CH4 Adsorption in Wet Metal-Organic Frameworks under Gas Hydrate Formation Conditions Using A Large Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Setup and Materials
2.2. Procedure and Data Processing
2.2.1. Gas Adsorption and Desorption Experiment
2.2.2. Data Processing Gas Calculations
3. Results
3.1. Reproducibility of the Experiment
3.2. Gas Adsorption for Dry Nanoporous Materials
3.3. Gas Absorption Behavior for Wet Nanoporous Materials
3.3.1. CH4 Gas Adsorption in Wet Hydrophobic Nanoporous Materials
3.3.2. CH4 Gas Adsorption in Wet Hydrophilic Nanoporous Materials
3.3.3. Experimental Methodology, Key Assumption, and the Way Forward
3.3.4. Effect of Material Properties on Gas Adsorption
3.4. Desorption Experiments
3.5. Material Stability and Characterization
3.5.1. Powdered Material Visualization before and after the Experiment
3.5.2. SEM Images Visualization
3.5.3. XRD Diffraction Pattern
3.6. Contribution to Sustainable Development
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koh, C.A. Towards a Fundamental Understanding of Natural Gas Hydrates. Chem. Soc. Rev. 2002, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Both, A.K.; Gao, Y.; Zeng, X.C.; Cheung, C.L. Gas Hydrates in Confined Space of Nanoporous Materials: New Frontier in Gas Storage Technology. Nanoscale 2021, 13, 7447–7470. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Shang, L.; Lv, Z.; He, J.; Yang, X.; Zhang, Z. Methane Hydrate Formation in Porous Media: Overview and Perspectives. J. Energy Chem. 2022, 74, 454–480. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V. “Nanoreactors” for Boosting Gas Hydrate Formation toward Energy Storage Applications. ACS Nano 2022, 16, 11504–11515. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.S.; von Solms, N. Metal–Organic Frameworks and Gas Hydrate Synergy: A Pandora’s Box of Unanswered Questions and Revelations. Energies 2023, 16, 111. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Metal–Organic Framework HKUST-1 Promotes Methane Hydrate Formation for Improved Gas Storage Capacity. ACS Appl. Mater. Interfaces 2020, 12, 53510–53518. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Collados, C.; Mouchaham, G.; Daemen, L.; Cheng, Y.; Ramirez-Cuesta, A.; Aggarwal, H.; Missyul, A.; Eddaoudi, M.; Belmabkhout, Y.; Silvestre-Albero, J. Quest for an Optimal Methane Hydrate Formation in the Pores of Hydrolytically Stable Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 13391–13397. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, K.; Gomes, C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Wu, Z.; Wee, V.; Ma, X.; Zhao, D. Adsorbed Natural Gas Storage for Onboard Applications. Adv. Sustain. Syst. 2021, 5, 2000200. [Google Scholar] [CrossRef]
- Alhasan, S.; Carriveau, R.; Ting, D.S.K. A Review of Adsorbed Natural Gas Storage Technologies. Int. J. Environ. Stud. 2016, 73, 343–356. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, H.-C. Gas Storage in Porous Metal–Organic Frameworks for Clean Energy Applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kökçam-Demir, Ü.; Goldman, A.; Esrafili, L.; Gharib, M.; Morsali, A.; Weingart, O.; Janiak, C. Coordinatively Unsaturated Metal Sites (Open Metal Sites) in Metal–Organic Frameworks: Design and Applications. Chem. Soc. Rev. 2020, 49, 2751–2798. [Google Scholar] [CrossRef] [PubMed]
- Kolle, J.M.; Fayaz, M.; Sayari, A. Understanding the Effect of Water on CO2 Adsorption. Chem. Rev. 2021, 121, 7280–7345. [Google Scholar] [CrossRef] [PubMed]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water Adsorption in MOFs: Fundamentals and Applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, X.; Wang, S.; Cui, H.; Li, C.; Zhu, G. Research Progress on the Water Stability of a Metal-Organic Framework in Advanced Oxidation Processes. Water. Air. Soil Pollut. 2021, 232, 18. [Google Scholar] [CrossRef]
- Rahman, M.M.; Muttakin, M.; Pal, A.; Shafiullah, A.Z.; Saha, B.B. A Statistical Approach to Determine Optimal Models for IUPAC-Classified Adsorption Isotherms. Energies 2019, 12, 4565. [Google Scholar] [CrossRef]
- Instruments, 3P Recording of Single and Multi-Component Isotherms Using Dynamic Methods. Available online: https://www.3p-instruments.com/single-and-multi-component-isotherms-with-dynamic-methods/#:~:text=Recording of single and multi-component isotherms using dynamic,theory%2C the ideal separation behavior is then calculated (accessed on 8 July 2024).
