Enhanced Solubility and Miscibility of CO2-Oil Mixture in the Presence of Propane under Reservoir Conditions to Improve Recovery Efficiency
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Viscosity of Trembley Oil
2.1.2. Density of Trembley Oil
2.2. Experimental Setup
2.3. Experimental Procedure
3. Mathematical Formulations
3.1. Oil Characterization
- A constant Watson factor Kw [32] is assumed based on the molecular weight and specific gravity of the oil sample:where M and are the molecular weight and specific gravity of the oil sample, respectively;
- Specific gravity for each SCN [33] can be calculated according to the Kw:for ,where , and are the specific gravity, molecular weight, and mole fraction of the ith SCN;
- The boiling temperature is calculated by using the Soreide [34] correlation, which is a function of andwhere is the boiling point temperature in °R;
- Then, the critical properties and acentric factor of SCN are estimated by the Kesler [35] correlations, which are the function of .where is the critical temperature in °R and is the critical pressure in psi;
- Acentric factor [36] can be calculated from as well:If ,If ,where , , , , , , , .
- Molecular weight of C42+ can be estimated from its known mass fraction and the number of moles:where , m, and n are the sample mass fraction, sample mass, and the number of moles of C42+, respectively.
3.2. Lumping
3.3. PR EOS Model
PR EOS
3.4. MMP
- Start with initial equations at the initial pressure and given temperature:where is the mole fraction of component i in the original reservoir oil, is the mole fraction of component i in injection gas, N is the number of components, and are the mole fractions of component i in the equilibrium liquid phase and gas phase, respectively, and is a parameter that can be anywhere in the interval from to . In this work, the first guesses of xi and yi are the mole fraction of C3H8 or CO2 or their mixture and Trembley oil;
- To obtain a set of intersecting tie lines, the following equations are applied:where is the fugacity coefficient of component i in the gas (V) or liquid (L) phase;
- Increase the pressure slightly. Equations (28) and (29) are solved again using the solution for the previous pressure;
- Repeat Step #c until one of the tie lines shrinks to a point at which condition is fulfilled for the nth tie line ifwhere Ln is the length of one of the tie lines between the injected fluid and oil. The pressure where this happens is the MMP. When the length is zero, the tie line connects two identical critical compositions. Finally, the MMP can be calculated by using the PR EOS with the determined gas fraction (xi) and liquid fraction (yi).
