Investigation of Arc Dynamic Behavior Change Induced by Various Parameter Configurations for C4F7N/CO2 Gas Mixture
Abstract
:1. Introduction
2. Modified Arc Model for C4F7N/CO2 Gas Mixture
2.1. Two-Dimensional MHD-Based Mathematical Arc Model
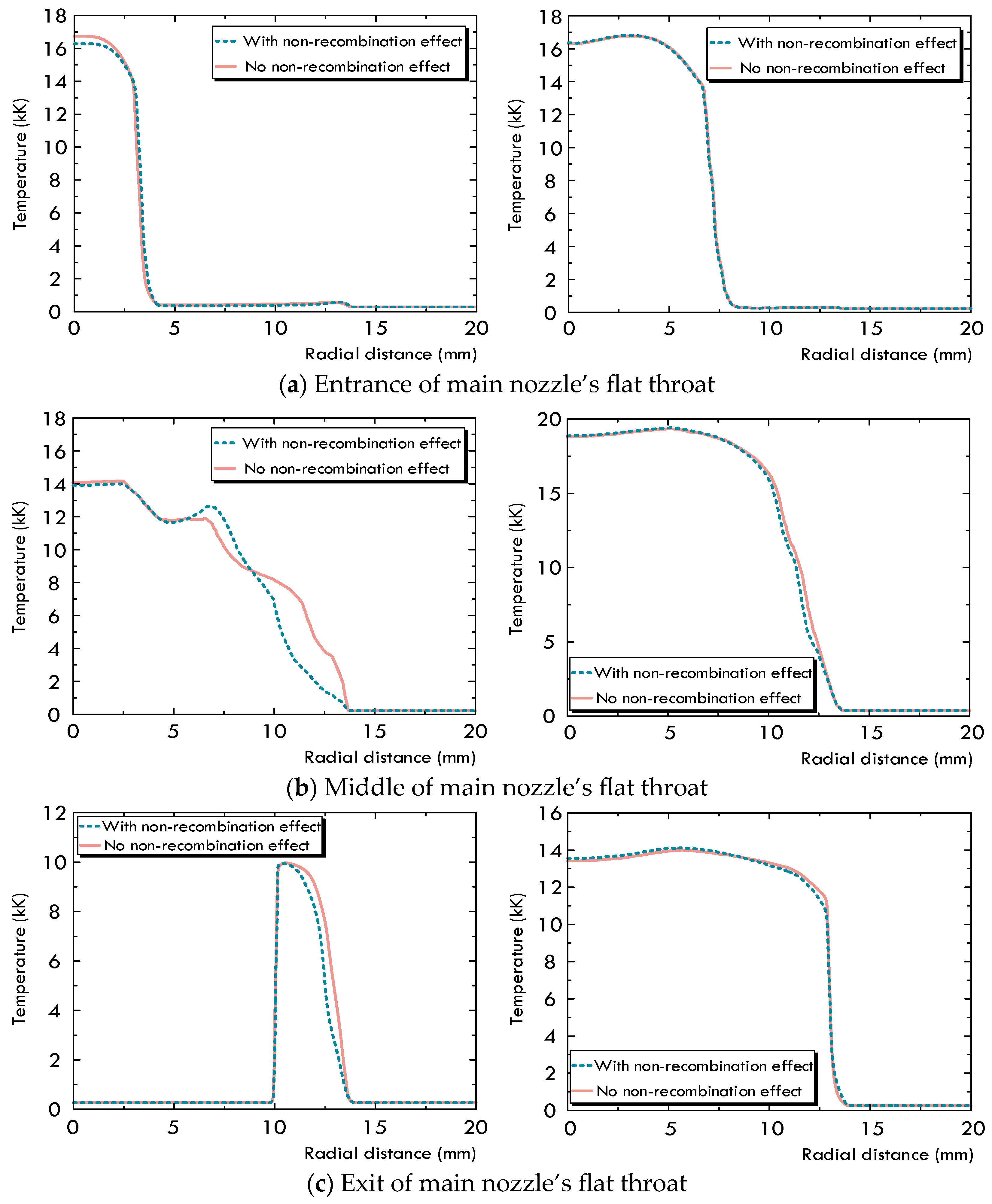
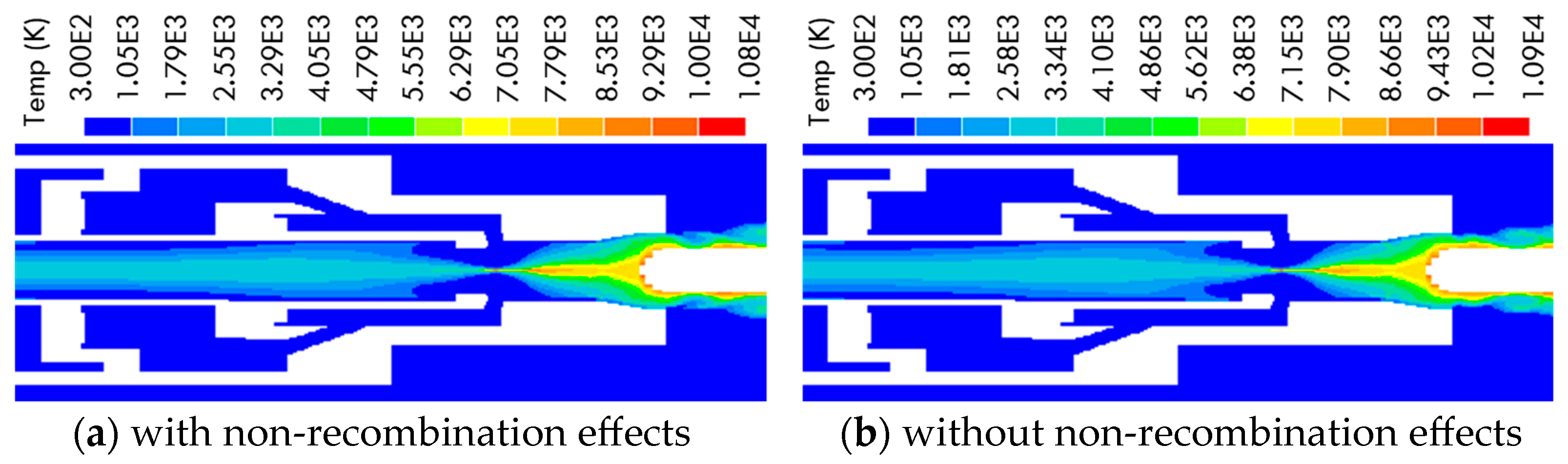
2.2. Difference of Arc Characteristics Caused by the Non-Recombination of C4F7N
3. Effects of Configuration Change on Arc Characteristics for C4F7N/CO2 Gas Mixture
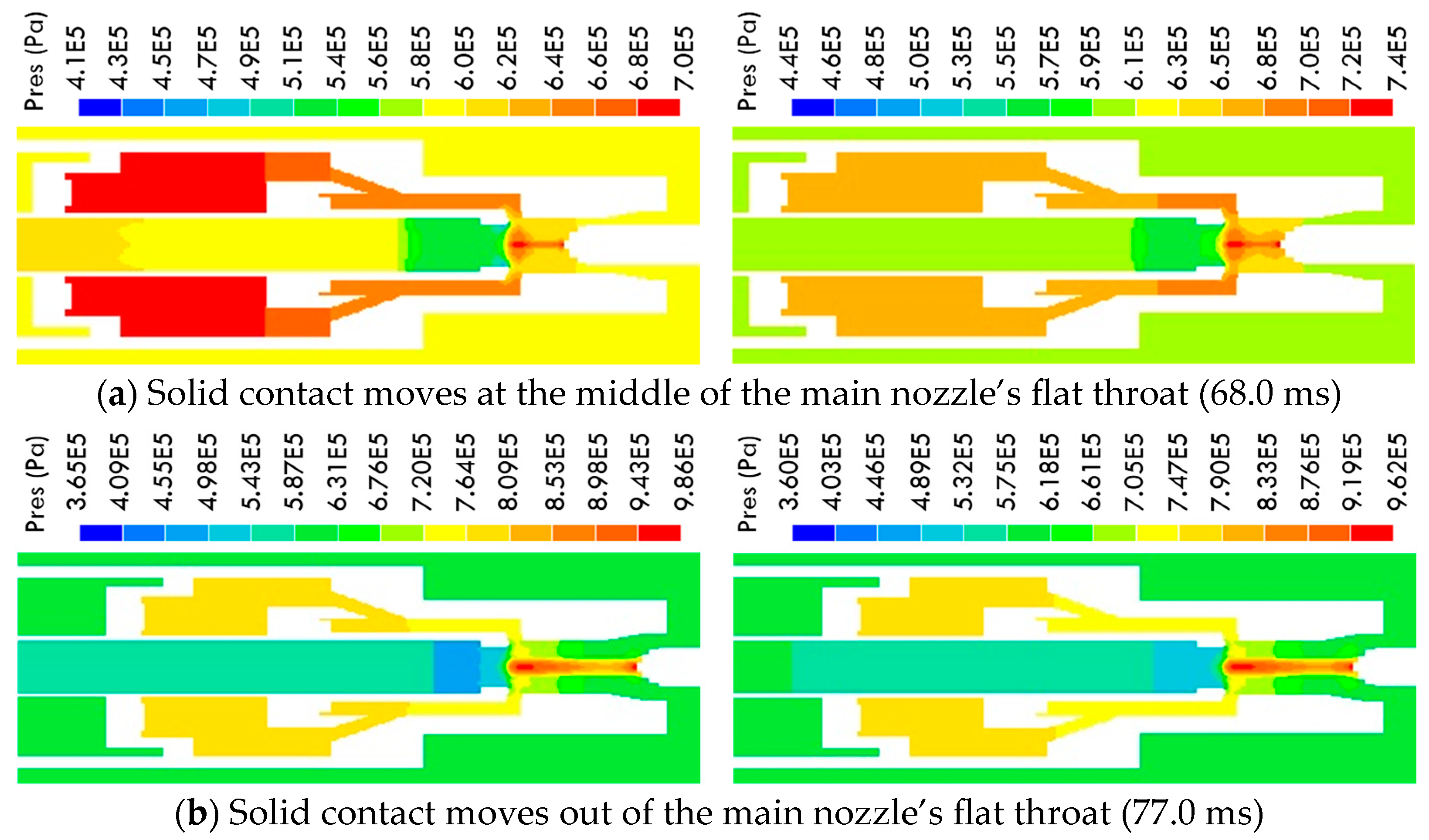
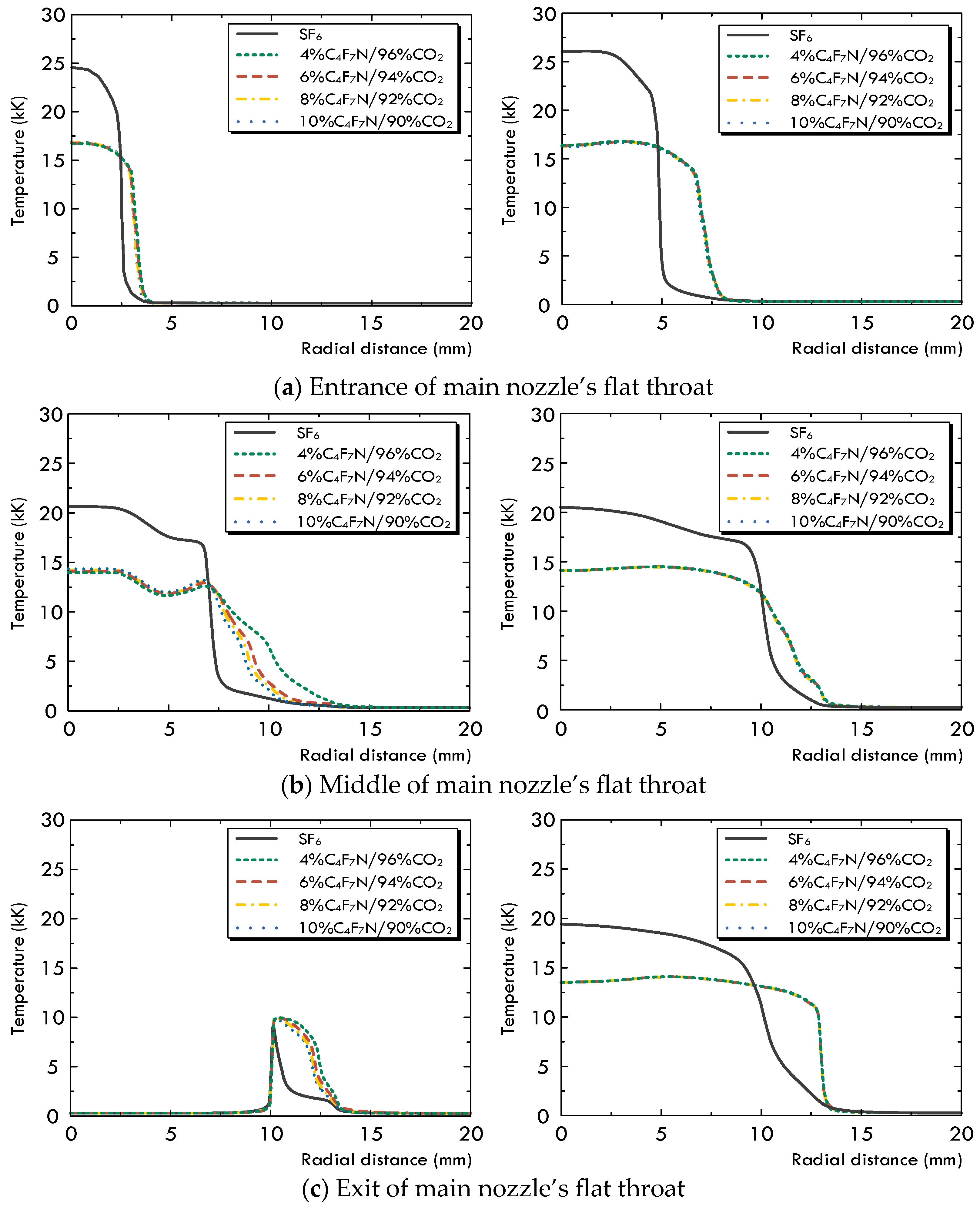
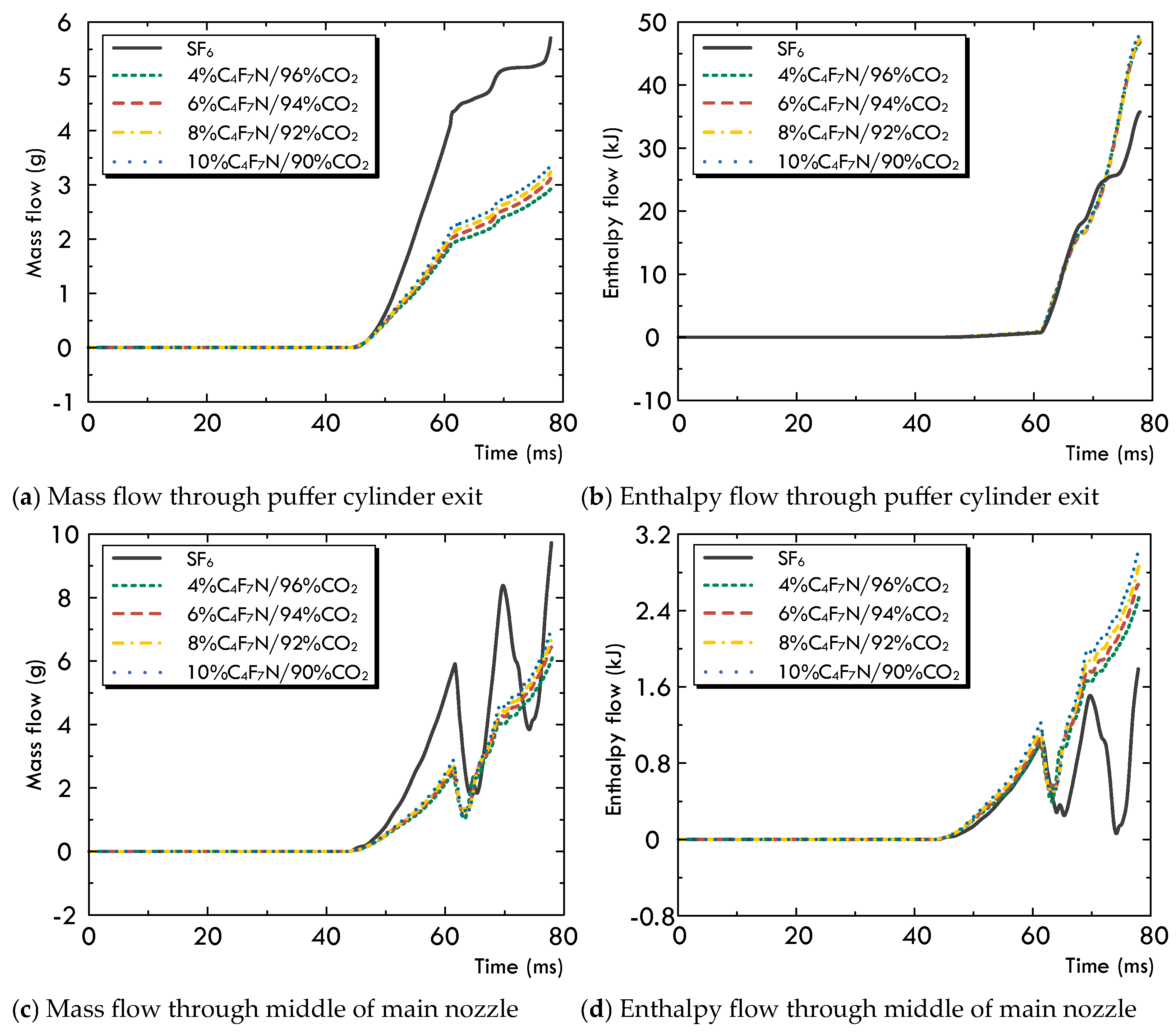
3.1. Variation in the Multi-Physical Fields under High-Current Phase
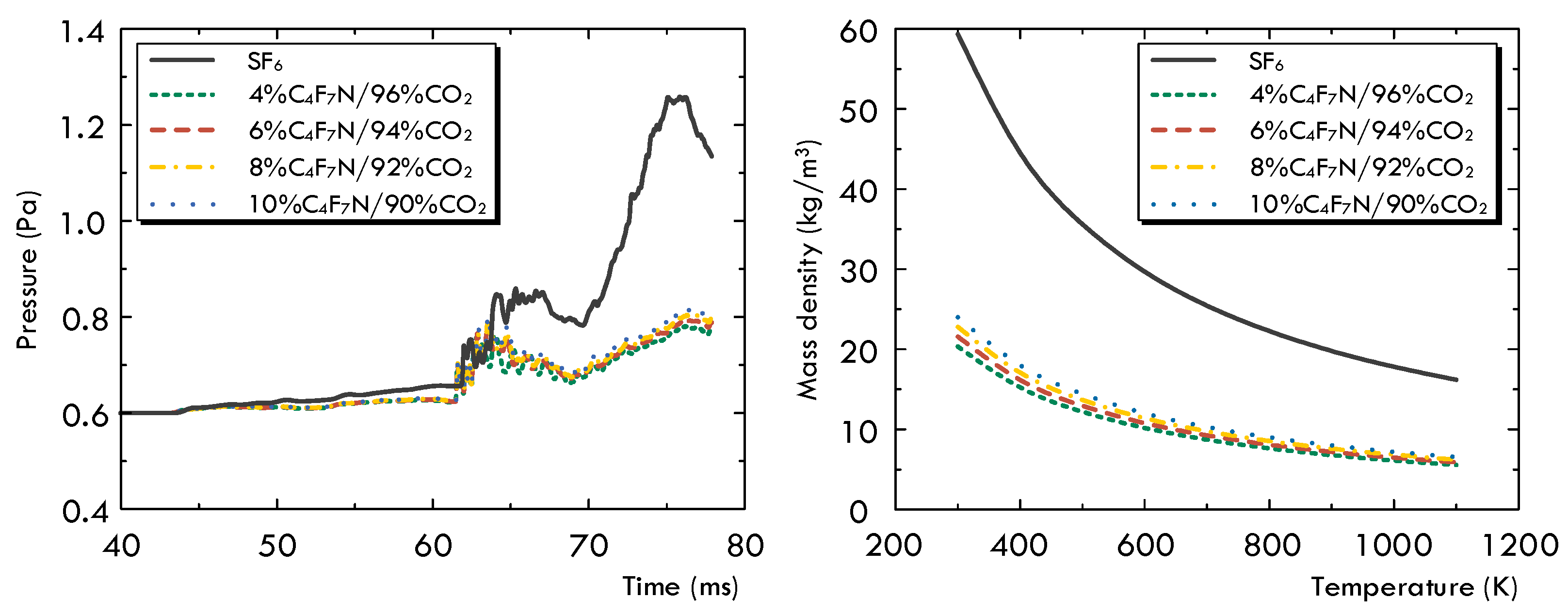
3.2. Arc Characteristics under Current Zero Phase
4. Conclusions
- (1)
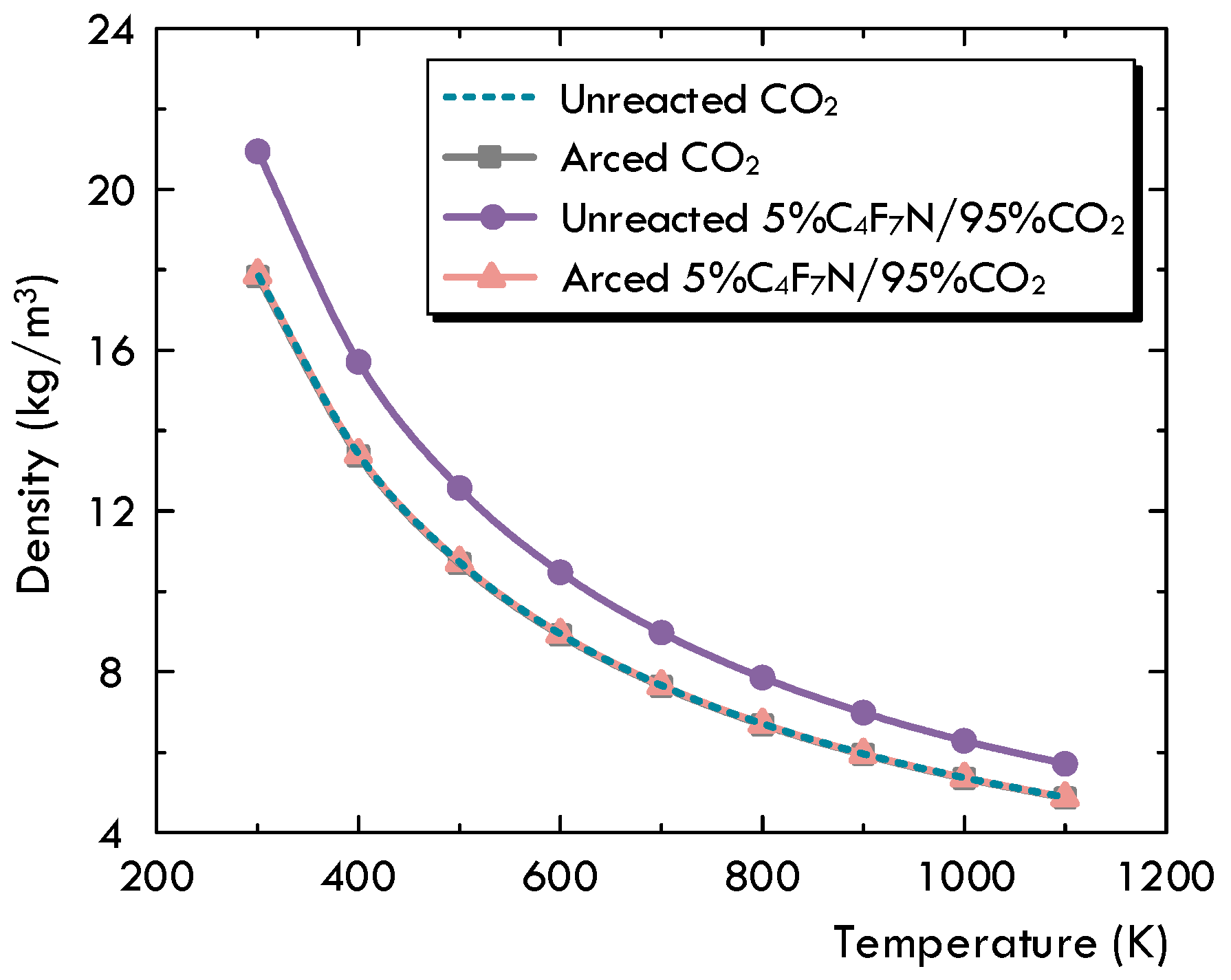
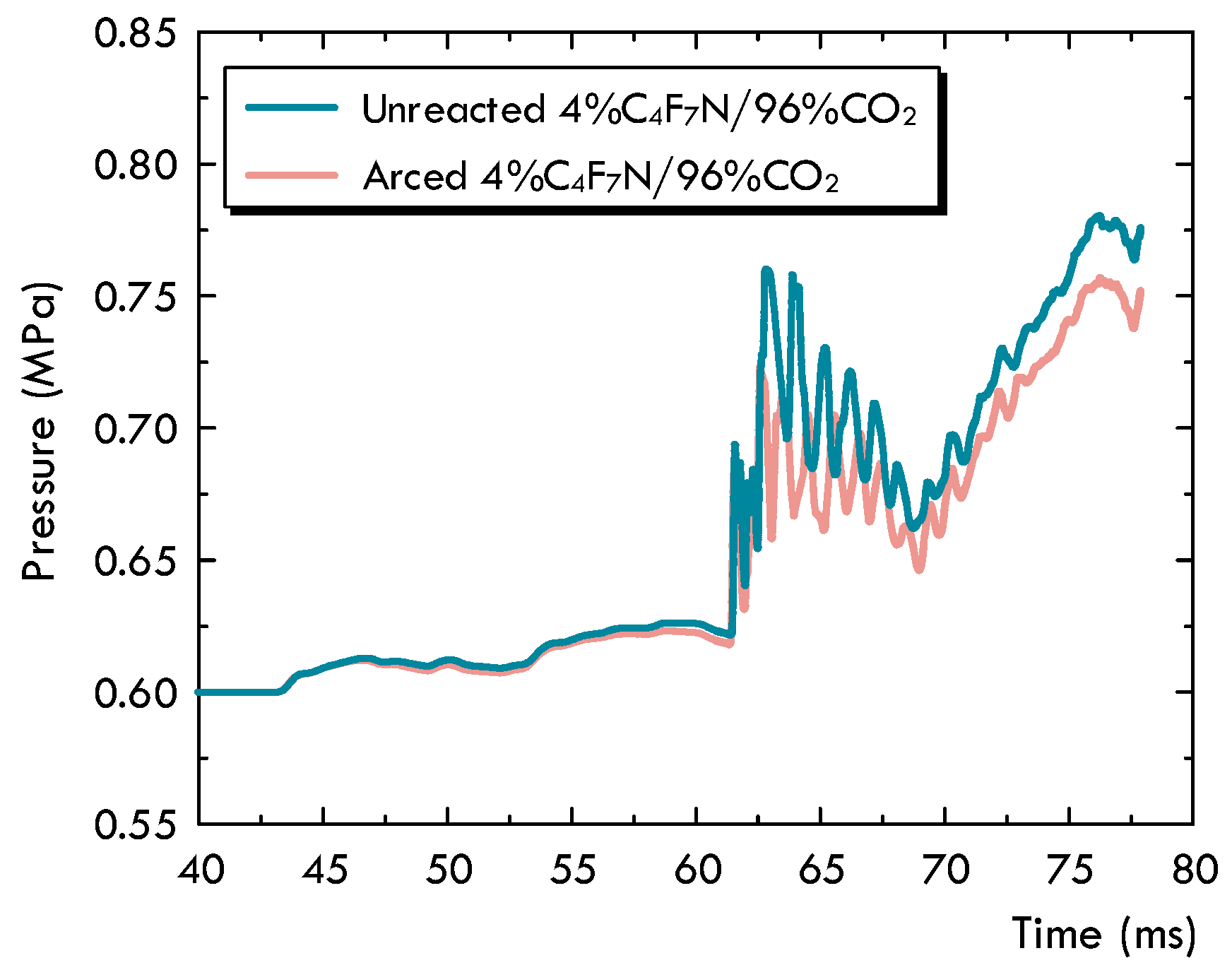
- Based on the decomposition characteristics for the C4F7N and C4F7N/CO2 gas mixture, the existing MHD-based two-dimensional arc model is innovatively modified to handle the non-recombination feature of C4F7N. It is proposed to differentiate the unreacted gas mixture, as initially filled in a circuit breaker without experiencing any significant dissociation, from the arced gas mixture, which is referred to as the LTE-LCE gas mixture. A species concentration equation is