Thermochemical Production of Hydrogen from Biomass: Pyrolysis and Gasification
Abstract
1. Introduction
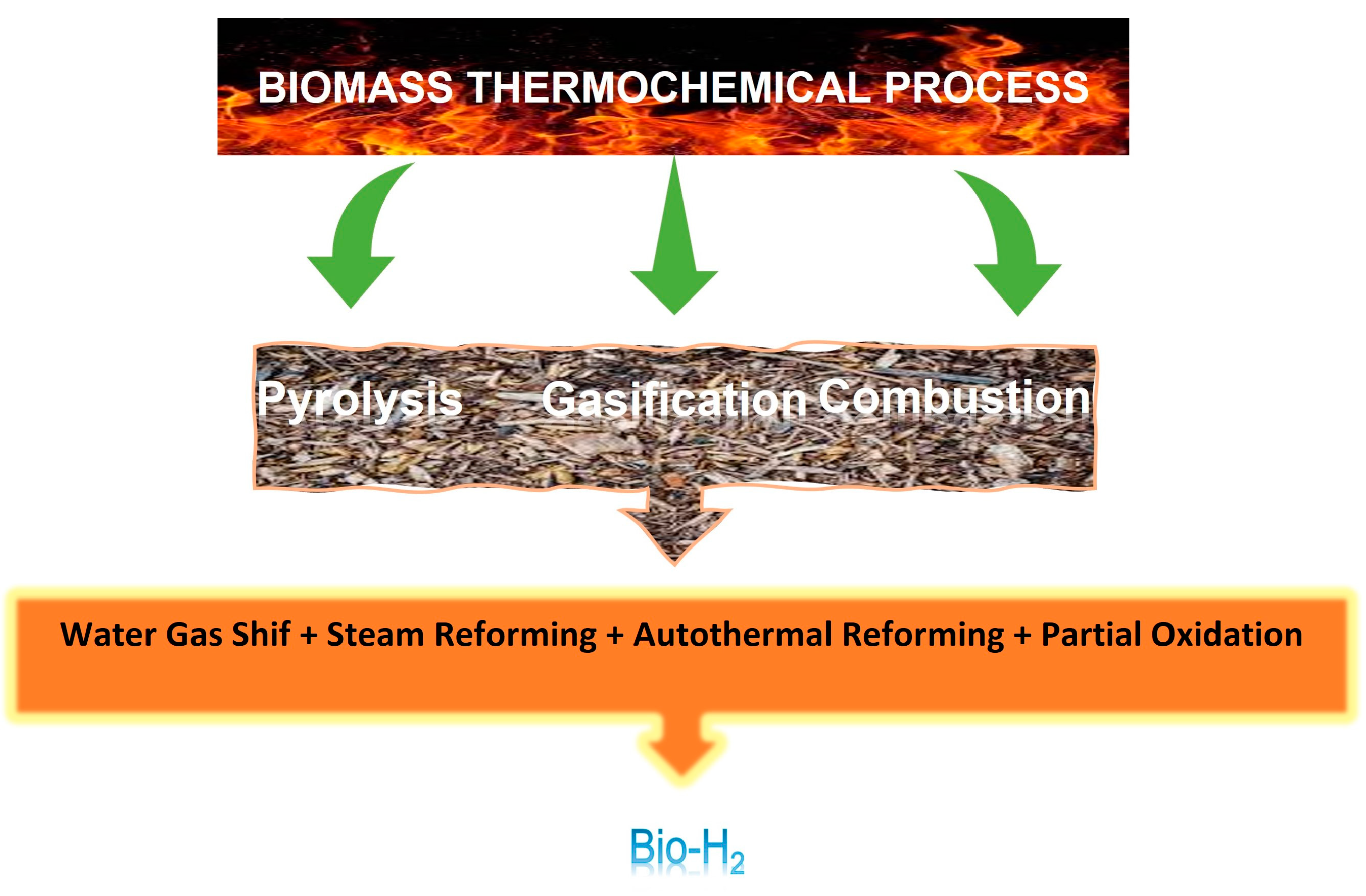
2. Thermochemical Processes: Pyrolysis and Gasification
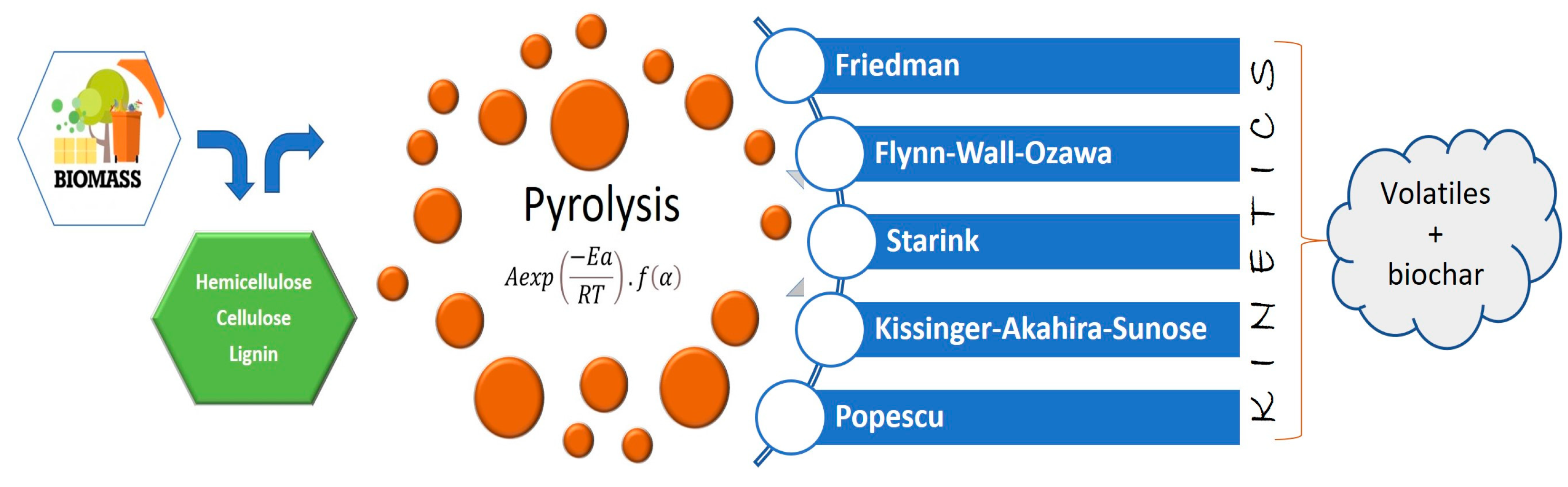
2.1. Generating Biohydrogen from Pyrolysis of Terrestrial Biomass
2.1.1. Catalysts for Generating Biohydrogen via Pyrolysis
2.1.2. Residual Water and Solid Residues to Generate Biohydrogen through Pyrolysis
2.1.3. Influence of the Reaction System
2.2. