Investigating the Effect of 2-Ethylhexyl Nitrate Cetane Improver (2-EHN) on the Autoignition Characteristics of a 1-Butanol–Diesel Blend
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Sample Characterization
2.2. Experimental Setup and Data Acquisition
3. Results and Discussion
3.1. Pressure Waveform in a Constant-Volume Combustion Chamber
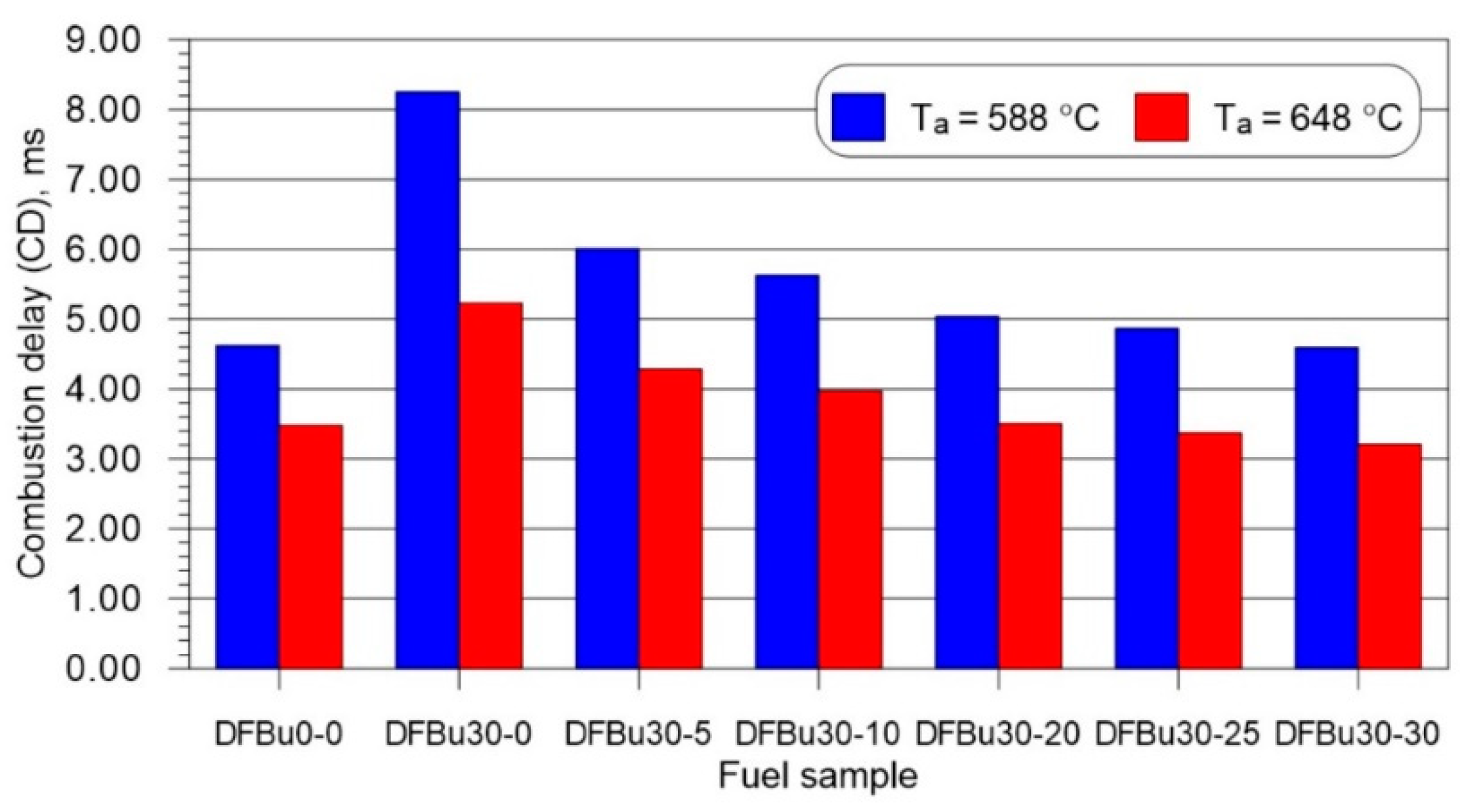
3.2. Ignition and Combustion Delay
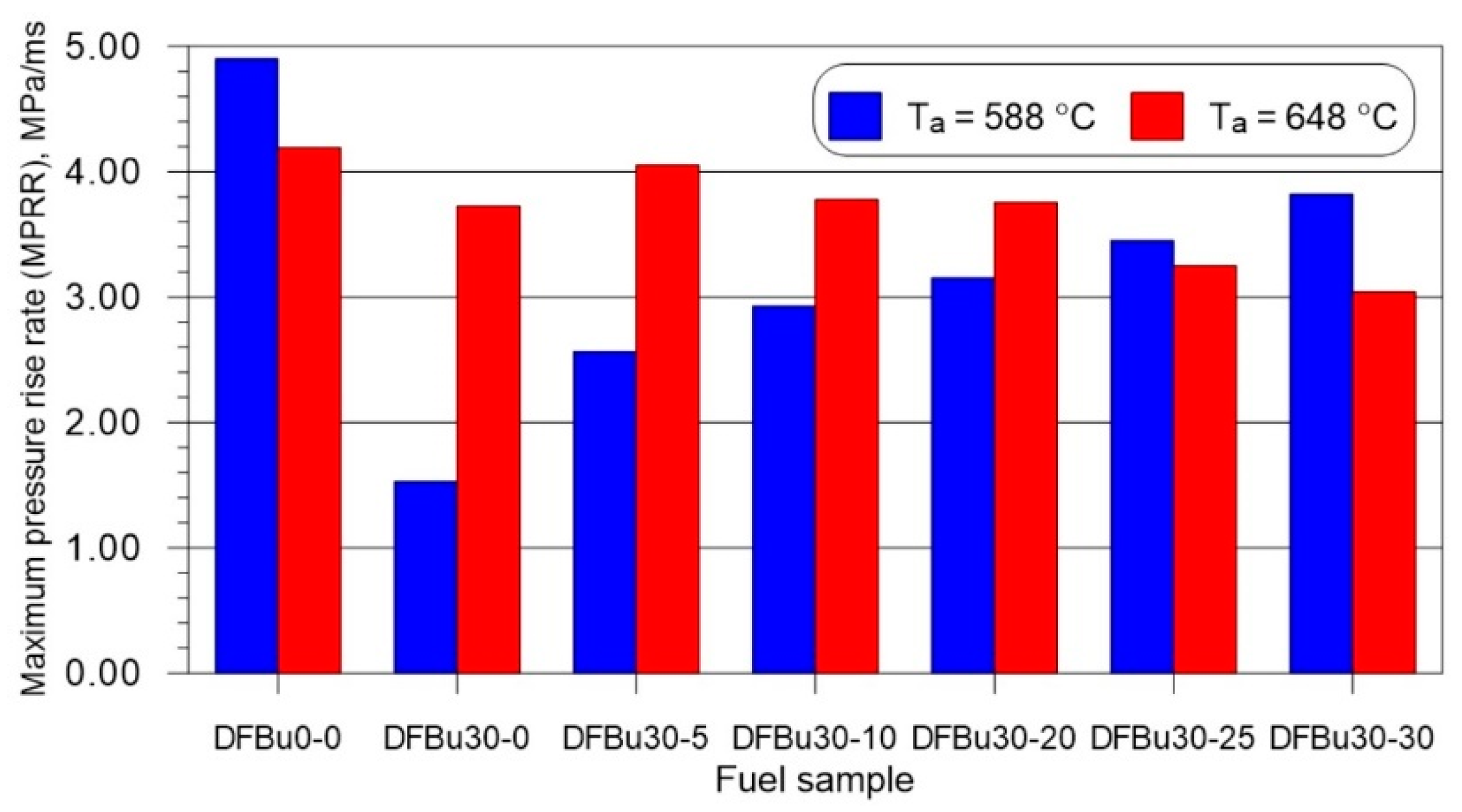
3.3. Average and Maximum Pressure Rise Rate
3.4. Maximum Pressure Rise
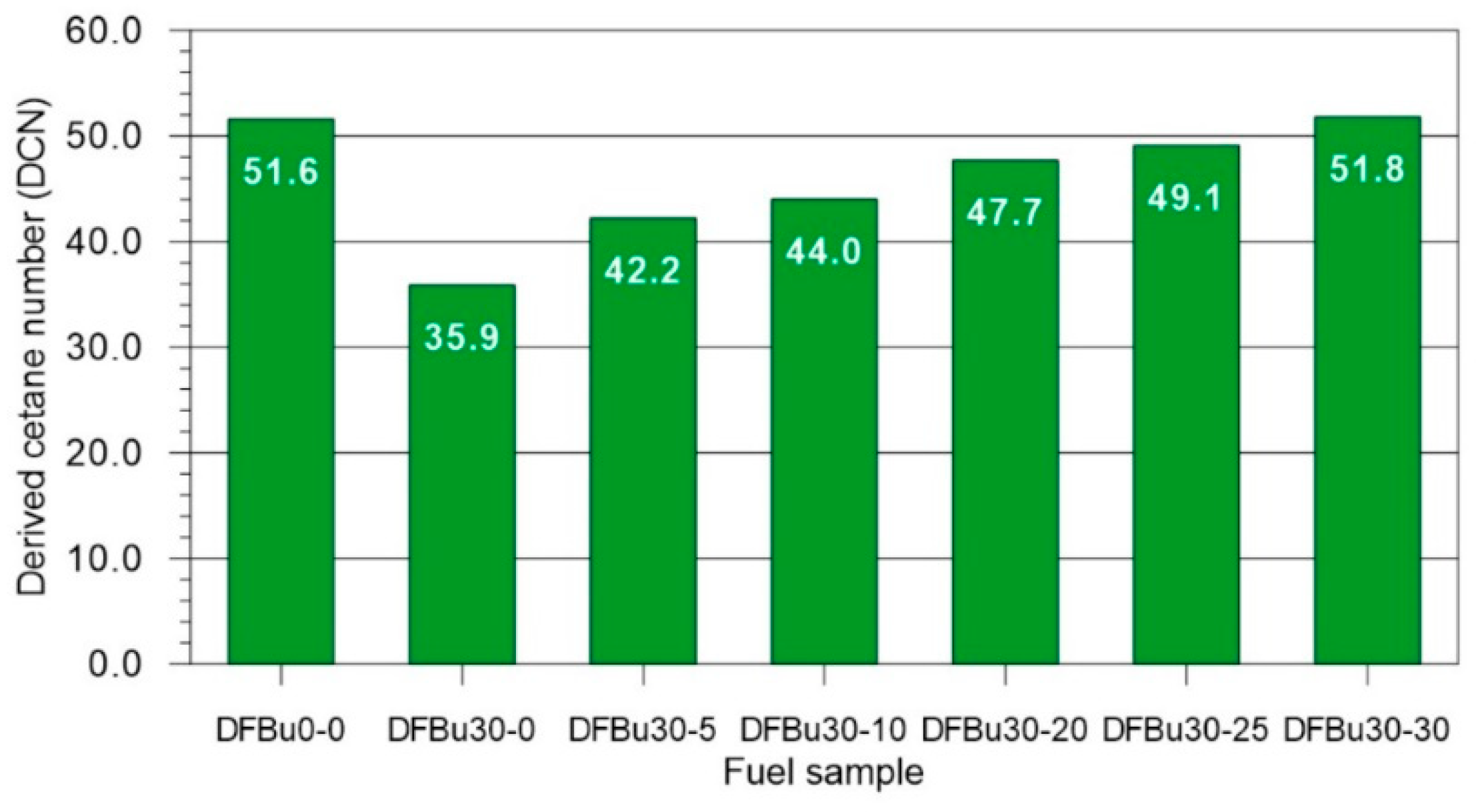
3.5. Derived Cetane Number
4. Conclusions
- As the mass fraction of 2-EHN in a 1-butanol–diesel blend at 30% (v/v) of 1-butanol increased, the ignition delay and combustion delay shortened, with noticeably lower values of these parameters recorded for the higher initial temperature in the combustion chamber.
- As the mass fraction of 2-EHN in a 1-butanol–diesel blend at 30% (v/v) of 1-butanol increased, for a lower initial temperature in the combustion chamber, the average pressure rise rate increased, and for the higher of these temperatures, the value of this parameter decreased.