solved to keep track of the concentration of the arced gas mixture, and a method is advised to allow the transfer between the two sets of material properties. From the comparison, it is found that the most noticeable impact of the gas’s non-recombination feature is the pressurization inside the puffer cylinder. With this consideration, the pressure for the cold gas becomes higher, and it will definitely establish better distributions of the flow field conditions during the arcing process.
- (2)
- The diffusing, multi-physical field characteristics and flow field conditions of the arc for the C4F7N/CO2 gas mixture are slightly affected by changing the volume concentration of C4F7N. An increase from 4% to 10% leads to a slightly higher pressure and larger mass and enthalpy fluxes through the arcing chamber, which improve its corresponding arc-extinguishing performance.
- (3)
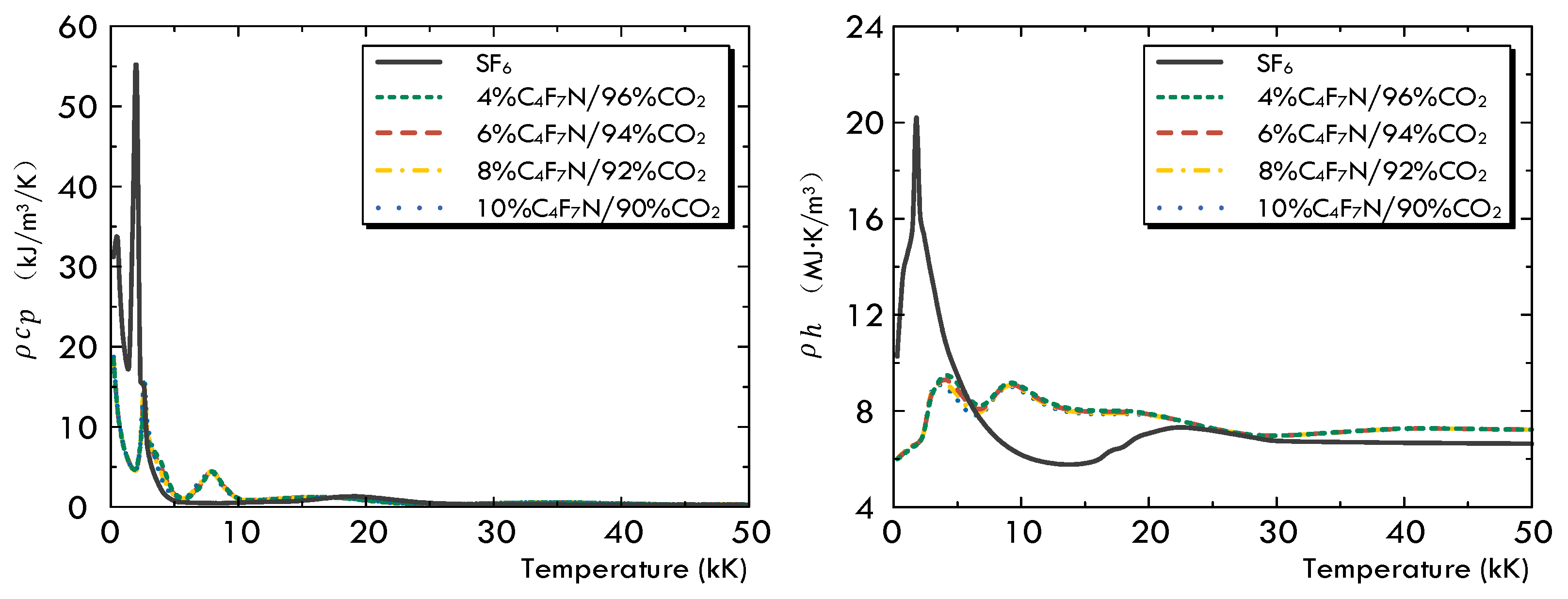
- In comparison with SF6 under the same operation conditions, the first difference is that the pressurization effect of the SF6 gas inside the upstream puffer cylinder is significantly superior to that of the C4F7N/CO2 gas mixture. Secondly, due to the noticeable higher values of both and at low temperatures, the arc diffusion in the SF6 gas atmosphere is well controlled both in the axial and radial directions. In the