Novel NSTEX System Based on Ti/CuO/NC Nanothermite Doped with NTO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
- —equivalence ratio of the nanothermite;
- nfuel/noxidising agent—the molar ratio between actual fuel (i.e., Ti)/oxidising agent (i.e., CuO) dispersed in the electrolyte;
- nstoichiometric fuel/nstoichiometric oxidising agent—the fuel/oxidising agent molar ratio in a stoichiometric reaction.
2.2. Friction and Impact Measurement
2.3. Laser Sensitivity Tests
2.4. SEM-EDS Tests
2.5. XRD Tests
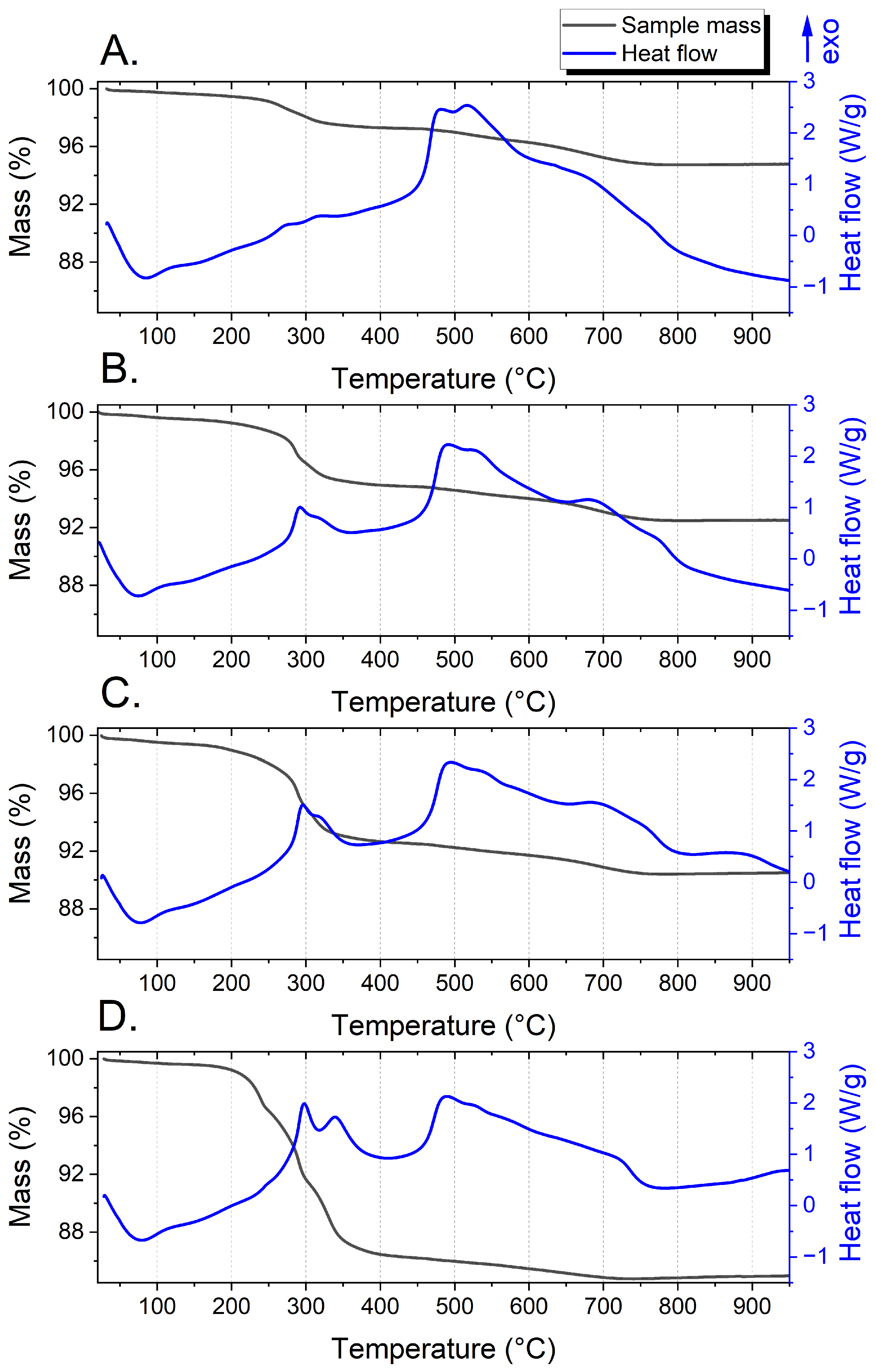
2.6. DSC/TG Tests
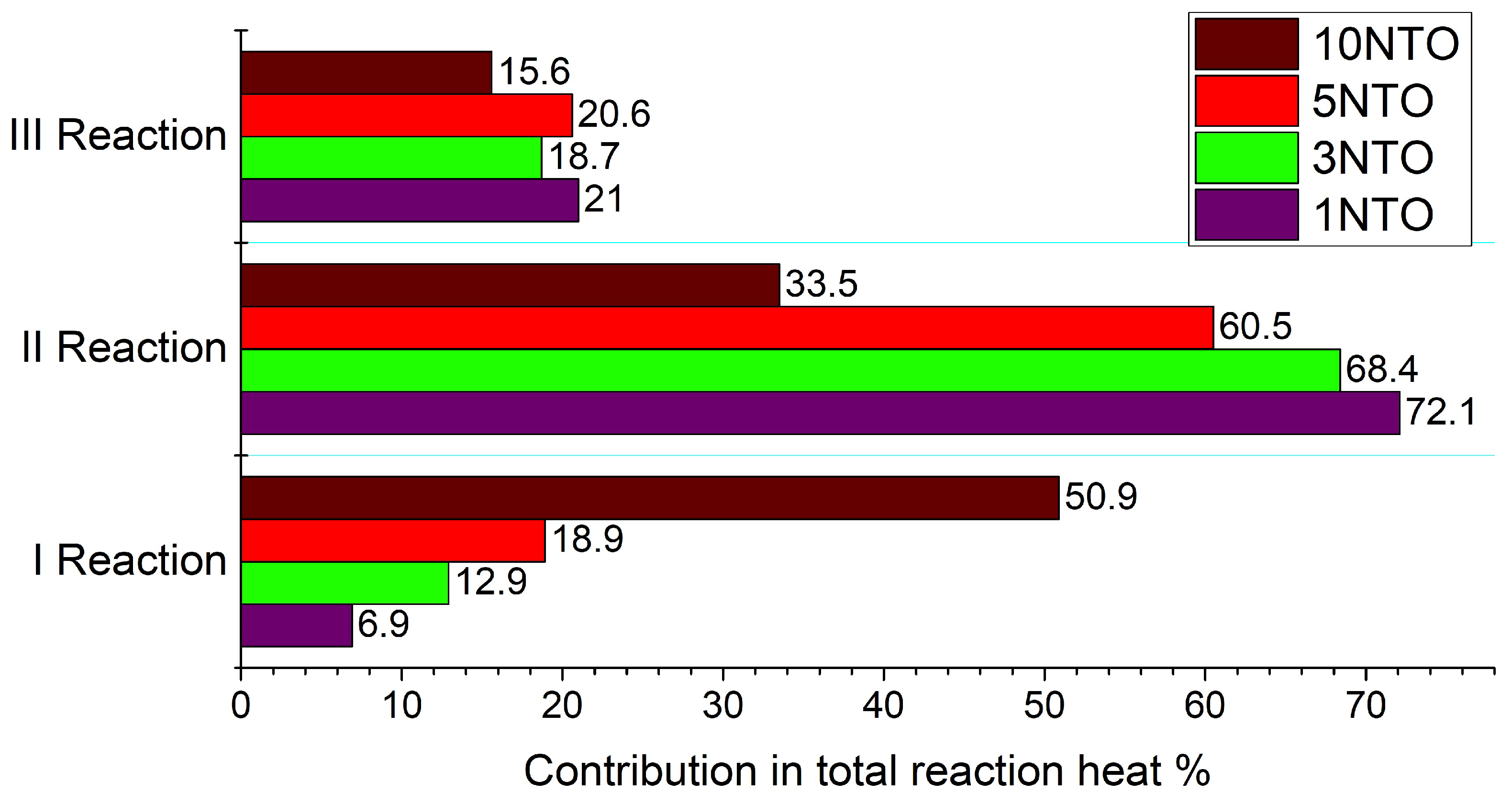
2.7. Closed Vessel Combustion
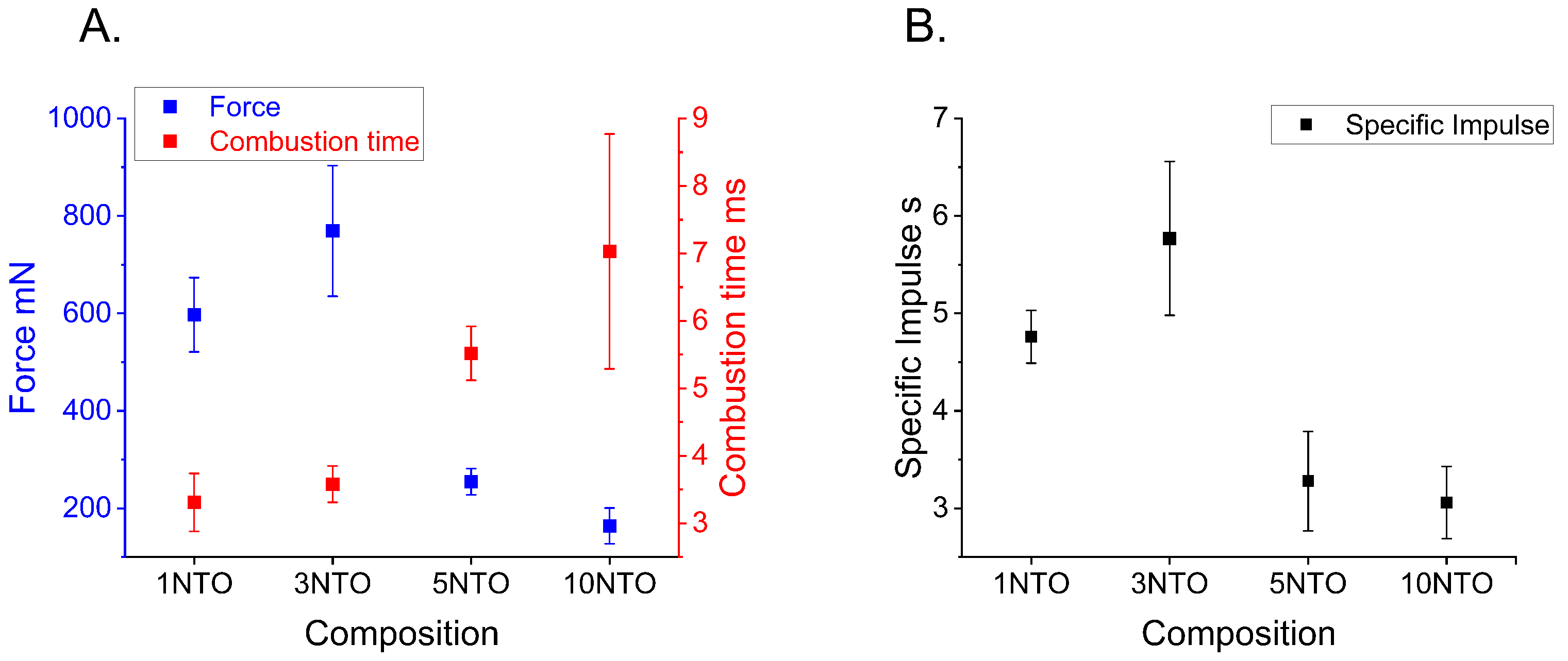
2.8. Thrust Force Measurements
- Isp —specific impulse [s];
- F—thrust force [N];
- Δt—combustion time [s];
- g—gravitational constant = 9.81 [m/s2];
- Δm—mass of combusted composition [kg].
