On the Use of the Multi-Site Langmuir Model for Predicting Methane Adsorption on Shale
Abstract
1. Introduction
2. Absolute Adsorption and Excess Adsorption
2.1. Equations for Absolute and Excess Adsorption
2.2. Bulk Phase Density
2.3. Adsorbed Phase Density
3. Single-Site Langmuir Adsorption Model
3.1. Single-Site Absolute Adsorption Model
3.2. Single-Site Excess Adsorption Model
4. Multi-Site Langmuir Adsorption Model
4.1. Heterogeneity for Methane Adsorption on Shale
4.2. Multi-Site Absolute Adsorption Model
- (1)
- Taking an average value of entropy , for adsorption to eliminate the parameter . Thus, Equation (12) becomes:
- (2)
- Assuming a Gaussian distribution is often the case with the adsorption heat for shales, as described by the equations below:
4.3. Multi-Site Excess Adsorption Model
5. Samples and Experiments
6. Results and Discussion
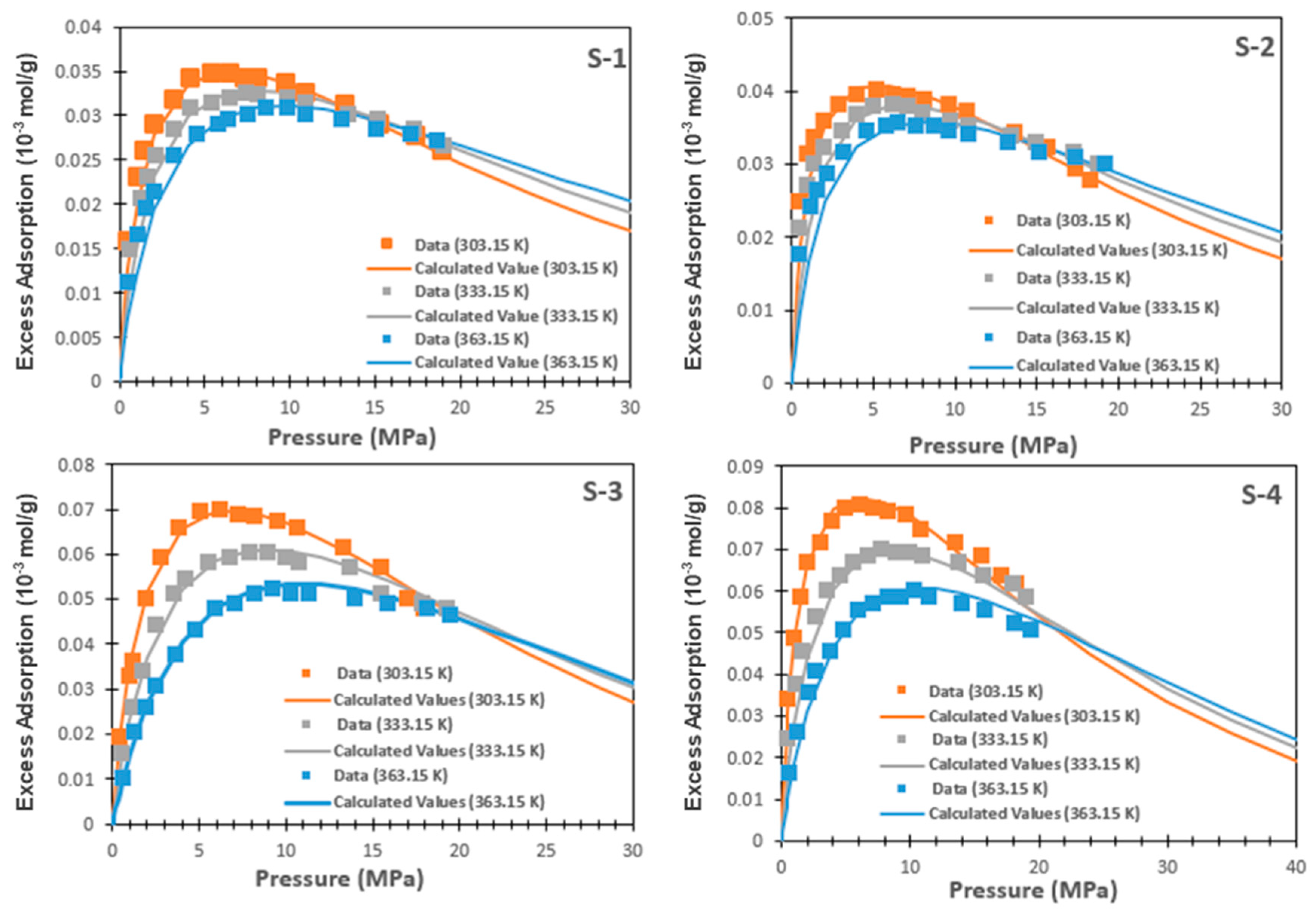
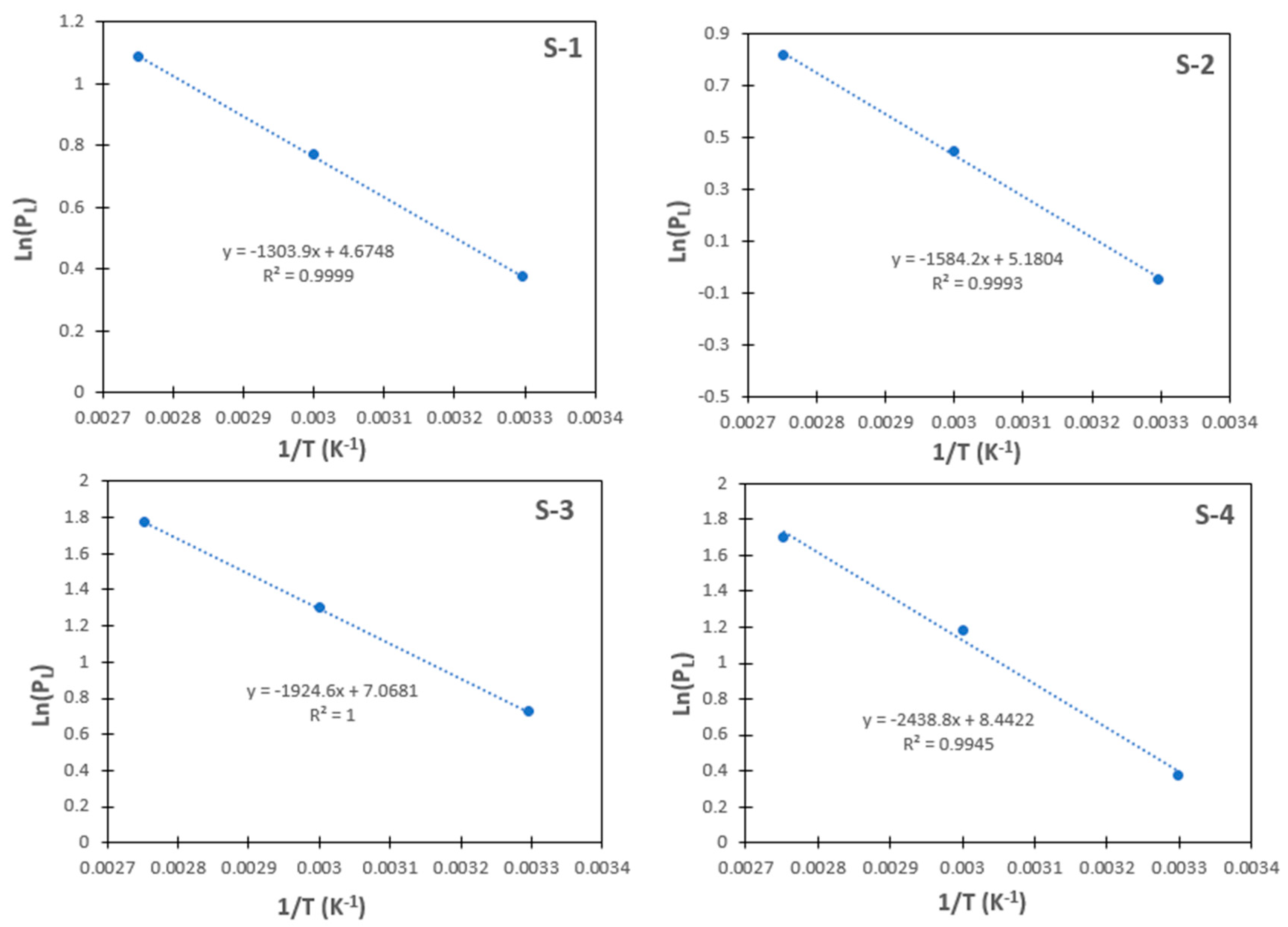
6.1. Adsorption Isotherms Fitting by Single-Site Model
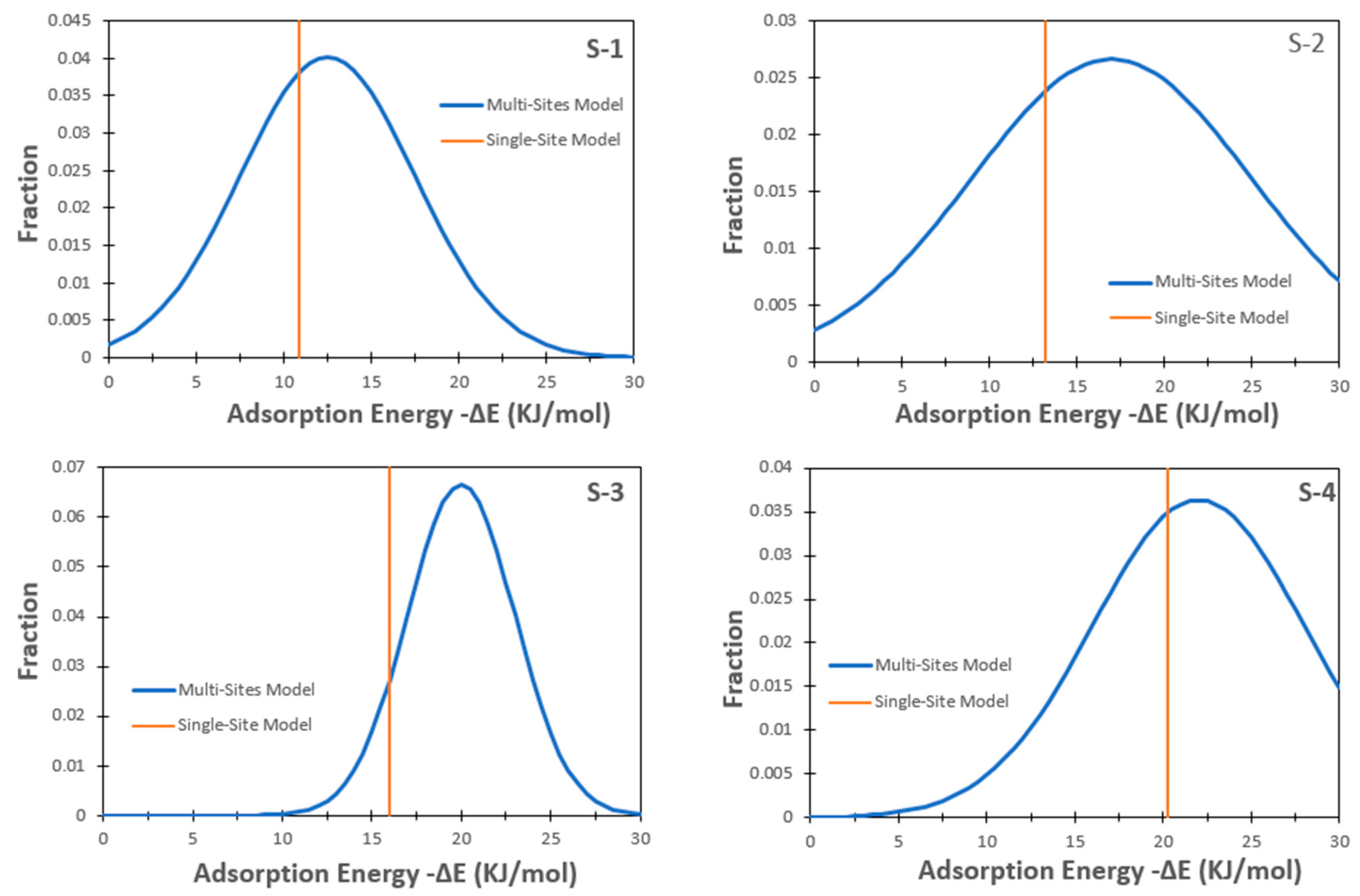
6.2. Adsorption Isotherms Fitting by Multi-Site Model
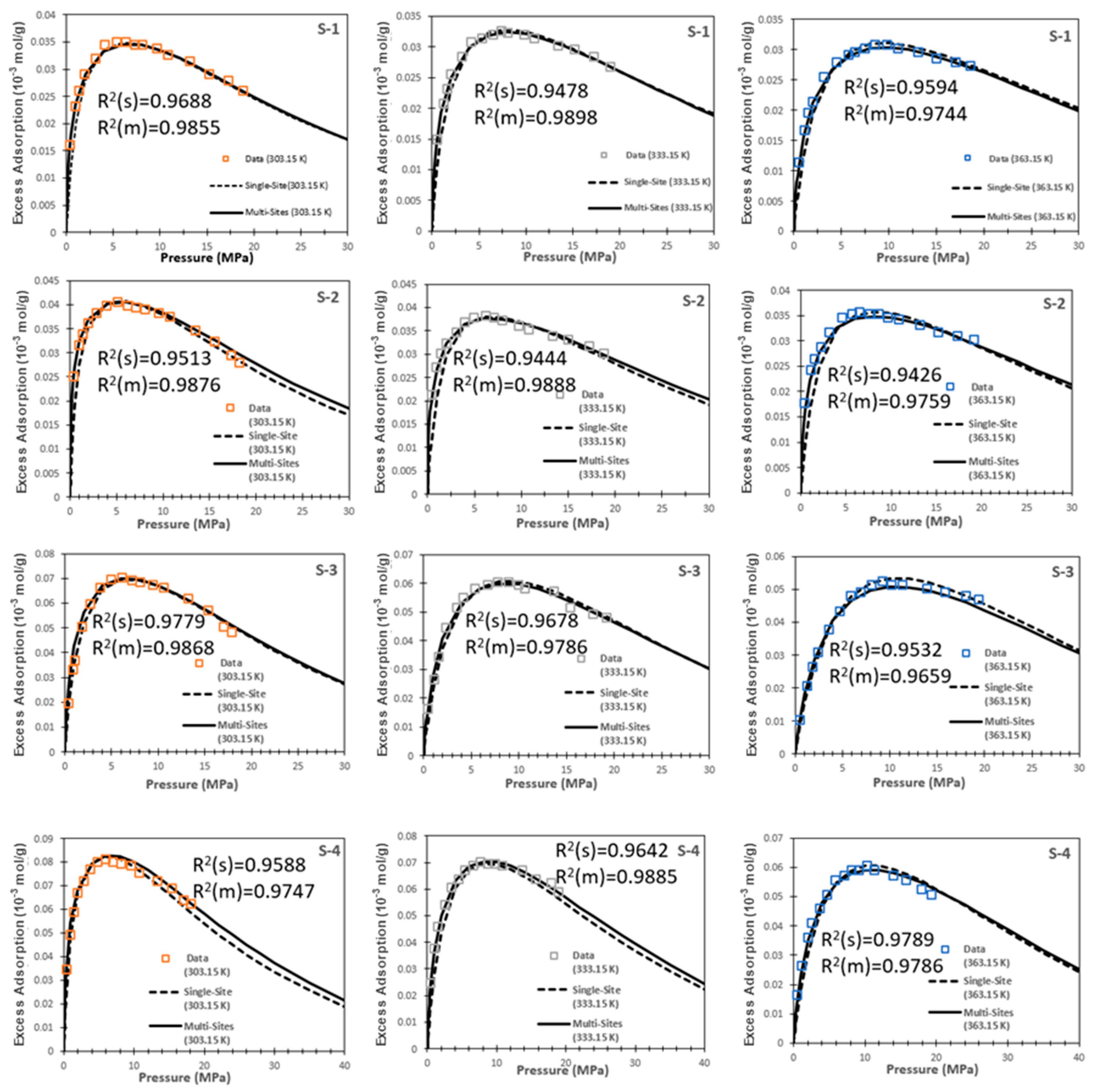
6.3. Comparison of Two Models
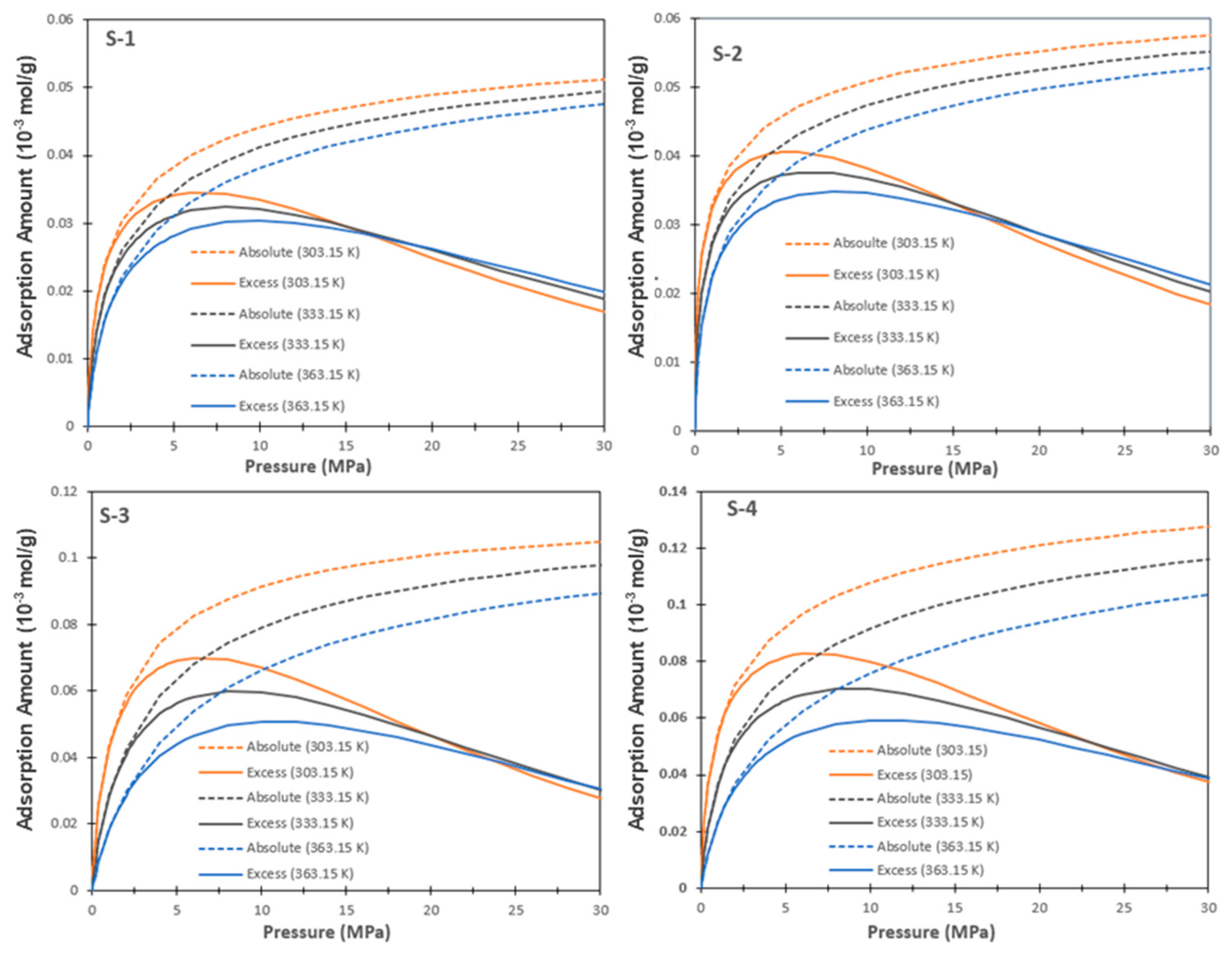
6.4. Absolute Adsorption and Excess Adsorption
7. Conclusions
- (1)
