Highly Efficient Layer-by-Layer Organic Photovoltaics Enabled by Additive Strategy
Highlights
- Developing an additive strategy for exciton and charge dynamics in acceptor films formation improves device performance in layer-by-layer organic photovoltaics (LbL OPVs).
- The additive strategy enhances photon harvesting in the long-wavelength range and improves the molecular arrangement of active layers.
- Volatile solid additives are efficient at individually optimizing the acceptor layer, which is beneficial for constructing efficient LbL OPVs.
- New insights on the dynamic process of active layers are required to better understand the working mechanisms of high-performance LbL OPVs.
Abstract
1. Introduction
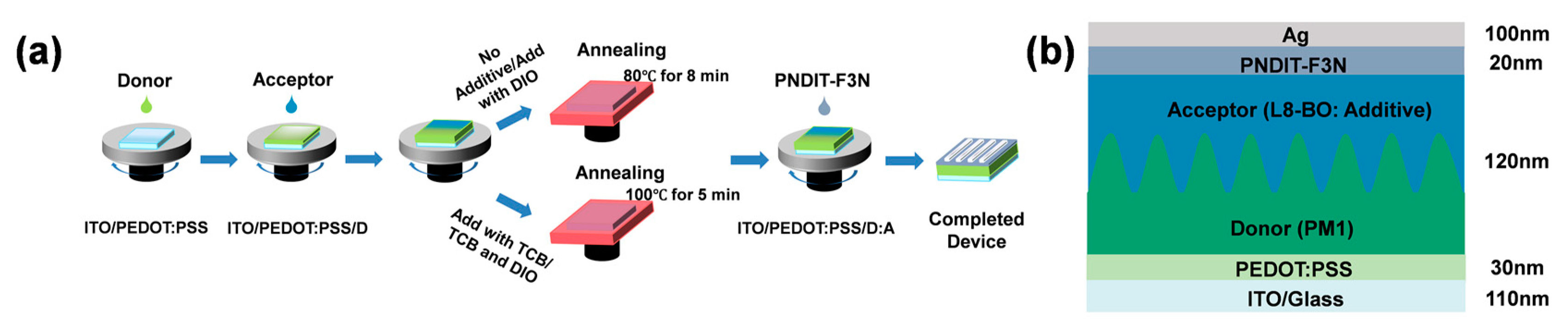
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Li, D.; Gao, Y.; Li, C. Recent Progress on Stability of Organic Solar Cells Based on Non-Fullerene Acceptors. Acta Phys. Chim. Sin. 2023, 40, 2306050. [Google Scholar] [CrossRef]
- Jee, M.; Ryu, H.; Lee, D.; Lee, W.; Woo, H. Recent Advances in Nonfullerene Acceptor-Based Layer-by-Layer Organic Solar Cells Using a Solution Process. Adv. Sci. 2022, 9, 2201876. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Zhao, D.; Wang, L.; Jiao, Z.; Huang, Q.; Wang, P.; Sun, M.; Yuan, G. Recent Progress in Organic Solar Cells: A Review on Materials from Acceptor to Donor. Molecules 2022, 27, 1800. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, C.; Wang, Y.; Zhang, M.; Liu, Z.; Wu, Y.; Chen, H. Theoretical study on organic photovoltaic heterojunction FTAZ/IDCIC. Chin. J. Chem. Phys. 2023, 36, 199–210. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Peng, Q. Ternary Blend Organic Solar Cells: Understanding the Morphology from Recent Progress. Adv. Mater. 2022, 34, 2107476. [Google Scholar] [CrossRef]
- Inganäs, O. Organic Photovoltaics over Three Decades. Adv. Mater. 2018, 30, 1800388. [Google Scholar] [CrossRef]