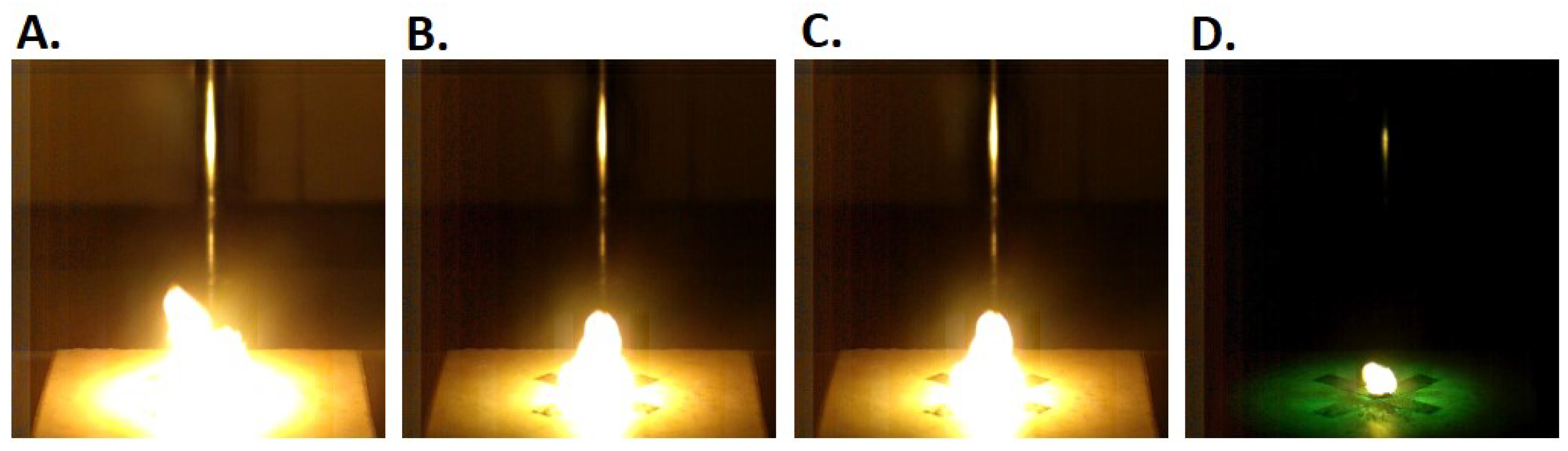
2.9. Open-Air Combustion
2.10. Combustion Velocity Measurements
3. Results and Discussion
3.1. Friction and Impact Sensitivity Measurements
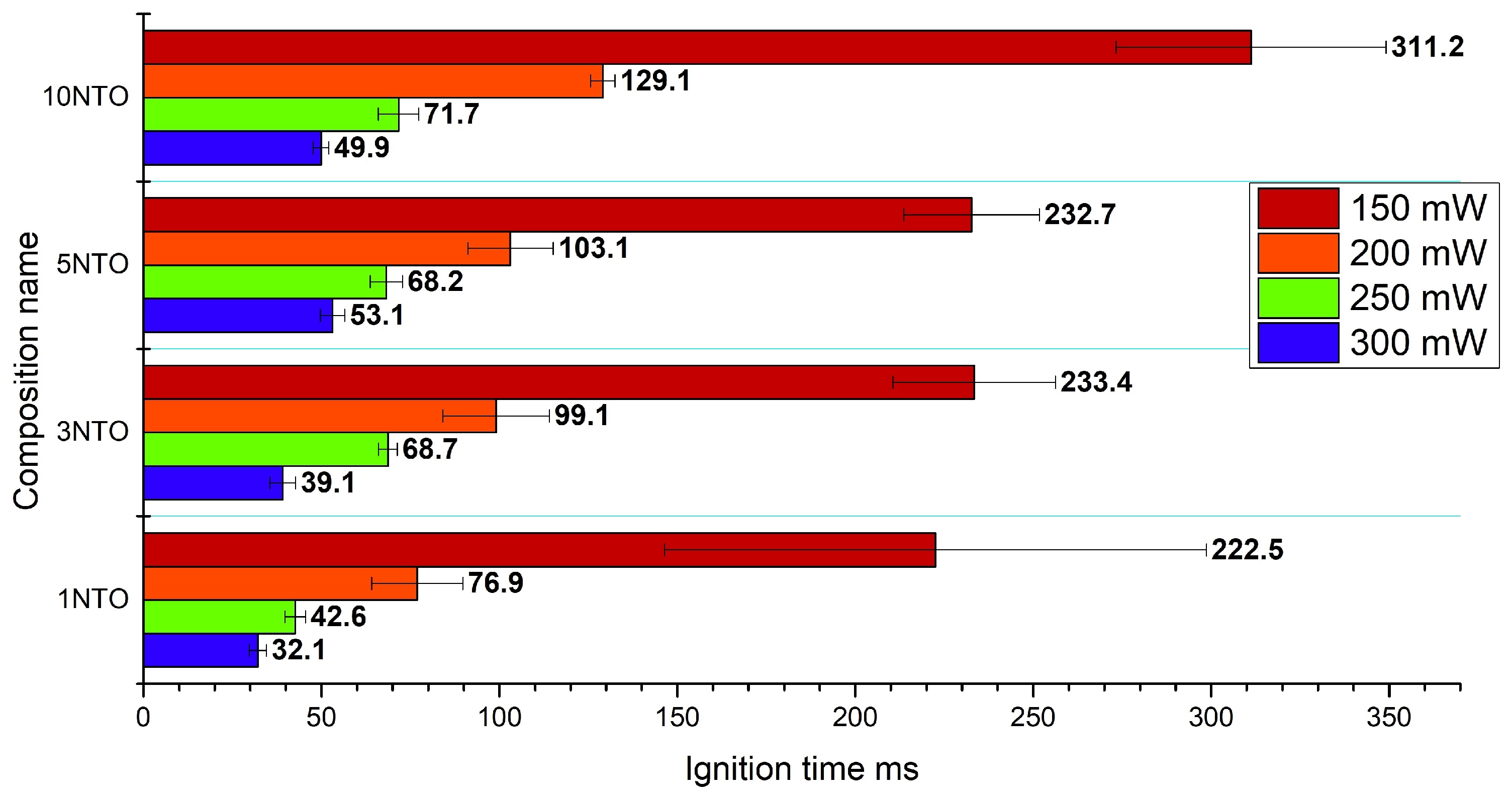
3.2. Laser Sensitivity Tests
3.3. SEM-EDS Tests
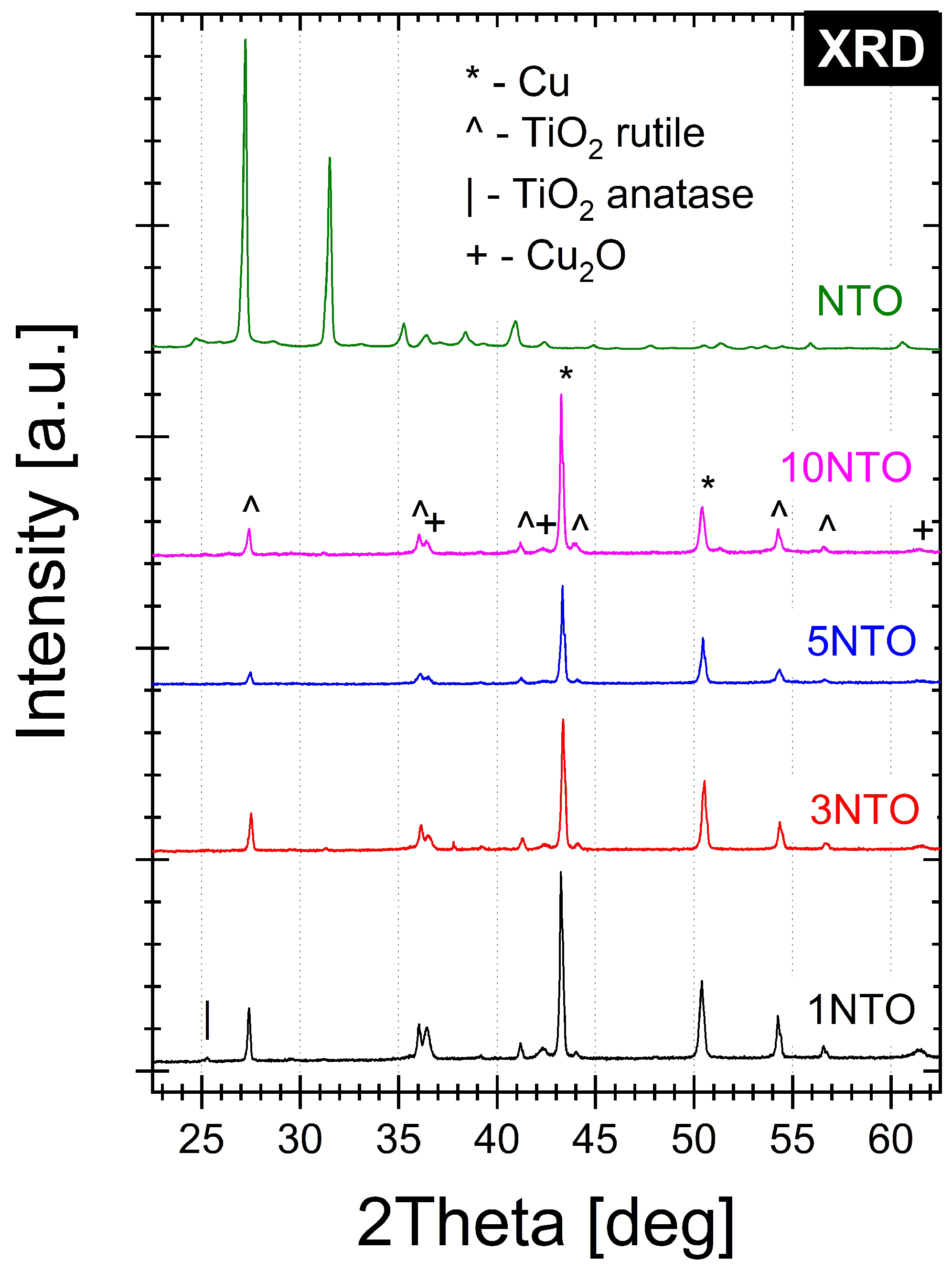
3.4. X-ray Diffractometry
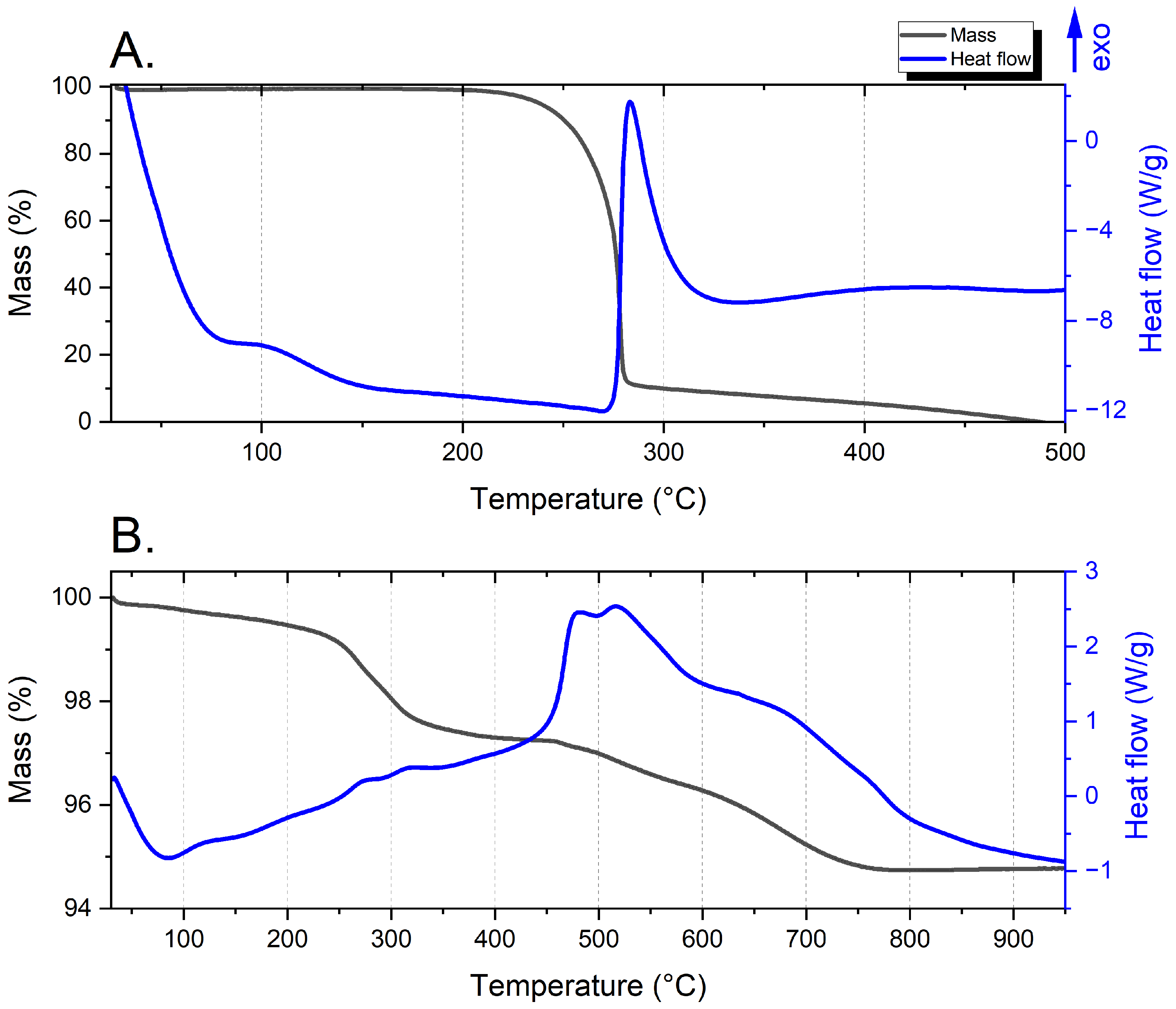
3.5. DSC/TG Tests
3.6. Closed Vessel Combustion
3.7. Thrust Force
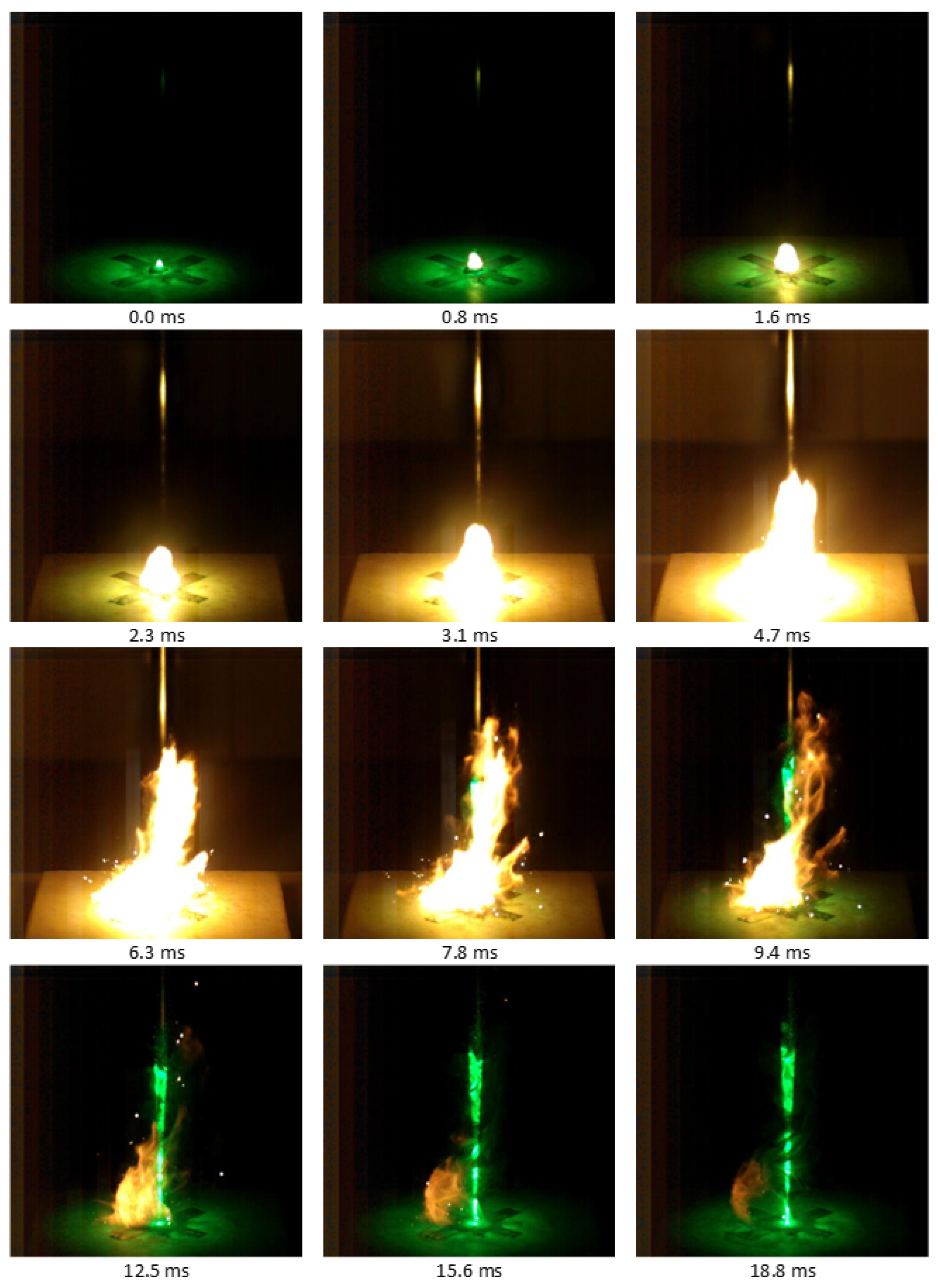
3.8. Open-Air Combustion
3.9. Combustion Velocity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Laser Sensitivity
| Composition | 300 mW | 250 mW | 200 mW | 150 mW |
|---|---|---|---|---|
| C3-1NC-1NTO | 32.09 ± 2.36 | 42.55 ± 2.95 | 76.85 ± 12.80 | 222.46 ± 76.07 |
| C3-1NC-3NTO | 39.11 ± 3.65 | 68.70 ± 2.58 | 99.05 ± 15.00 | 233.44 ± 22.76 |
| C3-1NC-5NTO | 52.97 ± 3.40 | 68.22 ± 4.48 | 103.09± 12.03 | 232.68 ± 18.99 |
| C3-1NC-10NTO | 49.82 ± 2.19 | 71.66 ± 5.71 | 129.10 ± 3.36 | 311.18 ± 37.94 |
Appendix A.2. SEM-EDS Tests

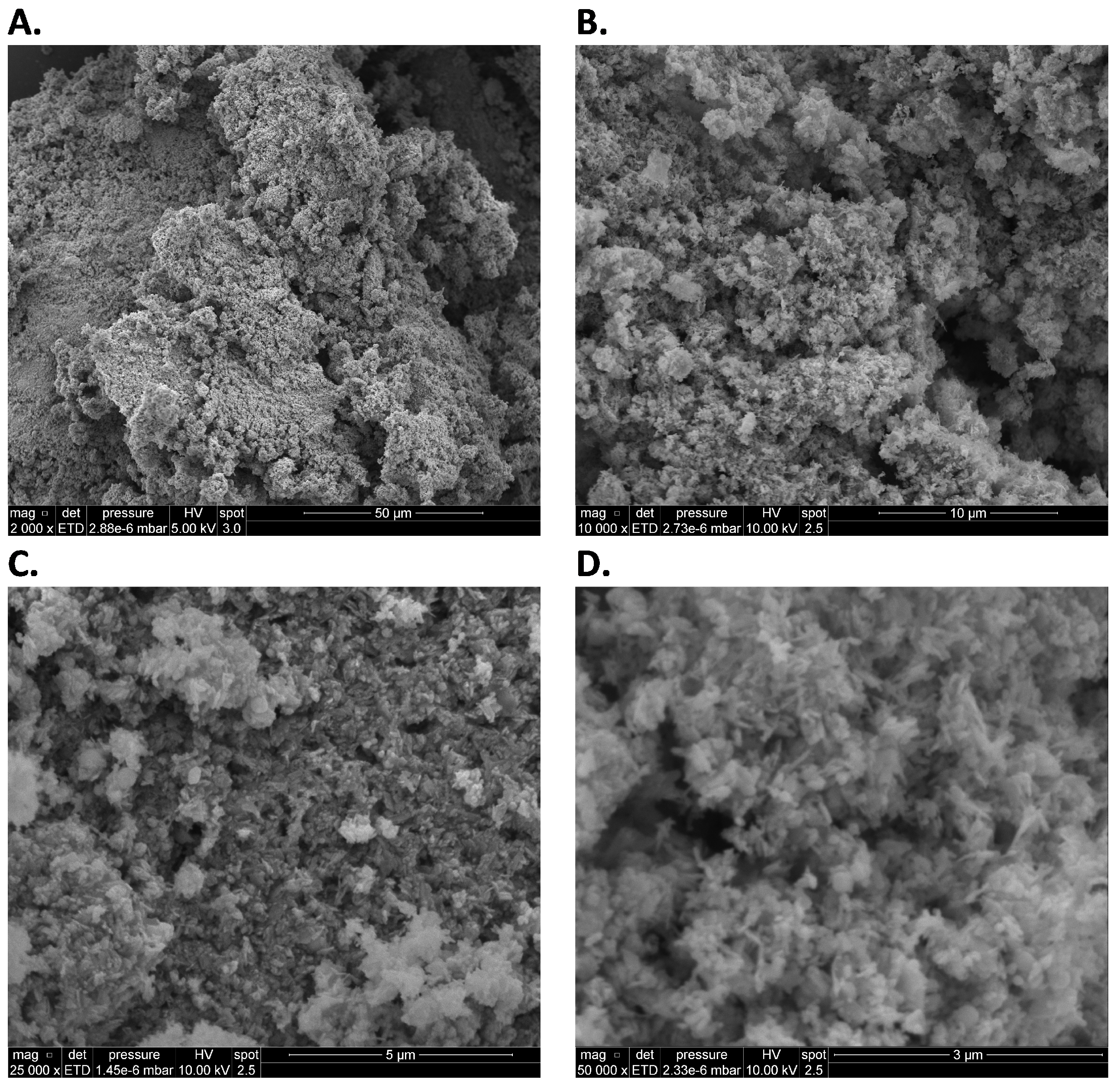
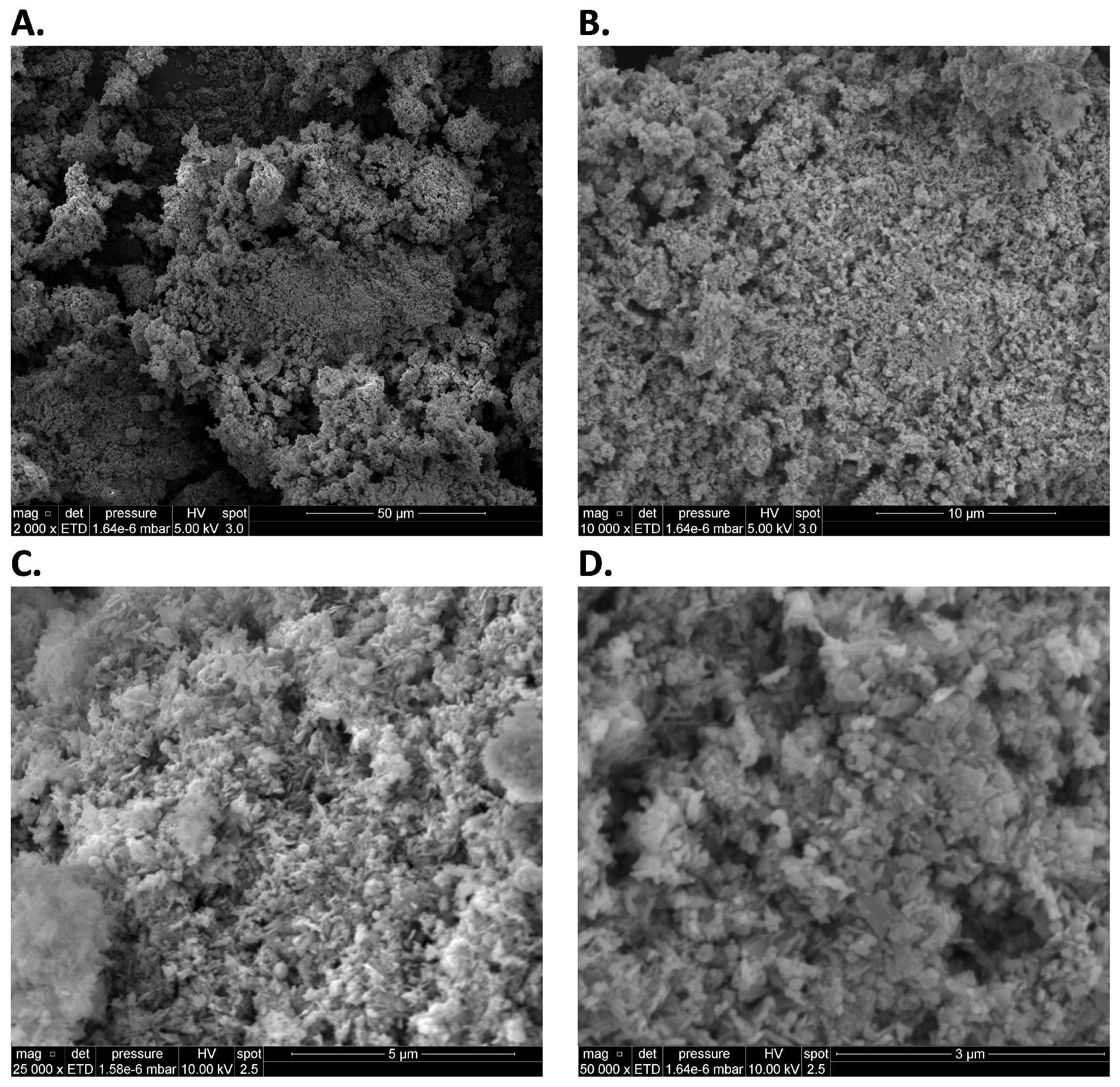
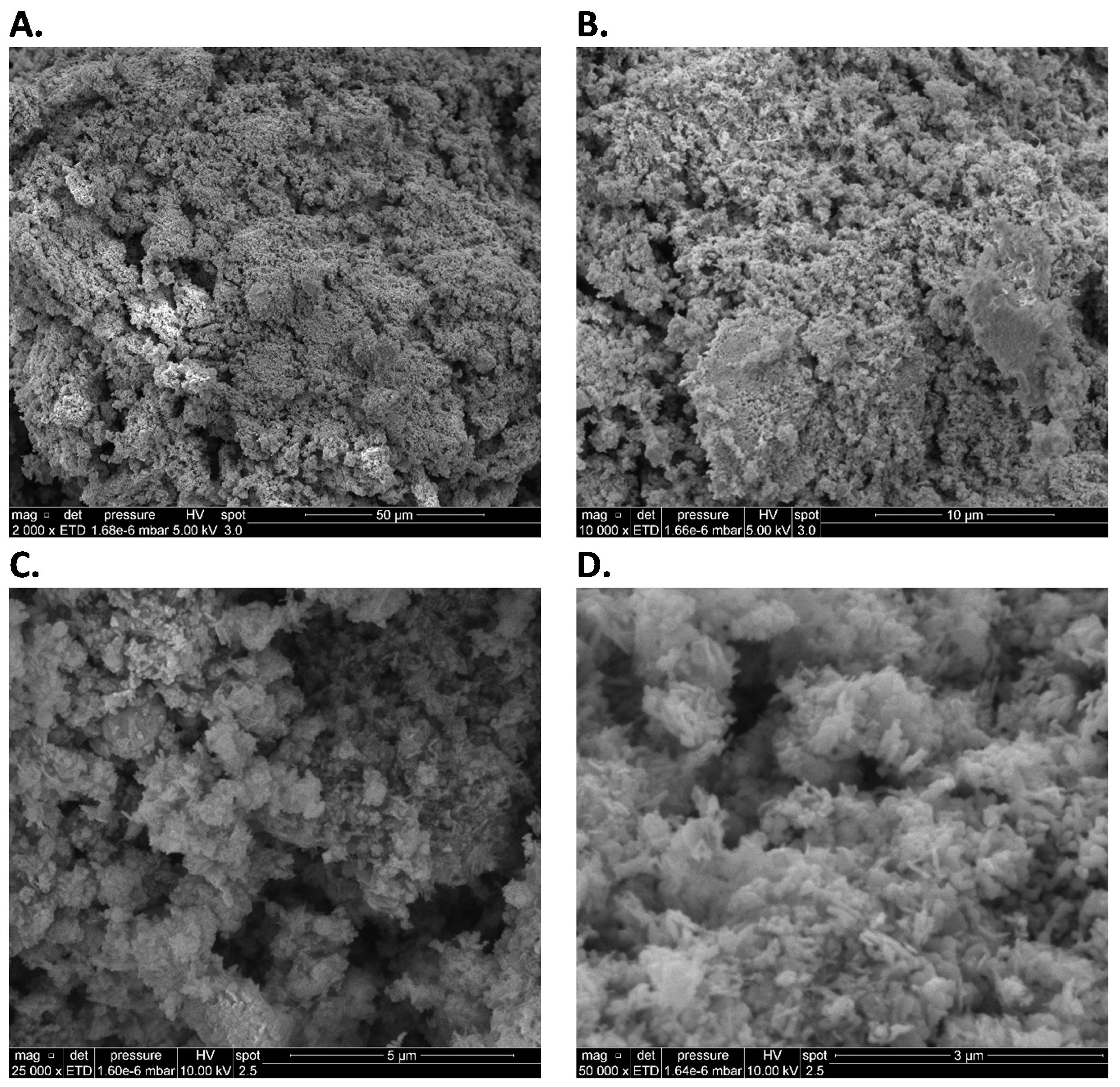
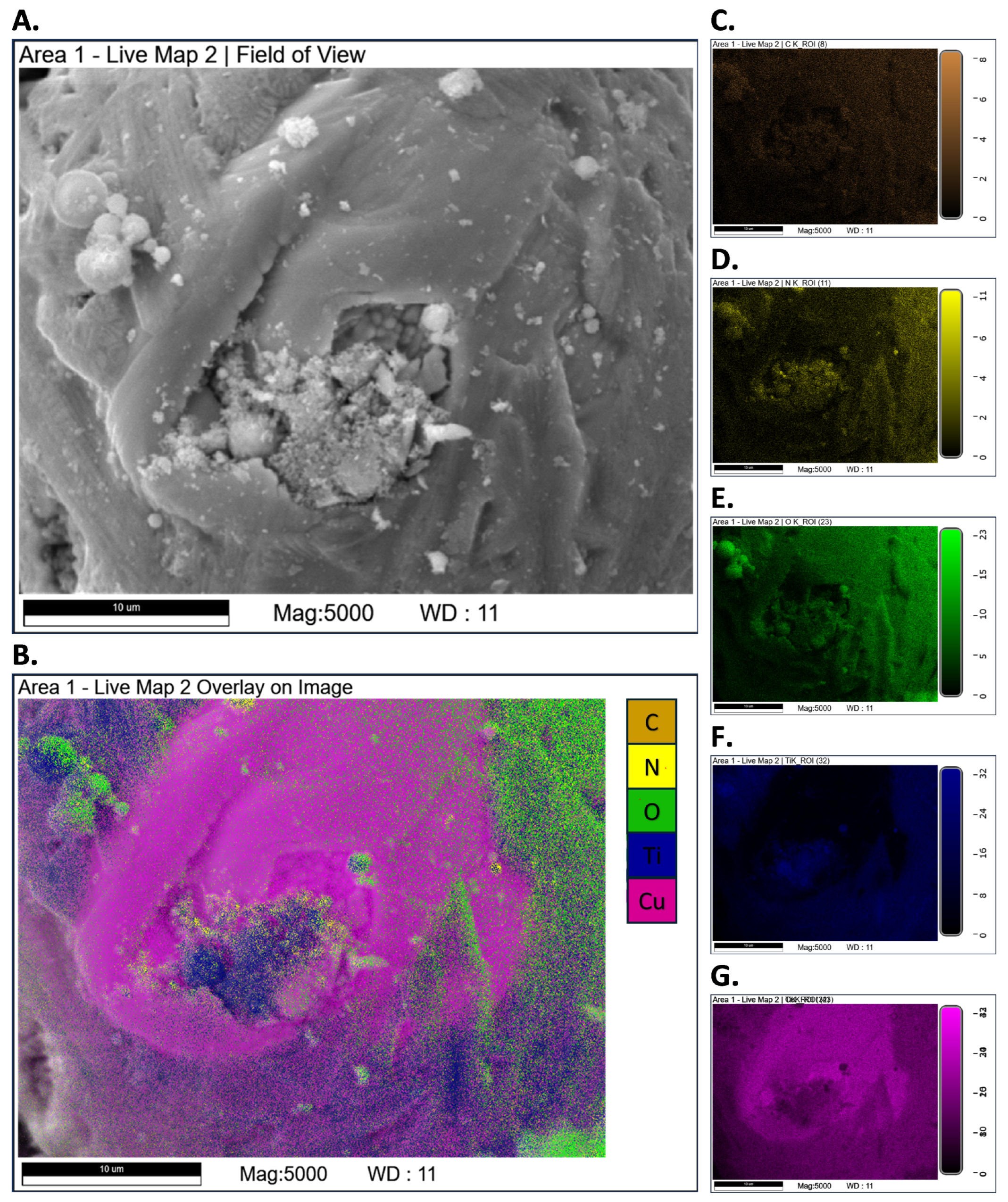
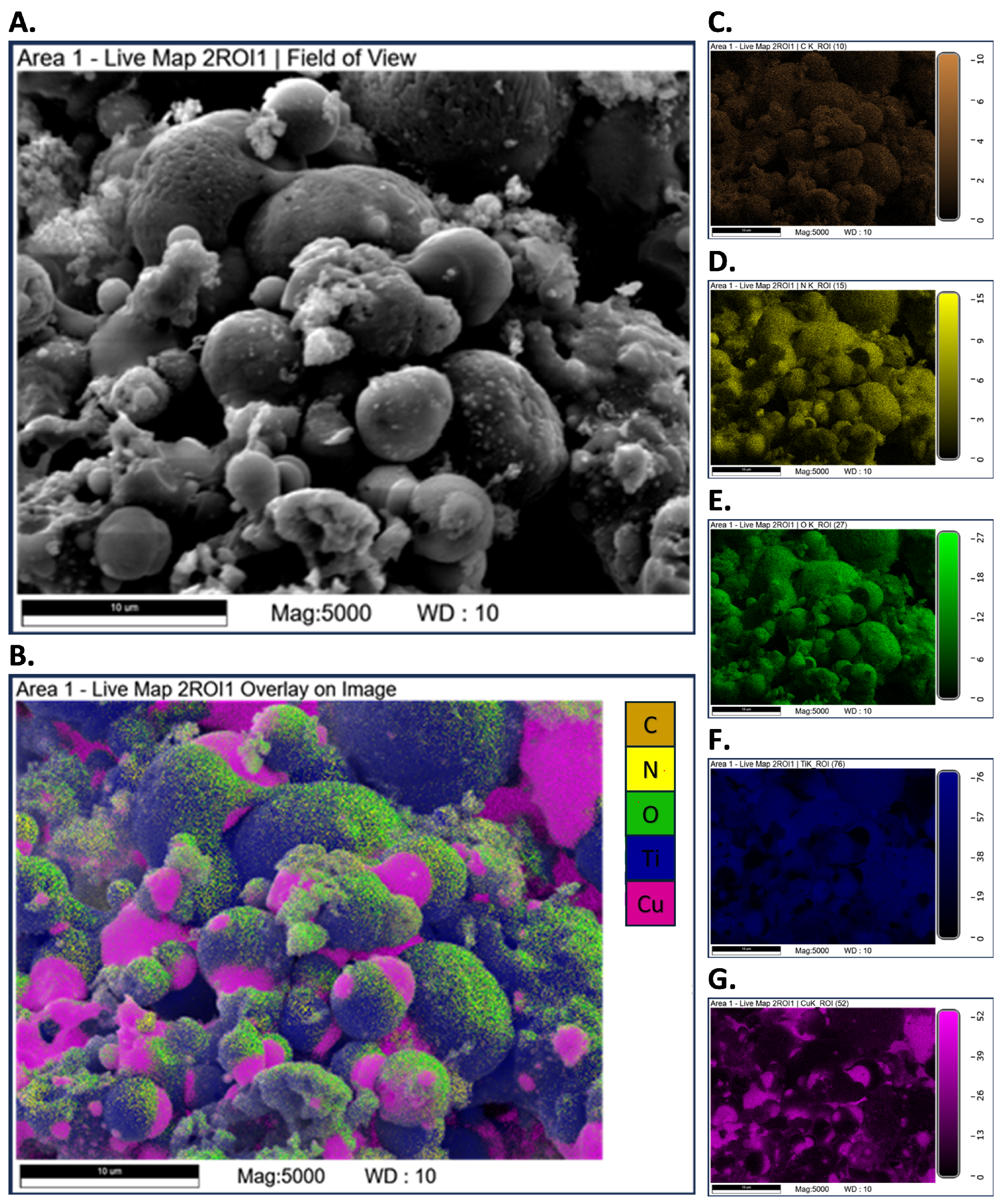
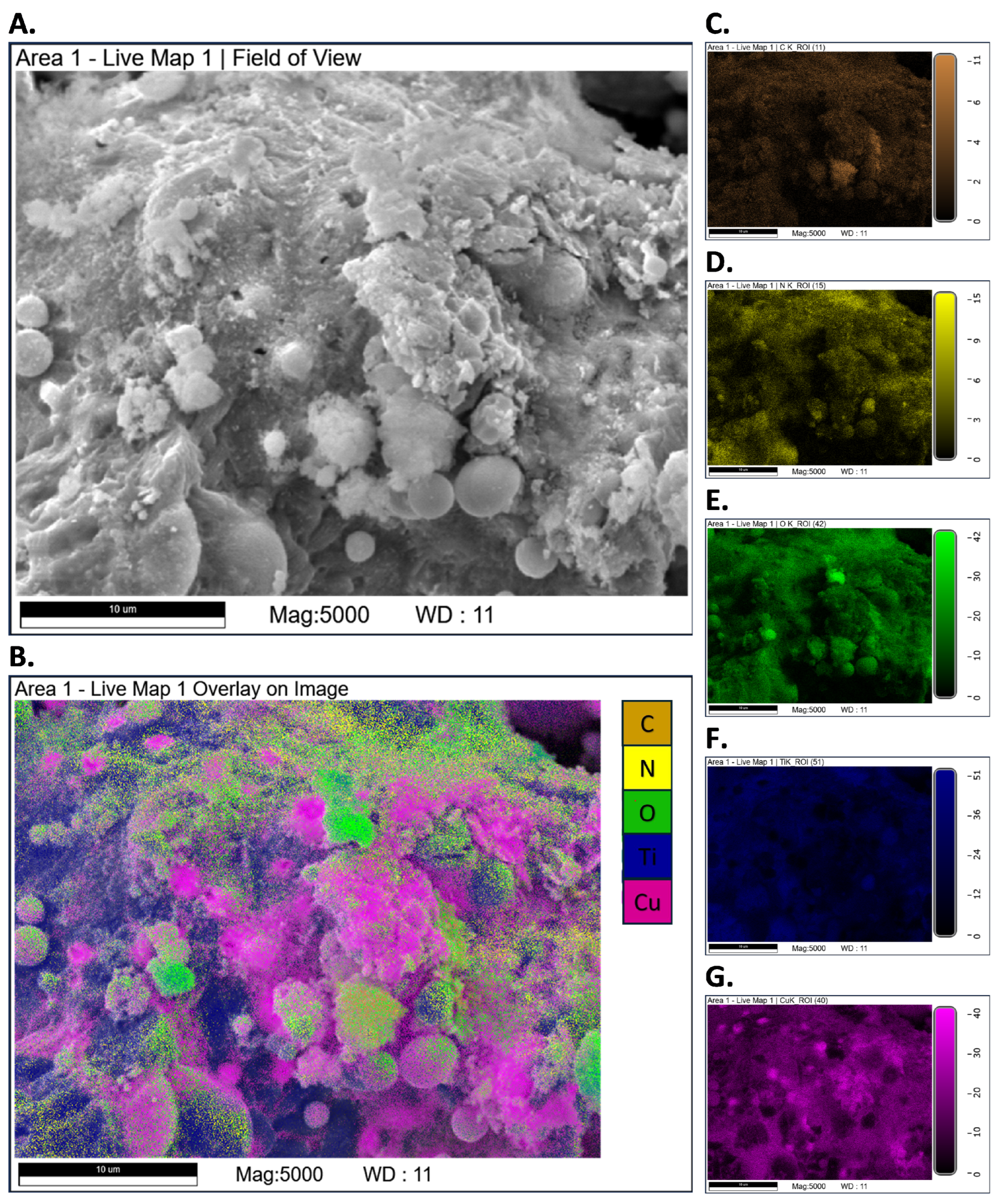
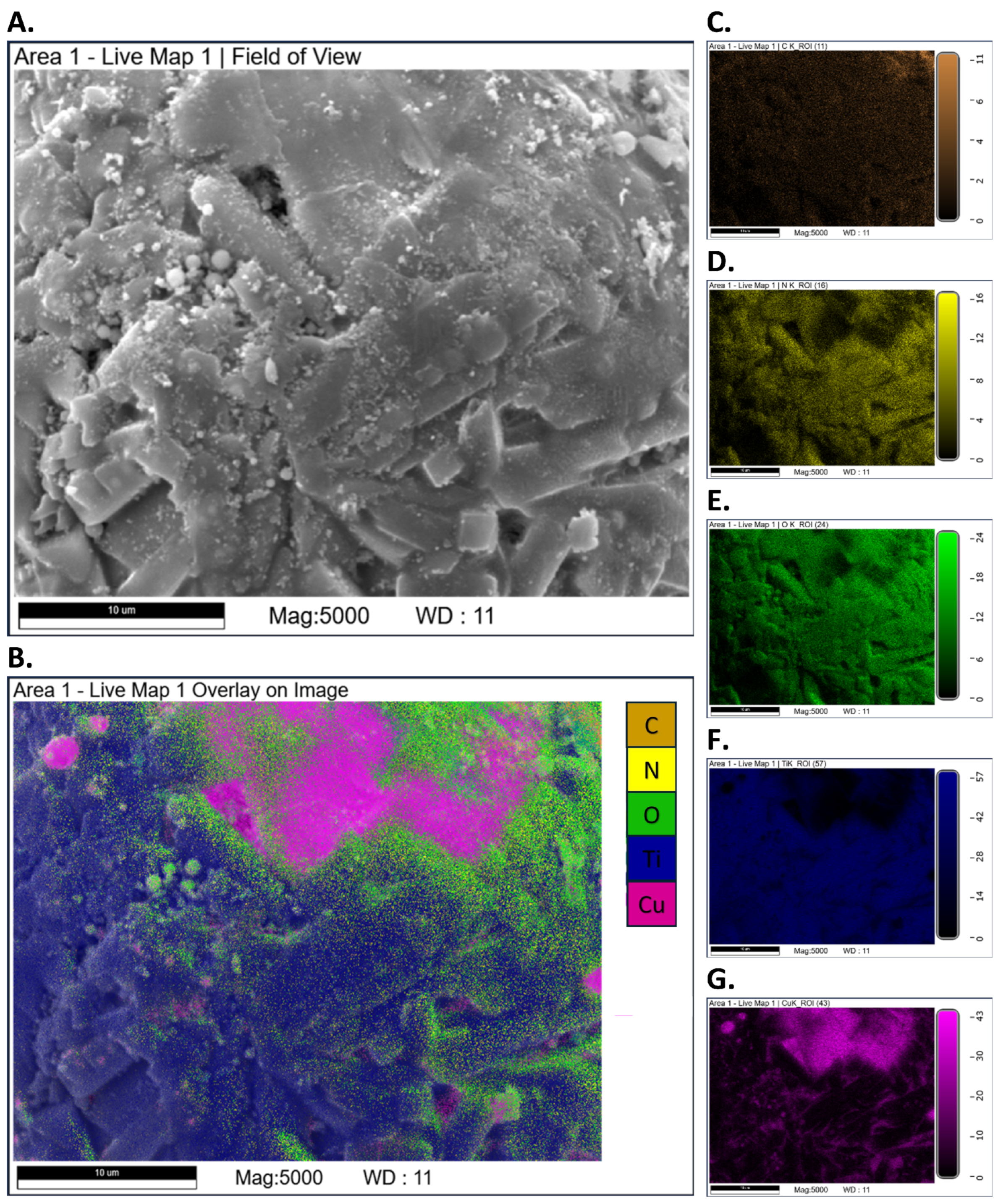
Appendix A.3. Closed-Vessel Combustion
| Composition | Combustion Time [ms] | Pmax [kPa] | [] |
|---|---|---|---|
| C3-1NTO | 106.5 ± 9.32 | 271.1 ± 4.27 | 69.0 ± 15.08 |
| C3-3NTO | 64.2 ± 5.75 | 278.1 ± 7.47 | 92.6 ± 10.52 |
| C3-5NTO | 109.2 ± 9.31 | 406.7 ± 11.54 | 81.1 ± 14.54 |
| C3-10NTO | 118.2 ± 9.22 | 422.5 ± 10.23 | 77.13 ± 1.69 |
Appendix A.4. Thrust Force
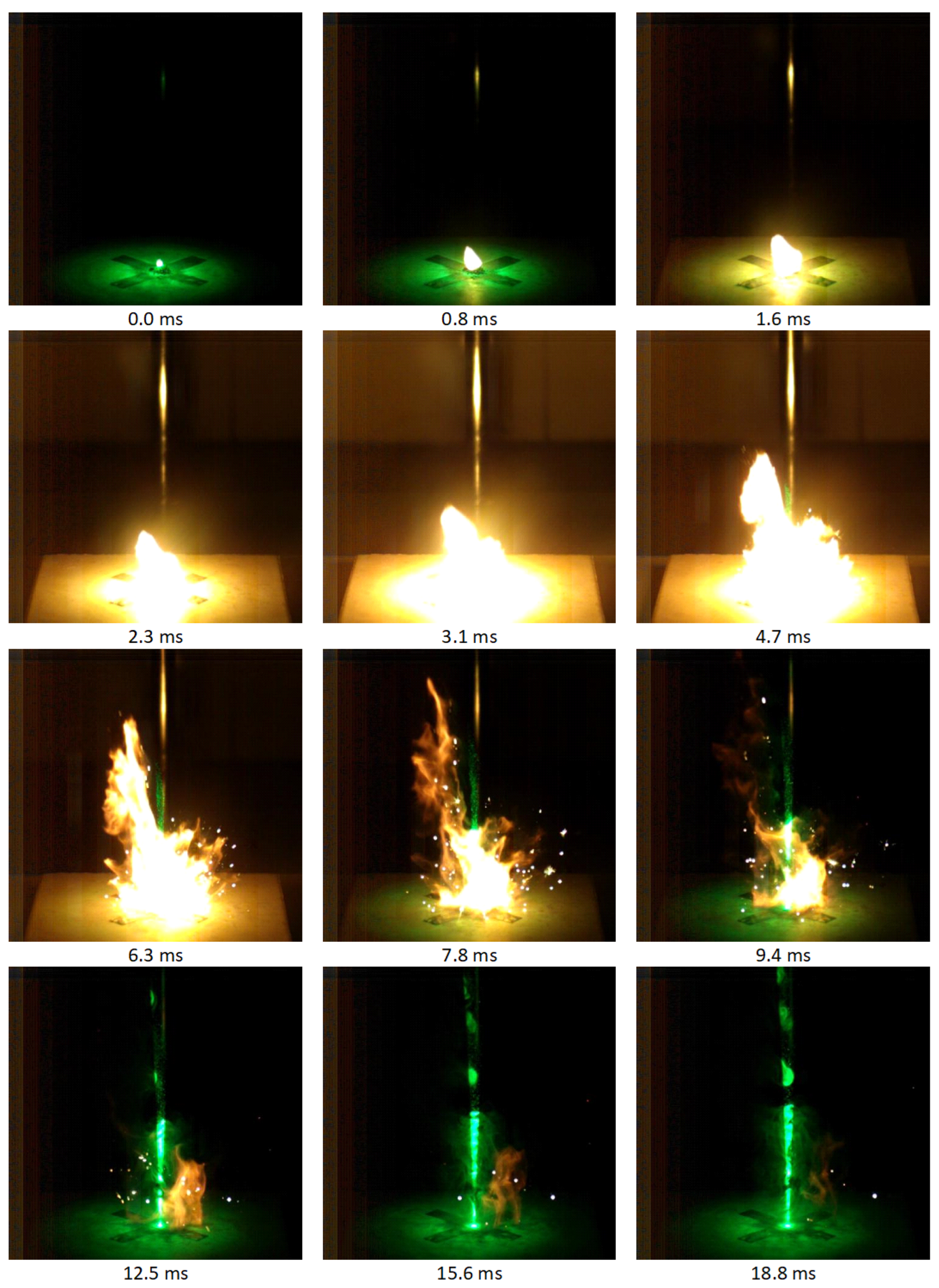
| Composition | Fmax [mN] | Combustion Time [ms] | Total Impulse [s] |
|---|---|---|---|
| C3-1NC-1NTO | 597.2 ± 76.3 | 3.31 ± 0.43 | 4.76 ± 0.27 |
| C3-1NC-3NTO | 769.2± 134.1 | 3.58 ± 0.27 | 5.77 ± 0.79 |