- Several laboratory tests have been conducted to understand the characteristics of these shale samples. The results have shown that these samples have many features in common, such as the primary compositions of the content being quartz, clay, and kerogen. Once the physical properties of these samples are studied, a series of adsorption isotherms analyses, with a wide range of pressures and temperatures, corresponding to the actual shale reservoir condition, are then carried out for these samples.
- (2)
- The traditional single-site Langmuir adsorption model is commonly applied to investigate the methane adsorption in shales. However, this model assumes that a surface is homogeneous, and thus it is incapable of addressing the heterogeneity (various mineral materials and pore sizes) of shales on methane adsorption. Therefore, in order to accurately analyze the methane adsorption behaviors on shales, an adsorption model with an assumption of a heterogeneous surface is needed.
- (3)
- Both the single-site and multi-site excess adsorption models achieved satisfying fitting results with the actual measured data. Nevertheless, the multi-site model is capable of addressing the heterogeneity of shales by a wide range of adsorption energy distributions (owing to the complex compositions and different pore sizes), which is different from the single-site model only characterized by single adsorption energy. Consequently, the multi-site model results have better accuracy against the experimental data.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aguilera, R. Flow units: From conventional to tight-gas to shale-gas to tight-oil to shale-oil reservoirs. SPE Reserv. Eval. Eng. 2014, 17, 190–208. [Google Scholar] [CrossRef]
- IEA (International Energy Agency): Outlook for unconventional gas. World Energy Outlook 2015, 2015, 229–268.
- Wood, D.A.; Hazra, B. Characterization of organic-rich shales for petroleum exploration & exploitation: A review-Part 3: Applied geomechanics, petrophysics and reservoir modeling. J. Earth Sci. 2017, 28, 779–803. [Google Scholar]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Lu, X.C.; Li, F.C.; Watson, A.T. Adsorption measurements in Devonian shales. Fuel 1995, 74, 599–603. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, X.; Li, Y.; Wu, K.; Shi, J.; Yang, L.; Feng, D.; Zhang, T.; Yu, P. Water distribution characteristic and effect on methane adsorption capacity in shale clay. Int. J. Coal Geol. 2016, 159, 135–154. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Ross, D.J.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Ambrose, R.J.; Hartman, R.C.; Diaz-Campos, M.; Akkutlu, I.Y.; Sondergeld, C.H. Shale Gas-in-Place Calculations Part I: New Pore-Scale Considerations. SPE J. 2012, 17, 219–229. [Google Scholar] [CrossRef]
