Diffusion Properties of Gas Molecules in Oil–Paper Insulation System Based on Molecular Dynamics Simulation
Abstract
1. Introduction
2. Molecular Dynamics Simulation
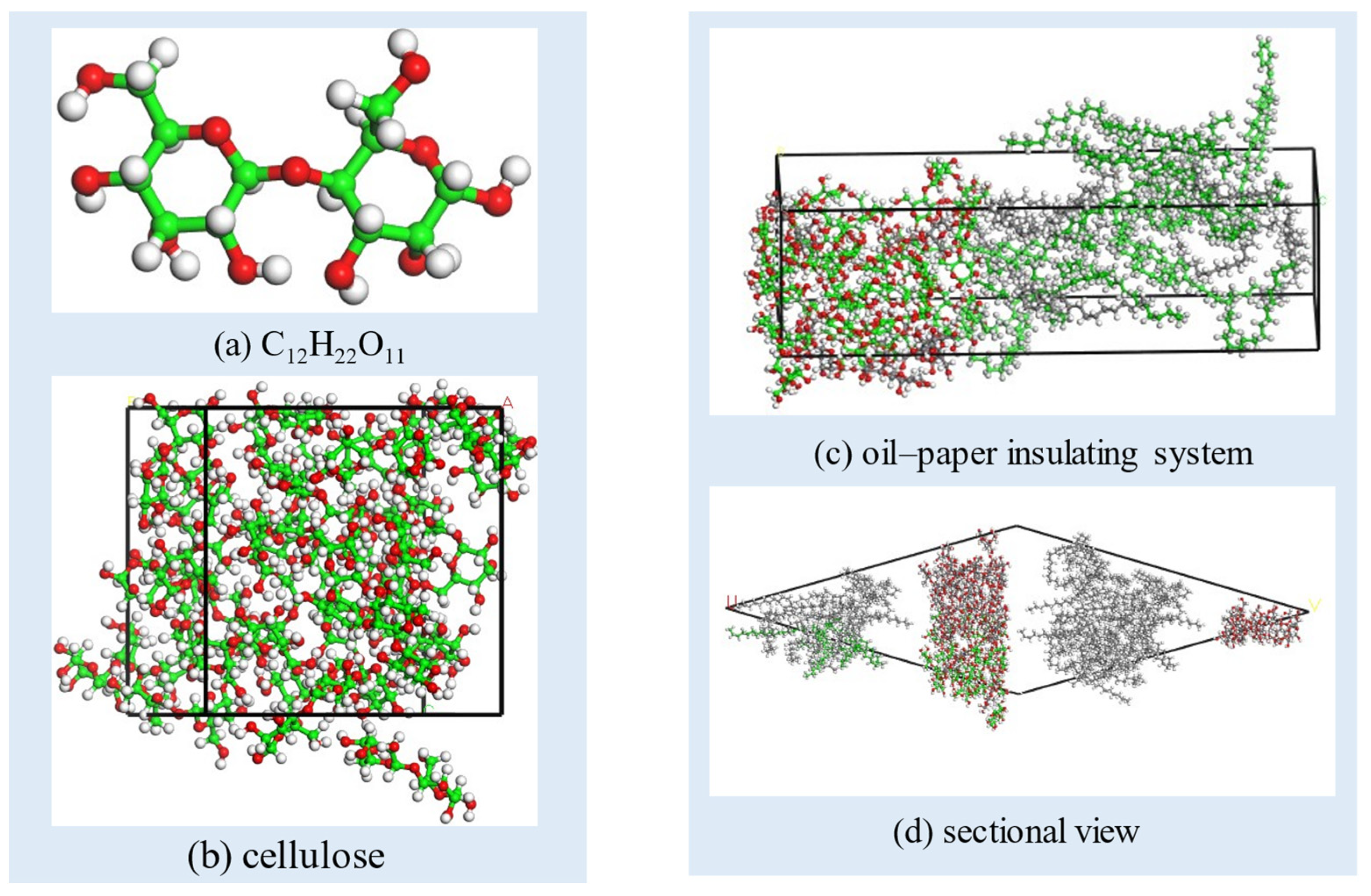
