Structural and Compositional Analysis of CZTSSe Thin Films by Varying S/(S+Se) Ratio
Abstract
:1. Introduction
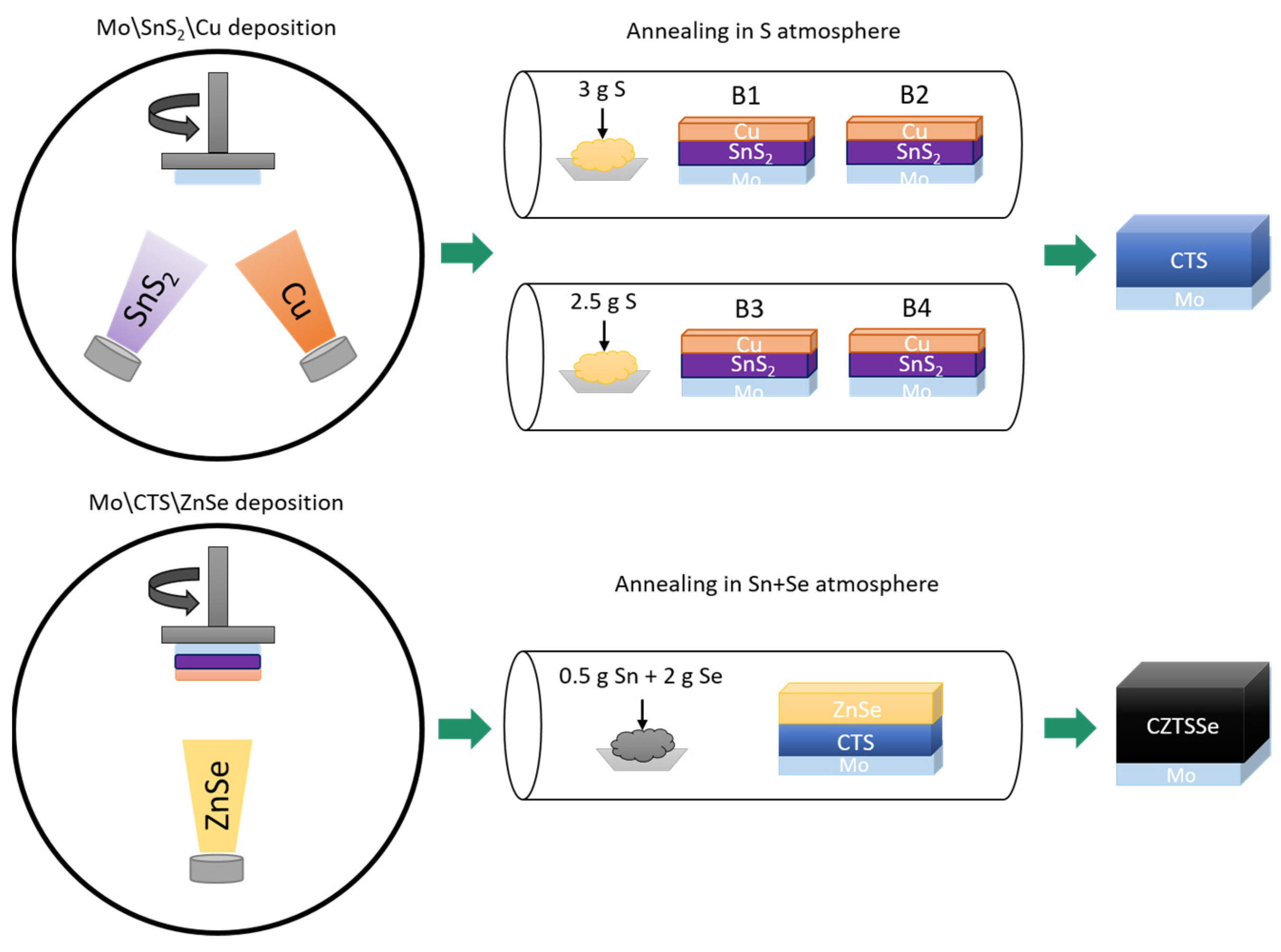
2. Materials and Methods
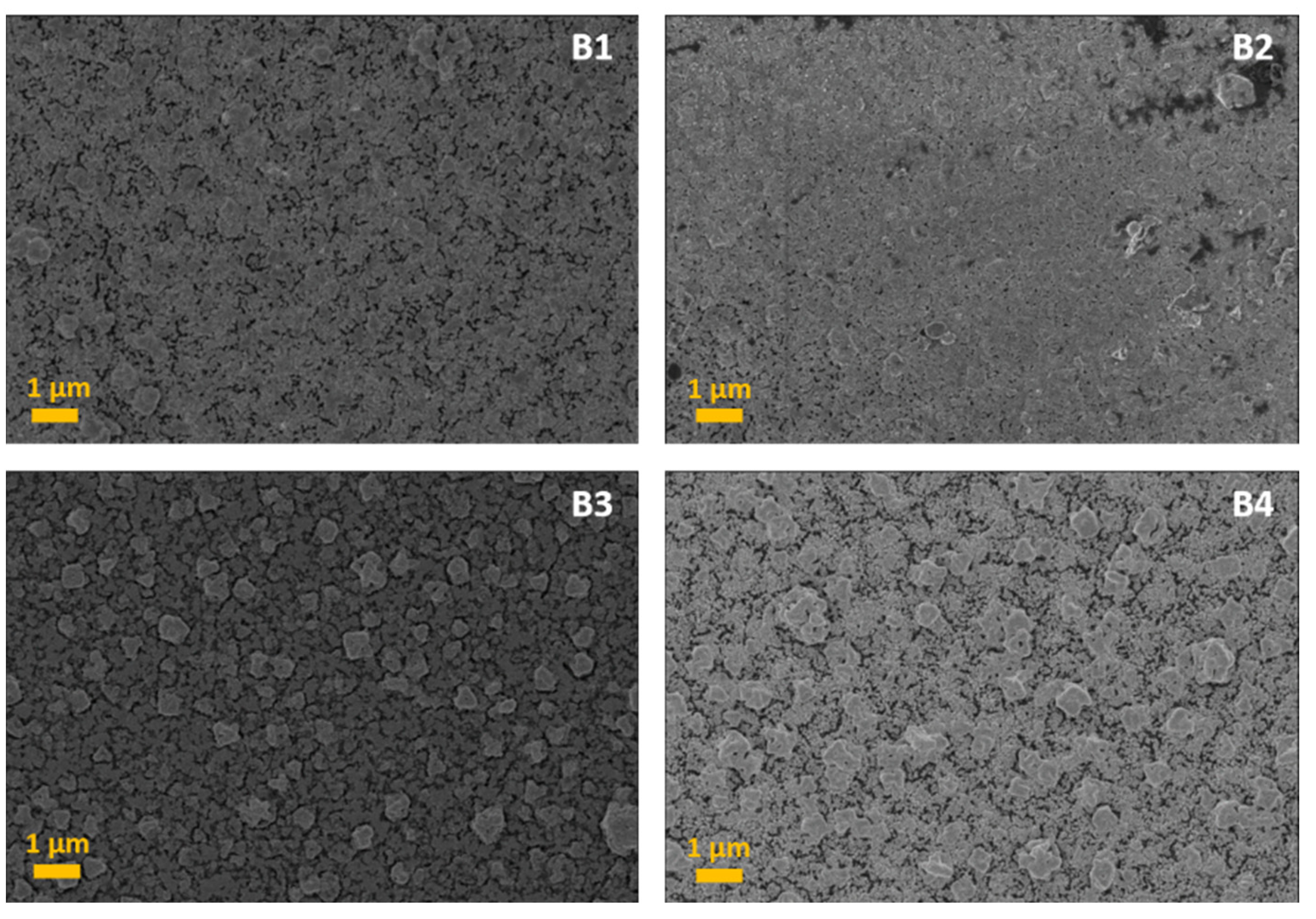
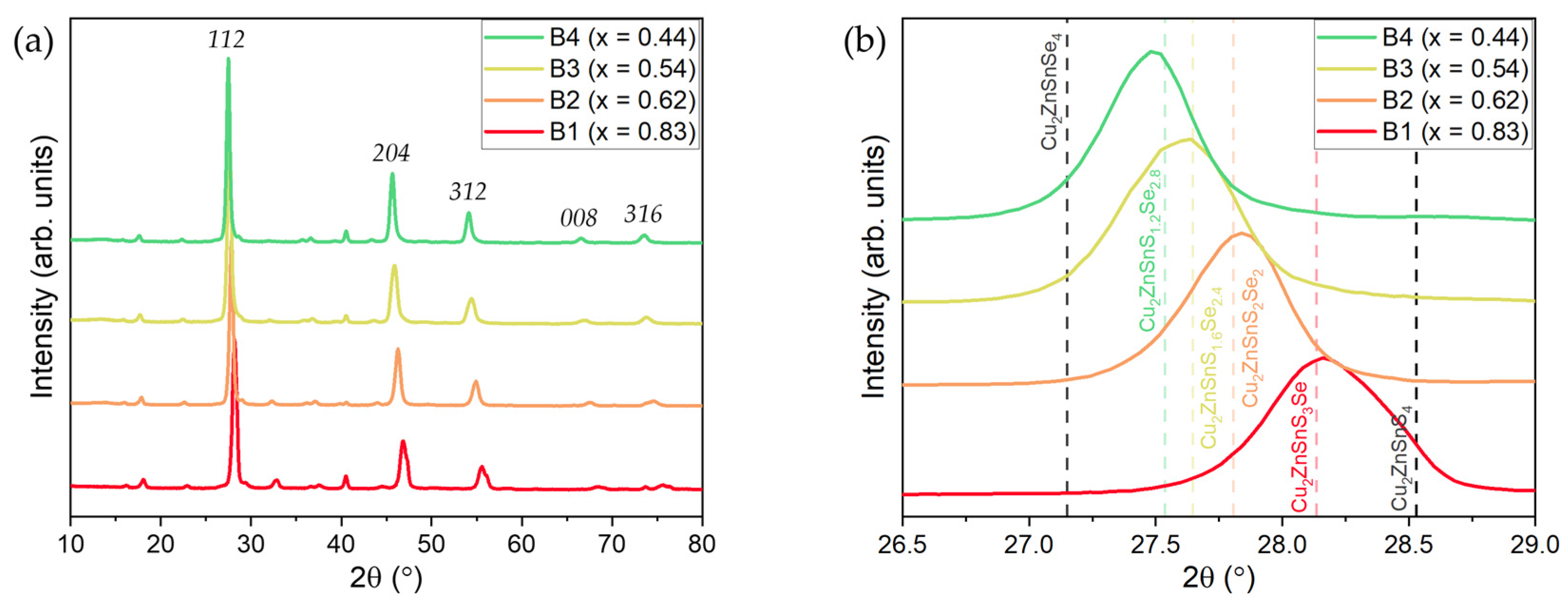
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, B.; Deng, Y.; Cao, L.; Zhu, J.; Zhou, Z. Doping of Sb into Cu2ZnSn(S,Se)4 Absorber Layer via Se&Sb2Se3 Co-Selenization Strategy for Enhancing Open-Circuit Voltage of Kesterite Solar Cells. Front. Chem. 2022, 10, 974761. [Google Scholar] [CrossRef]
- El Khouja, O.; Nouneh, K.; Ebn Touhami, M.; Matei, E.; Stancu, V.; Enculescu, M.; Galca, A.C. Growth and Characterization of Cu–Ni–Sn–S Films Electrodeposited at Different Applied Potentials. J. Mater. Sci. Mater. Electron. 2023, 34, 760. [Google Scholar] [CrossRef]
- Azmi, S.; Layachi, O.A.; Ouardi, M.E.; Khoumri, E.M.; Moujib, A.; Brouzi, A.E.; Nohair, M.; Pezzato, L.; Dabala, M. Growth of Cu2ZnSnS4 Thin Film Absorber Layer on Transparent Conductive Oxides and Molybdenum Substrates by Electrodeposition for Photovoltaic Application. Optik 2022, 250, 168320. [Google Scholar] [CrossRef]
- Azmi, S.; Khoumri, E.M.; Marrakchi, M.E.; Pezzato, L.; Nohair, M.; Dabala, M. Structural and Optical Annealing Route-Dependent Properties of CZTS Thin Films Grown by One-Step Electrodeposition with Free Annealing Sulfurization for Photovoltaic Application. J. Elec Mater. 2019, 48, 8254–8260. [Google Scholar] [CrossRef]