- As the mass fraction of 2-EHN in a 1-butanol–diesel blend at 30% (v/v) of 1-butanol increased, for a lower initial temperature in the combustion chamber, the maximum pressure rise rate increased, and for the higher of these temperatures, the value of this parameter decreased.
- As the mass fraction of 2-EHN in a 1-butanol–diesel blend at 30% (v/v) of 1-butanol increased, for both analyzed initial temperatures in the combustion chamber, the value of maximum pressure rise decreased marginally.
- As the mass fraction of 2-EHN in a 1-butanol–diesel blend at 30% (v/v) of 1-butanol increased, the value of the derived cetane number increased, with a value close to that obtained for the base fuel, which was diesel fuel, achieved only at a mass fraction of 2-EHN amounting to 30,000 ppm.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| 2-EHN | 2-ethylhexyl nitrate |
| ID | Ignition delay |
| CD | Combustion delay |
| DCN | Derived cetane number |
| BDB | 1-butanol–diesel blend |
| NOX | Nitrogen oxides |
| CN | Cetane number |
| NO3 | Nitrate |
| NO2 | Nitrogen dioxide |
| CH2O | Formaldehyde |
| OH | Hydroxyl |
| CVCC | Constant volume combustion chamber |
| FTIR | Fourier transform infrared spectroscopy |
| FAME | Fatty acid methyl esters |
| IBP | Initial boiling point |
| FBP | Final boiling point |
| tch | Combustion chamber wall temperature |
| tco | Temperature of injector coolant |
| p0 | Initial combustion chamber pressure |
| pinj | Fuel injection pressure |
| tinj | Injection pulse width (injection period) |
| pch | Pressure in the combustion chamber |
| MPR | Maximum pressure rise |
| MPRR | Maximum pressure rise rate |
| APRR | Average pressure rise rate |
| Ta | Initial temperature in the combustion chamber |
| ϕ | Symbol prefixed to the parameter indicating the value of the measurement uncertainty |
References
- Hasan, M.M.; Rahman, M.M. Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: A review. Renew. Sustain. Energy Rev. 2017, 74, 938–948. [Google Scholar] [CrossRef]
- Kanaveli, I.-P.; Atzemi, M.; Lois, E. Predicting the viscosity of diesel/biodiesel blends. Fuel 2017, 199, 248–263. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. Measurements and empirical correlations in predicting biodiesel-diesel blends’ viscosity and density. Fuel 2017, 199, 567–577. [Google Scholar] [CrossRef]
- No, S.-Y. Application of biobutanol in advanced CI engines—A review. Fuel 2016, 183, 641–658. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Fernández-Rodríguez, D.; Cova-Bonillo, A. Autoignition of blends of n-butanol and ethanol with diesel or biodiesel fuels in a constant volume combustion chamber. Energy 2017, 118, 613–621. [Google Scholar] [CrossRef]
- Pan, S.; Li, X.; Han, W.; Huang, Y. An experimental investigation on multi-cylinder RCCI engine fueled with 2-butanol/diesel. Energy Convers. Manag. 2017, 154, 92–101. [Google Scholar] [CrossRef]
- Tipanluisa, L.; Fonseca, N.; Casanova, J.; López, J.-M. Effect of n-butanol/diesel blends on performance and emissions of a heavy-duty diesel engine tested under the World Harmonised Steady-State cycle. Fuel 2021, 302, 121204. [Google Scholar] [CrossRef]
- Torres-Jimenez, E.; Jerman, M.S.; Gregorc, A.; Lisec, I.; Dorado, M.P.; Kegl, B. Physical and chemical properties of ethanol–diesel fuel blends. Fuel 2011, 90, 795–802. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Kakaras, E.C.; Giakoumis, E.G. Effects of ethanol–diesel fuel blends on the performance and exhaust emissions of heavy duty DI diesel engine. Energy Convers Manag. 2008, 49, 3155–3162. [Google Scholar] [CrossRef]
- Liu, J.; Yao, A.; Yao, C. Effects of diesel injection pressure on the performance and emissions of a HD common-rail diesel engine fueled with diesel/methanol dual fuel. Fuel 2015, 140, 192–200. [Google Scholar] [CrossRef]
- Soni, D.K.; Gupta, R. Numerical investigation of emission reduction techniques applied on methanol blended diesel engine. Alex. Eng. J. 2016, 55, 1867–1879. [Google Scholar] [CrossRef]
- Geng, P.; Yao, C.; Wei, L.; Liu, J.; Wang, Q.; Pan, W.; Wang, J. Reduction of PM emissions from a heavy-duty diesel engine with diesel/methanol dual fuel. Fuel 2014, 123, 1–11. [Google Scholar] [CrossRef]
- Szulczyk, K.R. Which is a better transportation fuel—Butanol or ethanol? Int. J. Energy Environ. 2010, 1, 11. [Google Scholar]
- Kumar, S.; Cho, J.H.; Park, J.; Moon, I. Advances in diesel–alcohol blends and their effects on the performance and emissions of diesel engines. Renew. Sustain. Energy Rev. 2013, 22, 46–72. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol combustion chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vendharaj, S.; Yang, W.M.; Roberts, W.L.; Dibble, R.W. Feasibility of using less viscous and lower cetane (LVLC) fuels in a diesel engine: A review. Renew. Sustain. Energy Rev. 2015, 51, 1166–1190. [Google Scholar] [CrossRef]
- Kumar, B.R.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Choi, B.; Jiang, X.; Kim, Y.K.; Jung, G.; Lee, C.; Choi, I.; Song, C.S. Effect of diesel fuel blend with n-butanol on the emission of a turbocharged common rail direct injection diesel engine. Appl. Energy 2015, 146, 20–28. [Google Scholar] [CrossRef]
- Yan, F.; Cheng, X.; Qiu, L.; Huang, R.; Huang, S.; Liu, B. Spray flame soot sampling and morphology analysis of butanol–diesel blends. J. Energy Inst. 2017, 90, 855–863. [Google Scholar] [CrossRef]
- Choi, B.; Jiang, X. Individual hydrocarbons and particulate matter emission from a turbocharged CRDI diesel engine fueled with n-butanol/diesel blends. Fuel 2015, 154, 188–195. [Google Scholar] [CrossRef]
- Cheng, X.; Li, S.; Yang, J.; Liu, B. Investigation into partially premixed combustion fueled with N-butanol-diesel blends. Renew. Energy 2016, 86, 723–732. [Google Scholar] [CrossRef]