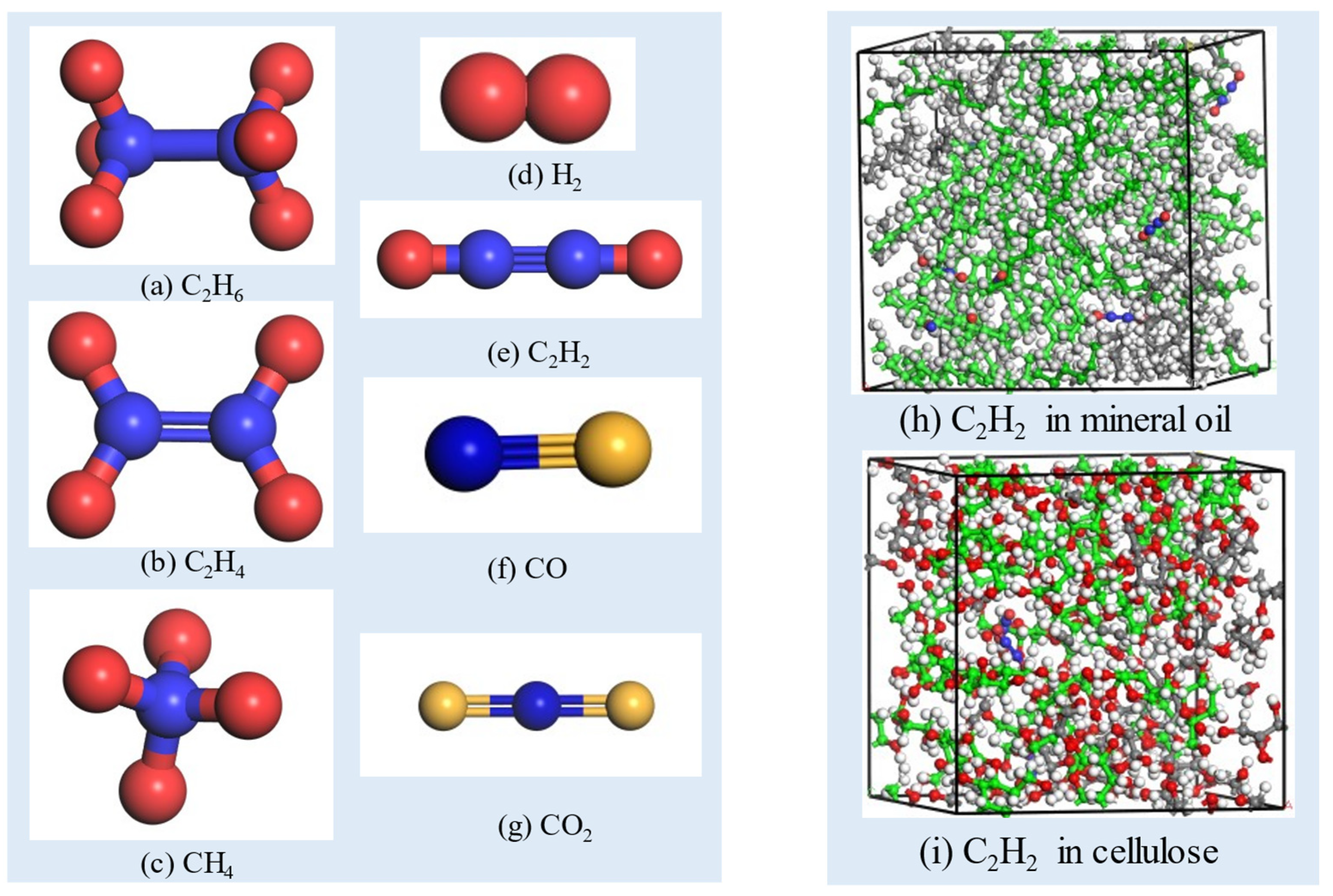
2.1. Model Establishment
- (1)
- The Forcite/Geometry Optimization module is used to perform geometric optimization on each unit. The cutoff radius was set to 12.5 Å.