- Hyun, S.M.; Lee, J.H.; Jung, G.Y.; Kim, Y.K.; Kim, T.K.; Jeoung, S.; Kwak, S.K.; Moon, D.; Moon, H.R. Exploration of Gate-Opening and Breathing Phenomena in a Tailored Flexible Metal-Organic Framework. Inorg. Chem. 2016, 55, 1920–1925. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Cao, J. Methane Hydrate Formation in Hollow ZIF-8 Nanoparticles for Improved Methane Storage Capacity. Catalysts 2022, 12, 485. [Google Scholar] [CrossRef]
- Casco, M.E.; Silvestre-Albero, J.; Ramírez-Cuesta, A.J.; Rey, F.; Jordá, J.L.; Bansode, A.; Urakawa, A.; Peral, I.; Martínez-Escandell, M.; Kaneko, K.; et al. Methane Hydrate Formation in Confined Nanospace Can Surpass Nature. Nat. Commun. 2015, 6, 6432. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Methane Hydrate Growth Promoted by Microporous Zeolitic Imidazolate Frameworks ZIF-8 and ZIF-67 for Enhanced Methane Storage. ACS Sustain. Chem. Eng. 2021, 9, 9001–9010. [Google Scholar] [CrossRef]
- Farrando-Perez, J.; Balderas-Xicohtencatl, R.; Cheng, Y.; Daemen, L.; Cuadrado-Collados, C.; Martinez-Escandell, M.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Rapid and Efficient Hydrogen Clathrate Hydrate Formation in Confined Nanospace. Nat. Commun. 2022, 13, 5953. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ahn, Y.-H.; Lee, H. Phase Equilibria of CO 2 and CH 4 Hydrates in Intergranular Meso/Macro Pores of MIL-53 Metal Organic Framework. J. Chem. Eng. Data 2015, 60, 2178–2185. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, S.; Guo, P.; Fan, S.; Zhang, S. Understanding the Characteristic of Methane Hydrate Equilibrium in Materials and Its Potential Application. Chem. Eng. J. 2018, 349, 775–781. [Google Scholar] [CrossRef]
- Casco, M.E.; Rey, F.; Jordá, J.L.; Rudić, S.; Fauth, F.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Fernández, E.V.; Silvestre-Albero, J. Paving the Way for Methane Hydrate Formation on Metal-Organic Frameworks (MOFs). Chem. Sci. 2016, 7, 3658–3666. [Google Scholar] [CrossRef]
- Babaei, H.; DeCoster, M.E.; Jeong, M.; Hassan, Z.M.; Islamoglu, T.; Baumgart, H.; McGaughey, A.J.H.; Redel, E.; Farha, O.K.; Hopkins, P.E.; et al. Observation of Reduced Thermal Conductivity in a Metal-Organic Framework Due to the Presence of Adsorbates. Nat. Commun. 2020, 11, 4010. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Audu, C.O.; Liao, Y.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T.; Grayson, M. Thermal Conductivity of ZIF-8 Thin-Film under Ambient Gas Pressure. ACS Appl. Mater. Interfaces 2017, 9, 28139–28143. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.A.; Kaiser, A.; Andreassen, K.A. Methane Storage in Metal-Organic Framework HKUST-1 with Enhanced Heat Management Using 3D Printed Metal Lattices. Chem. Eng. Res. Des. 2023, 192, 362–370. [Google Scholar] [CrossRef]