C4F7N/CO2 gas mixture, the longer axial diffusion of the arc significantly deteriorates its post-arc recovery characteristics. This indicates that using the C4F7N/CO2 gas mixture as an arc-extinguishing medium requires the optimization of the mechanical characteristics of the driving mechanism, such as increasing its moving velocity, as well as improvements in the design of the puffer cylinder or nozzle geometry of the arc chamber.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Intergovernmental Panel on Climate Change (IPCC). FAR Climate Change: Scientific Assessment of Climate Change; IPCC: Geneva, Switzerland, 2010. [Google Scholar]
- Forster, P.; Storelvmo, T.; Armour, K.; Collins, W.; Dufresne, J.-L.; Frame, D.; Lunt, D.J.; Mauritsen, T.; Palmer, M.D.; Watanabe, M. IPCC Sixth Assessment Report Working Group I: The Physical Science Basis—Chapter 07: The Earth’s Energy Budget, Climate Feedbacks, and Climate Sensitivity; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Mantilla, J.; Claessens, M.; Kriegel, M. Environmentally Friendly Perfluoroketones-Based Mixture as Switching Medium in High Voltage Circuit Breakers; A3-113; CIGRE: Paris, France, 2016. [Google Scholar]
- Gautschi, D.; Ficheux, A.; Walter, M.; Vuachet, J. Application of a Fluoronitrile Gas in GIS and GIL as an Environmental Friendly Alternative to SF6; B3-106; CIGRE: Paris, France, 2016. [Google Scholar]