- Zhou, M.; Liao, C.; Duan, Y.; Xu, X.; Yu, L.; Li, R.; Peng, Q. 19.10% Efficiency and 80.5% Fill Factor Layer-by-Layer Organic Solar Cells Realized by 4-Bis (2-Thienyl) Pyrrole-2,5-Dione Based Polymer Additives for Inducing Vertical Segregation Morphology. Adv. Mater. 2023, 35, 2208279. [Google Scholar] [CrossRef]
- Ning, H.; Jiang, Q.; Han, P.; Lin, M.; Zhang, G.; Chen, J.; Chen, H.; Zeng, S.; Gao, J.; Liu, J.; et al. Manipulating the solubility properties of polymer donors for high-performance layer-by-layer processed organic solar cells. Energy Environ. Sci. 2021, 14, 5919–5928. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Liu, X.; Zhao, X.; Hu, Y.; Yang, Z.; Yang, C.; Yuan, Z.; Chen, Y. Layer-by-Layer Solution-Processed Organic Solar Cells with Perylene Diimides as Acceptors. ACS Appl. Mater. Interfaces 2021, 13, 29876–29884. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Z.; Lu, G.; Yu, N.; Li, C.; Gao, J.; Gu, X.; Hao, X.; Lu, G.; Tang, Z.; et al. Binary Organic Solar Cells Breaking 19% via Manipulating the Vertical Component Distribution. Adv. Mater. 2022, 34, 2204718. [Google Scholar] [CrossRef]
- Sun, R.; Guo, J.; Sun, C.; Wang, T.; Luo, Z.; Zhang, Z.; Jiao, X.; Tang, W.; Yang, C.; Li, Y.; et al. A universal layer-by-layer solution-processing approach for efficient non-fullerene organic solar cells. Energy Environ. Sci. 2019, 12, 384–395. [Google Scholar] [CrossRef]
- Liu, M.; Yao, Q.; Li, S.; Qin, Y.; Jeong, S.Y.; Ma, Y.; Shen, L.; Ma, X.; Yang, K.; Yuan, G.; et al. Interfacial Engineering for Photomultiplication Type Organic Photodetectors with Signal-Noise-Ratio over 89,000. Adv. Opt. Mater. 2024, 12, 2303216. [Google Scholar] [CrossRef]
- Hong, L.; Yao, H.; Cui, Y.; Bi, P.; Zhang, T.; Cheng, Y.; Zu, Y.; Qin, J.; Yu, R.; Ge, Z.; et al. 18.5% Efficiency Organic Solar Cells with a Hybrid Planar/Bulk Heterojunction. Adv. Mater. 2021, 33, 2103091. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tian, H.; Ni, Y.; Xu, Y.; Zhang, L.; Zhang, F.; Wu, S.; Jeong, S.; Huang, T.; Du, X.; et al. Eco-friendly solvent-processed layer-by-layer ternary all-polymer solar cells exhibiting over 18.5% efficiency. Chem. Eng. J. 2024, 493, 152558. [Google Scholar] [CrossRef]