- Paudel, H.P.; Shi, W.; Hopkinson, D.; Steckel, J.A.; Duan, Y. Computational Modelling of Adsorption and Diffusion Properties of CO2 and CH4in ZIF-8 for Gas Separation Applications: A Density Functional Theory Approach. React. Chem. Eng. 2021, 6, 990–1001. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of Metal-Organic Frameworks by Water Adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Xian, S.; Peng, J.; Pandey, H.; Thonhauser, T.; Wang, H.; Li, J. Robust Metal–Organic Frameworks with High Industrial Applicability in Efficient Recovery of C3H8 and C2H6 from Natural Gas Upgrading. Engineering 2023, 23, 56–63. [Google Scholar] [CrossRef]
- Fomkin, A.A.; Dubovik, B.A.; Limonov, N.V.; Pribylov, A.A.; Pulin, A.L.; Men’shchikov, I.E.; Shkolin, A.V. Methane Adsorption on Microporous Carbon Adsorbent Prepared from Thermochemically Activated Wood. Prot. Met. Phys. Chem. Surfaces 2021, 57, 17–21. [Google Scholar] [CrossRef]
- Tian, T.; Zeng, Z.; Vulpe, D.; Casco, M.E.; Divitini, G.; Midgley, P.A.; Silvestre-Albero, J.; Tan, J.C.; Moghadam, P.Z.; Fairen-Jimenez, D. A Sol-Gel Monolithic Metal-Organic Framework with Enhanced Methane Uptake. Nat. Mater. 2018, 17, 174–179. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Fielder-Dunton, L.; Jian, Q.; Ferrari, M.C. The Effect of Solution Casting Temperature and Ultrasound Treatment on PEBAX MH-1657/ZIF-8 Mixed Matrix Membranes Morphology and Performance. Membranes 2022, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Nguyen, H.L.; Hanikel, N.; Li, K.K.-Y.; Zhou, Z.; Ma, T.; Yaghi, O.M. High-Yield, Green and Scalable Methods for Producing MOF-303 for Water Harvesting from Desert Air. Nat. Protoc. 2023, 18, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air, Anywhere, Anytime. ACS Cent. Sci. 2020, 6, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, L.G.; Tu, Y.D.; Pan, Q.; Palash, M.L.; Saha, B.B.; Aristov, Y.I.; Wang, R.Z. Metal-Organic Frameworks for Energy Conversion and Water Harvesting: A Bridge between Thermal Engineering and Material Science. Nano Energy 2021, 84, 105946. [Google Scholar] [CrossRef]
- Chen, S.; Wang, D.; Wang, Z.; Fu, Y.; Xu, Y.; Liu, D. Kinetic Study of Methane Storage in Hydrophobic ZIF-8 by Adsorption-Hydration Hybrid Technology. Energy 2023, 283, 129013. [Google Scholar] [CrossRef]
- Li, Z.; Li, N.; Kan, J.; Liu, B.; Chen, G. Competitive and Synergistic Mechanisms of Adsorption-Hydration during Methane Storage in the Wet ZIF-8 Fixed Bed. Fuel 2023, 351, 129055. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, X.; Fu, Y.; Chen, S.; Zi, M. Molecular Insights into CH4/H2O Transport and Hydrate Formation in Hydrophobic Metal-Organic Frameworks ZIF-8: Implication for CH4 Storage by Adsorption-Hydration Hybrid Method. Fuel 2023, 337, 126851. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Silvestre-Albero, J. Methane Storage on Nanoporous Carbons. In Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 209–226. ISBN 9789811335044. [Google Scholar]