4. Results and Discussions
4.1. PVT Equipment Validation
4.2. Characterization of Trembley Oil
4.3. Validation of the Developed BIP Correlations
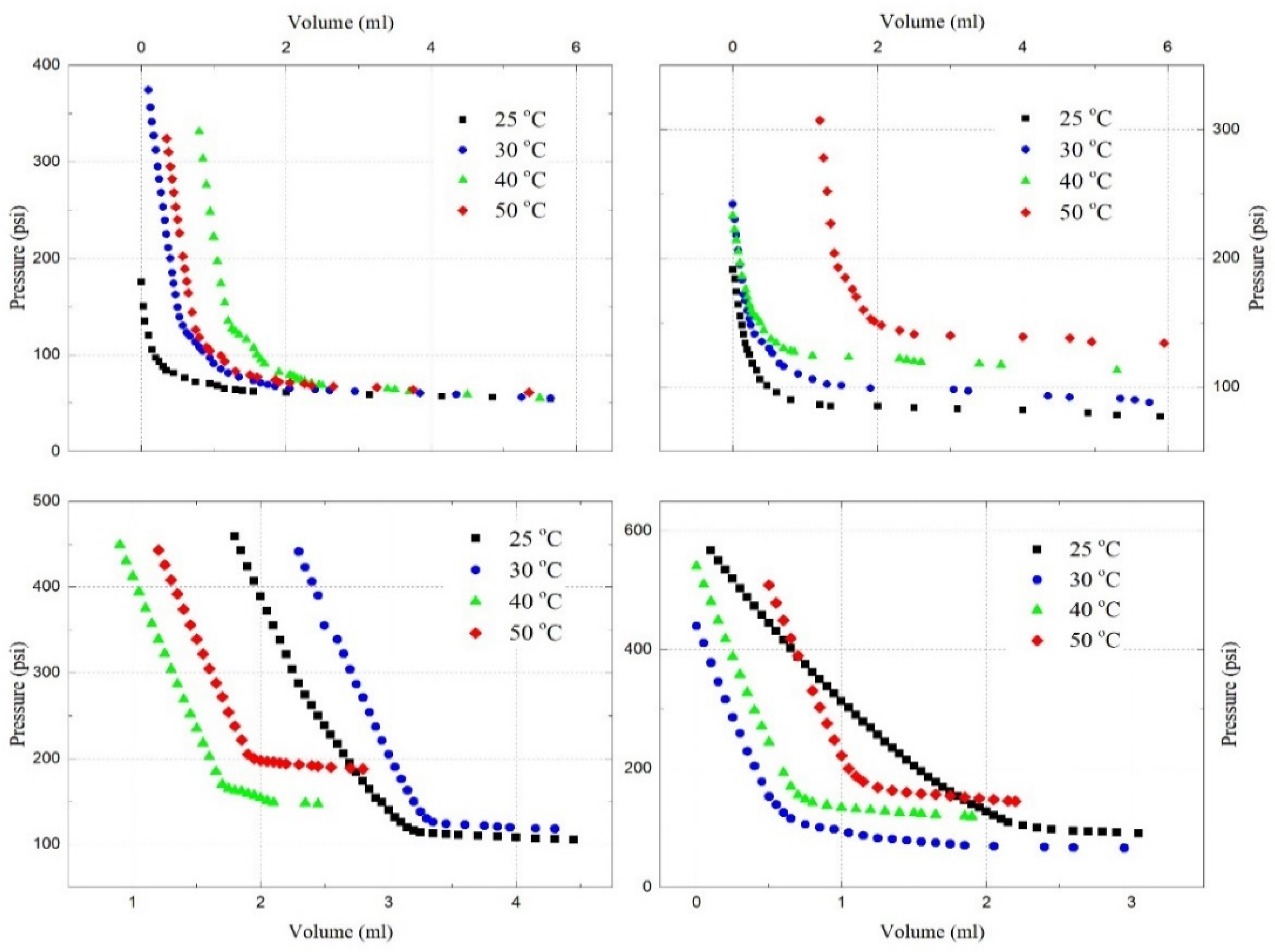
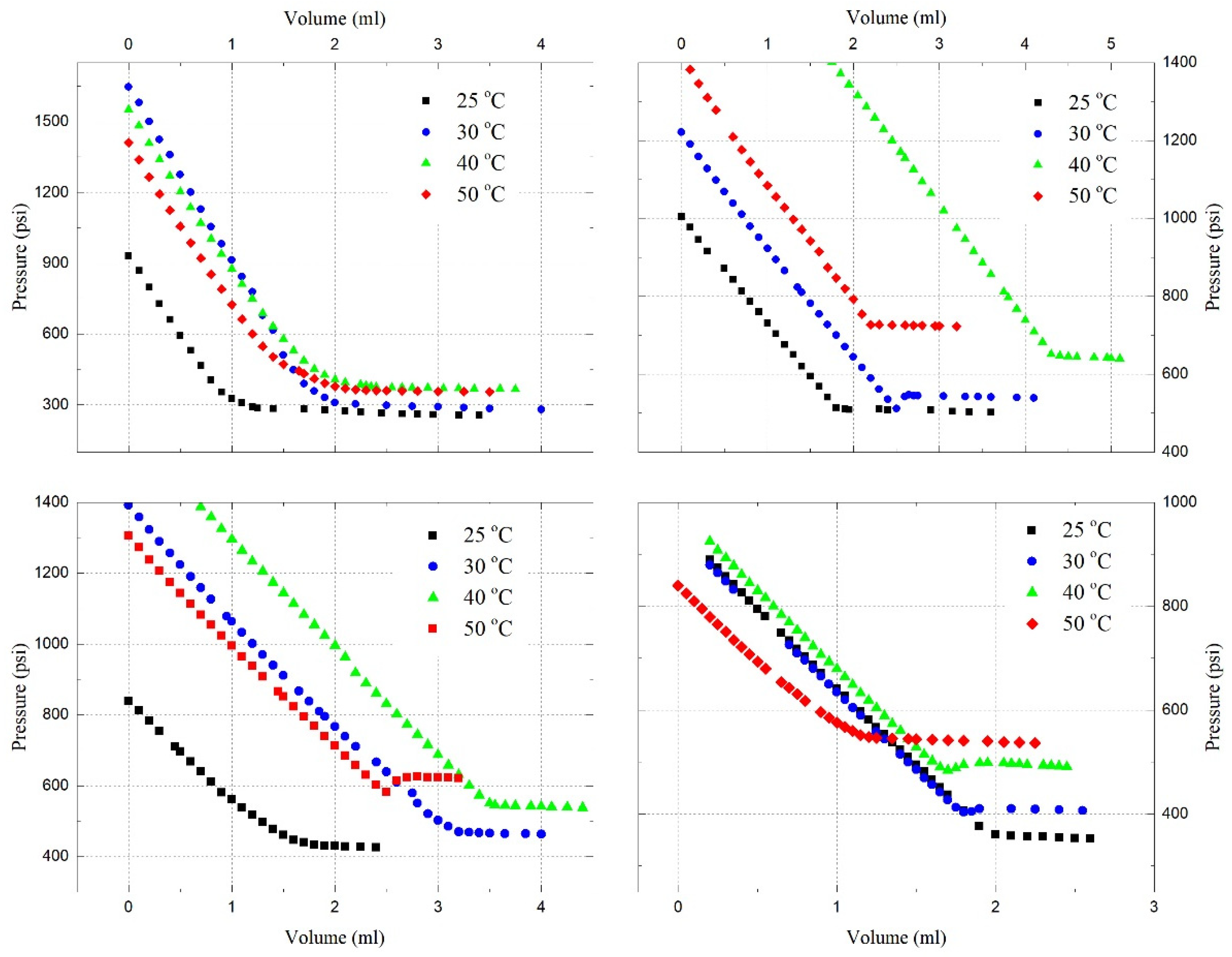
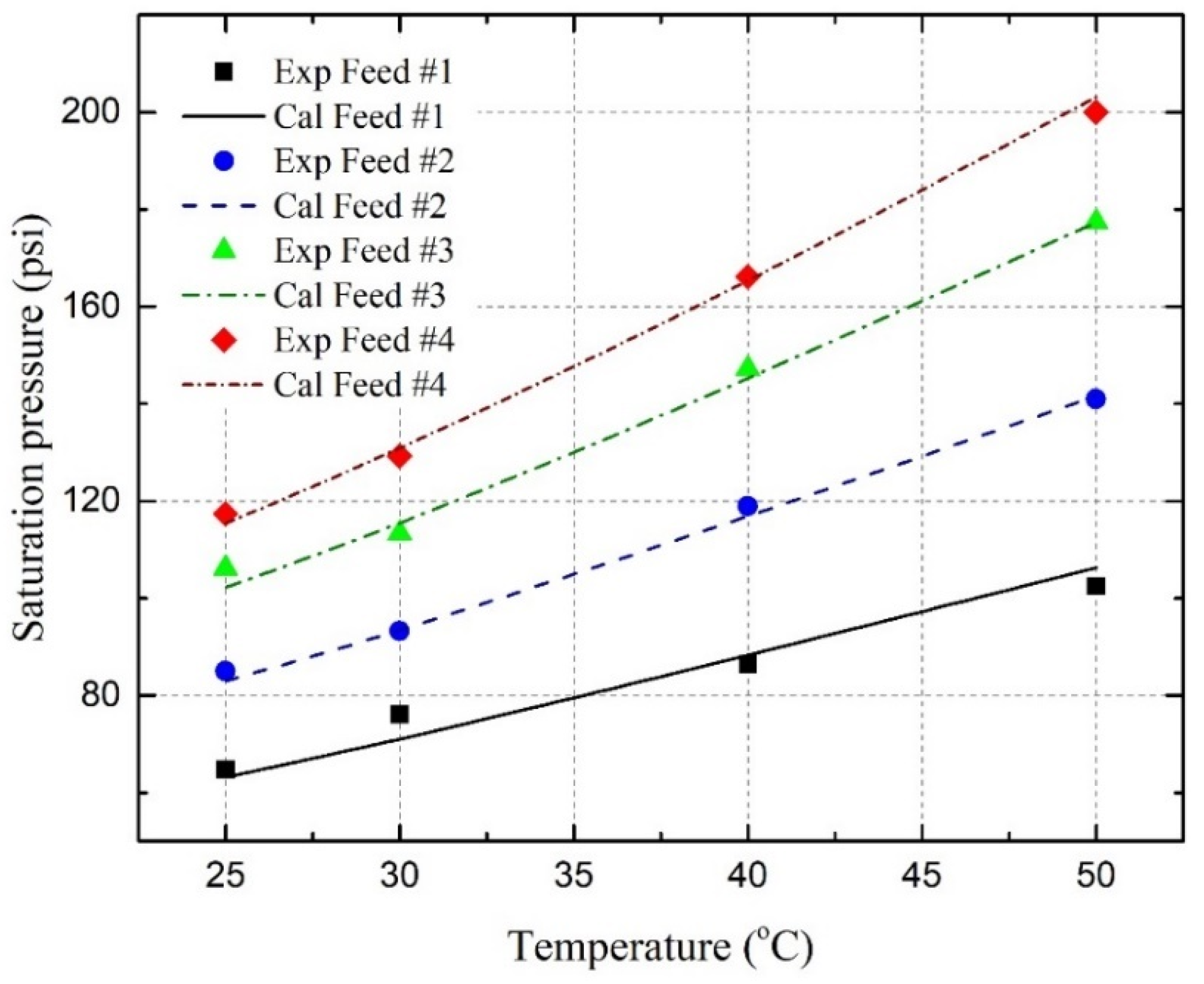
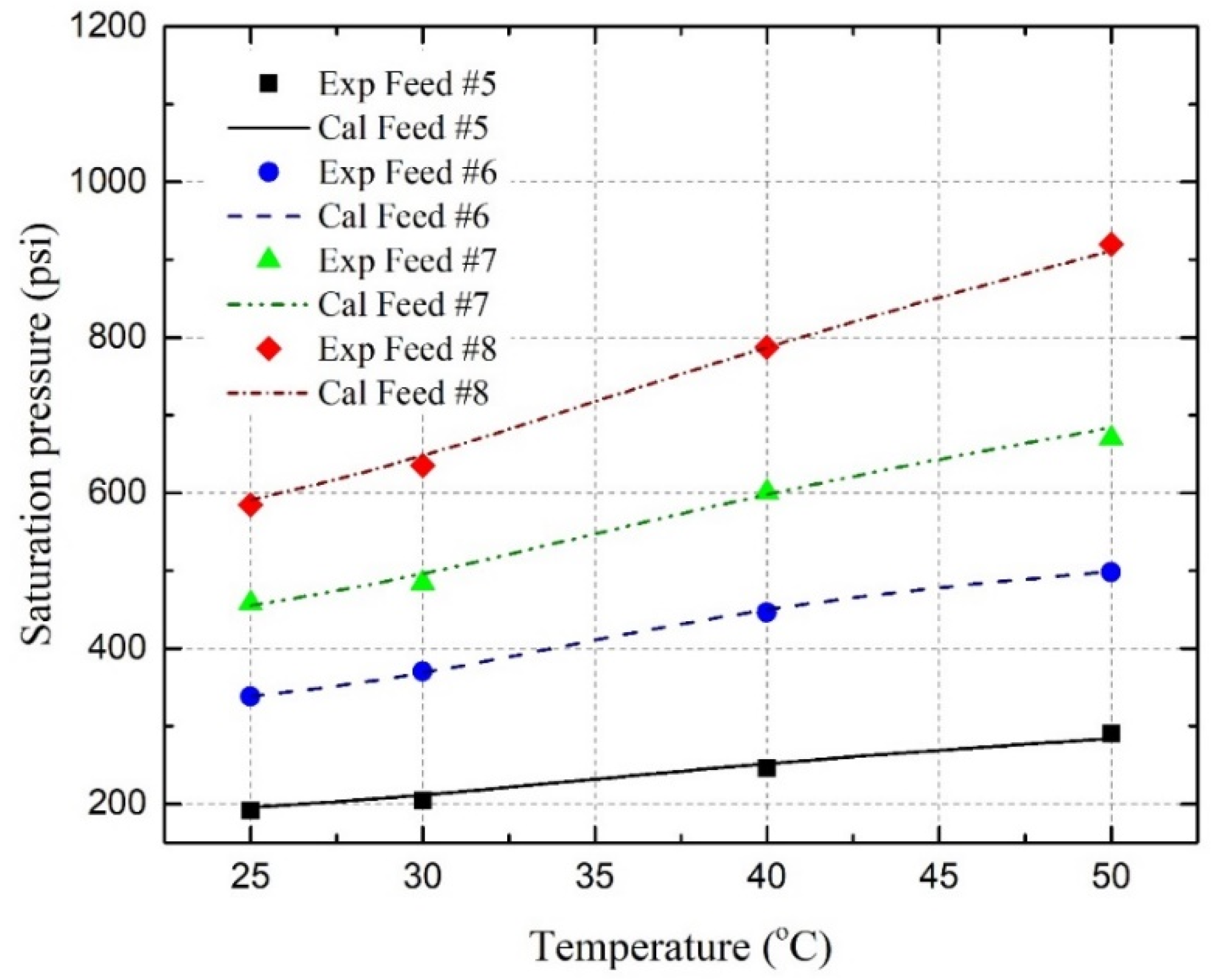
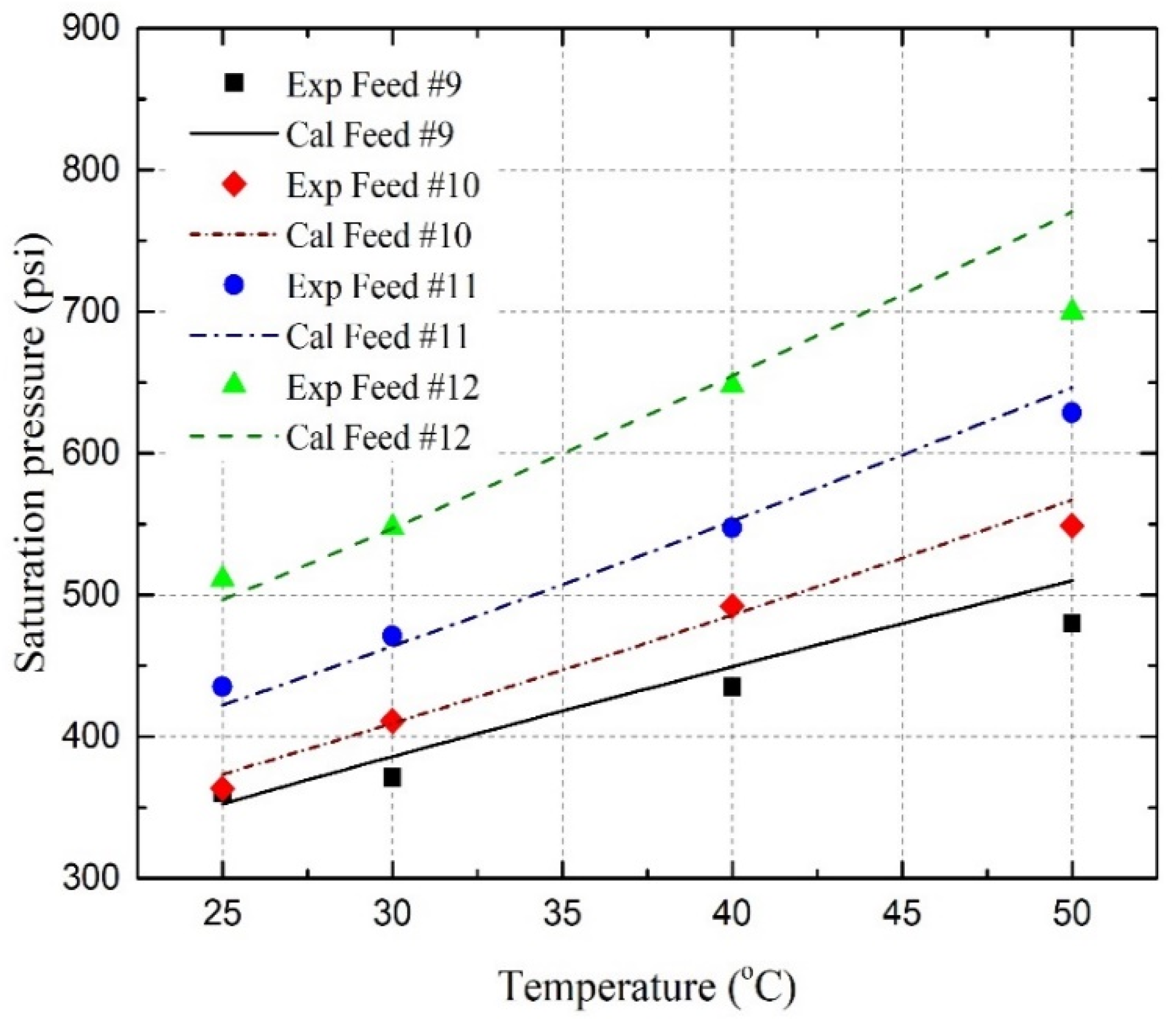
4.4. Saturation Pressure and Solubility
4.5. Viscosity of the Gas-Saturated Oil at High Pressures
4.6. Miscibility between Gas and Oil
5. Conclusions
- (1)
- Under reservoir conditions, the saturation pressures of the C3H8-oil mixture, CO2-oil mixture, and C3H8-CO2-oil mixture have been experimentally and theoretically determined. It has been found that the saturation pressure increases with the temperature, as well as with the amount of C3H8, CO2, or CO2-C3H8 gas mixture in the oil;
- (2)
- The saturation pressures are successfully predicted by using the well-developed PR EOS model, together with two newly developed BIP correlations with an overall AARD of 2.39%, where three pseudo-component lumping schemes are applied;
- (3)
- The correlations of Trembley oil’s viscosity and density have been successfully generated for temperatures ranging from 20 °C to 75 °C. The measured viscosity and saturation pressure data confirm that the presence of C3H8 in the CO2 stream can enhance oil-recovery performance by achieving miscibility at lower pressures and delaying gas-breakthrough time, ultimately leading to more CO2 being stored in the reservoir;
- (4)
- The enhancement in miscibility through C3H8 addition can effectively delay gas breakthrough and increase incremental oil recovery compared to immiscible or near-miscible CO2 flooding, thus minimizing CO2 breakthrough and improving the overall EOR efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| a | Attraction parameter in the PR-EOS |
| ac | Constant in the PR EOS |
| b | Van der Waals volume, m3/kmol |
| Kw | Watson factor, dimensionless |
| T | Temperature, °C |
| TbR | Normal boiling point at 1 atm, °R |
| TC | Critical temperature, K |
| Mi | Molecular weight of component i, g/mol |
| m | Sample mass |
| n | Number of moles |
| P | Pressure, psi |
| PC | Critical pressure, psi |
| R | Universal gas constant |
| SG | Specific gravity, dimensionless |
| V | Original molar volume calculated by the PR EOS, m3/kmol |
| VC | Critical molar volume, m3/kmol |
| Calculated saturation pressure | |