| C3-1NC-5NTO | 254.6 ± 26.9 | 5.52 ± 0.40 | 3.28 ± 0.51 |
| C3-1NC-10NTO | 163.8± 36.7 | 7.03 ± 1.74 | 3.06 ± 0.37 |


References
- Fahd, A.; Zorainy, M.Y.; Dubois, C.; Boffito, D.C.; Chaouki, J.; Wen, J.Z. Combustion characteristics of EMOFs/oxygenated salts novel thermite for green energetic applications. Thermochim. Acta 2021, 704, 179019. [Google Scholar] [CrossRef]
- Nicollet, A.; Salvagnac, L.; Baijot, V.; Estève, A.; Rossi, C. Fast circuit breaker based on integration of Al/CuO nanothermites. Sens. Actuators A Phys. 2018, 273, 249–255. [Google Scholar] [CrossRef]
- Sikder, A.; Sikder, N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard. Mater. 2004, 112, 1–15. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–general aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A. Nanotechnology innovations, industrial applications and patents. Environ. Chem. Lett. 2017, 15, 185–191. [Google Scholar] [CrossRef]
- Trache, D.; DeLuca, L.T. Nanoenergetic Materials: Preparation, Properties, and Applications. Nanomaterials 2020, 10, 2347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Torabi, M.; Lu, J.; Shen, R.; Zhang, K. Nanostructured energetic composites: Synthesis, ignition/combustion modeling, and applications. ACS Appl. Mater. Interfaces 2014, 6, 3058–3074. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Chen, S.; Zhang, W.; Cui, H.; Qin, Z.; Xu, K. Self-assembly preparation of advanced metastable MCo2O4/GO/Al (M = Cu, Mg, Zn, Ni) nanothermites to realize large heat release, stable combustion and high safety. Ceram. Int. 2022, 48, 20825–20837. [Google Scholar] [CrossRef]
- Emam, A.F.E. Synthesis and Characterization of Nanothermites for Energetic Applications. Ph.D. Thesis, Ecole Polytechnique, Montreal, QC, Canada, 2021. [Google Scholar]