- Lin, K.S.; Adhikari, A.K.; Ku, C.N.; Chiang, C.L.; Kuo, H. Synthesis and Characterization of Porous HKUST-1 Metal Organic Frameworks for Hydrogen Storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Senkovska, I.; Kaskel, S. High pressure methane adsorption in the metal-organic frameworks Cu3(btc)2, Zn2(bdc)2dabco, and Cr3F(H2O)2O(bdc)3. Microporous Mesoporous Mater. 2008, 112, 108–115. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane Storage in Metal-Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S. Local Structure of Framework Cu(II) in HKUST-1 Metallorganic Framework: Spectroscopic Characterization upon Activation and Interaction with Adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic Imidazolate Framework Materials: Recent Progress in Synthesis and Applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Bao, Q.; Lou, Y.; Xing, T.; Chen, J. Rapid Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) in Aqueous Solution via Microwave Irradiation. Inorg. Chem. Commun. 2013, 37, 170–173. [Google Scholar] [CrossRef]
- Sann, E.E.; Pan, Y.; Gao, Z.; Zhan, S.; Xia, F. Highly Hydrophobic ZIF-8 Particles and Application for Oil-Water Separation. Sep. Purif. Technol. 2018, 206, 186–191. [Google Scholar] [CrossRef]
- Hu, F.H.; Chi, L.T.; Syu, G.B.; Yu, T.Y.; Lin, M.P.; Chen, J.J.; Yu, W.Y.; Kang, D.Y. Mixed-Linker MOF-303 Membranes for Pervaporation. J. Membr. Sci. Lett. 2023, 3, 100053. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Wang, J.; Wang, Z.; Kapteijn, F.; Liu, X. Highly Water-Permeable Metal–Organic Framework MOF-303 Membranes for Desalination. J. Am. Chem. Soc. 2021, 143, 20055–20058. [Google Scholar] [CrossRef]
- Mehio, N.; Dai, S.; Jiang, D. Quantum Mechanical Basis for Kinetic Diameters of Small Gaseous Molecules. J. Phys. Chem. A 2014, 118, 1150–1154. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Azzan, H.; Pini, R.; Petit, C. H2, N2, CO2, and CH4 Unary Adsorption Isotherm Measurements at Low and High Pressures on Zeolitic Imidazolate Framework ZIF-8. J. Chem. Eng. Data 2022, 67, 1674–1686. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [PubMed]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High Pressure Adsorption of Hydrogen, Nitrogen, Carbon Dioxide and Methane on the Metal-Organic Framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Gadea-Ramos, E.; Kaneko, K.