- Zhang, B.Y.; Zhou, R.; Wang, K.; Guo, Z.; Li, X.W.; Cao, M.C.; Deng, J.W.; Wang, D.B. Arc Interruption Performance of C4F7N-CO2 Mixture in a 126 kV Disconnector. IEEE Trans. Power Deliv. 2023, 38, 1197–1207. [Google Scholar] [CrossRef]
- Uchii, T.; Shinkai, T.; Suzuki, K. Thermal interruption capability of carbon dioxide in a puffer-type circuit breaker utilizing polymer ablation. In Proceedings of the IEEE/PES Transmission and Distribution Conference and Exhibition, Yokohama, Japan, 6–10 October 2002; pp. 1750–1754. [Google Scholar]
- Meijer, S.; Smit, J.J.; Girodet, A. Comparison of the breakdown strength of N2, CO2 and SF6 using the extended up-and-down method. In Proceedings of the IEEE 8th International Conference on Properties & Applications of Dielectric Material, Bali, Indonesia, 26–30 June 2006; pp. 653–656. [Google Scholar]
- Stoller, P.C.; Seeger, M.; Iordanidis, A.A.; Naidis, G.V. CO2 as an Arc interruption medium in gas circuit breakers. IEEE Trans. Plasma Sci. 2013, 41, 2359–2369. [Google Scholar] [CrossRef]
- Chervy, B.; Gonzalez, J.J.; Gleizes, A. Calculation of the interruption capability of SF6-CF4 and SF6-C2F6 mixtures-Part II: Arc decay modeling. IEEE Trans. Plasma Sci. 1996, 24, 198–209. [Google Scholar] [CrossRef]