- Sui, H.; Atashin, S.; Wen, J.Z. Thermo-chemical and energetic properties of layered nano-thermite composites. Thermochim. Acta 2016, 642, 17–24. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Li, G.; Luo, Y. Tuning the reactivity of Al/Fe2O3 nanoenergetic materials via an approach combining soft template self-assembly with sol–gel process process. J. Solid State Chem. 2015, 230, 1–7. [Google Scholar] [CrossRef]
- Zaky, M.G.; Abdalla, A.M.; Sahu, R.P.; Puri, I.K.; Radwan, M.; Elbasuney, S. Nanothermite colloids: A new prospective for enhanced performance. Def. Technol. 2019, 15, 319–325. [Google Scholar] [CrossRef]
- Song, J.; Guo, T.; Yao, M.; Ding, W.; Zhang, X.; Bei, F.; Tang, J.; Huang, J.; Yu, Z. Thermal behavior and combustion of Al nanoparticles/MnO 2-nanorods nanothermites with addition of potassium perchlorate. RSC Adv. 2019, 9, 41319–41325. [Google Scholar] [CrossRef] [PubMed]
- Elbasuney, S.; Yehia, M.; Ismael, S.; Saleh, A.; Fahd, A.; El-Shaer, Y. Nitrocellulose catalyzed with nanothermite particles: Advanced energetic nanocomposite with superior decomposition kinetics. J. Energetic Mater. 2023, 41, 550–565. [Google Scholar] [CrossRef]
- Fahd, A.; Baranovsky, A.; Dubois, C.; Chaouki, J.; Wen, J.Z. Superior performance of quaternary NC/GO/Al/KClO4 nanothermite for high speed impulse small-scale propulsion applications. Combust. Flame 2021, 232, 111527. [Google Scholar] [CrossRef]
- Kim, W.D.; Lee, S.; Lee, D.C. Nanothermite of Al nanoparticles and three-dimensionally ordered macroporous CuO: Mechanistic insight into oxidation during thermite reaction. Combust. Flame 2018, 189, 87–91. [Google Scholar] [CrossRef]
- Guo, X.; Liang, T.; Islam, M.L.; Chen, X.; Wang, Z. Highly reactive thermite energetic materials: Preparation, characterization, and applications: A review. Molecules 2023, 28, 2520. [Google Scholar] [CrossRef]
- Fahd, A.; Baranovsky, A.; Dubois, C.; Chaouki, J.; Elbasuney, S.; Shokry, S. Thrust characteristics of nano-carbon/Al/oxygenated salt nanothermites for micro-energetic applications. Def. Technol. 2023, 30, 55–69. [Google Scholar] [CrossRef]