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. High-Pressure Methane Storage in Porous Materials: Are Carbon Materials in the Pole Position? Chem. Mater. 2015, 27, 959–964. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Liu, C.; Zhou, L. Comparative Studies of CO2 and CH4 Sorption on Activated Carbon in Presence of Water. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 322, 14–18. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Farrando-Pérez, J.; Martínez-Escandell, M.; Missyul, A.; Silvestre-Albero, J. Effect of Additives in the Nucleation and Growth of Methane Hydrates Confined in a High-Surface Area Activated Carbon Material. Chem. Eng. J. 2020, 388, 124224. [Google Scholar] [CrossRef]
- Celzard, A.; Marêché, J.F. Optimal Wetting of Active Carbons for Methane Hydrate Formation. Fuel 2006, 85, 957–966. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, R.; Wang, F. Fast Formation Kinetics of Methane Hydrates Loaded by Silver Nanoparticle Coated Activated Carbon (Ag-NP@AC). Chem. Eng. J. 2021, 417, 129206. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, B.; Xu, L.; Zhang, R.; He, Y.; Wang, F. How Porous Surfaces Influence the Nucleation and Growth of Methane Hydrates. Fuel 2021, 291, 120142. [Google Scholar] [CrossRef]
- Mu, L.; Liu, B.; Liu, H.; Yang, Y.; Sun, C.; Chen, G. A Novel Method to Improve the Gas Storage Capacity of ZIF-8. J. Mater. Chem. 2012, 22, 12246. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, J.; Chen, S.; Fu, Y.; Zhang, Y.; Wang, D.; Pei, J.; Liu, D. Molecular Insights into Hybrid CH4 Physisorption-Hydrate Growth in Hydrophobic Metal–Organic Framework ZIF-8: Implications for CH4 Storage. Chem. Eng. J. 2022, 430, 132901. [Google Scholar] [CrossRef]
- Elaouni, A.; El Ouardi, M.; Zbair, M.; BaQais, A.; Saadi, M.; Ait Ahsaine, H. ZIF-8 Metal Organic Framework Materials as a Superb Platform for the Removal and Photocatalytic Degradation of Organic Pollutants: A Review. RSC Adv. 2022, 12, 31801–31817. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.; Nouali, H.; Marichal, C.; Chaplais, G.; Patarin, J. Energetic Performances of the Metal–Organic Framework ZIF-8 Obtained Using High Pressure Water Intrusion–Extrusion Experiments. Phys. Chem. Chem. Phys. 2013, 15, 4888. [Google Scholar] [CrossRef] [PubMed]
- Khay, I.; Chaplais, G.; Nouali, H.; Marichal, C.; Patarin, J. Water Intrusion–Extrusion Experiments in ZIF-8: Impacts of the Shape and Particle Size on the Energetic Performances. RSC Adv. 2015, 5, 31514–31518. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Yang, Y.; Li, X.; Li, J.; Li, Z. Competitive Adsorption and Selectivity of Benzene and Water Vapor on the Microporous Metal Organic Frameworks (HKUST-1). Chem. Eng. J. 2015, 259, 79–89. [Google Scholar] [CrossRef]