- Yang, W.H.; Wang, Z.J.; Wang, D.B.; Chen, Q.L.; Zhao, S.C.; Zhuo, R. Arc interruption performance of SF6/N2 gas and dielectric recovery in disconnector. In Proceedings of the 2023 4th International Symposium on Insulation and Discharge Computation for Power Equipment, IDCOMPU2023, Wuhan, China, 26–28 May 2023; pp. 149–156. [Google Scholar]
- Zhao, H.; Li, X.W.; Zhu, K.; Wang, Q.; Lin, H.; Guo, X.X. Study of the arc interruption performance of SF6-CO2 mixtures as a substitute for SF6. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2657–2667. [Google Scholar] [CrossRef]
- Nechmi, H.E.; Beroual, A.; Girodet, A.; Vinson, P. Fluoronitriles/CO2 gas mixture as promising substitute to SF6 for insulation in high voltage applications. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2587–2593. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.X.; Chen, Q.; Fu, M.L.; Zhuo, R.; Xiao, S.; Chen, D.C.; Tang, J. Study on the Dielectric Properties of C4F7N/N2 Mixture Under Highly Non-Uniform Electric Field. IEEE Access 2018, 6, 42868–42876. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, W.J.; Li, H.; Yan, X.L.; Li, Z.B.; Chen, W.J.; Bian, K. Influence of conductor surface roughness on insulation performance of C4F7N/CO2 mixed gas. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 922–929. [Google Scholar] [CrossRef]