- Boerasu, I.; Vasile, B.S. Current Status of the Open-Circuit Voltage of Kesterite CZTS Absorber Layers for Photovoltaic Applications—Part I, a Review. Materials 2022, 15, 8427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-C.; Tang, J.-Y.; Niu, Y.-H.; Huang, R.; Chen, L.; Jiang, M.-Y. Study the Best Ratio of S and Se in CZTSSe Solar Cells with Nontoxic Buffer Layer. J. Renew. Sustain. Energy 2021, 13, 033701. [Google Scholar] [CrossRef]
- Zein, W.; Alanazi, T.I.; Salah, M.M.; Saeed, A. Concurrent Design of Alloy Compositions of CZTSSe and CdZnS Using SCAPS Simulation: Potential Routes to Overcoming VOC Deficit. Energies 2023, 16, 5754. [Google Scholar] [CrossRef]
- Khare, A.; Himmetoglu, B.; Cococcioni, M.; Aydil, E.S. First Principles Calculation of the Electronic Properties and Lattice Dynamics of Cu2ZnSn(S1−xSex)4. J. Appl. Phys. 2012, 111, 123704. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhou, Z.; Zhou, W.; Kou, D.; Meng, Y.; Qi, Y.; Yuan, S.; Han, L.; Wu, S. Progress and Prospectives of Solution-Processed Kesterite Absorbers for Photovoltaic Applications. Nanoscale 2023, 15, 8900–8924. [Google Scholar] [CrossRef]
- Jeong, W.-L.; Min, J.-H.; Kim, H.-S.; Kim, J.-H.; Kim, J.-H.; Lee, D.-S. Quantification of Effective Thermal Conductivity in the Annealing Process of Cu2ZnSn(S,Se)4 Solar Cells with 9.7% Efficiency Fabricated by Magnetron Sputtering. Sustain. Energy Fuels 2018, 2, 999–1006. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, U.P. Formation of CZTSSe Absorber Layer Using Thiourea Treatment of CZTSe. Mater. Today Proc. 2021, 39, 1838–1842. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Mali, S.S.; Agwane, G.L.; Kamble, A.; Kim, I.Y.; Patil, P.S.; Kim, J.Y.; Kim, J.H. Influence of Laser Repetition Rate on the Cu2ZnSn(SSe)4 Thin Films Synthesized via Pulsed Laser Deposition Technique. Sol. Energy Mater. Sol. Cells 2016, 157, 331–336. [Google Scholar] [CrossRef]