- Ma, X.; Xu, W.; Liu, Z.; Jeong, S.; Xu, C.; Zhang, J.; Woo, H.; Zhou, Z.; Zhang, F. Over 18.1% Efficiency of Layer-by-Layer Polymer Solar Cells by Enhancing Exciton Utilization near the ITO Electrode. ACS Appl. Mater. Interfaces 2023, 15, 7247–7254. [Google Scholar] [CrossRef]
- Tian, H.; Xu, W.; Liu, Z.; Xie, Y.; Zhang, W.; Xu, Y.; Jeong, S.; Zhang, F.; Weng, N.; Zhang, Z.; et al. Over 18.8% Efficiency of Layer-By-Layer Organic Photovoltaics Enabled by Ameliorating Exciton Utilization in Acceptor Layer. Adv. Funct. Mater. 2024, 34, 2313751. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Ni, Y.; Xu, W.; Zhou, H.; Ke, S.; Tian, H.; Jeong, S.; Wong, W.; Ma, X.; et al. Over 17.1% or 18.2% Efficiency of Layer-by-Layer All-Polymer Solar Cells via Incorporating Efficient Pt Complexes as Energy Donor Additive. ACS Mater. Lett. 2024, 6, 2964–2973. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, H.; Hu, D.; Zhang, C.; Xu, X.; Lu, H.; Wu, Y.; Yang, C.; Bo, Z. Improving the Performance of Layer-by-Layer Processed Organic Solar Cells via Introducing a Wide-Bandgap Dopant into the Upper Acceptor Layer. Adv. Mater. 2023, 35, 2211372. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Li, Y.; Chen, Z.; Wu, H.; Lin, Y.; Gao, Y.; Wang, M.; Ding, G.; Min, J.; et al. Compromising Charge Generation and Recombination of Organic Photovoltaics with Mixed Diluent Strategy for Certified 19.4% Efficiency. Adv. Mater. 2023, 35, 2300400. [Google Scholar] [CrossRef]
- Li, C.; Gu, X.; Chen, Z.; Han, X.; Yu, N.; Wei, Y.; Gao, J.; Chen, H.; Zhang, M.; Wang, A.; et al. Achieving Record-Efficiency Organic Solar Cells upon Tuning the Conformation of Solid Additives. J. Am. Chem. Soc. 2022, 144, 14731–14739. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, Y.; Liu, F.; Ran, G.; Huang, F.; Wang, W.; Zhang, W.; Zhang, C.; Hou, J.; Zhu, X. Organic Solar Cells with Over 19% Efficiency Enabled by a 2D-Conjugated Non-fullerene Acceptor Featuring Favorable Electronic and Aggregation Structures. Adv. Mater. 