| Experimentally measured saturation pressure | |
| wi | Mass fraction |
| xi | Mole fraction of component i in the equilibrium liquid phase |
| yi | Mole fraction of component i in the equilibrium gas phase |
| zi | Mole fraction of the component i |
| α | α function in the PR EOS |
| β | Adjustable exponent defined in Equation (21) |
| σ | Adjustable exponent defined in Equation (25) |
| ρ | Density, g/cm3 |
| μ | Viscosity, cP |
| Binary interaction parameter (BIP) between ith and jth component. | |
| ω | Acentric factor, dimensionless |
| Fugacity coefficient of component i, dimensionless | |
| Generic symbol for any property | |
| Specific gravity, dimensionless | |
| Specific gravity of component i, dimensionless |
References
- Green, D.W.; Willhite, P.G. Enhanced Oil Recovery; Henry, L., Ed.; Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Al-Abri, A.; Amin, R. Phase Behaviour, Fluid Properties and Recovery Efficiency of Immiscible and Miscible Condensate Displacements by SCCO2 Injection: Experimental Investigation. Transp. Porous Media 2010, 85, 743–756. [Google Scholar] [CrossRef]
- Du, X.; Salasakar, S.; Thakur, G. A Comprehensive Summary of the Application of Machine Learning Techniques for CO2-Enhanced Oil Recovery Projects. Mach. Learn. Knowl. Extr. 2024, 6, 917–943. [Google Scholar] [CrossRef]
- Wallace, M. The U.S. CO2 Enhanced Oil Recovery Survey An Interim Update of Enhanced Oil Production Totals and CO2 Supplies for Active CO2 EOR Projects in the U.S. as of End-of-Year 2020; Advanced Resources International, Inc.: Arlington, VA, USA, 2020. [Google Scholar]
- Gozalpour, F.; Ren, S.R.; Tohidi, B. CO2 EOR and Storage in Oil Reservoir. Oil Gas. Sci. Technol. 2005, 60, 537–546. [Google Scholar] [CrossRef]
- Talbi, K.; Kaiser, T.M.V.; Maini, B.B. Experimental Investigation of CO-Based VAPEX for Recovery of Heavy Oils and Bitumen. J. Can. Pet. Technol. 2008, 47. [Google Scholar] [CrossRef]
- Badamchi-Zadeh, A.; Yarranton, H.W.; Maini, B.B.; Satyro, M.A. Phase Behaviour and Physical Property Measurements for VAPEX Solvents: Part II. Propane, Carbon Dioxide and Athabasca Bitumen. J. Can. Pet. Technol. 2009, 48, 57–65. [Google Scholar] [CrossRef]
- Li, H.; Yang, D.; Tontiwachwuthikul, P. Experimental and Theoretical Determination of Equilibrium Interfacial Tension for the Solvent(s)–CO2–Heavy Oil Systems. Energy Fuels 2012, 26, 1776–1786. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Y.; Wang, X.; Huang, S. Propane-Enriched CO2 Immiscible Flooding for Improved Heavy Oil Recovery. Energy Fuels 2012, 26, 2124–2135. [Google Scholar] [CrossRef]