- Shen, X.; Tang, C.; Zhang, C.; Li, G.; Zhao, Y.; Li, W.; Chen, G.; Yang, T. CuZnSn(SxSe1−x)4 Solar Cell Prepared by the Sol-Gel Method Following a Modified Three-Step Selenization Process. Crystals 2019, 9, 474. [Google Scholar] [CrossRef]
- Qin, X.; Xu, B.; Lin, J.; Chen, J.; Tong, H.; Chen, Y.; Yang, P.; Chu, J.; Sun, L. Above 10% Efficient Electrodeposited Cu2ZnSn(S,Se)4 Solar Cell Achieved by Modifying Precursor. Sol. Energy Mater. Sol. Cells 2022, 242, 111781. [Google Scholar] [CrossRef]
- Son, D.-H.; Kim, Y.-I.; Kim, S.-H.; Nam, D.; Cheong, H.; Kang, J.-K.; Yang, K.-J.; Kim, D.-H. Effects of S and Se Contents on the Physical and Photovoltaic Properties of Cu2ZnSn(SX,Se1−X)4 Thin Films: Achieving a PCE of 9.47%. J. Mater. Chem. A 2019, 7, 22986–22995. [Google Scholar] [CrossRef]
- Taskesen, T.; Pareek, D.; Hauschild, D.; Haertel, A.; Weinhardt, L.; Yang, W.; Pfeiffelmann, T.; Nowak, D.; Heske, C.; Gütay, L. Steep Sulfur Gradient in CZTSSe Solar Cells by H2S-Assisted Rapid Surface Sulfurization. RSC Adv. 2021, 11, 12687–12695. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Impact of Selenium Composition Variation in CZTS Solar Cell. Optik 2021, 234, 166421. [Google Scholar] [CrossRef]