- Anderson, R.; Llamedo, M.; Tohidi, B.; Burgass, R.W. Experimental Measurement of Methane and Carbon Dioxide Clathrate Hydrate Equilibria in Mesoporous Silica. J. Phys. Chem. B 2003, 107, 3507–3514. [Google Scholar] [CrossRef]
- Tsalaporta, E.; MacElroy, J.M.D. A Comparative Study of the Physical and Chemical Properties of Pelletized HKUST-1, ZIF-8, ZIF-67 and UiO-66 Powders. Heliyon 2020, 6, e04883. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Velazquez-Garcia, J.; Bennett, T.D.; Fairen-Jimenez, D. Mechanically and Chemically Robust ZIF-8 Monoliths with High Volumetric Adsorption Capacity. J. Mater. Chem. A 2015, 3, 2999–3005. [Google Scholar] [CrossRef]
- Qi, X.; Liu, K.; Chang, Z. Beyond Powders: Monoliths on the Basis of Metal-Organic Frameworks (MOFs). Chem. Eng. J. 2022, 441, 135953. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. 3D-Printed Metal-Organic Framework Monoliths for Gas Adsorption Processes. ACS Appl. Mater. Interfaces 2017, 9, 35908–35916. [Google Scholar] [CrossRef] [PubMed]
- Montazerolghaem, M.; Aghamiri, S.F.; Talaie, M.R.; Tangestaninejad, S. A Comparative Investigation of CO2 Adsorption on Powder and Pellet Forms of MIL-101. J. Taiwan Inst. Chem. Eng. 2017, 72, 45–52. [Google Scholar] [CrossRef]
- Policicchio, A.; Filosa, R.; Abate, S.; Desiderio, G.; Colavita, E. Activated Carbon and Metal Organic Framework as Adsorbent for Low-Pressure Methane Storage Applications: An Overview. J. Porous Mater. 2017, 24, 905–922. [Google Scholar] [CrossRef]
- Decoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The Effect of Water Adsorption on the Structure of the Carboxylate Containing Metal-Organic Frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 2013, 1, 11922–11932. [Google Scholar] [CrossRef]
- Bazer-Bachi, D.; Assié, L.; Lecocq, V.; Harbuzaru, B.; Falk, V. Towards Industrial Use of Metal-Organic Framework: Impact of Shaping on the MOF Properties. Powder Technol. 2014, 255, 52–59. [Google Scholar] [CrossRef]
- Domán, A.; Czakkel, O.; Porcar, L.; Madarász, J.; Geissler, E.; László, K. Role of Water Molecules in the Decomposition of HKUST-1: Evidence from Adsorption, Thermoanalytical, X-ray and Neutron Scattering Measurements. Appl. Surf. Sci. 2019, 480, 138–147. [Google Scholar] [CrossRef]
- Majano, G.; Martin, O.; Hammes, M.; Smeets, S.; Baerlocher, C.; Pérez-Ramírez, J. Solvent-Mediated Reconstruction of the Metal-Organic Framework HKUST-1 (Cu3(BTC)2). Adv. Funct. Mater. 2014, 24, 3855–3865. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Li, Y.Y.; Xu, F.; Xiao, J.; Xia, Q.; Li, Y.Y.; Li, Z. A Novel Mechanochemical Method for Reconstructing the Moisture-Degraded HKUST-1. Chem. Commun. 2015, 51, 10835–10838. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven Chemical Separations to Change the World. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Angelini, P.; Armstrong, T.; Counce, R.; Griffith, W.; Klasson, T.; Muralidharan, G.; Closset, G.; Keller, G.; Watson, J. Materials for Separation Technologies: Energy and Emission Reduction Opportunities; U.S. Department of Energy: Washington, DC, USA, 2005. [Google Scholar]