- Shen, C.; Zhou, X.; Zou, B.; Wang, B.; Zhang, K.; Zeng, F. Experimental Study on the Effect of Pressure Decline Rate on Foamy Oil Flow Characteristics in a Heavy Oil– CO2-C3H8 System. Can. J. Chem. Eng. 2022, 100, 2707–2717. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Robinson, D.B.; Peng, D.Y. The Characterization of the Heptanes and Heavier Fractions for the GP Peng-Robinson Programs; Gas Processors Association: Tulsa, OK, USA, 1978. [Google Scholar]
- Perez, A.G.; Coquelet, C.; Paricaud, P.; Chapoy, A. Comparative Study of Vapour-Liquid Equilibrium and Density Modelling of Mixtures Related to Carbon Capture and Storage with the SRK, PR, PC-SAFT and SAFT-VR Mie Equations of State for Industrial Uses. Fluid. Phase Equilib. 2017, 440, 19–35. [Google Scholar] [CrossRef]
- Maghari, A.; Hosseinzadeh-Shahri, L. Evaluation of the Performance of Cubic Equations of State in Predicting the Regularities in Dense Fluids. Fluid. Phase Equilib. 2003, 206, 287–311. [Google Scholar] [CrossRef]
- Jaubert, J.-N.; Mutelet, F. VLE Predictions with the Peng–Robinson Equation of State and Temperature Dependent Kij Calculated through a Group Contribution Method. Fluid. Phase Equilib. 2004, 224, 285–304. [Google Scholar] [CrossRef]
- Boukouvalas, C.J.; Magoulas, K.G.; Stamataki, S.K.; Tassios, D.P. Prediction of Vapor−Liquid Equilibria with the LCVM Model: Systems Containing Light Gases with Medium and High Molecular Weight Compounds. Ind. Eng. Chem. Res. 1997, 36, 5454–5460. [Google Scholar] [CrossRef]
- Li, H.; Yang, D. Modified α Function for the Peng−Robinson Equation of State to Improve the Vapor Pressure Prediction of Non-Hydrocarbon and Hydrocarbon Compounds. Energy Fuels 2011, 25, 215–223. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium Constants from a Modified Redlich-Kwong Equation of State. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Gasem, K.A.M.; Gao, W.; Pan, Z.; Robinson, R.L. A Modified Temperature Dependence for the Peng–Robinson Equation of State. Fluid. Phase Equilib. 2001, 181, 113–125. [Google Scholar] [CrossRef]
- Nji, G.N.; Svrcek, W.Y.; Yarranton, H.; Satyro, M.A. Characterization of Heavy Oils and Bitumens 2. Improving the Prediction of Vapor Pressures for Heavy Hydrocarbons at Low Reduced Temperatures Using the Peng−Robinson Equation of State. Energy Fuels 2009, 23, 366–373. [Google Scholar] [CrossRef]