- Kieffel, Y.; Biquez, F.; Vigouroux, D.; Ponchon, P.; Schlernitzauer, A.; Magous, R.; Cros, G.; Owens, J.G. Characteristics of g3—An alternative to SF6. In Proceedings of the 2016 IEEE International Conference on Dielectrics (ICD), Montpellier, France, 3–7 July 2016; pp. 54–57. [Google Scholar]
- Kieffel, Y.; Biquez, F. SF6 alternative development for high voltage switchgears. In Proceedings of the Electrical Insulation Conference, Denver, CO, USA, 26–30 July 2015; pp. 379–383. [Google Scholar]
- Fu, L.J.; Shao, X.J.; Xin, Z.Z.; Xie, C. Experimental Research on Breaking Performance of C4F7N/CO2 Mixed Gas in Medium Voltage Load switch. High Volt. Appar. 2020, 56, 86–91. [Google Scholar]
- Song, Y.; Lin, X.; Xu, J.Y.; Zhang, J. Interruption Performance of C4F7N/CO2 Gas in High-voltage Circuit Breaker. High Volt. Eng. 2023, 49, 971–981. [Google Scholar]
- Lv, Q.S.; Guo, Z.; Xu, H.Y.; Wang, L.; Li, X.W.; Xiang, Z. Experimental Study on the Free Arc-burning Arc Characteristics of C4F7N/CO2 Mixture. High Volt. Eng. 2019, 445, 3804–3809. [Google Scholar]
- Fang, M.T.C.; Brannen, D. A Current-Zero Arc Model Based on Forced Convection. IEEE Trans. Plasma Sci. 1979, 7, 217–229. [Google Scholar] [CrossRef]
- Yan, J.D.; Fang, M.T.C.; Hall, W. The development of PC based CAD tools for auto-expansion circuit breaker design. IEEE Trans. Power Deliv. 1999, 14, 176–181. [Google Scholar] [CrossRef]