- Roque-Malherbe, R.M. Adsorption and Diffusion in Nanoporous Materials; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar]
- Yang, F.; Ning, Z.; Zhang, R.; Zhao, H.; Krooss, B.M. Investigations on the methane sorption capacity of marine shales from Sichuan Basin, China. Int. J. Coal Geol. 2015, 146, 104–117. [Google Scholar] [CrossRef]
- Do, D.D.; Do, H.D.; Fan, C.; Nicholson, D. On the existence of negative excess isotherms for argon adsorption on graphite surfaces and in graphitic pores under supercritical conditions at pressures up to 10,000 atm. Langmuir 2010, 26, 4796–4806. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L. Thermodynamics of adsorption in porous materials. AIChE J. 2002, 48, 145–160. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Z.; Li, X.; Dong, X. Methane storage in nanoporous material at supercritical temperature over a wide range of pressures. Sci. Rep. 2016, 6, 33461. [Google Scholar] [CrossRef]
- Nikolaev, K.M.; Dubinin, M.M. Concerning adsorptional properties of carbon adsorbents 3. A study of adsorption isotherms of gases and vapors on active carbons over a wide interval of temperatures, including the critical region. Russ. Chem. Bull. 1958, 7, 1124–1133. [Google Scholar] [CrossRef]