- Li, H.; Zheng, S.; Yang, D. Enhanced Swelling Effect and Viscosity Reduction of Solvent(s)/CO2/Heavy-Oil Systems. SPE J. 2013, 18, 695–707. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.; Fan, Z. Vapor-Liquid Phase Boundaries and Swelling Factors of C3H8-n-C4H10-CO2-Heavy Oil Systems under Reservoir Conditions. Fluid. Phase Equilib. 2017, 434, 211–221. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Yang, D. Determination of Multiphase Boundaries and Swelling Factors of Solvent(s)–CO2–Heavy Oil Systems at High Pressures and Elevated Temperatures. Energy Fuels 2013, 27, 1293–1306. [Google Scholar] [CrossRef]
- Li, X.; Han, H.; Yang, D.; Liu, X.; Qin, J. Phase Behavior of C3H8–CO2–Heavy Oil Systems in the Presence of Aqueous Phase under Reservoir Conditions. Fuel 2017, 209, 358–370. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Badamchi-Zadeh, A.; Satyro, M.A.; Maini, B.B. Phase Behaviour and Physical Properties of Athabasca Bitumen, Propane and CO2. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 17–19 June 2008. [Google Scholar]
- Eghbali, S.; Dehghanpour, H.; Dragani, J.; Zhang, X. Phase Behaviour and Viscosity of Bitumen-CO2/Light Hydrocarbon Mixtures at Elevated Temperatures: A Cold Lake Case Study. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 13–14 March 2018. [Google Scholar]
- Eghbali, S.; Dehghanpour, H. An Experimental and Modeling Study of Solvent-Bitumen Phase Behavior at Elevated Temperatures Using Cold Lake Bitumen. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. [Google Scholar]
- Nourozieh, H. Phase Partitioning and Thermo-Physical Properties of Athabasca Bitumen/Solvent Mixtures. Ph.D. Dissertation, University of Calgary, Calgary, AB, Canada, 2013. [Google Scholar]
- Luo, Y.; Yang, S.; Zhang, Y.; Kou, G.; Zhao, S.; Zhao, X.; Zhang, X.; Chen, H.; Wang, X.; Xiao, Z.; et al. Characteristics and Mechanisms of CO2 Flooding with Varying Degrees of Miscibility in Reservoirs Composed of Low-Permeability Conglomerate Formations. Processes 2024, 12, 1203. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Tao, Z.; Wang, S.; Fan, J.; Feng, Q.; Xue, Q. The Miscible Behaviors and Mechanism of CO2/CH4/C3H8/N2 and Crude Oil in Nanoslits: A Molecular Dynamics Simulation Study. Fuel 2021, 304, 121461. [Google Scholar] [CrossRef]
- Huang, E.T.S.; Tracht, J.H. The Displacement of Residual Oil By Carbon Dioxide. In Proceedings of the Improved Oil Recovery Symposium of the Society of Petroleum Engineers of AIME, Tulsa, OK, USA, 22–24 April 1974. [Google Scholar]
- Whitson, C.H. Characterizing Hydrocarbon Plus Fractions. Soc. Pet. Eng. J. 1983, 23, 683–694. [Google Scholar] [CrossRef]
- Whitson, C.H.; Brule, M.R. Phase Behavior; Society of Petroleum Engineers: Houston, TX, USA, 2000; Volume 20. [Google Scholar]