- Thakur, P.; Sharma, V.; Thakur, N. Study of energy release in Fe2O3/Al nano-thermite with graphene as an additional fuel. Phys. B Condens. Matter 2021, 610, 412803. [Google Scholar] [CrossRef]
- Song, J.; Duan, F.; Chen, J.; Wang, G.; Tian, Q.; Feng, J. Thermal and combustion behaviors of aluminum/manganese dioxide/fluoroelastomer terpolymer nanothermite. Eng. Rep. 2023, 5, e12587. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, S.; Li, G.; Luo, Y. Effect of fluoropolymer content on thermal and combustion performance of direct writing high-solid nanothermite composite. RSC Adv. 2022, 12, 5612–5618. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Singh, V.; Julien, B.; Tenailleau, C.; Estève, A.; Rossi, C. Pioneering insights into the superior performance of titanium as a fuel in energetic materials. Chem. Eng. J. 2023, 453, 139922. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Energetic nanoparticles and nanomaterials for future defense applications. Hum. Factors Mech. Eng. Def. Saf. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Dang, J.; Ma, Y.; Ma, H. Hematite: A good catalyst for the thermal decomposition of energetic materials and the application in nano-thermite. Molecules 2023, 28, 2035. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Huang, Q.; Liu, F.; Xie, Q.; Hu, M.; Su, J.; Zhang, C.; Zeng, Y.; Zhu, C. A novel nano-thermite system with BiOF as fluorine-containing oxidant for enhanced energy release performance. Chem. Eng. J. 2023, 468, 143591. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, G.; Zhu, M.; Jiang, X. Constant volume combustion properties of Al/Fe2O3/RDX nanocomposite: The effects of its particle size and chemical constituents. Combust. Flame 2022, 238, 111938. [Google Scholar] [CrossRef]