- (2)
- The internal relaxation of the model. In the first place, the Forcite/Geometry Optimization module is used to minimize the energy of the entire system. Then, in order to stabilize the total energy of the model and adjust the density of the model, the Forcite/Dynamics is used under the isothermal and isobaric ensemble (NPT) at 298 K, the processing time is set as 100 ps and the pressure is set as 0.01 Gpa–0.0001 GPa. After the entire model system reaches stability, the optimized density of the insulation oil molecule model is approximately 0.83 g/cm3, the insulation paper model’s density is around 1.2 g/cm3, and the oil–paper insulation system model’s density is approximately 0.95 g/cm3, all close to the actual densities. This result proves that the whole simulation process is effective. The equilibrium state oil–paper insulation model before molecular dynamics processing is shown in Figure 2, Figure 3 and Figure 4.
- (3)
- The Forcite/Dynamics module is used to perform molecular dynamics simulations of liquid systems. Electrostatic and Van der Waals, respectively, are set to Ewald- and Atom-based calculations. The temperature is set to the normal operating temperature of the transformer, which is 343 K. The canonical ensemble (NVT) is used in molecular dynamics and the simulation time is 100 ps, with data collected every 1 ps for the entire trajectory of molecular dynamics.
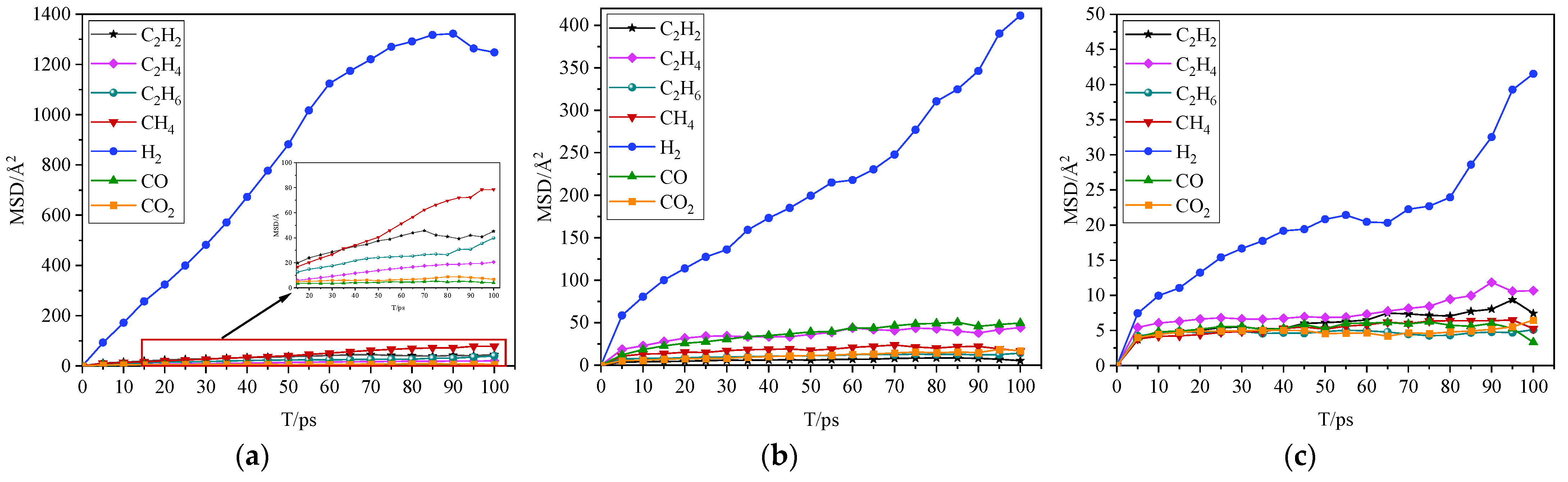
2.2. Simulation Results and Analysis
3. Discussions
- (1)
- The gas molecules exhibit continuous “jumping” motion characteristics in three insulating mediums. The migration displacement of gas molecules in the oil–paper composite insulation is generally smaller than that in cellulose, and the motion migration displacement in insulating oil is the largest. This difference is most obvious in the motion displacement trajectory of H2. The transition displacement of the six gas molecules except H2 are not significantly different in the same insulating medium, and the MSD curves have crossovers. The different properties of insulating materials have an important impact on the transition motion of gas molecules.