- SAE International. Internal Combustion Engine Handbook. Basics, Components, System and Perspectives, 2nd ed.; van Basshuysen, R., Schäfer, F., Eds.; TechTrans, Translator; SAE International: Warrendale, PA, USA, 2016. [Google Scholar]
- Yanowitz, J.; Ratcliff, M.A.; McCormick, R.L.; Taylor, J.D.; Murphy, M.J. Compendium of Experimental Cetane Numbers; Technical Report NREL/TP-5400-61693; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2014. [Google Scholar]
- Chukwuezie, O.C.; Nwakuba, N.R.; Asoegwu, S.N.; Nwaigwe, K.N. Cetane number effect on engine performance and gas emission: A review. Am. J. Eng. Res. 2017, 6, 56–67. [Google Scholar]
- Kurtz, E.; Polonowski, C. The influence of fuel cetane number on catalyst light-off operation in a modern diesel engine. SAE Int. J. Fuels Lubr. 2017, 10, 664–671. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, Z.; Wu, Y.; Wang, C.; Chang, W.; Zheng, Z.; Yao, M. Effects of 2-ethylhexyl nitrate (EHN) on combustion and emissions on a compression ignition engine fueling high-pressure direct-injection pure methanol fuel. Fuel 2023, 341, 127684. [Google Scholar] [CrossRef]
- Almodovar, C.A.; Goldsmith, C.F. Laser schlieren study of the thermal decomposition of 2-ethylhexyl-nitrate. Proc. Combust. Inst. 2021, 38, 997–1005. [Google Scholar] [CrossRef]
- Pan, M.; Qian, W.; Huang, R.; Tong, C.; Huang, H.; Xu, L.; Hao, B. Effects of dimethyl carbonate and 2-ethylhexyl nitrate on energy distribution, combustion and emissions in a diesel engine under different load conditions. Energy Convers. Manag. 2019, 199, 111985. [Google Scholar] [CrossRef]
- Ileri, E. Experimental study of 2-ethylhexyl nitrate effects on engine performance and exhaust emissions of a diesel engine fueled with n-butanol or 1-pentanol diesel–sunflower oil blends. Energy Convers. Manag. 2016, 118, 320–330. [Google Scholar] [CrossRef]
- İleri, E.; Koçar, G. Effects of antioxidant additives on engine performance and exhaust emissions of a diesel engine fueled with canola oil methyl ester–diesel blend. Energy Convers. Manag. 2013, 76, 145–154. [Google Scholar] [CrossRef]
- Ruina, L.; Zhong, W.; Peiyong, N.; Yang, Z.; Mingdi, L.; Lilin, L. Effects of cetane number improvers on the performance of diesel engine fuelled with methanol/biodiesel blend. Fuel 2014, 128, 180–187. [Google Scholar]
- Kuszewski, H. Effect of adding 2-ethylhexyl nitrate cetane improver on the autoignition properties of ethanol–diesel fuel blend—Investigation at various ambient gas temperatures. Fuel 2018, 224, 57–67. [Google Scholar] [CrossRef]
- Imdadul, H.; Sebayang, A.; Silitonga, A.; Masjuki, H.; Kalam, M.; Milano, J.; Ibrahim, H.; Shamsuddin, A. Effect of Nitrate-based Cetane Improver on Ternary Higher Alcohol Biodiesel Blends and Diesel Engine Exhaust Emissions. In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), Samarinda, Indonesia, 23–24 October 2021; pp. 995–1002. [Google Scholar] [CrossRef]
- Sendilvelan, S.; Rajan, K. Effect of butanol-diesel blends in a compression ignition engine to reduce emission. Rasayan J. Chem. 2017, 10, 190–194. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Zhou, S.; Zhu, Y.; Feng, Y. Numerical Investigation of n-Butanol Addition on the Performance and Emission Characteristics of CI Diesel Engine. Int. Energy J. 2018, 18, 25–38. [Google Scholar]
- Labeckas, G.; Slavinskas, S. The effect of n-butanol additions to diesel fuel on engine performance and exhaust emissions. Mech. Agric. Conserv. Resour. 2020, 66, 211–214. [Google Scholar]
- Kim, H.Y.; Ge, J.C.; Choi, N.J. Effects of Ethanol–Diesel on the Combustion and Emissions from a Diesel Engine at a Low Idle Speed. Appl. Sci. 2020, 10, 4153. [Google Scholar] [CrossRef]
- Uyaroglu, A.; Unaldi, M. Analysis of the Performance and Emission Characteristics of Biokerosene with 2-Ethylhexyl Nitrate Additive. Eurasia Proc. Sci. Technol. Eng. Math. (EPSTEM) 2022, 17, 60–68. [Google Scholar] [CrossRef]
- Şimşek, S.; Uslu, S. Analysis of the effects of cetane improver addition to diesel on engine performance and emissions. International Journal of Automotive Engineering and Technologies. Mart 2021, 10, 26–32. [Google Scholar] [CrossRef]
- Zahos-Siagos, I.; Karathanassis, V.; Karonis, D. Exhaust Emissions and Physicochemical Properties of n-Butanol/Diesel Blends with 2-Ethylhexyl Nitrate (EHN) or Hydrotreated Used Cooking Oil (HUCO) as Cetane Improvers. Energies 2018, 11, 3413. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jaworski, A.; Ustrzycki, A.; Lejda, K.; Balawender, K.; Woś, P. Use of the constant volume combustion chamber to examine the properties of autoignition and derived cetane number of mixtures of diesel fuel and ethanol. Fuel 2017, 200, 564–575. [Google Scholar] [CrossRef]
- Kuszewski, H. Experimental investigation of the effect of ambient gas temperature on the autoignition properties of ethanol–diesel fuel blends. Fuel 2018, 214, 26–38. [Google Scholar] [CrossRef]
- Kuszewski, H. Effect of Injection Pressure and Air–Fuel Ratio on the Self-ignition Properties of 1-butanol–Diesel Fuel Blends: Study Using a Constant-Volume Combustion Chamber. Energy Fuels 2019, 33, 2335–2347. [Google Scholar] [CrossRef]
- Kuszewski, H. Experimental study of the autoignition properties of n-butanol–diesel fuel blends at various ambient gas temperatures. Fuel 2019, 235, 1316–1326. [Google Scholar] [CrossRef]
- Kuszewski, H. Experimental investigation of the autoignition properties of ethanol–biodiesel fuel blends. Fuel 2019, 235, 1301–1308. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jaworski, A.; Mądziel, M.; Woś, P. The investigation of auto-ignition properties of 1-butanol–biodiesel blends under various temperatures conditions. Fuel 2023, 346, 128388. [Google Scholar] [CrossRef]
- PN-EN 590:2022-08; Automotive Fuels–Diesel–Requirements and Test Methods. Polish Standardization Committee: Warsaw, Poland, 2022.