- Nie, H.q.; Chan, H.Y.; Pisharath, S.; Hng, H.H. Combustion characteristic and aging behavior of bimetal thermite powders. Def. Technol. 2021, 17, 755–762. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Lin, K.; Hao, D.; Qiu, L.; Wang, D.; Fan, R.; Xia, D. Ammonium perchlorate encapsulating nanothermites as high energetic composites: Preparation, thermal decomposition and combustion properties. Chem. Eng. Sci. 2019, 207, 334–343. [Google Scholar] [CrossRef]
- Kim, S.; Johns, A.A.; Wen, J.Z.; Deng, S. Burning structures and propagation mechanisms of nanothermites. Proc. Combust. Inst. 2023, 39, 3593–3604. [Google Scholar] [CrossRef]
- Gandhi, P.M.; Schoenitz, M.; Dreizin, E.L. Evaluation and design of metal-based gas-generating energetic materials. Combust. Flame 2023, 249, 112615. [Google Scholar] [CrossRef]
- EN 13631-3:2005; Explosives for Civil Uses—High Explosives—Part 3: Determination of Sensitiveness to Friction of Explosives. European Committee for Standardization: Belgium, Brussels, 2005.
- EN 13631-4:2002; Explosives for Civil Uses—High Explosives—Part 4: Determination of Sensitiveness to Impact of Explosives. European Committee for Standardization: Belgium, Brussels, 2002.
- Shi, L.; Zhang, W.; Cheng, J.; Yu, C.; Shen, R.; Ye, J.; Qin, Z.; Chao, Y. A high energy output and low onset temperature nanothermite based on three-dimensional ordered macroporous nano-NiFe2O4. RSC Adv. 2016, 6, 93330–93334. [Google Scholar] [CrossRef]
- Gibot, P.; Goetz, V. SnO2–polyaniline composites for the desensitization of Al/SnO2 thermite composites. J. Appl. Polym. Sci. 2020, 137, 48947. [Google Scholar] [CrossRef]
- Chen, L.; Ru, C.; Zhang, H.; Zhang, Y.; Chi, Z.; Wang, H.; Li, G. Assembling hybrid energetic materials with controllable interfacial microstructures by electrospray. ACS Omega 2021, 6, 16816–16825. [Google Scholar] [CrossRef] [PubMed]