- Lee, Y.; Seo, D.; Lee, S.; Park, Y. Advances in Nanomaterials for Sustainable Gas Separation and Storage: Focus on Clathrate Hydrates. Acc. Chem. Res. 2023, 56, 3111–3120. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K. Recent Trends in the Development of Adsorption Technologies for Carbon Dioxide Capture: A Brief Literature and Patent Reviews (2014–2018). J. Clean. Prod. 2020, 253, 119707. [Google Scholar] [CrossRef]
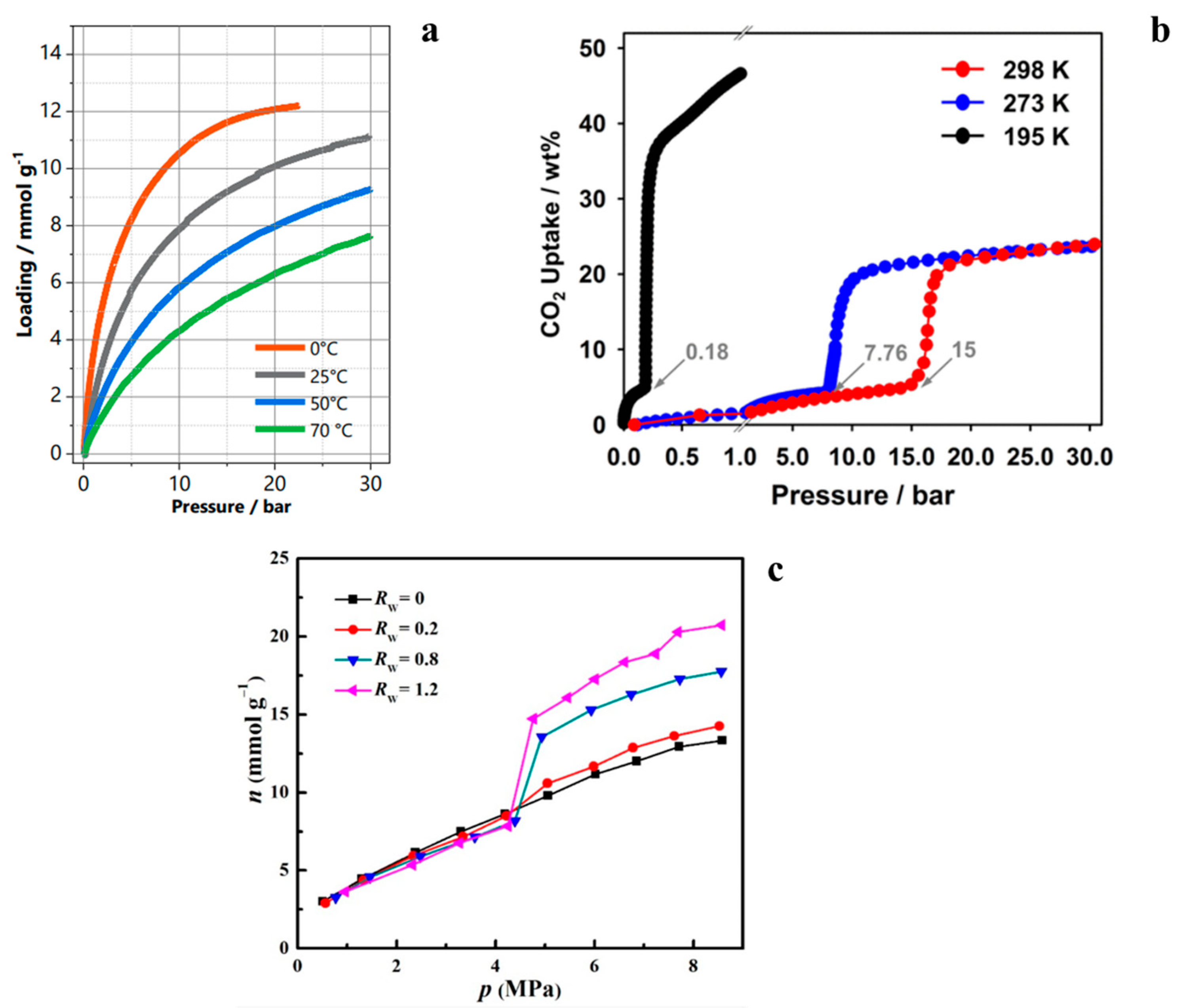
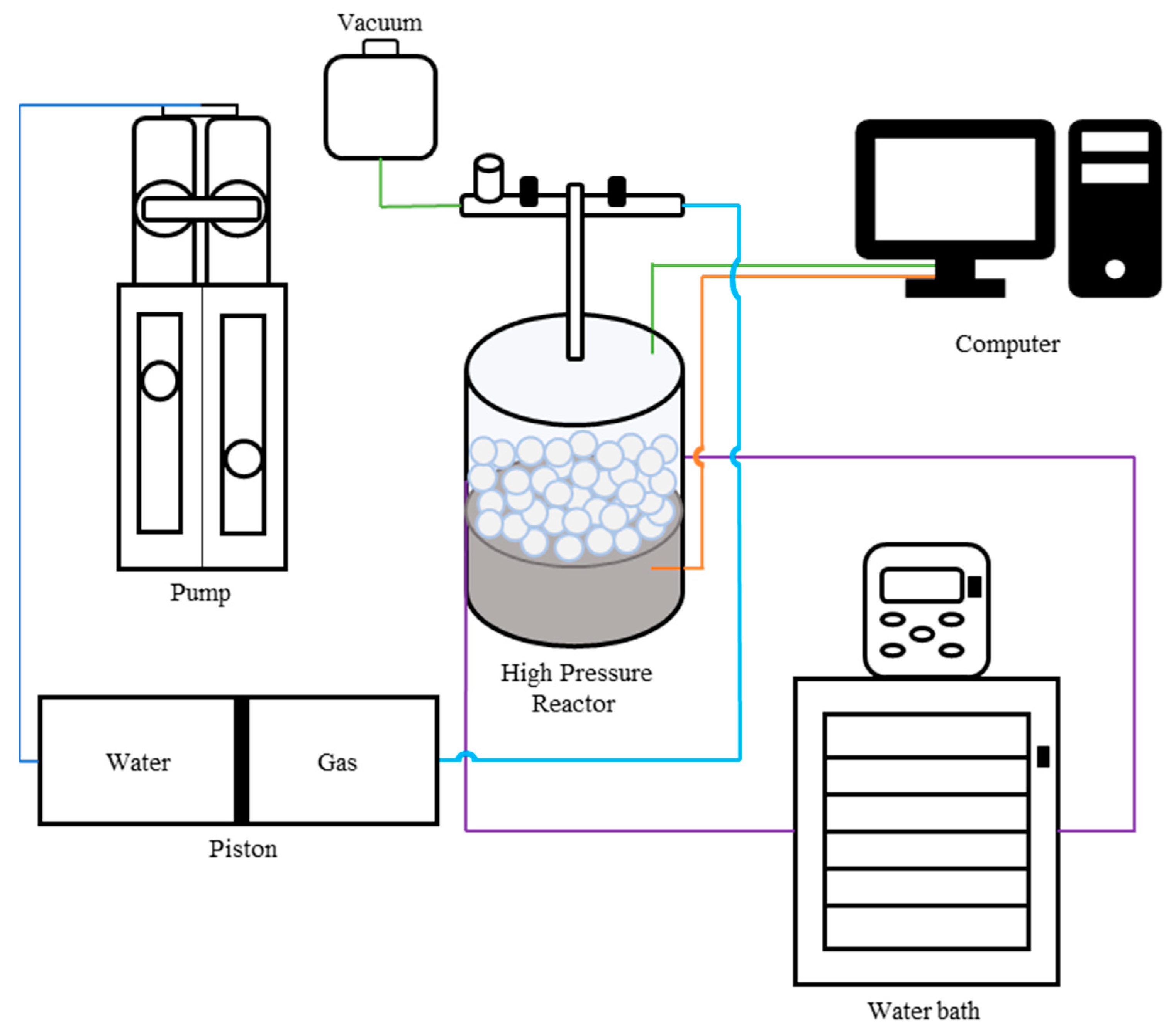

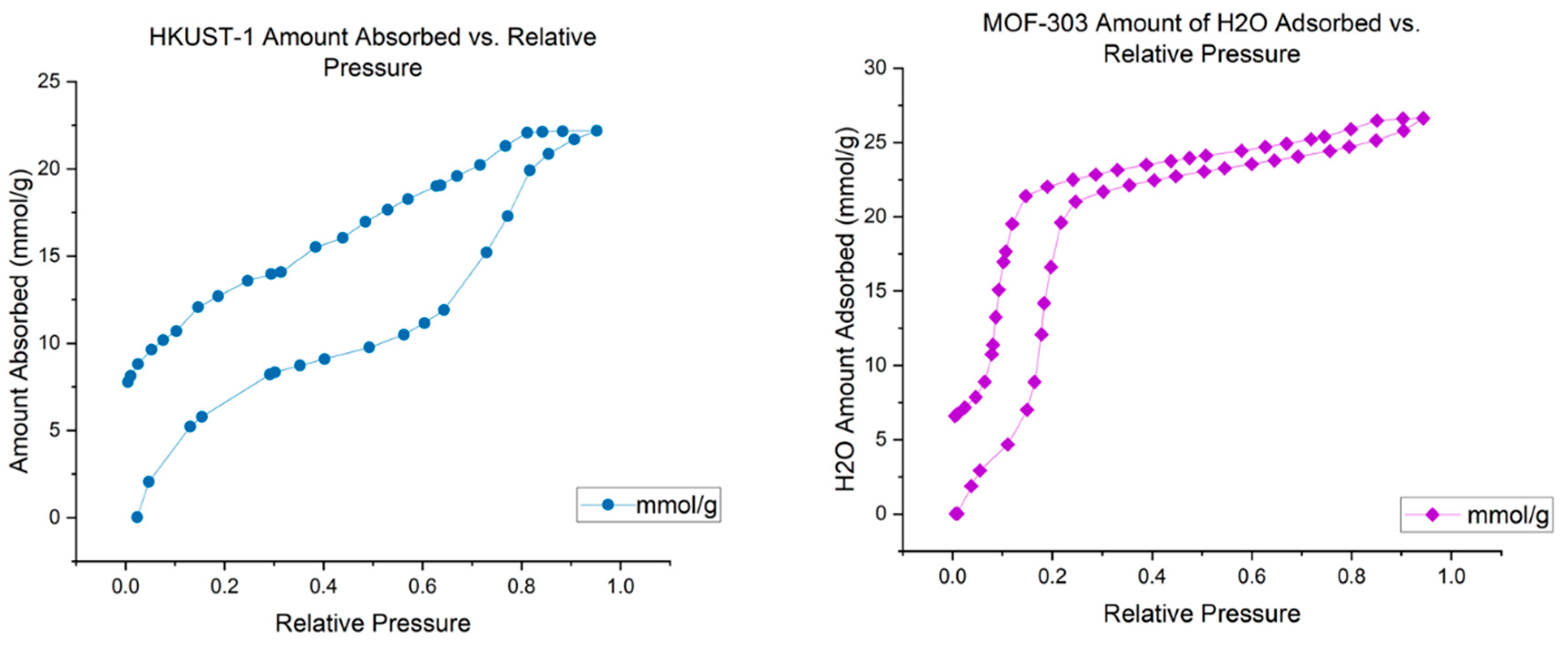



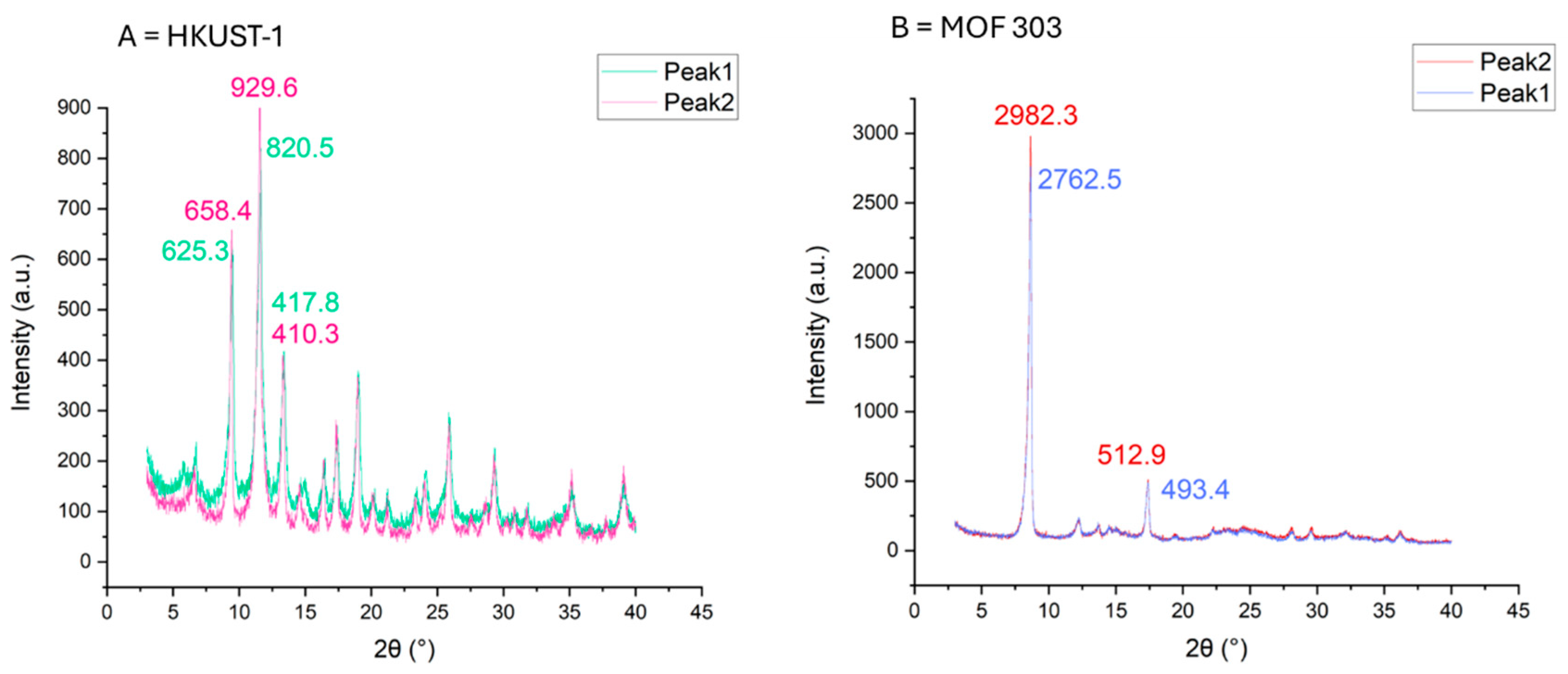
| Sample Type | HKUST-1 | ZIF-8 | MOF-303 | NuChar Carbon |
|---|---|---|---|---|
| BET Surface area (m2/g) * | 641 | 1550 | 1133 | 1366 |
| Adsorption energy *** for CH4 (kJ/mol) | 15–30 [29] | 18.7–44.7 [30,31] | 19 [32] | 12–14 [33] |
| Density (g/cm3) | 0.88 [34] | 0.95 [35] | 0.40 ** | 0.40 |
| Procurement | Sigma Aldrich | Sigma Aldrich | NovoMOF | Nuchar |
| Purity | 99% | 99% | 99% | 99% |
| CH4 Amount Adsorbed (Reactor Volume = 210 mL) | ||
|---|---|---|
| Exp.1# | Exp.2# | |
| Temp (°C) | 274.15 K | 274.15 K |
| Weight (g) | 15 | 6.3 |
| Pressure (bars) | (mmol/g) | (mmol/g) |
| 0 | 0.00 | 0.00 |
| 20 | 24.03 | 22.92 |
| 25 | 28.72 | 27.54 |
| 30 | 32.74 | 31.87 |
| 35 | 36.47 | 36.01 |
| 40 | 39.91 | 39.59 |
| 45 | 42.85 | 43.17 |
| 50 | 45.66 | 46.35 |
| 55 | 48.49 | 49.24 |
| 60 | 51.26 | 52.09 |
| 65 | 53.35 | 54.98 |
| 70 | 55.53 | 57.60 |
| 75 | XX ** | 59.99 |
| 80 | XX ** | 62.28 |
| CH4 Amount Adsorbed (mmol/g) (RW= 0) | ||||
|---|---|---|---|---|
| Temp (K) | 274.15 | 274.15 | 274.15 | 274.15 |
| Weight (g) | 6.3 | 42.1 | 20.5 | 17.3 |
| Pressure (bar) | ZIF-8 | NuChar-AC | HKUST-1 | MOF-303 |
| 0 | 0.00 | 0.00 | 0.0 | 0.0 |
| 20 | 22.92 | 2.15 | 5.1 | 7.2 |
| 25 | 27.54 | 2.58 | 5.7 | 8.5 |
| 30 | 31.87 | 3.16 | 6.0 | 9.7 |
| 35 | 36.01 | 3.66 | 6.2 | 10.8 |
| 40 | 39.59 | 4.13 | 6.8 | 11.7 |
| 45 | 43.17 | 4.60 | 7.5 | 12.6 |
| 50 | 46.35 | 5.08 | 7.7 | 13.3 |
| 55 | 49.24 | 5.52 | 8.4 | 14.0 |
| 60 | 52.09 | 6.01 | 9.1 | 14.7 |
| 65 | 54.98 | 6.49 | 15.3 | |
| 70 | 57.60 | 6.78 | 15.9 | |
| 75 | 59.99 | 6.81 | 16.0 | |
| 80 | 62.28 | 6.81 | 16.0 | |
| CH4 Amount Adsorbed in Wet Hydrophobic Material (mmol/g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| T (K) | 274.15 | 274.15 | ||||||
| W (g) | 42.2 | 42.2 | 42.2 | 42.2 | 42.2 | 6.3 | 6.3 | 6.3 |