- ASTM D7668; Standard Test Method for Determination of Derived Cetane Number (DCN) of Diesel Fuel Oils—Ignition Delay and Combustion Delay Using a Constant Volume Combustion Chamber Method. ASTM: West Conshohocken, PA, USA, 2017.
- EN 16715; Liquid Petroleum Products—Determination of Ignition Delay and Derived Cetane Number (DCN) of Middle Distillate Fuels—Ignition Delay and Combustion Delay Determination Using a Constant Volume Combustion Chamber with Direct Fuel Injection. BSI: London, UK, 2017.
- Lapuerta, M.; Sanz-Argent, J.; Raine, R. Ignition Characteristics of Diesel Fuel in a Constant Volume Bomb under Diesel-Like Conditions. Effect of the Operation Parameters. Energy Fuels 2014, 28, 5445–5454. [Google Scholar] [CrossRef]
- Lapuerta, M.; Sanz-Argent, J.; Raine, R. Heat release determination in a constant volume combustion chamber from the instantaneous cylinder pressure. Appl. Therm. Eng. 2014, 63, 520–527. [Google Scholar] [CrossRef]
- Kuszewski, H. Physical and Chemical Properties of 1-Butanol–Diesel Fuel Blends. Energy Fuel 2018, 32, 11619–11631. [Google Scholar] [CrossRef]
- Jaworski, A.; Kuszewski, H.; Longwic, R.; Sander, P. Assessment of Self-Ignition Properties of Canola Oil–n-Hexane Blends in a Constant Volume Combustion Chamber and Compression Ignition Engine. Appl. Sci. 2023, 13, 10558. [Google Scholar] [CrossRef]
- Lakshminarayanan, P.A.; Aghav, Y.V. Ignition Delay in a Diesel Engine. In Modelling Diesel Combustion; Mechanical Engineering Series; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Stanik, W.; Mazanek, A.; Jakóbiec, J. Study of diesel oil containing 7% (v/v) of FAME and cetane boost additive for the assessment its utility and purity of injectors. Combust. Engines 2015, 162, 933–943. [Google Scholar]