2023, 35, 2300363. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wu, Q.; Guo, J.; Wang, T.; Wu, Y.; Qiu, B.; Luo, Z.; Yang, W.; Hu, Z.; Guo, J.; et al. A Layer-by-Layer Architecture for Printable Organic Solar Cells Overcoming the Scaling Lag of Module Efficiency. Joule 2020, 4, 407–419. [Google Scholar] [CrossRef]
- Ding, G.; Chen, T.; Wang, M.; Xia, X.; He, C.; Zheng, X.; Li, Y.; Zhou, D.; Lu, X.; Zuo, L.; et al. Solid Additive-Assisted Layer-by-Layer Processing for 19% Efficiency Binary Organic Solar Cells. Nano Micro Lett. 2023, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ni, Y.; Zhang, W.; Xu, Y.; Zheng, B.; Jeong, S.; Wu, S.; Ma, Z.; Du, X.; Hao, X.; et al. Over 19.2% efficiency of layer-by-layer organic photovoltaics enabled by a highly crystalline material as an energy donor and nucleating agent. Energy Environ. Sci. 2024, 17, 5173–5182. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Xu, X.; Qin, W.; Yin, S. Improved efficiency of flexible polymer solar cells with a non-annealing active layer. J. Renew. Sustain. Energy 2014, 6, 023128. [Google Scholar] [CrossRef]
- Fu, H.; Peng, Z.; Fan, Q.; Lin, F.R.; Qi, F.; Ran, Y.; Wu, Z.; Fan, B.; Jiang, K.; Woo, H.Y.; et al. A Top-Down Strategy to Engineer Active Layer Morphology for Highly Efficient and Stable All-Polymer Solar Cells. Adv. Mater. 2022, 34, 2202608. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Gurney, R.; Lidzey, D.; Wang, T. A review of non-fullerene polymer solar cells: From device physics to morphology control. Rep. Prog. Phys. 2019, 82, 036601. [Google Scholar] [CrossRef]
- Liu, W.; Sun, S.; Zhou, L.; Cui, Y.; Zhang, W.; Hou, J.; Liu, F.; Xu, S.; Zhu, X. Design of Near-Infrared Nonfullerene Acceptor with Ultralow Nonradiative Voltage Loss for High-Performance Semitransparent Ternary Organic Solar Cells. Angew. Chem. Int. Edit. 2022, 61, e202116111. [Google Scholar] [CrossRef]