- Paszula, J.M.; Kowalewski, E. Badanie procesu rozwoju detonacji nieidealnych materiałów wybuchowych. Mater. Wysokoenergetyczne 2015, 7, 95–105. [Google Scholar]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermites: A short review. Factsheet for experimenters, present and future challenges. Propellants Explos. Pyrotech. 2019, 44, 18–36. [Google Scholar] [CrossRef]
- Chernai, A.V. On the mechanism of ignition of condensed secondary explosives by a laser pulse. Combust. Explos. Shock Waves 1996, 32, 8–15. [Google Scholar] [CrossRef]
- Lawless, Z.D.; Hobbs, M.L.; Kaneshige, M.J. Thermal conductivity of energetic materials. J. Energetic Mater. 2020, 38, 214–239. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley Online Library: Hoboken, NJ, USA, 2006. [Google Scholar]
- Jegadheeswaran, S.; Sundaramahalingam, A.; Pohekar, S.D. High-conductivity nanomaterials for enhancing thermal performance of latent heat thermal energy storage systems. J. Therm. Anal. Calorim. 2019, 138, 1137–1166. [Google Scholar] [CrossRef]
- Shouman, A.R. A review of one aspect of the thermal-explosion theory. J. Eng. Math. 2006, 56, 179–184. [Google Scholar] [CrossRef]
- Gouma, P.I.; Mills, M.J. Anatase-to-rutile transformation in titania powders. J. Am. Ceram. Soc. 2001, 84, 619–622. [Google Scholar] [CrossRef]
- Bolotina, N.; Kirschbaum, K.; Pinkerton, A.A. Energetic materials: α-NTO crystallizes as a four-component triclinic twin. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Pourmortazavi, S.M.; Rahimi-Nasrabadi, M.; Kohsari, I.; Hajimirsadeghi, S.S. Non-isothermal kinetic studies on thermal decomposition of energetic materials: KNF and NTO. J. Therm. Anal. Calorim. 2012, 110, 857–863. [Google Scholar] [CrossRef]
- Lee, J.S.; Jaw, K.S. Thermal decomposition properties and compatibility of CL-20, NTO with silicone rubber. J. Therm. Anal. Calorim. 2006, 85, 463–467. [Google Scholar] [CrossRef]
- Nie, H.; Tan, L.P.; Pisharath, S.; Hng, H.H. Nanothermite composites with a novel cast curable fluoropolymer. Chem. Eng. J. 2021, 414, 128786. [Google Scholar] [CrossRef]