- Wu, Y.; Rong, M.Z.; Yang, Q.; Hu, G.X. Simulation on dynamic characteristics of arc in low voltage circuit breaker modelling. Proc. CSEE 2005, 25, 143–148. [Google Scholar]
- Pei, Y.Q.; Zhong, J.Y.; Zhang, J.M.; Yan, J.D. A comparative study of arc behaviour in an auto-expansion circuit breaker with different arc durations. J. Phys. D Appl. Phys. 2014, 47, 335201. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.L.; Sun, H.; Murphy, A.B.; Rong, M.Z.; Yang, F.; Chen, Z.X.; Niu, C.P.; Wang, X.H. Properties of C4F7N–CO2 thermal plasmas: Thermodynamic properties, transport coefficients and emission coefficients. J. Phys. D Appl. Phys. 2018, 51, 1555206. [Google Scholar] [CrossRef]
- Fang, M.T.C.; Zhuang, Q.; Guo, X.J. Current-Zero Behaviour of an SF6 Gas- Blast Arc. II. Turbulent Flow. J. Phys. D Appl. Phys. 1994, 27, 74–83. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, J.D.; Fang, M.T.C. The modelling of an SF 6 arc in a supersonic nozzle: I. Cold flow features and dc arc characteristics. J. Phys. D Appl. Phys. 2014, 47, 215201. [Google Scholar] [CrossRef]
- Dixon, C.M.; Yan, J.D.; Fang, M.T.C. A comparison of three radiation models for the calculation of nozzle arcs. J. Phys. D Appl. Phys. 2004, 37, 3309–3318. [Google Scholar] [CrossRef]