- Firdaus, Y.; Le Corre, V.; Karuthedath, S.; Liu, W.; Markina, A.; Huang, W.; Chattopadhyay, S.; Nahid, M.; Nugraha, M.; Lin, Y.; et al. Long-range exciton diffusion in molecular non-fullerene acceptors. Nat. Commun. 2020, 11, 5220. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Du, X.; Lin, H.; Yang, G.; Zheng, C.; Tao, S. High-Performance Layer-by-Layer organic solar cells enabled by Non-Halogenated solvent with 17.89% efficiency. Chem. Eng. J. 2023, 452, 139496. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Liu, W.; Zhou, Z.; Yue, Q.; Zheng, W.; Sun, R.; Liu, W.; Xu, S.; Fan, H.; et al. Organic Solar Cells with 18% Efficiency Enabled by an Alloy Acceptor: A Two-in-One Strategy. Adv. Mater. 2021, 33, 2100830. [Google Scholar] [CrossRef]
- Fu, J.; Fong, P.; Liu, H.; Huang, C.; Lu, X.; Lu, S.; Abdelsamie, M.; Kodalle, T.; Sutter-Fella, C.; Yang, Y.; et al. 19.31% binary organic solar cell and low non-radiative recombination enabled by non-monotonic intermediate state transition. Nat. Commun. 2023, 14, 1760. [Google Scholar] [CrossRef]
- Chen, H.; Jeong, S.; Tian, J.; Zhang, Y.; Naphade, D.; Alsufyani, M.; Zhang, W.; Griggs, S.; Hu, H.; Barlow, S.; et al. A 19% efficient and stable organic photovoltaic device enabled by a guest nonfullerene acceptor with fibril-like morphology. Energy Environ. Sci. 2023, 16, 1062–1070. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, E.; Li, H.; Shen, Z.; Liu, W.; Guo, J.; Luo, Q.; Zhang, J.; Lu, G.; Ma, C.; et al. Layer-by-layer slot-die coated high-efficiency organic solar cells processed using twin boiling point solvents under ambient condition. Nano Res. 2021, 14, 4236–4242. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Hong, L.; Zhang, T.; Xu, Y.; Xian, K.; Gao, B.; Qin, J.; Zhang, J.; Wei, Z.; et al. Achieving Over 15% Efficiency in Organic Photovoltaic Cells via Copolymer Design. Adv. Mater. 2019, 31, 1808356. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Hu, L.; Xie, Z.; Mao, H.; Tan, L.; Zhang, Y.; Chen, Y. High-Efficiency (16.93%) Pseudo-Planar Heterojunction Organic Solar Cells Enabled by Binary Additives Strategy. Adv. Funct. Mater. 2021, 31, 2102291. [Google Scholar] [CrossRef]