- Soreide, I. Improved Phase Behavior Predictions of Petroleum Reservoir Fluids from a Cubic Equation of State. Ph.D. Dissertation, Norwegian Inst. of Technology, Trondheim, Norway, 1989. [Google Scholar]
- Kesler, M.G.; BI, L. Improve Prediction of Enthalpy of Fractions. Hydrocarb. Process. 1976, 153, 153–158. [Google Scholar]
- Lee, B.I.; Kesler, M.G. A Generalized Thermodynamic Correlation Based on Three-parameter Corresponding States. AIChE J. 1975, 21, 510–527. [Google Scholar] [CrossRef]
- Al-Meshari, A.A.; McCain, W.D. New Strategic Method to Tune Equation-of-State for Compositional Simulation. In Proceedings of the SPE Technical Symposium of Saudi Arabia Section, Dhahran, Saudi Arabia, 14–16 May 2005. [Google Scholar]
- Danesh, A.; Xu, D.; Todd, A.C. A Grouping Method To Optimize Oil Description for Compositional Simulation of Gas-Injection Processes. SPE Reserv. Eng. 1992, 7, 343–348. [Google Scholar] [CrossRef]
- Hong, K.C. Lumped-Component Characterization of Crude Oils for Compositional Simulation. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 4–7 April 1982. [Google Scholar]
- Zabaloy, M.S.; Vera, J.H. The Peng−Robinson Sequel. An Analysis of the Particulars of the Second and Third Generations. Ind. Eng. Chem. Res. 1998, 37, 1591–1597. [Google Scholar] [CrossRef]
- Michelsen, M.L. The Isothermal Flash Problem. Part I. Stability. Fluid. Phase Equilib. 1982, 9, 1–19. [Google Scholar] [CrossRef]
- Johns, R.T.; Orr, F.M. Miscible Gas Displacement of Multicomponent Oils. SPE J. 1996, 1, 39–50. [Google Scholar] [CrossRef]
- Wang, Y.; Orr, F.M. Analytical Calculation of Minimum Miscibility Pressure. Fluid. Phase Equilib. 1997, 139, 101–124. [Google Scholar] [CrossRef]
- Wang, Y.; Orr, F.M. Calculation of Minimum Miscibility Pressure. J. Pet. Sci. Eng. 2000, 27, 151–164. [Google Scholar] [CrossRef]







| Feed # | Composition, mol% | Temperature, °C | ||
|---|---|---|---|---|
| C3H8 | CO2 | Trembley Oil | ||
| 1 | 34.62 | 0.0 | 65.38 | 25, 30, 40, 50 |
| 2 | 46.65 | 0.0 | 53.35 | 25, 30, 40, 50 |
| 3 | 60.08 | 0.0 | 39.92 | 25, 30, 40, 50 |
| 4 | 72.21 | 0.0 | 27.79 | 25, 30, 40, 50 |
| 5 | 0.0 | 29.29 | 70.71 | 25, 30, 40, 50 |
| 6 | 0.0 | 47.35 | 52.65 | 25, 30, 40, 50 |
| 7 | 0.0 | 60.39 | 39.61 | 25, 30, 40, 50 |
| 8 | 0.0 | 73.74 | 26.26 | 25, 30, 40, 50 |
| 9 | 13.03 | 46.04 | 40.57 | 25, 30, 40, 50 |
| 10 | 20.54 | 41.18 | 38.28 | 25, 30, 40, 50 |
| 11 | 14.34 | 51.05 | 34.61 | 25, 30, 40, 50 |
| 12 | 16.16 | 57.57 | 26.27 | 25, 30, 40, 50 |