- Xie, H.; Dimitrievska, M.; Fontané, X.; Sánchez, Y.; López-Marino, S.; Izquierdo-Roca, V.; Bermúdez, V.; Pérez-Rodríguez, A.; Saucedo, E. Formation and Impact of Secondary Phases in Cu-Poor Zn-Rich Cu2ZnSn(S1−ySey)4 (0 ≤ y ≤ 1) Based Solar Cells. Sol. Energy Mater. Sol. Cells 2015, 140, 289–298. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Xie, H.; Fairbrother, A.; Fontané, X.; Gurieva, G.; Saucedo, E.; Pérez-Rodríguez, A.; Schorr, S.; Izquierdo-Roca, V. Multiwavelength Excitation Raman Scattering of Cu2ZnSn(SxSe1−x)4 (0 ≤ x ≤ 1) Polycrystalline Thin Films: Vibrational Properties of Sulfoselenide Solid Solutions. Appl. Phys. Lett. 2014, 105, 031913. [Google Scholar] [CrossRef]
- Shi, J.J.; Wang, J.; Meng, F.; Zhou, J.; Xu, X.; Yin, K.; Lou, L.; Jiao, M.; Zhang, B.; Wu, H.; et al. Multinary alloying for facilitated cation exchange and suppressed defect formation in kesterite solar cells with above 14% certified efficiency. Nat. Energy 2024. [Google Scholar] [CrossRef]
- Woo, K.; Kim, Y.; Yang, W.; Kim, K.; Kim, I.; Oh, Y.; Kim, J.Y.; Moon, J. Band-Gap-Graded Cu2ZnSn(S1−x,Sex)4 Solar Cells Fabricated by an Ethanol-Based, Particulate Precursor Ink Route. Sci. Rep. 2013, 3, 3069. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Jeong, H.-J.; Lee, S.K.; Kim, S.-T.; Jang, J.-H. The Effect of S/(S+Se) Ratios on the Formation of Secondary Phases in the Band Gap Graded Cu2ZnSn(S,Se)4 Thin Film Solar Cells. J. Alloys Compd. 2019, 793, 289–294. [Google Scholar] [CrossRef]
- Zeng, C.; Liang, Y.; Zeng, L.; Zhang, L.; Zhou, J.; Huang, P.; Hong, R. Effect of S/(S+Se) Ratio during the Annealing Process on the Performance of Cu2ZnSn(S,Se)4 Solar Cells Prepared by Sputtering from a Quaternary Target. Sol. Energy Mater. Sol. Cells 2019, 203, 110167. [Google Scholar] [CrossRef]