- Liu, S.; Zhu, Z.; Zhang, Z.; Gao, G.; Wei, Y. Effect of a cetane number (CN) improver on combustion and emission characteristics of a compression-ignition (CI) engine fueled with an ethanol-diesel blend. Energy Fuels 2010, 24, 2449–2454. [Google Scholar] [CrossRef]
- Worldwide Fuel Charter 2019—Gasoline and Diesel Fuel, 6th ed.; Prepared by ACEA, AUTO ALLIANCE, EMA and JAMA; ACEA: Austin, TX, USA, 2019.
- ASTM D975; Standard Specification for Diesel Fuel Oils. ASTM: West Conshohocken, PA, USA, 2022.
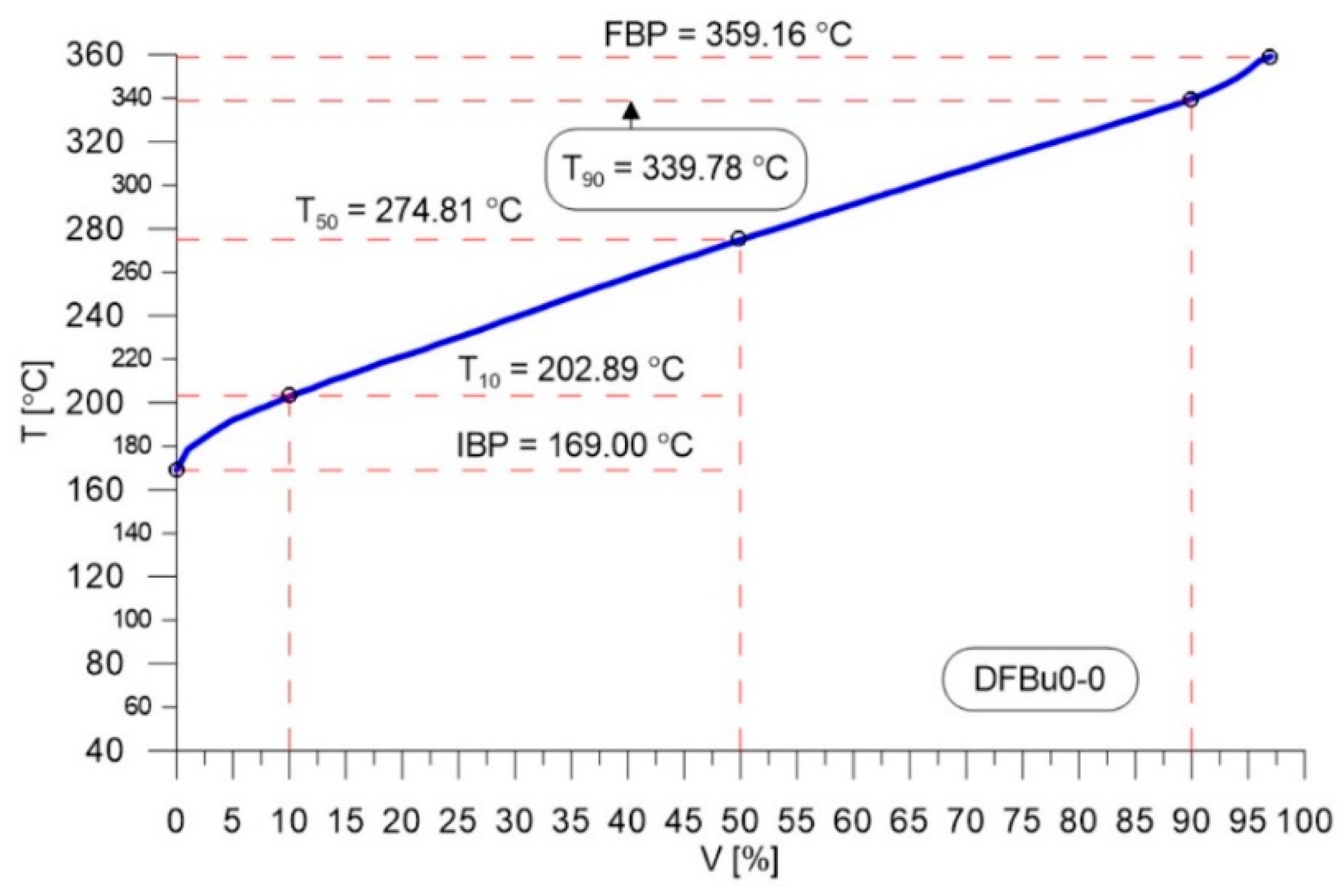
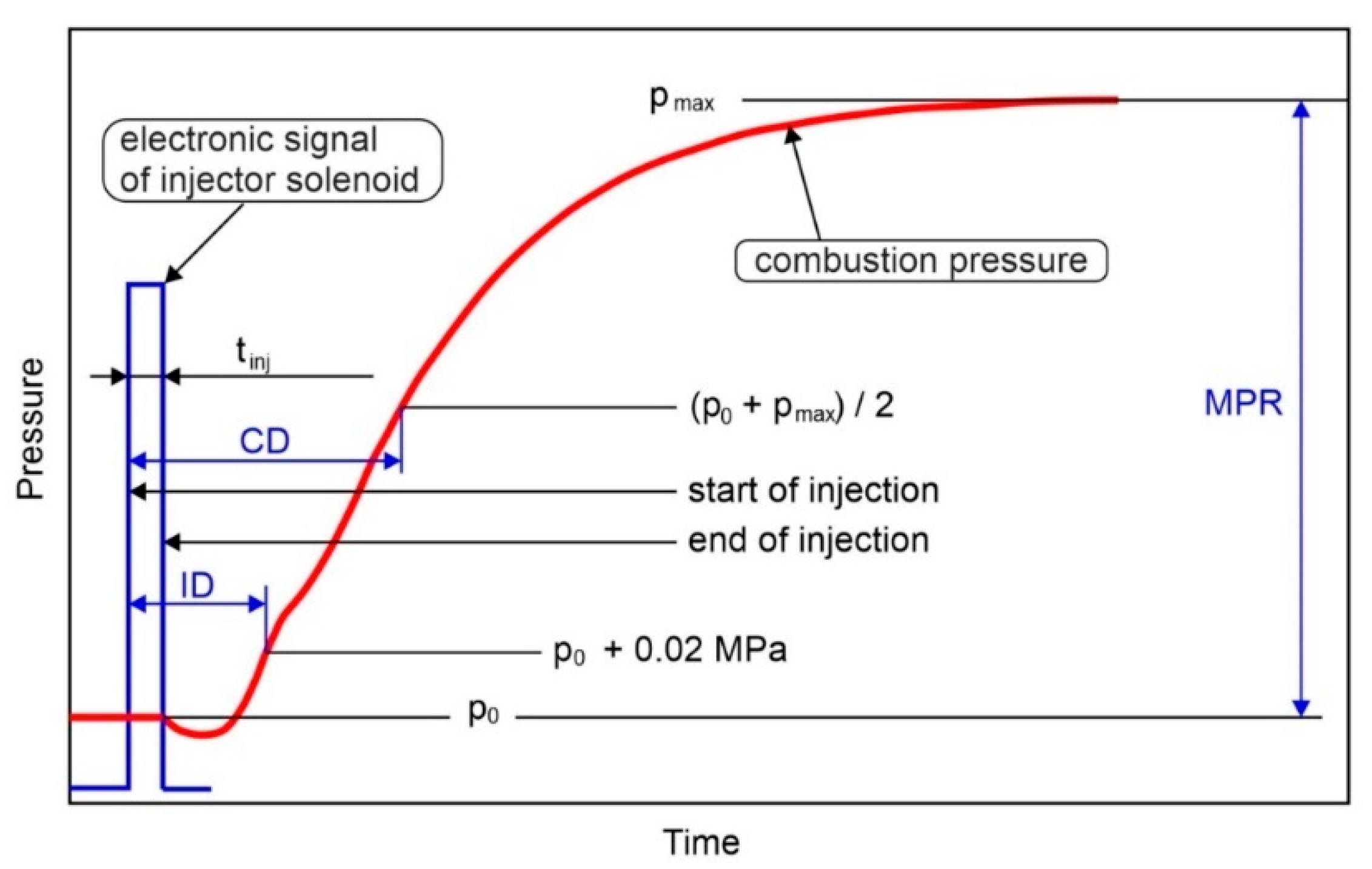

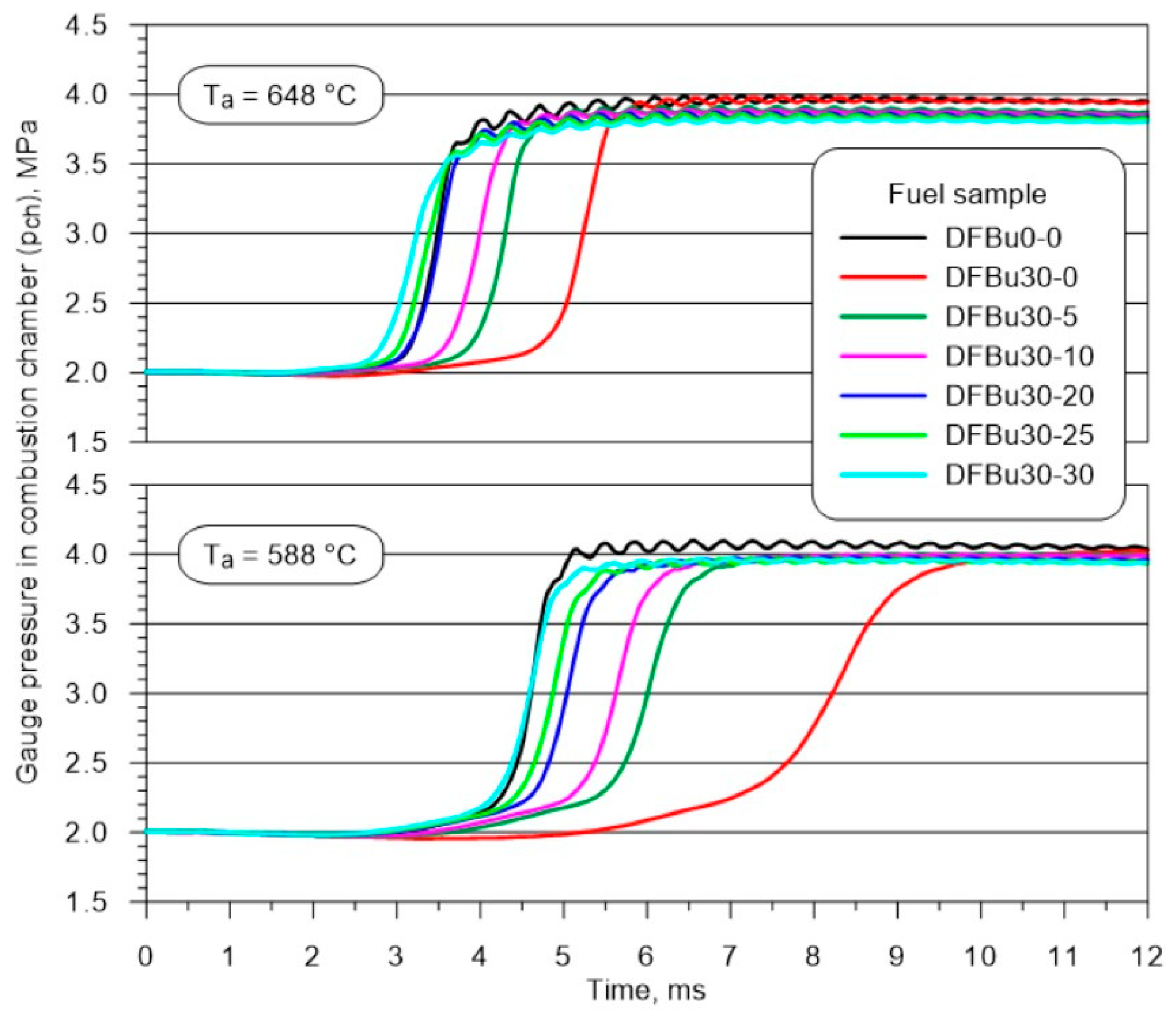
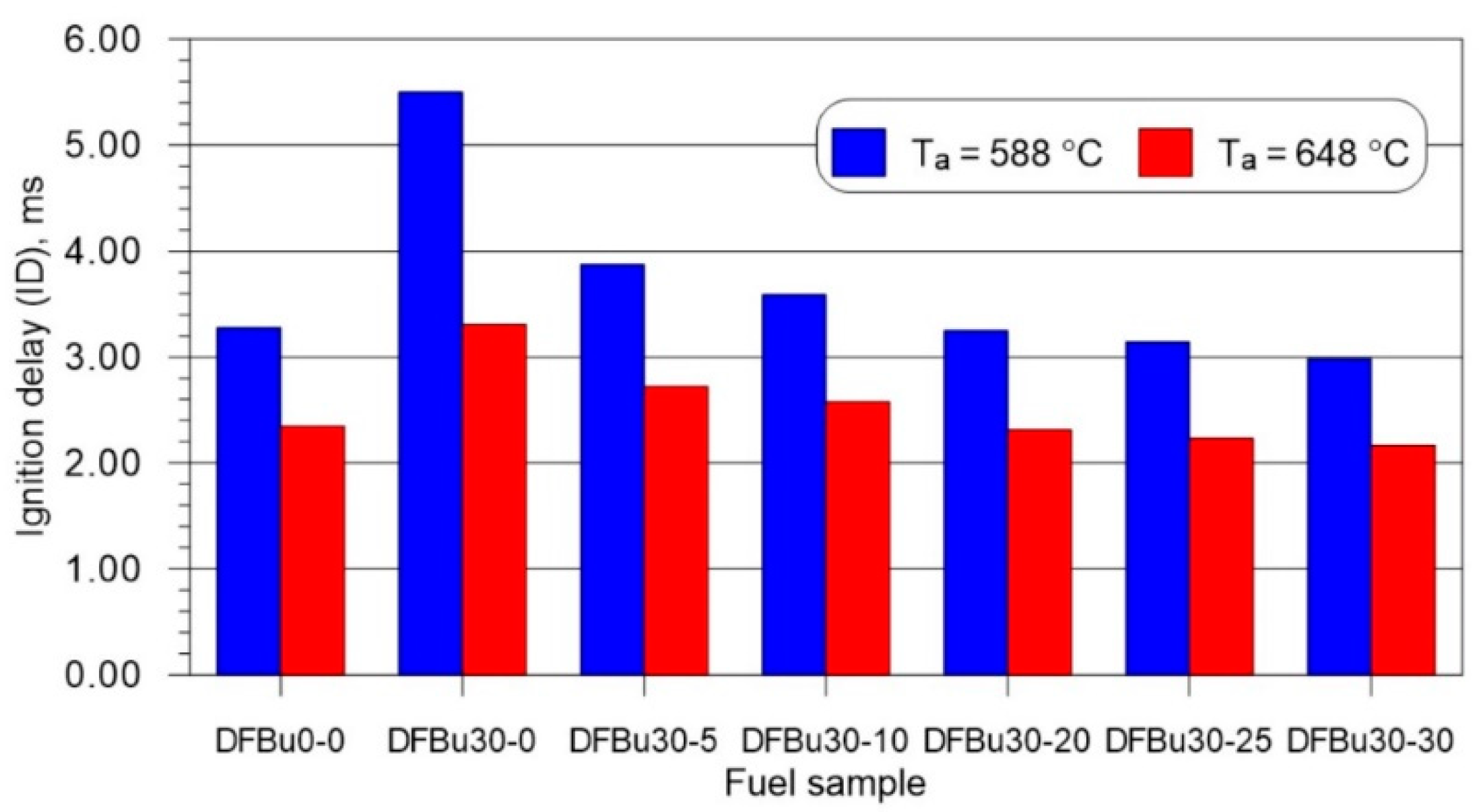


| Parameter | Unit | Value |
|---|---|---|
| FAME | % (v/v) | 6.4 |
| DCN | - | 51.6 |
| Cetane index | - | 53.5 |
| IBP | °C | 169.0 |
| T10 | °C | 202.9 |
| T50 | °C | 274.8 |
| T90 | °C | 339.8 |
| E250 | % (v/v) | 35.0 |
| E350 | % (v/v) | 94.0 |
| FBP | °C | 359.2 |
| Kinematic viscosity at 40 °C | mm2/s | 2.7 |
| Dynamic viscosity at 40 °C | cP | 2.6 |
| Density at 15 °C | g/cm3 | 0.834 |
| 2-EHN | ppm (m/m) | 0.0 |
| Mono Aromatics | % (m/m) | 17.6 |
| Di Aromatics | % (m/m) | 2.3 |
| Tri+ Aromatics | % (m/m) | 0.2 |
| Total Aromatics | % (m/m) | 20.1 |
| Polycyclic Aromatics | % (m/m) | 2.5 |
| Symbols of Fuel Samples | Fraction, % (by Volume) | Fraction, ppm (by Mass) | |