| Carbon No. | wt % | mol% | Carbon No. | wt% | mol% |
|---|---|---|---|---|---|
| C1 | 0.0000 | 0.0000 | C23 | 2.4646 | 1.7589 |
| C2 | 0.0000 | 0.0000 | C24 | 2.4059 | 1.6459 |
| C3 | 0.0000 | 0.0000 | C25 | 2.1876 | 1.4370 |
| C4 | 0.0000 | 0.0000 | C26 | 2.0541 | 1.2977 |
| C5 | 0.0000 | 0.0000 | C27 | 2.0280 | 1.2340 |
| C6 | 0.5450 | 1.4653 | C28 | 1.8724 | 1.0989 |
| C7 | 3.2287 | 7.4656 | C29 | 1.8359 | 1.0405 |
| C8 | 3.9144 | 7.9396 | C30 | 1.9715 | 1.0802 |
| C9 | 5.8007 | 10.4787 | C31 | 1.8066 | 0.9581 |
| C10 | 4.5822 | 7.4615 | C32 | 1.4699 | 0.7553 |
| C11 | 3.8471 | 5.7023 | C33 | 1.4436 | 0.7194 |
| C12 | 3.3481 | 4.5539 | C34 | 1.2218 | 0.5910 |
| C13 | 4.7586 | 5.9800 | C35 | 1.3866 | 0.6517 |
| C14 | 4.6292 | 5.4060 | C36 | 0.9497 | 0.4340 |
| C15 | 3.9684 | 4.3283 | C37 | 1.3118 | 0.5833 |
| C16 | 3.5751 | 3.6578 | C38 | 1.1327 | 0.4905 |
| C17 | 5.1290 | 4.9415 | C39 | 0.6638 | 0.2801 |
| C18 | 4.1833 | 3.8083 | C40 | 0.8432 | 0.3469 |
| C19 | 3.3873 | 2.9225 | C41 | 0.7403 | 0.2972 |
| C20 | 2.4659 | 2.0220 | C42 | 0.8318 | 0.3260 |
| C21 | 3.0100 | 2.3514 | C42+ | 6.8063 | 0.8485 |
| C22 | 2.1989 | 1.6402 | Total | 100 | 100 |
| Temperature (°C) | Reference Value (psi) | Continuous Method | Stepwise Method | ||
|---|---|---|---|---|---|
| Measured (psi) | Error (%) | Measured (psi) | Error (%) | ||
| 30 | 158.4 | 163.2 | 3.03 | 161.6 | 2.02 |
| 40 | 190.3 | 195.4 | 2.67 | 192.7 | 1.26 |
| 50 | 226.8 | 232.6 | 2.56 | 230.1 | 1.45 |
| No. of PCs * | MW, g/mol | PC, atm | TC, K | γ | Tb, K | ω |
|---|---|---|---|---|---|---|
| 1 | 234.667 | 17.008 | 753.715 | 0.863 | 573.479 | 0.114 |
| 2 | 150.212 | 23.711 | 641.937 | 0.794 | 459.751 | 0.212 |
| 490.845 | 14.631 | 869.390 | 0.954 | 687.960 | 0.636 | |
| 3 | 129.247 | 25.914 | 610.064 | 0.774 | 427.981 | 0.176 |
| 213.037 | 18.350 | 727.432 | 0.846 | 546.376 | 0.312 | |
| 580.628 | 13.101 | 910.548 | 0.982 | 728.647 | 0.739 | |
| 4 | 116.781 | 27.578 | 588.588 | 0.760 | 407.150 | 0.153 |
| 163.012 | 21.979 | 664.193 | 0.807 | 481.339 | 0.236 | |
| 231.871 | 17.262 | 748.773 | 0.860 | 568.598 | 0.337 | |
| 604.239 | 12.911 | 919.991 | 0.990 | 737.828 | 0.766 | |
| 5 | 116.782 | 27.579 | 588.589 | 0.761 | 407.517 | 0.153 |
| 163.013 | 21.980 | 664.193 | 0.808 | 481.773 | 0.237 | |
| 219.448 | 17.853 | 735.917 | 0.852 | 555.609 | 0.322 | |
| 296.820 | 14.796 | 808.874 | 0.899 | 632.341 | 0.408 | |
| 745.441 | 12.270 | 966.642 | 1.030 | 782.285 | 0.933 | |
| 6 | 107.672 | 28.999 | 571.533 | 0.750 | 390.998 | 0.136 |
| 134.178 | 25.045 | 619.963 | 0.780 | 437.200 | 0.186 | |