- Reich, R.; Ziegler, W.T.; Rogers, K.A. Adsorption of methane, ethane, and ethylene gases and their binary and ternary mixtures and carbon dioxide on activated carbon at 212-301 K and pressures to 35 atmospheres. Ind. Eng. Chem. Process Des. Dev. 1980, 19, 336–344. [Google Scholar] [CrossRef]
- Mehta, S.D.; Danner, R.P. An improved potential theory method for predicting gas-mixture adsorption equilibria. Ind. Eng. Chem. Fundam. 1985, 24, 325–330. [Google Scholar] [CrossRef]
- Ozawa, S.; Kusumi, S.; Ogino, Y. Physical adsorption of gases at high pressure. IV. An improvement of the Dubinin—Astakhov adsorption equation. J. Colloid Interface Sci. 1976, 56, 83–91. [Google Scholar] [CrossRef]
- Yang, F.; Xie, C.; Xu, S.; Ning, Z.; Krooss, B.M. Supercritical methane sorption on organic-rich shales over a wide temperature range. Energy Fuels 2017, 31, 13427–13438. [Google Scholar] [CrossRef]
- Setzmann, U.; Wagner, W. A new equation of state and tables of thermodynamic properties for methane covering the range from the melting line to 625 K at pressures up to 100 MPa. J. Phys. Chem. Ref. Data 1991, 20, 1061–1155. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Wu, K.; Wang, K.; Luo, J.; Feng, D.; Qu, S.; Li, X. A multi-site model to determine supercritical methane adsorption in energetically heterogeneous shales. Chem. Eng. J. 2018, 349, 438–455. [Google Scholar] [CrossRef]