|---|---|---|---|
| Diesel Fuel | 1-Butanol | 2-EHN | |
| DFBu0-0 | 100 | 0 | 0 |
| DFBu30-0 | 70 | 30 | 0 |
| DFBu30-5 | 70 | 30 | 5000 |
| DFBu30-10 | 70 | 30 | 10,000 |
| DFBu30-20 | 70 | 30 | 20,000 |
| DFBu30-25 | 70 | 30 | 25,000 |
| DFBu30-30 | 70 | 30 | 30,000 |
| Calibration Parameter | Setting | Tolerance |
|---|---|---|
| tch | 588.0 °C | ±0.2 °C |
| tco | 50 °C | ±2 °C |
| p0 | 2.00 MPa | ±0.02 MPa |
| pinj | 100.0 MPa | ±1.5 MPa |
| tinj | 2.5 ms | not defined |
| Symbols of Fuel Samples | tinj, ms | pinj, MPa | p0, MPa | tch (Ta), °C | ϕID, ms | ϕCD, ms | ϕDCN | ϕMPR, MPa | ϕAPRR, MPa/ms | ϕMPRR, MPa/ms |
|---|---|---|---|---|---|---|---|---|---|---|
| DFBu0-0 | 2.5 | 99.4 | 2.00 | 587.6 | 0.0226 | 0.0254 | 0.3 | 0.003 | 0.0234 | 0.1515 |
| DFBu30-0 | 2.5 | 99.6 | 2.00 | 587.5 | 0.0475 | 0.0967 | 0.2 | 0.006 | 0.0044 | 0.0287 |
| DFBu30-5 | 2.5 | 98.9 | 2.00 | 587.7 | 0.0118 | 0.0307 | 0.1 | 0.005 | 0.0107 | 0.1506 |
| DFBu30-10 | 2.5 | 99.6 | 2.00 | 587.7 | 0.0239 | 0.0239 | 0.1 | 0.005 | 0.0077 | 0.0771 |
| DFBu30-20 | 2.5 | 99.0 | 2.00 | 588.8 | 0.0136 | 0.0226 | 0.2 | 0.006 | 0.0408 | 0.1579 |
| DFBu30-25 | 2.5 | 99.1 | 2.00 | 588.7 | 0.0228 | 0.0224 | 0.2 | 0.004 | 0.0102 | 0.1175 |
| DFBu30-30 | 2.5 | 100.5 | 2.00 | 588.8 | 0.0333 | 0.0368 | 0.4 | 0.002 | 0.0160 | 0.1580 |
| Symbols of Fuel Samples | tinj, ms | pinj, MPa | p0, MPa | tch (Ta), °C | ϕID, ms | ϕCD, ms | ϕMPR, MPa | ϕAPRR, MPa/ms | ϕMPRR, MPa/ms |
|---|---|---|---|---|---|---|---|---|---|
| DFBu0-0 | 2.5 | 100.2 | 2.00 | 648.3 | 0.0121 | 0.0136 | 0.001 | 0.0256 | 0.2302 |