- Singh, A.K.; Aggarwal, G.; Singh, R.K.; Klein, T.R.; Das, C.; Neergat, M.; Kavaipatti, B.; van Hest, M.F.A.M. Synthesis of CZTS/Se and Their Solid Solution from Electrodeposited Cu–Sn–Zn Metal Precursor: A Study of S and Se Replacement Reaction. ACS Appl. Energy Mater. 2018, 1, 3351–3358. [Google Scholar] [CrossRef]
- Enkhbat, T.; Kim, S.; Kim, J. Device Characteristics of Band Gap Tailored 10.04% Efficient CZTSSe Solar Cells Sprayed from Water-Based Solution. ACS Appl. Mater. Interfaces 2019, 11, 36735–36741. [Google Scholar] [CrossRef] [PubMed]
- Jirage, S.; Pagare, P.; Garadkar, K.; Delekar, S.; Bhuse, V. Novel Chemical Approach to Synthesis and Characterization of Greener and Rock-like Cu2ZnSnS3Se (CZTSSe) Thin Films of Kasterite Structure. Mater. Today: Proc. 2021, 43, 2774–2779. [Google Scholar] [CrossRef]
- Kokal, R.K.; Saha, S.; Deepa, M.; Ghosal, P. Non-Toxic Configuration of Indium Selenide Nanoparticles- Cu2ZnSnS2Se2 /Carbon Fabric in a Quasi Solid-State Solar Cell. Electrochim. Acta 2018, 266, 373–383. [Google Scholar] [CrossRef]
- Franckevičius, M.; Pakštas, V.; Grincienė, G.; Kamarauskas, E.; Giraitis, R.; Nekrasovas, J.; Selskis, A.; Juškėnas, R.; Niaura, G. Efficiency Improvement of Superstrate CZTSSe Solar Cells Processed by Spray Pyrolysis Approach. Sol. Energy 2019, 185, 283–289. [Google Scholar] [CrossRef]