- Hu, Y.; Devegowda, D.; Striolo, A.; Phan, A.; Ho, T.A.; Civan, F.; Sigal, R.F. Microscopic dynamics of water and hydrocarbon in shale-kerogen pores of potentially mixed wettability. Spe J. 2014, 20, 112–124. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, T.; Wiggins-Camacho, J.D.; Ellis, G.S.; Lewan, M.D.; Zhang, X. Experimental investigation of changes in methane adsorption of bitumen-free Woodford Shale with thermal maturation induced by hydrous pyrolysis. Mar. Pet. Geol. 2015, 59, 114–128. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-pressure methane sorption isotherms of black shales from the Netherlands. Energy Fuels 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Qi, R.; Zeng, Y.; Qin, H.; Ye, H.; Zhang, W. Molecular simulation of adsorption behaviors of methane, carbon dioxide and their mixtures on kerogen: Effect of kerogen maturity and moisture content. Fuel 2018, 211, 159–172. [Google Scholar] [CrossRef]
- Blanco AA, G.; Vallone, A.F.; Korili, S.A.; Gil, A.; Sapag, K. A comparative study of several microporous materials to store methane by adsorption. Microporous Mesoporous Mater. 2016, 224, 323–331. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, X.; Liang, L.; Zeng, Q. Adsorption of methane in organic-rich shale nanopores: An experimental and molecular simulation study. Fuel 2017, 200, 299–315. [Google Scholar] [CrossRef]