| Rw= | 0 | 0.5 | 1 | 1.5 | 2 | 0 | 0.5 | 1.0 |
| P (bar) | NuChar AC (mmol/g) | ZIF-8 (mmol/g) | ||||||
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 20 | 2.1 | 1.7 | 1.2 | 0.7 | 0.3 | 22.9 | 22.5 | 22.0 |
| 25 | 2.6 | 2.0 | 1.5 | 0.9 | 0.4 | 27.5 | 26.9 | 26.3 |
| 30 | 3.2 | 2.5 | 1.8 | 1.1 | 0.4 | 31.9 | 31.0 | 30.4 |
| 35 | 3.7 | 2.9 | 2.1 | 1.3 | 0.5 | 36.0 | 35.3 | 34.7 |
| 40 | 4.1 | 3.3 | 2.4 | 1.5 | 0.6 | 39.6 | 38.8 | 38.3 |
| 45 | 4.6 | 3.7 | 2.6 | 1.7 | 0.7 | 43.2 | 42.4 | 41.9 |
| 50 | 5.1 | 4.1 | 2.9 | 1.9 | 0.8 | 46.4 | 45.4 | 45.0 |
| 55 | 5.5 | 4.4 | 3.2 | 2.1 | 0.9 | 49.2 | 48.3 | 48.0 |
| 60 | 6.0 | 4.8 | 3.5 | 2.2 | 1.0 | 52.1 | 51.2 | 50.9 |
| 65 | 6.5 | 5.2 | 3.8 | 2.4 | 1.0 | 55.0 | 53.8 | 53.7 |
| 70 | 6.8 | 5.6 | 4.0 | 2.6 | 1.1 | 57.6 | 56.3 | 56.2 |
| 75 | 6.8 | 5.7 | 4.3 | 2.8 | 1.2 | 60.0 | 58.6 | 58.6 |
| 80 | 6.8 | 6.1 | 4.6 | 3.0 | 1.3 | 62.3 | 60.8 | 60.9 |
| CH4 Amount Adsorbed in Wet and Hydrophobic Material (mmol/g) | ||||||
|---|---|---|---|---|---|---|
| T (K) | 274.15 | 274.15 | ||||
| W (g) | 20.5 | 20.5 | 20.5 | 17.3 | 17.3 | 17.3 |
| Rw= | 0 | 0.85 | 2.36 | 0 | 1 | 1.5 |
| P (bar) | HKUST-1 (mmol/g) | MOF-303 (mmol/g) | ||||
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 20 | 5.1 | 4.9 | 3.5 | 7.2 | 6.3 | 5.9 |
| 25 | 5.7 | 5.5 | 4.2 | 8.5 | 7.6 | 7.1 |
| 30 | 6.0 | 6.0 | 5.3 | 9.7 | 8.8 | 8.3 |
| 35 | 6.2 | 6.6 | 7.2 | 10.8 | 10.2 | 9.9 |
| 40 | 6.8 | 7.5 | 9.2 | 11.7 | 11.8 | 11.7 |
| 45 | 7.5 | 8.3 | 11.1 | 12.6 | 13.0 | 13.0 |
| 50 | 7.7 | 9.1 | 14.2 | 13.3 | 14.0 | 14.1 |
| 55 | 8.4 | 9.8 | 18.5 | 14.0 | 15.0 | 15.2 |
| 60 | 9.1 | 10.6 | 24.6 | 14.7 | 15.9 | 16.1 |
| 65 | 15.3 | 16.8 | 17.0 | |||
| 70 | 15.9 | 17.6 | 17.9 | |||
| 75 | 16.0 | 18.4 | 18.8 | |||
| 80 | 16.0 | 19.0 | 19.6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, J.S.; Öncü, N.; von Solms, N. CH4 Adsorption in Wet Metal-Organic Frameworks under Gas Hydrate Formation Conditions Using A Large Reactor. Energies 2024, 17, 3509. https://doi.org/10.3390/en17143509
Pandey JS, Öncü N, von Solms N. CH4 Adsorption in Wet Metal-Organic Frameworks under Gas Hydrate Formation Conditions Using A Large Reactor. Energies. 2024; 17(14):3509. https://doi.org/10.3390/en17143509
Chicago/Turabian StylePandey, Jyoti Shanker, Nehir Öncü, and Nicolas von Solms. 2024. "CH4 Adsorption in Wet Metal-Organic Frameworks under Gas Hydrate Formation Conditions Using A Large Reactor" Energies 17, no. 14: 3509. https://doi.org/10.3390/en17143509
APA StylePandey, J. S., Öncü, N., & von Solms, N. (2024). CH4 Adsorption in Wet Metal-Organic Frameworks under Gas Hydrate Formation Conditions Using A Large Reactor. Energies, 17(14), 3509. https://doi.org/10.3390/en17143509