- Lesnyak, V.; George, C.; Genovese, A.; Prato, M.; Casu, A.; Ayyappan, S.; Scarpellini, A.; Manna, L. Alloyed Copper Chalcogenide Nanoplatelets via Partial Cation Exchange Reactions. ACS Nano 2014, 8, 8407–8418. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Khot, K.V.; Mali, S.S.; Hong, C.K.; Bhosale, P.N. Investigating the Role of Selenium-Ion Concentration on Optoelectronic Properties of the Cu2ZnSn(S1–xSex)4 Thin Films. Ind. Eng. Chem. Res. 2020, 59, 10868–10881. [Google Scholar] [CrossRef]
- López-Vergara, F.; Galdámez, A.; Barahona, P.; Manríquez, V. Effect of the Selenium Content in the Optiacal Properties of the Kesterite Cu2ZnSnS4−xSex Phases. J. Chil. Chem. Soc. 2016, 61, 3291–3294. [Google Scholar] [CrossRef]
- Pakštas, V.; Grincienė, G.; Selskis, A.; Balakauskas, S.; Talaikis, M.; Bruc, L.; Curmei, N.; Niaura, G.; Franckevičius, M. Improvement of CZTSSe Film Quality and Superstrate Solar Cell Performance through Optimized Post-Deposition Annealing. Sci. Rep. 2022, 12, 16170. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.Y.; Nouneh, K.; Touhami, M.E.; Matei, E.; Badica, P.; Burdusel, M.; Negrila, C.C.; Baibarac, M.; Pintilie, L.; Galca, A.C. Influence of Boric Acid Concentration on the Properties of Electrodeposited CZTS Absorber Layers. Phys. Scr. 2020, 95, 054001. [Google Scholar] [CrossRef]
- Delgado-Sanchez, J.M.; Lillo-Bravo, I. High Vapor Transport Deposition: A Novel Process to Develop Cu2ZnSn(SxSe1–x)4 Thin Film Solar Cells. Sol. RRL 2022, 6, 2100835. [Google Scholar] [CrossRef]
- Jiang, X.; Shao, L.; Zhang, J.; Chen, J. Preparation and Characterization of Cu2ZnSn(SxSe1−x)4 Thin Films by Synchronous Sulfo-Selenization of Single-Source Evaporated Metallic Precursors. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 689–693. [Google Scholar] [CrossRef]
