi, J.-Y.; Chen, Z. Toward Practical Applications in Proton Exchange Membrane Fuel Cells with Gram-Scale PGM-Free Catalysts. J. Power Sources 2023, 586, 233534. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinaiou, I.; Daletou, M.K. Enhancing Electrode Efficiency in Proton Exchange Membrane Fuel Cells with PGM-Free Catalysts: A Mini Review. Energies 2024, 17, 3443. https://doi.org/10.3390/en17143443
Martinaiou I, Daletou MK. Enhancing Electrode Efficiency in Proton Exchange Membrane Fuel Cells with PGM-Free Catalysts: A Mini Review. Energies. 2024; 17(14):3443. https://doi.org/10.3390/en17143443
Chicago/Turabian StyleMartinaiou, Ioanna, and Maria K. Daletou. 2024. "Enhancing Electrode Efficiency in Proton Exchange Membrane Fuel Cells with PGM-Free Catalysts: A Mini Review" Energies 17, no. 14: 3443. https://doi.org/10.3390/en17143443