| Sample | Grain Density (g/cm3) | Bulk Density (g/cm3) | Total Pore Volume (cm3/g) | Total Porosity (%) |
|---|---|---|---|---|
| S-1 | 2.25 | 2.49 | 0.0141 | 3.5 |
| S-2 | 2.30 | 2.21 | 0.0186 | 4.1 |
| S-3 | 2.56 | 2.43 | 0.0210 | 5.1 |
| S-4 | 2.74 | 2.49 | 0.0365 | 9.1 |
| Sample | Temperature T (K) | (10−3 mol/g) | Langmuir Pressure PL (MPa) | Expansion Coefficient (k−1) | (J/mol/K) | (KJ/mol) |
|---|---|---|---|---|---|---|
| S-1 | 303.15 | 0.050 | 1.45 | 0.0015 | −58.01 | −10.84 |
| 333.15 | 2.15 | |||||
| 363.15 | 2.95 | |||||
| S-2 | 303.15 | 0.055 | 0.95 | 0.0018 | −62.21 | −13.17 |
| 333.15 | 1.55 | |||||
| 363.15 | 2.25 | |||||
| S-3 | 303.15 | 0.110 | 2.05 | 0.0022 | −77.91 | −16.00 |
| 333.15 | 3.65 | |||||
| 363.15 | 5.85 | |||||
| S-4 | 303.15 | 0.120 | 1.45 | 0.0020 | −89.33 | −20.27 |
| 333.15 | 3.25 | |||||
| 363.15 | 5.45 |
| Sample | (10−3 mol/g) | (J/mol/K) | (kJ/mol) | Standard Deviation (Dimensionless) | Expansion Coefficient (K−1) |
|---|---|---|---|---|---|
| S-1 | 0.053 | −66 | −12.5 | 5 | 0.0015 |
| S-2 | 0.060 | −76 | −17 | 8 | 0.0018 |
| S-3 | 0.108 | −90.5 | −20 | 3 | 0.0022 |
| S-4 | 0.124 | −98 | −22 | 6 | 0.0020 |