- Yan, J.D.; Nuttall, K.I.; Fang, M.T.C. A comparative study of turbulence models for SF6 arcs in a supersonic nozzle. J. Phys. D Appl. Phys. 1999, 32, 1401–1406. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yan, J.D.; Murphy, A.B.; Hall, W.; Fang, M.T.C. Computational investigation of arc behavior in an auto-expansion circuit breaker contaminated by ablated nozzle vapor. IEEE Trans. Plasma Sci. 2002, 30, 706–719. [Google Scholar] [CrossRef]




| Equation | |||
|---|---|---|---|
| Mass | 1 | 0 | 0 |
| x-Momentum | v | viscous terms | |
| y-Momentum | w | viscous terms | |
| Energy | h | viscous dissipation | |
| Arced gas concentration | 0 | ||
| Current continuity | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yan, X.; Liu, B.; Bian, Y. Investigation of Arc Dynamic Behavior Change Induced by Various Parameter Configurations for C4F7N/CO2 Gas Mixture. Energies 2024, 17, 3485. https://doi.org/10.3390/en17143485
Wang W, Yan X, Liu B, Bian Y. Investigation of Arc Dynamic Behavior Change Induced by Various Parameter Configurations for C4F7N/CO2 Gas Mixture. Energies. 2024; 17(14):3485. https://doi.org/10.3390/en17143485
Chicago/Turabian StyleWang, Wen, Xianglian Yan, Beiyang Liu, and Yalin Bian. 2024. "Investigation of Arc Dynamic Behavior Change Induced by Various Parameter Configurations for C4F7N/CO2 Gas Mixture" Energies 17, no. 14: 3485. https://doi.org/10.3390/en17143485