| DFBu30-0 | 2.5 | 100.0 | 2.00 | 648.2 | 0.0280 | 0.0448 | 0.004 | 0.0223 | 0.1425 |
| DFBu30-5 | 2.5 | 99.2 | 1.99 | 648.4 | 0.0144 | 0.0254 | 0.003 | 0.0540 | 0.1631 |
| DFBu30-10 | 2.5 | 99.9 | 2.00 | 648.3 | 0.0158 | 0.0413 | 0.003 | 0.0323 | 0.1701 |
| DFBu30-20 | 2.5 | 100.0 | 2.00 | 648.3 | 0.0119 | 0.0163 | 0.002 | 0.0196 | 0.0857 |
| DFBu30-25 | 2.5 | 100.2 | 2.00 | 648.3 | 0.0213 | 0.0266 | 0.004 | 0.0206 | 0.1126 |
| DFBu30-30 | 2.5 | 99.9 | 2.00 | 648.4 | 0.0188 | 0.0334 | 0.002 | 0.0201 | 0.1091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuszewski, H.; Jaworski, A. Investigating the Effect of 2-Ethylhexyl Nitrate Cetane Improver (2-EHN) on the Autoignition Characteristics of a 1-Butanol–Diesel Blend. Energies 2024, 17, 4085. https://doi.org/10.3390/en17164085
Kuszewski H, Jaworski A. Investigating the Effect of 2-Ethylhexyl Nitrate Cetane Improver (2-EHN) on the Autoignition Characteristics of a 1-Butanol–Diesel Blend. Energies. 2024; 17(16):4085. https://doi.org/10.3390/en17164085
Chicago/Turabian StyleKuszewski, Hubert, and Artur Jaworski. 2024. "Investigating the Effect of 2-Ethylhexyl Nitrate Cetane Improver (2-EHN) on the Autoignition Characteristics of a 1-Butanol–Diesel Blend" Energies 17, no. 16: 4085. https://doi.org/10.3390/en17164085
APA StyleKuszewski, H., & Jaworski, A. (2024). Investigating the Effect of 2-Ethylhexyl Nitrate Cetane Improver (2-EHN) on the Autoignition Characteristics of a 1-Butanol–Diesel Blend. Energies, 17(16), 4085. https://doi.org/10.3390/en17164085