- Sun, R.; Wu, Y.; Yang, X.; Gao, Y.; Chen, Z.; Li, K.; Qiao, J.; Wang, T.; Guo, J.; Liu, C.; et al. Single-Junction Organic Solar Cells with 19.17% Efficiency Enabled by Introducing One Asymmetric Guest Acceptor. Adv. Mater. 2022, 34, 2110147. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, M.; Liu, Z.; Tian, H.; Zhang, W.; Sun, S.; Jeong, S.; Zhang, F.; Li, X.; Sun, Q.; et al. A nonfullerene acceptor as a solid additive realizing a record efficiency of 17.74% in quasi-layered all-polymer solar cells. J. Mater. Chem. A 2024, 12, 4077–4085. [Google Scholar] [CrossRef]
- Kaewprajak, A.; Kumnorkaew, P.; Sagawa, T. Influence of binary additives into the solvent for preparation of polymer and fullerene bulk heterojunction solar cells by convective deposition method. Org. Electron. 2019, 73, 18–25. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Wang, J.; Yang, K.; Zhang, H.; Jeong, S.; Ma, X.; Woo, H.; Zhang, F. Dual-Band Photomultiplication-Type Organic Photodetectors with Ultrahigh Signal-to-Noise Ratios. ACS Appl. Mater. Interfaces 2024, 16, 35400–35409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Zheng, S.; Wang, H.; Dong, J.; Li, J. Application and Development of Electrochemical Spectroscopy Methods. Acta Phys. Chim. Sin. 2023, 40, 2304040. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Liu, M.; Zhao, Z.; Yang, K.; Liu, P.; Zhang, H.; Li, J.; Ma, X.; Yao, Q.; et al. Photomultiplication-Type All-Polymer Photodetectors and Their Applications in Photoplethysmography Sensor. Acta Phys. Chim. Sin. 2024, 40, 2311021. [Google Scholar] [CrossRef]
- Sun, Y.; Nian, L.; Kan, Y.; Ren, Y.; Chen, Z.; Zhu, L.; Zhang, M.; Yin, H.; Xu, H.; Li, J.; et al. Rational control of sequential morphology evolution and vertical distribution toward 17.18% efficiency all-small-molecule organic solar cells. Joule 2022, 6, 2835–2848. [Google Scholar] [CrossRef]