- (2)
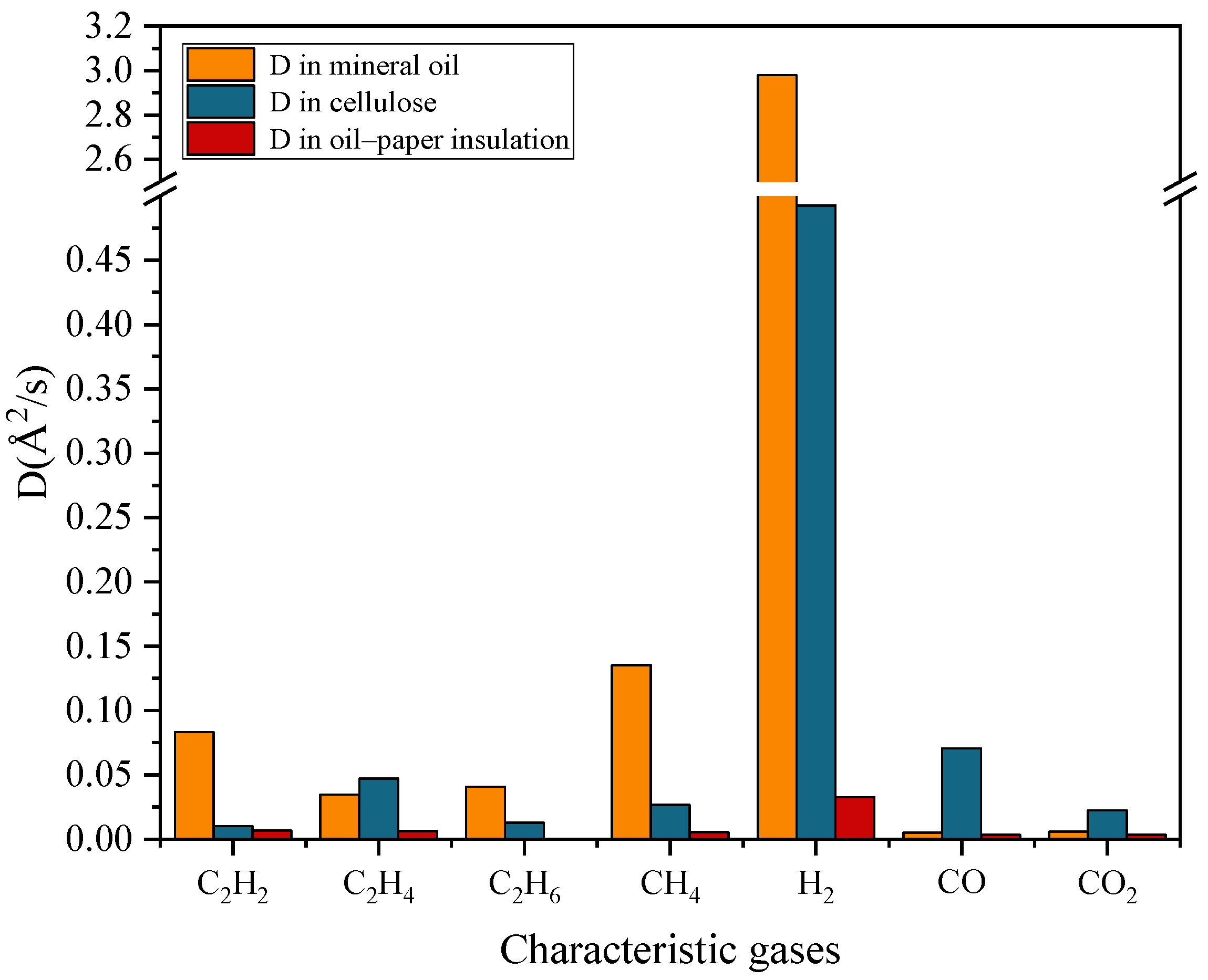
- The diffusion coefficient of characteristic gas molecules is highest in insulating oil, followed by cellulose. In oil–paper composite insulation systems, the diffusion coefficient is minimal. The diffusion coefficient of H2 is the largest among the three insulating mediums. The differences in diffusion coefficients of other gas molecules among various mediums are relatively small, and there is no absolute regularity in the ranking of diffusion coefficients among different mediums. Due to differences in molecular structure, different insulating materials will hinder the diffusion of gas molecules to varying degrees.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Hu, W.; Hao, S.; Gao, C.; Liu, Y.; Wu, W.; Li, L. A fast computational method for internal temperature field in Oil-Immersed power transformers. Appl. Therm. Eng. 2024, 236, 121558. [Google Scholar] [CrossRef]
- Feng, D.; Chen, G.; Yan, X.; Hao, J.; Liao, R. Molecular pyrolysis process and gas production characteristics of 3-element mixed insulation oil under thermal fault. High Volt. 2022, 7, 1130–1140. [Google Scholar] [CrossRef]
- Rangel Bessa, A.; Farias Fardin, J.; Marques Ciarelli, P.; Frizera Encarnação, L. Conventional Dissolved Gases Analysis in Power Transformers: Review. Energies 2023, 16, 7219. [Google Scholar] [CrossRef]
- Ali, M.S.; Bakar, A.H.A.; Omar, A.; Jaafar, A.S.A.; Mohamed, S.H. Conventional methods of dissolved gas analysis using oil-immersed power transformer for fault diagnosis: A review. Electr. Power Syst. Res. 2023, 216, 109064. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, H.B.; Kuhnke, M.; Werle, P.; Gockenbach, E.; Borsi, H. Evolution and discharge pattern of creeping discharge at aged oil/pressboard interface. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Toronto, ON, Canada, 16–19 October 2016; pp. 1053–1056. [Google Scholar]
- Kulyk, S.O.; Shutenko, V.O. Analysis of Gas Content in Oil-Filled Equipment with Spark Discharges and Discharges with High Energy Density. Trans. Electr. Electron. Mater. 2019, 20, 437–447. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, Z.; Cheng, H.; Yang, L.; Meng, X. Study on Relationship between Cumulative Discharge Energy and Discharge Marks on Pressboard under High Field Strength. In Proceedings of the IEEE 1st China International Youth Conference on Electrical Engineering (CIYCEE), Wuhan, China, 1–4 November 2020; pp. 1–5. [Google Scholar]