| 171.067 | 21.184 | 676.108 | 0.814 | 493.328 | 0.250 | |
| 219.447 | 17.852 | 735.916 | 0.852 | 555.108 | 0.322 | |
| 290.818 | 14.952 | 804.197 | 0.896 | 626.856 | 0.403 | |
| 718.796 | 12.343 | 958.868 | 1.022 | 774.496 | 0.901 |
| Binary and Ternary Systems | Feed No. | No. of PCs | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 1 | 2 | 3 | 4 | 5 | 6 | ||
| C3H8-oil | 1 | 25 | 1.99 | 1.21 | 1.32 | 1.47 | 1.22 | 1.31 |
| 2 | 30 | 2.21 | 1.30 | 1.48 | 1.52 | 1.25 | 1.34 | |
| 3 | 40 | 2.44 | 1.54 | 1.55 | 1.57 | 1.29 | 1.39 | |
| 4 | 50 | 2.68 | 1.41 | 1.54 | 1.63 | 1.33 | 1.44 | |
| CO2-oil | 5 | 25 | 0.19 | 0.27 | 0.04 | 0.05 | 0.03 | 0.05 |
| 6 | 30 | 0.09 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | |
| 7 | 40 | 0.92 | 0.06 | 0.10 | 0.11 | 0.08 | 0.12 | |
| 8 | 50 | 0.38 | 0.00 | 0.01 | 0.02 | 0.00 | 0.03 | |
| C3H8-CO2-oil (β for C3H8-PC) | 9 | 25 | 1.60 | 1.36 | 1.36 | 1.36 | 1.22 | 1.36 |
| 10 | 30 | 1.69 | 1.30 | 1.40 | 1.42 | 1.28 | 1.39 | |
| 11 | 40 | 2.68 | 1.36 | 1.36 | 1.36 | 1.29 | 1.34 | |
| 12 | 50 | 1.72 | 1.36 | 1.37 | 1.36 | 1.33 | 1.20 | |
| C3H8-CO2-oil (β for CO2-PC) | 9 | 25 | 0.22 | 0.01 | 0.01 | 0.01 | 0.03 | 0.01 |
| 10 | 30 | 0.31 | 0.01 | 0.02 | 0.04 | 0.03 | 0.06 | |
| 11 | 40 | 0.50 | 0.01 | 0.01 | 0.01 | 0.08 | 0.01 | |
| 12 | 50 | 0.34 | 0.01 | 0.02 | 0.01 | 0.00 | 0.01 | |
| System | μ (cP) |
|---|---|
| C3H8-oil (Feed #1) | 2.33 |
| CO2-oil (Feed #8) | 3.03 |
| C3H8-CO2-oil (Feed #12) | 1.89 |
| Dead oil at 1600 psi | 9.50 |
| Dead oil at room pressure | 9.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Li, X.; Thakur, G.C. Enhanced Solubility and Miscibility of CO2-Oil Mixture in the Presence of Propane under Reservoir Conditions to Improve Recovery Efficiency. Energies 2024, 17, 4790. https://doi.org/10.3390/en17194790
Du X, Li X, Thakur GC. Enhanced Solubility and Miscibility of CO2-Oil Mixture in the Presence of Propane under Reservoir Conditions to Improve Recovery Efficiency. Energies. 2024; 17(19):4790. https://doi.org/10.3390/en17194790
Chicago/Turabian StyleDu, Xuejia, Xiaoli Li, and Ganesh C. Thakur. 2024. "Enhanced Solubility and Miscibility of CO2-Oil Mixture in the Presence of Propane under Reservoir Conditions to Improve Recovery Efficiency" Energies 17, no. 19: 4790. https://doi.org/10.3390/en17194790
APA StyleDu, X., Li, X., & Thakur, G. C. (2024). Enhanced Solubility and Miscibility of CO2-Oil Mixture in the Presence of Propane under Reservoir Conditions to Improve Recovery Efficiency. Energies, 17(19), 4790. https://doi.org/10.3390/en17194790