| Sample | Temperature (K) | Percentage of Error (%) | |
|---|---|---|---|
| Single-Site Model | Multi-Site Model | ||
| S-1 | 303.15 | 3.12 | 1.45 |
| 333.15 | 5.22 | 1.02 | |
| 363.15 | 4.06 | 2.56 | |
| S-2 | 303.15 | 4.87 | 1.24 |
| 333.15 | 5.56 | 1.12 | |
| 363.15 | 5.74 | 2.41 | |
| S-3 | 303.15 | 2.21 | 1.32 |
| 333.15 | 3.22 | 2.14 | |
| 363.15 | 4.68 | 3.41 | |
| S-4 | 303.15 | 4.12 | 2.53 |
| 333.15 | 3.58 | 1.15 | |
| 363.15 | 2.11 | 2.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Ji, Y.; Zhang, K.; Jing, L.; Zhao, T. On the Use of the Multi-Site Langmuir Model for Predicting Methane Adsorption on Shale. Energies 2024, 17, 4990. https://doi.org/10.3390/en17194990
Wu Z, Ji Y, Zhang K, Jing L, Zhao T. On the Use of the Multi-Site Langmuir Model for Predicting Methane Adsorption on Shale. Energies. 2024; 17(19):4990. https://doi.org/10.3390/en17194990
Chicago/Turabian StyleWu, Zhe, Yuan Ji, Ke Zhang, Li Jing, and Tianyi Zhao. 2024. "On the Use of the Multi-Site Langmuir Model for Predicting Methane Adsorption on Shale" Energies 17, no. 19: 4990. https://doi.org/10.3390/en17194990
APA StyleWu, Z., Ji, Y., Zhang, K., Jing, L., & Zhao, T. (2024). On the Use of the Multi-Site Langmuir Model for Predicting Methane Adsorption on Shale. Energies, 17(19), 4990. https://doi.org/10.3390/en17194990