- Xu, Z.; Yang, L.; Wei, Y. Partial Discharge Characteristics of Fast Developing Discharge in Oil-pressboard Insulation. Proc. CSEE 2021, 41, 7529–7540. [Google Scholar]
- Shahsiah, A. Modeling Dynamic Propagation of Characteristic Gases in Transformer Oil/Paper Insulation and Transformer Fault Diagnostics. Ph.D. Thesis, Rensselaer Polytechnic Institute, Troy, NY, USA, 2006; pp. 81–93. [Google Scholar]
- Shahsiah, A.; Degeneff, R.C.; Nelson, J.K. A study of the temperature based dynamic nature of characteristic gases in oil-cellulose insulation systems. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 471–479. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Korobeynikov, S.M.; Ryzhkina, A.Y. Determination of the hydrogen diffusion coefficient in transformer oil. Tech. Phys. 2011, 56, 421–422. [Google Scholar] [CrossRef]
- Yang, L.; Qi, C.; Wu, G.; Liao, R.; Wang, Q.; Gong, C.; Gao, J. Molecular dynamics simulation of diffusion behaviour of gas molecules within oil–paper insulation system. Mol. Simul. 2013, 39, 988–999. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, G.; Li, Z.; Lin, H. Diffusion Motion Analysis of gas Molecules in Oil—Paper Insulation System Based on Molecular Dynamics Simulation. In Proceedings of the 2022 IEEE 5th International Electrical and Energy Conference (CIEEC), Nangjing, China, 27–29 May 2022; pp. 4205–4210. [Google Scholar]
- Huang, Z.; He, J.; Xiang, C.; Li, J.; Wang, F.; Zhou, J.; Jiang, T. Gas diffusion behavior in green camellia insulating oils. AIP Adv. 2018, 8, 115127. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Zhou, J.; Wu, G.; Wang, Q.; Li, Y.; Li, Y.; Wang, Q.; Han, L.; Wu, X. Dissolved Gas Diffusion Coefficients and Properties in Camellia Insulating Oil. In Proceedings of the 2018 IEEE International Conference on High Voltage Engineering and Application (ICHVE), IEEE, Athens, Greece, 10–13 September 2018; pp. 1–4. [Google Scholar]
- Ye, W.; Hao, J.; Chen, Y.; Zhu, M.; Pan, Z.; Hou, F. Difference analysis of gas molecules diffusion behavior in natural ester and mineral oil based on molecular dynamic simulation. Molecules 2019, 24, 4463. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I.R.; Rasco, J.; Lu, S.T. Chemical characterization of transformer mineral-insulating oils. Environ. Forensics 2010, 11, 117–145. [Google Scholar] [CrossRef]