- Apperson, S.J. Characterization and MEMS Applications of Nanothermite Materials; University of Missouri-Columbia: Columbia, MI, USA, 2010. [Google Scholar]
- Apperson, S.; Shende, R.; Subramanian, S.; Tappmeyer, D.; Gangopadhyay, S.; Chen, Z.; Gangopadhyay, K.; Redner, P.; Nicholich, S.; Kapoor, D. Generation of fast propagating combustion and shock waves with copper oxide/aluminum nanothermite composites. Appl. Phys. Lett. 2007, 91, 2787972. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, J.; Xu, J.; Shen, Y.; Wang, C.a.; Ye, Y.; Shen, R. Experimental and numerical investigations of the effect of charge density and scale on the heat transfer behavior of Al/CuO nano-thermite. Vacuum 2021, 184, 109878. [Google Scholar] [CrossRef]











| Composition Name | NTO wt.% | Ti wt.% | CuO wt.% | NC wt.% |
|---|---|---|---|---|
| 1NTO | 1 | 29 | 69 | 1 |
| 3NTO | 3 | 28 | 68 | 1 |
| 5NTO | 5 | 28 | 66 | 1 |
| 10NTO | 10 | 26 | 63 | 1 |
| Sample | Friction Sensitivity [N] | Impact Sensitivity [J] |
|---|---|---|
| 1NTO | 128 | >50 |
| 3NTO | 96 | >50 |
| 5NTO | 84 | >50 |
| 10NTO | 40 | >50 |
| NTO | >360 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polis, M.; Stolarczyk, A.; Szydło, K.; Lisiecka, B.; Procek, M.; Sławski, S.; Gołofit, T.; Hawelek, Ł.; Jarosz, T. Novel NSTEX System Based on Ti/CuO/NC Nanothermite Doped with NTO. Energies 2024, 17, 3675. https://doi.org/10.3390/en17153675
Polis M, Stolarczyk A, Szydło K, Lisiecka B, Procek M, Sławski S, Gołofit T, Hawelek Ł, Jarosz T. Novel NSTEX System Based on Ti/CuO/NC Nanothermite Doped with NTO. Energies. 2024; 17(15):3675. https://doi.org/10.3390/en17153675
Chicago/Turabian StylePolis, Mateusz, Agnieszka Stolarczyk, Konrad Szydło, Barbara Lisiecka, Marcin Procek, Sebastian Sławski, Tomasz Gołofit, Łukasz Hawelek, and Tomasz Jarosz. 2024. "Novel NSTEX System Based on Ti/CuO/NC Nanothermite Doped with NTO" Energies 17, no. 15: 3675. https://doi.org/10.3390/en17153675