- He, C.; Pan, Y.; Ouyang, Y.; Shen, Q.; Gao, Y.; Yan, K.; Fang, J.; Chen, Y.; Ma, C.; Min, J.; et al. Manipulating the D: A interfacial energetics and intermolecular packing for 19.2% efficiency organic photovoltaics. Energy Environ. Sci. 2022, 15, 2537–2544. [Google Scholar] [CrossRef]
- Song, J.; Zhu, L.; Li, C.; Xu, J.; Wu, H.; Zhang, X.; Zhang, Y.; Tang, Z.; Liu, F.; Sun, Y. High-efficiency organic solar cells with low voltage loss induced by solvent additive strategy. Matter 2021, 4, 2542–2552. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, M.; Yang, C.; Duan, L.; Yang, R. Efficient P3HT:PC61BM solar cells employing 1,2,4-trichlorobenzene as the processing additives. Chin. J. Polym. Sci. 2016, 35, 302–308. [Google Scholar] [CrossRef]
- Liang, Q.; Chang, Y.; Liang, C.; Zhu, H.; Guo, Z.; Liu, J. Application of Crystallization Kinetics Strategy in Morphology Control of Solar Cells Based on Nonfullerene Blends. Acta Phys. Chim. Sin. 2023, 39, 2212006. [Google Scholar] [CrossRef]
- Liang, Q.; Li, W.; Lu, H.; Yu, Z.; Zhang, X.; Dong, Q.; Song, C.; Miao, Z.; Liu, J. Recent Advances of Solid Additives Used in Organic Solar Cells: Toward Efficient and Stable Solar Cells. ACS Appl. Energy Mater. 2023, 6, 31–50. [Google Scholar] [CrossRef]
- Cui, F.; Chen, Z.; Qiao, J.; Wang, T.; Lu, G.; Yin, H.; Hao, X. Ternary-Assisted Sequential Solution Deposition Enables Efficient All-Polymer Solar Cells with Tailored Vertical-Phase Distribution. Adv. Funct. Mater. 2022, 32, 2200478. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Q.; Lu, G.; Ryu, H.; Li, Y.; Jin, H.; Chen, Z.; Tang, Z.; Lu, G.; Hao, X.; et al. Vertically optimized phase separation with improved exciton diffusion enables efficient organic solar cells with thick active layers. Nat. Commun. 2022, 13, 2369. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lee, T.; Chin, Y.; Pacalaj, R.; Labanti, C.; Park, S.; Dong, Y.; Cho, H.; Kim, J.; Minami, D.; et al. Molecular orientation-dependent energetic shifts in solution-processed non-fullerene acceptors and their impact on organic photovoltaic performance. Nat. Commun. 2023, 14, 1870. [Google Scholar] [CrossRef] [PubMed]