- Mazeau, K.; Heux, L. Molecular dynamics simulations of bulk native crystalline and amorphous structures of cellulose. J. Phys. Chem. B 2003, 107, 23942403. [Google Scholar] [CrossRef]


| Main Components | C20H42 | C20H38 | C20H26 |
|---|---|---|---|
| Content proportion | Around 60% | 10–40% | 5–15% |
| Gas Molecules | C2H2 | C2H4 | C2H6 | CH4 | H2 | CO | CO2 |
|---|---|---|---|---|---|---|---|
| a | 0.49962 | 0.20772 | 0.24419 | 0.81177 | 17.87845 | 0.03019 | 0.03503 |
| R2 | 0.98109 | 0.99334 | 0.93657 | 0.98948 | 0.99384 | 0.96189 | 0.85117 |
| D (Å2/s) | 0.08327 | 0.03462 | 0.040698 | 0.135295 | 2.979742 | 0.005032 | 0.005838 |
| Gas Molecules | C2H2 | C2H4 | C2H6 | CH4 | H2 | CO | CO2 |
|---|---|---|---|---|---|---|---|
| a | 0.06096 | 0.28352 | 0.07723 | 0.16114 | 2.95479 | 0.42381 | 0.13473 |
| R2 | 0.94855 | 0.86356 | 0.96678 | 0.90714 | 0.98375 | 0.98264 | 0.98612 |
| D (Å2/s) | 0.01016 | 0.047253 | 0.012872 | 0.026857 | 0.492465 | 0.070635 | 0.022455 |
| Gas Molecules | C2H2 | C2H4 | C2H6 | CH4 | H2 | CO | CO2 |
|---|---|---|---|---|---|---|---|
| a | 0.04036 | 0.03755 | 0.00106 | 0.03373 | 0.19665 | 0.02107 | 0.02065 |
| R2 | 0.90097 | 0.83083 | 0.01092 | 0.97429 | 0.90467 | 0.69893 | 0.69548 |
| D (Å2/s) | 0.006727 | 0.006258 | 0.000177 | 0.005622 | 0.032775 | 0.003512 | 0.003442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Zhan, H.; Luo, C.; Hu, S.; Duan, X.; Liao, M. Diffusion Properties of Gas Molecules in Oil–Paper Insulation System Based on Molecular Dynamics Simulation. Energies 2024, 17, 3811. https://doi.org/10.3390/en17153811
Tao J, Zhan H, Luo C, Hu S, Duan X, Liao M. Diffusion Properties of Gas Molecules in Oil–Paper Insulation System Based on Molecular Dynamics Simulation. Energies. 2024; 17(15):3811. https://doi.org/10.3390/en17153811
Chicago/Turabian StyleTao, Jia, Hao Zhan, Chuanxian Luo, Shengnan Hu, Xiongying Duan, and Minfu Liao. 2024. "Diffusion Properties of Gas Molecules in Oil–Paper Insulation System Based on Molecular Dynamics Simulation" Energies 17, no. 15: 3811. https://doi.org/10.3390/en17153811
APA StyleTao, J., Zhan, H., Luo, C., Hu, S., Duan, X., & Liao, M. (2024). Diffusion Properties of Gas Molecules in Oil–Paper Insulation System Based on Molecular Dynamics Simulation. Energies, 17(15), 3811. https://doi.org/10.3390/en17153811