- Zaki, M.Y.; Sava, F.; Simandan, I.D.; Buruiana, A.T.; Bocirnea, A.E.; Stavarache, I.; Velea, A.; Galca, A.C.; Pintilie, L. From Non-Stoichiometric CTSe to Single Phase and Stoichiometric CZTSe Films by Annealing under Sn+Se Atmosphere. Ceram. Int. 2023, 49, 33692–33702. [Google Scholar] [CrossRef]
- Zaki, M.Y.; Sava, F.; Simandan, I.-D.; Buruiana, A.T.; Stavarache, I.; Bocirnea, A.E.; Mihai, C.; Velea, A.; Galca, A.-C. A Two-Step Magnetron Sputtering Approach for the Synthesis of Cu2ZnSnS4 Films from Cu2SnS3\ZnS Stacks. ACS Omega 2022, 7, 23800–23814. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, Z.; Luo, M.; Zhao, J.; Chen, J.; Wang, L.; Liu, X.; Xue, D.-J.; Cheng, Y.-B.; Song, H.; et al. Sulfurization Induced Surface Constitution and Its Correlation to the Performance of Solution-Processed Cu2ZnSn(S,Se)4 Solar Cells. Sci. Rep. 2014, 4, 6288. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y. Investigation of S/Se Ratio on the Properties of Solution-Processed CZTSSe Solar Cell. Chalcogenide Lett. 2016, 13, 397–402. [Google Scholar]
- Yu, J.; Deng, H.; Zhang, Q.; Tao, J.; Sun, L.; Yang, P.; Chu, J. The Role of Tuning Se/(S+Se) Ratio in the Improvement of Cu2MnSn(S,Se)4 Thin Films Properties and Photovoltaic Device Performance. Sol. Energy 2019, 179, 279–285. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, X.; Li, W.; Liu, L.; Tanaka, T. Synthesis of the Cu2ZnSn(S,Se)4 Alloys with Tunable Phase Structure and Composition via a Novel Non-Toxic Solution Method. RSC Adv. 2013, 3, 26160–26165. [Google Scholar] [CrossRef]