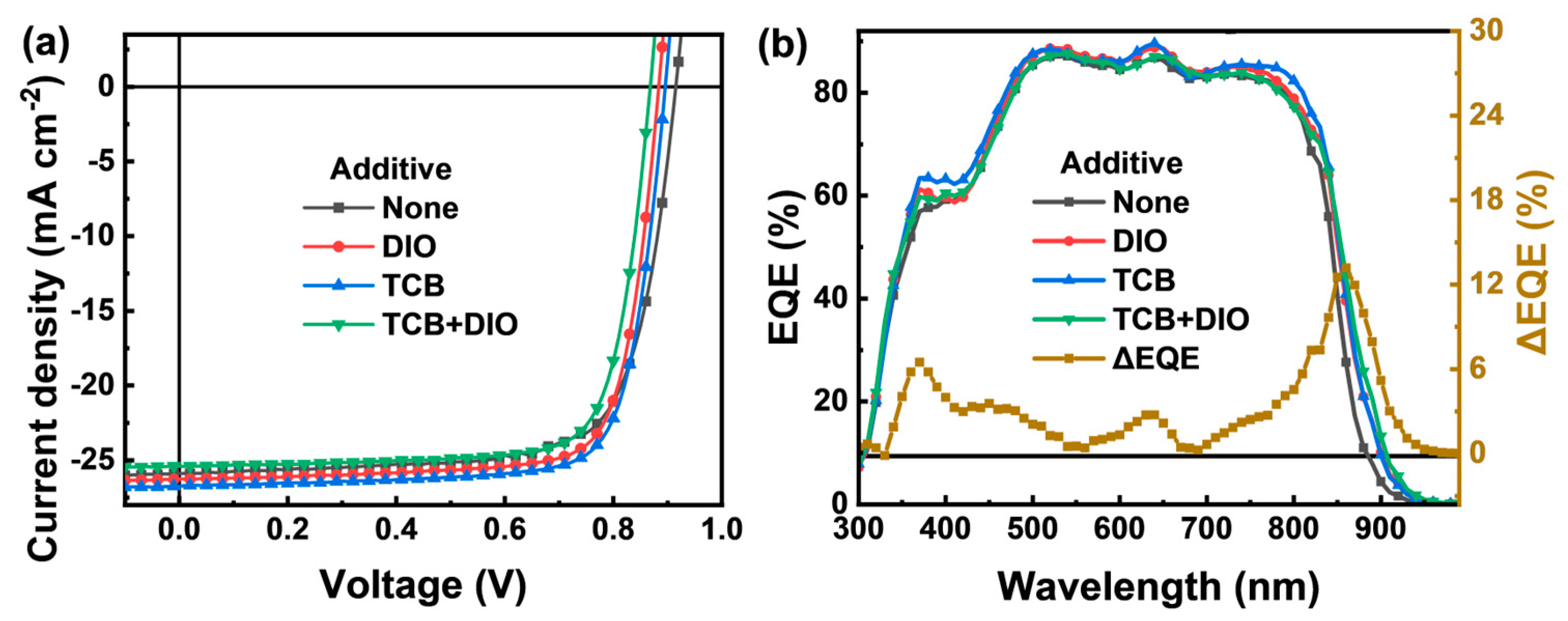
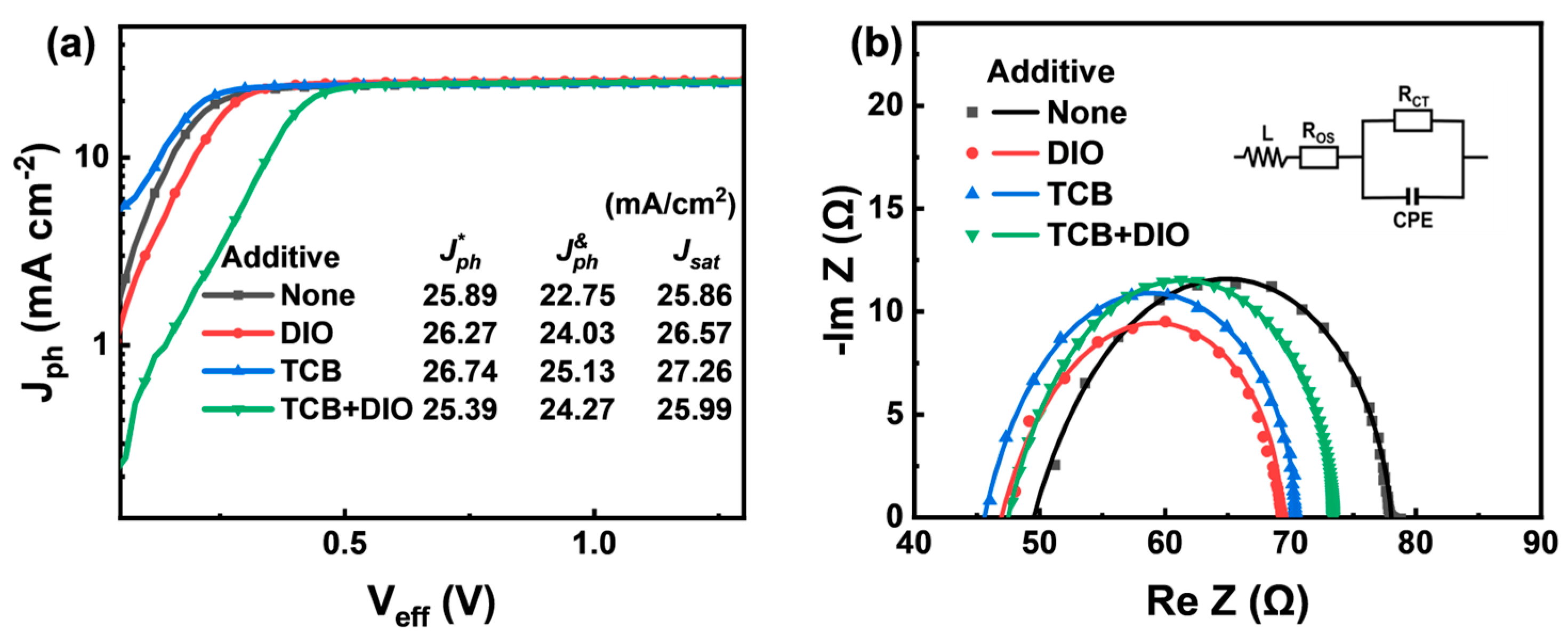
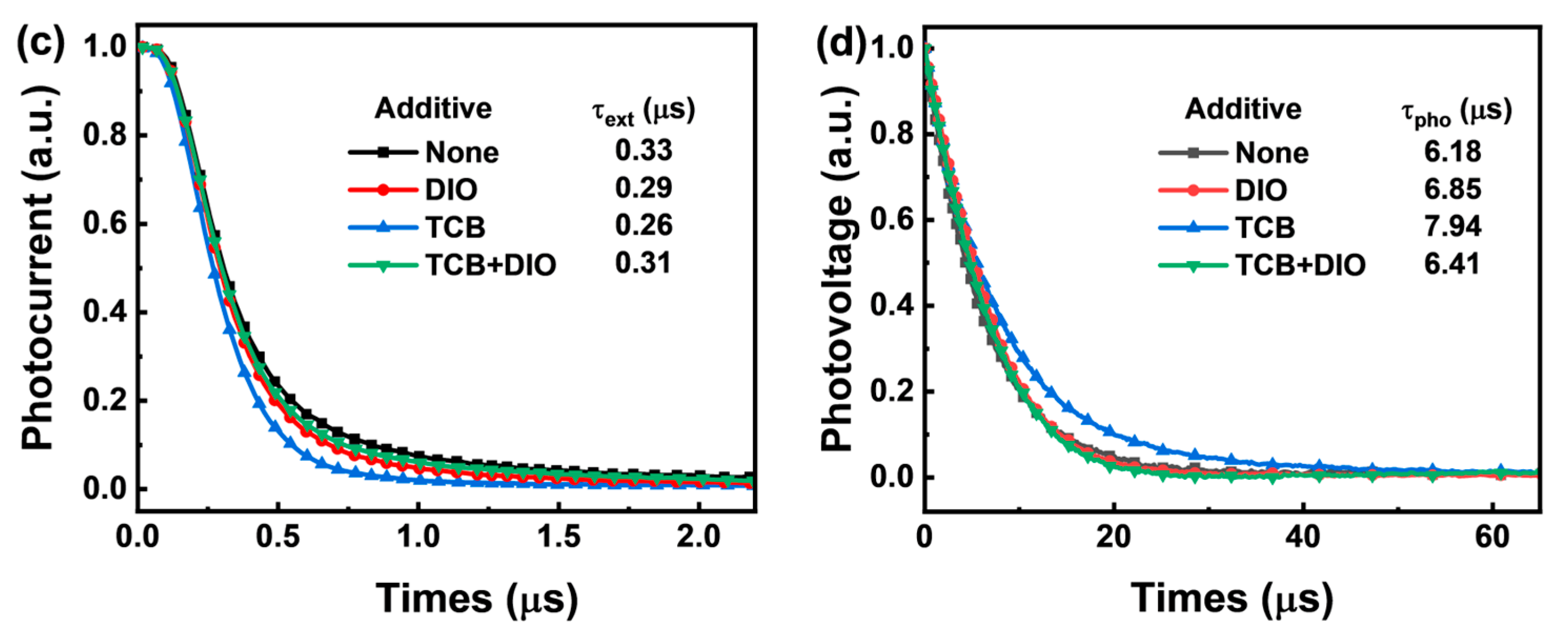
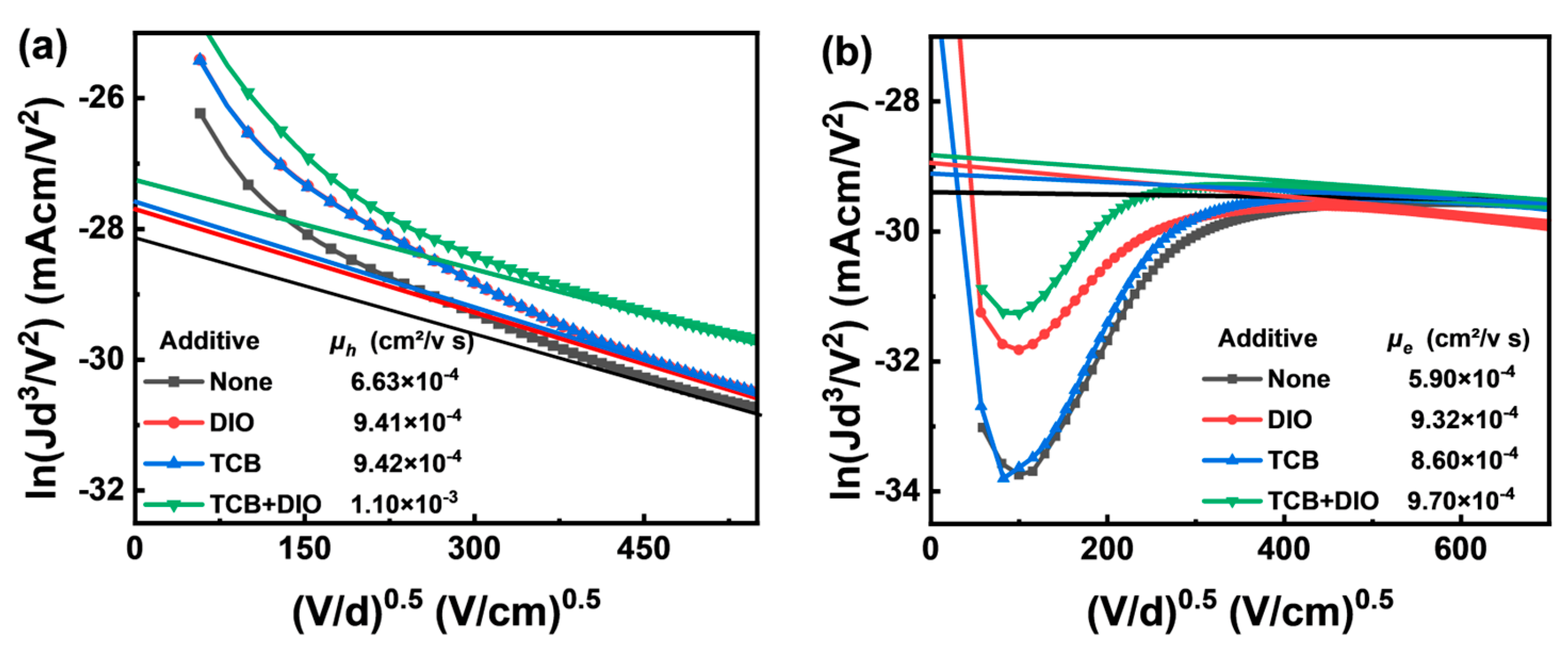
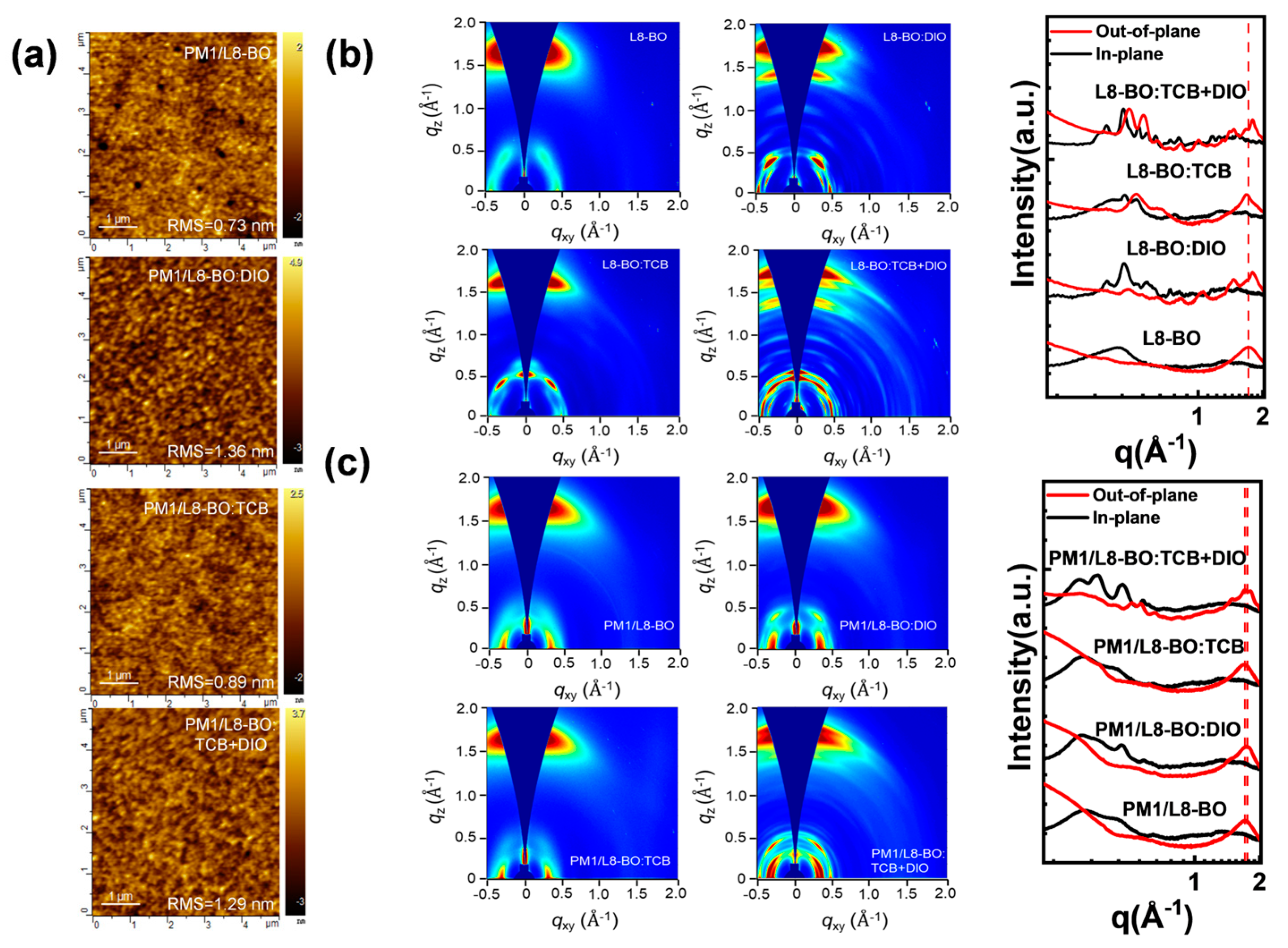
| Additive | JSC (Ave. ± Dev.) a (mA/cm2) | Cal. JSC (mA/cm2) | VOC (Ave. ± Dev.) a (V) | FF (Ave. ± Dev.) a (%) | PCE (Ave. ± Dev.) a (%) | RS (Ω cm2) | RSH (Ω cm2) |
|---|---|---|---|---|---|---|---|
| None | 25.89 (25.55 ± 0.34) | 24.57 | 0.92 (0.917 ± 0.005) | 73.16 (72.71 ± 0.45) | 17.43 (17.29 ± 0.14) | 2.8 | 650 |
| DIO | 26.27 (26.10 ± 0.17) | 25.18 | 0.88 (0.879 ± 0.002) | 77.77 (77.55 ± 0.22) | 17.98 (17.87 ± 0.11) | 2.4 | 1090 |
| TCB | 26.74 (26.46 ± 0.28) | 25.43 | 0.90 (0.898 ± 0.004) | 76.88 (76.45 ± 0.43) | 18.50 (18.38 ± 0.12) | 2.5 | 910 |
| TCB + DIO | 25.39 (25.14 ± 0.25) | 25.05 | 0.88 (0.875 ± 0.006) | 77.21 (76.83 ± 0.38) | 17.06 (16.93 ± 0.13) | 2.7 | 770 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.; Tian, H.; Gong, R.; Zhou, H.; Xu, W.; Wang, J.; Ma, X.; Zhang, F. Highly Efficient Layer-by-Layer Organic Photovoltaics Enabled by Additive Strategy. Energies 2024, 17, 4022. https://doi.org/10.3390/en17164022
Ni Y, Tian H, Gong R, Zhou H, Xu W, Wang J, Ma X, Zhang F. Highly Efficient Layer-by-Layer Organic Photovoltaics Enabled by Additive Strategy. Energies. 2024; 17(16):4022. https://doi.org/10.3390/en17164022
Chicago/Turabian StyleNi, Yuheng, Hongyue Tian, Ruifeng Gong, Hang Zhou, Wenjing Xu, Jian Wang, Xiaoling Ma, and Fujun Zhang. 2024. "Highly Efficient Layer-by-Layer Organic Photovoltaics Enabled by Additive Strategy" Energies 17, no. 16: 4022. https://doi.org/10.3390/en17164022
APA StyleNi, Y., Tian, H., Gong, R., Zhou, H., Xu, W., Wang, J., Ma, X., & Zhang, F. (2024). Highly Efficient Layer-by-Layer Organic Photovoltaics Enabled by Additive Strategy. Energies, 17(16), 4022. https://doi.org/10.3390/en17164022