- Singh, O.P.; Vijayan, N.; Sood, K.N.; Singh, B.P.; Singh, V.N. Controlled Substitution of S by Se in Reactively Sputtered CZTSSe Thin Films for Solar Cells. J. Alloys Compd. 2015, 648, 595–600. [Google Scholar] [CrossRef]
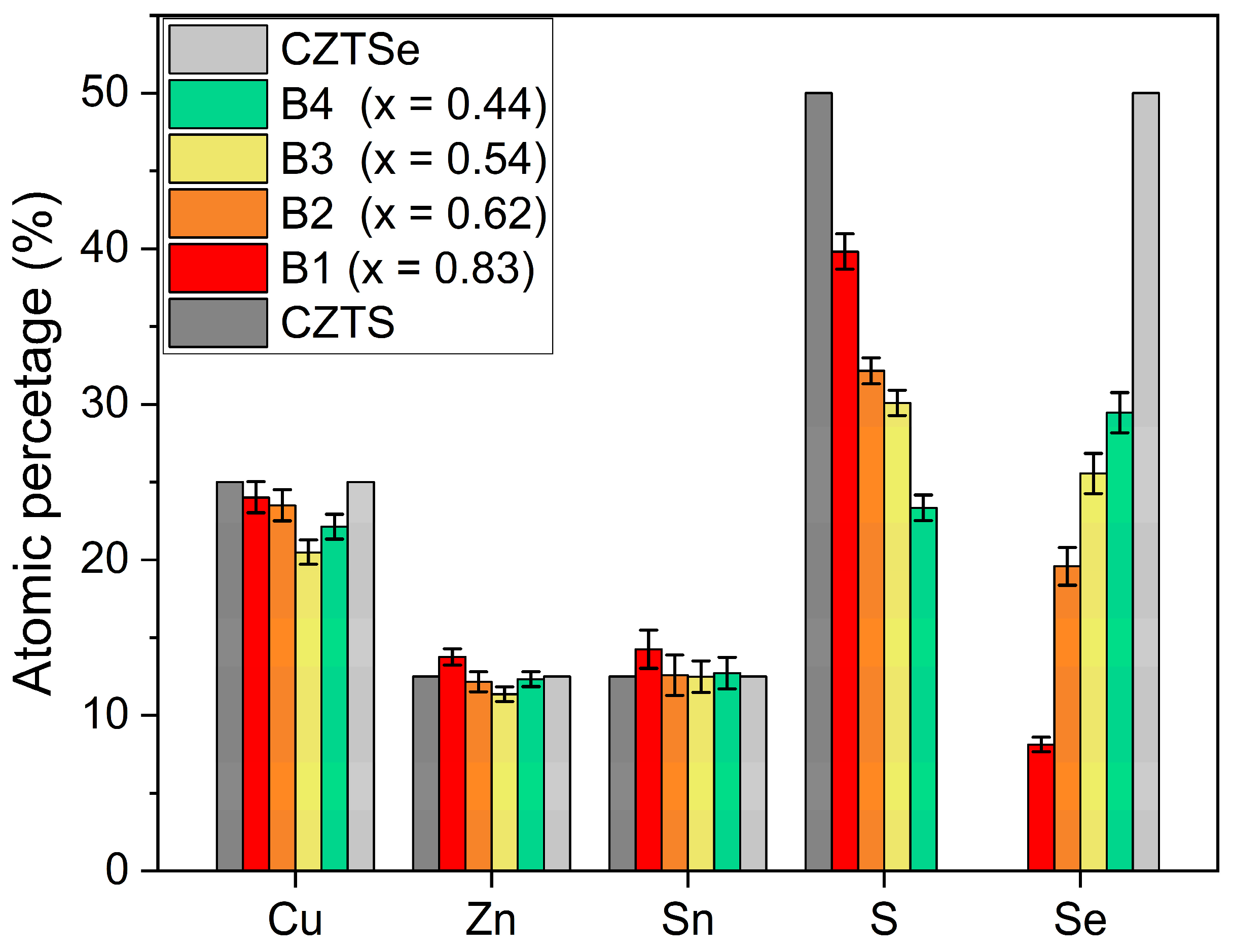

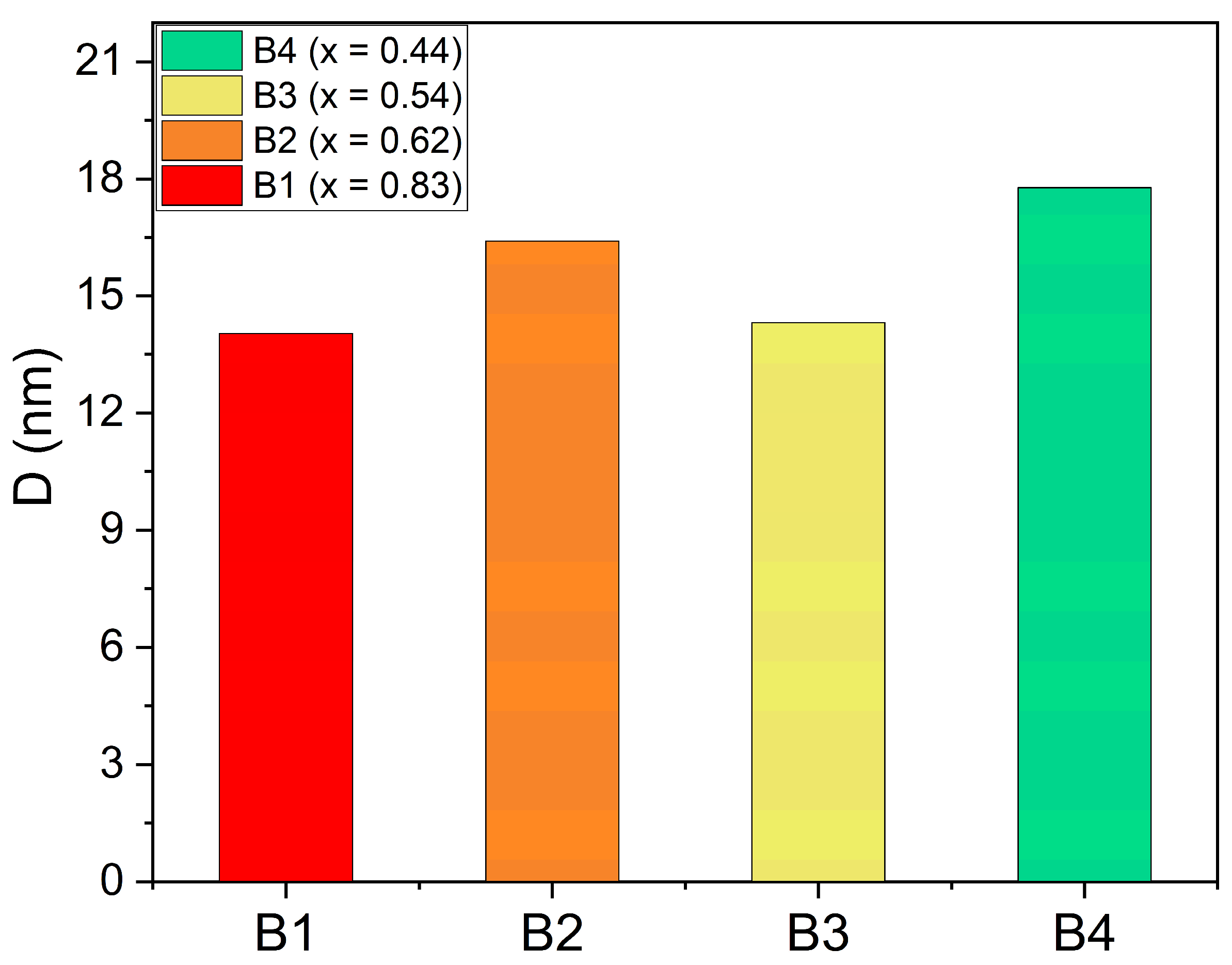
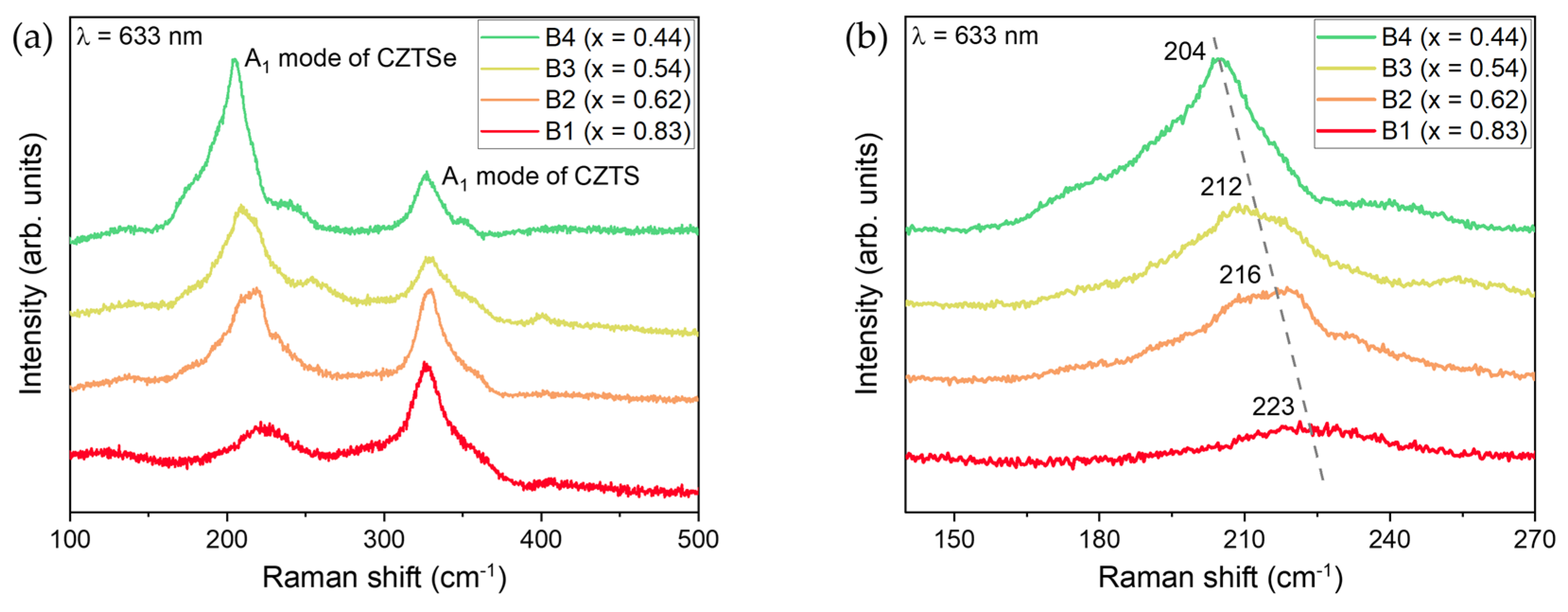
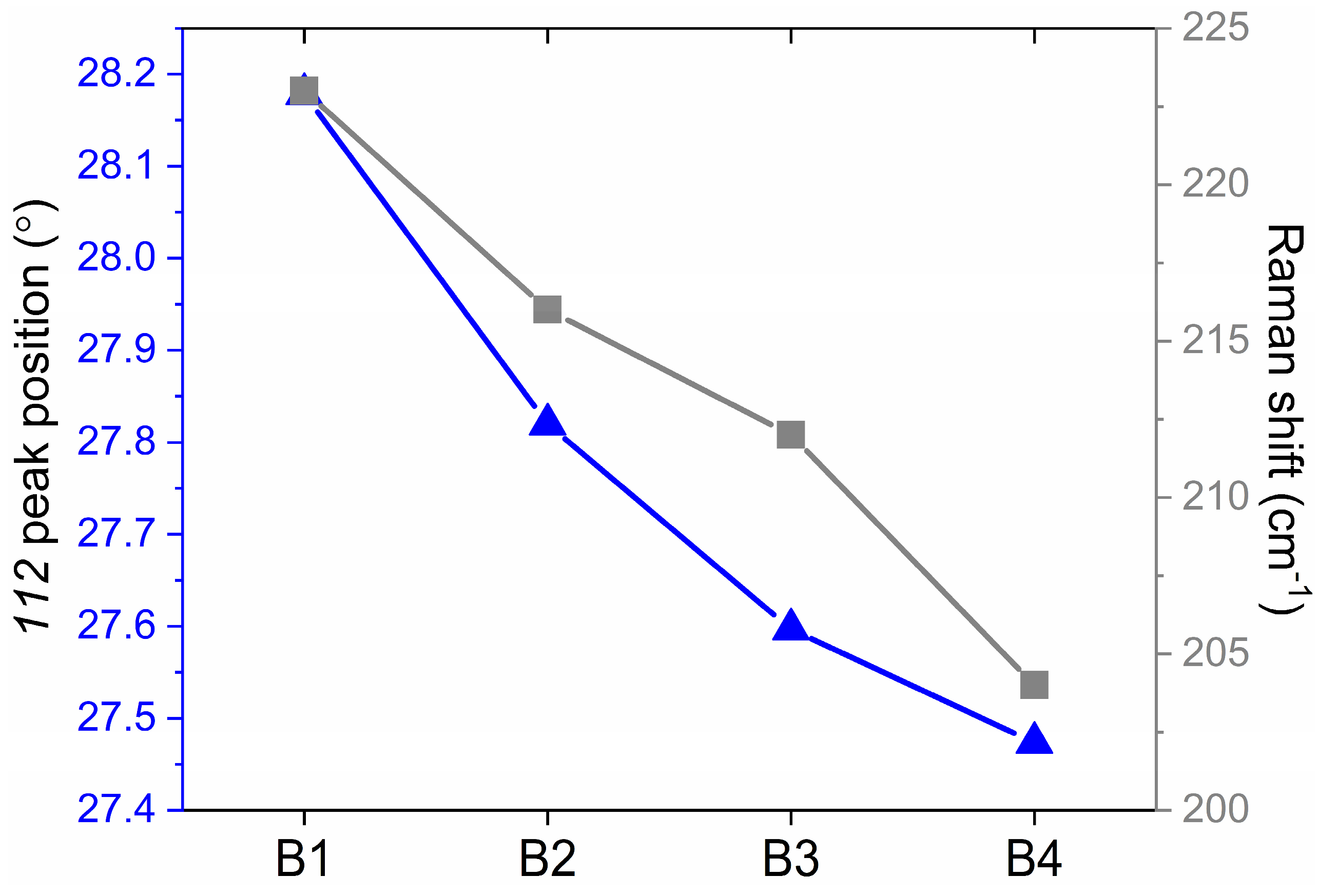
| Atomic Percentage (%) | |||||||
|---|---|---|---|---|---|---|---|
| Cu | Zn | Sn | S | Se | S/(S+Se) | Stoichiometry | |
| B1 | 24.02 | 13.77 | 14.25 | 39.82 | 8.14 | 0.83 | Cu1.92Zn1.10Sn1.14S3.18Se0.66 |
| B2 | 23.51 | 12.16 | 12.59 | 32.15 | 19.59 | 0.62 | Cu1.89Zn0.97Sn1.00S2.57Se1.57 |
| B3 | 20.49 | 11.37 | 12.49 | 30.09 | 25.56 | 0.54 | Cu1.65Zn0.91Sn1.00S2.40Se2.04 |
| B4 | 22.13 | 12.33 | 12.72 | 23.35 | 29.46 | 0.44 | Cu1.77Zn0.99Sn1.02S1.86Se2.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, M.Y.; Sava, F.; Simandan, I.D.; Mihai, C.; Velea, A. Structural and Compositional Analysis of CZTSSe Thin Films by Varying S/(S+Se) Ratio. Energies 2024, 17, 3684. https://doi.org/10.3390/en17153684
Zaki MY, Sava F, Simandan ID, Mihai C, Velea A. Structural and Compositional Analysis of CZTSSe Thin Films by Varying S/(S+Se) Ratio. Energies. 2024; 17(15):3684. https://doi.org/10.3390/en17153684
Chicago/Turabian StyleZaki, Mohamed Yassine, Florinel Sava, Iosif Daniel Simandan, Claudia Mihai, and Alin Velea. 2024. "Structural and Compositional Analysis of CZTSSe Thin Films by Varying S/(S+Se) Ratio" Energies 17, no. 15: 3684. https://doi.org/10.3390/en17153684






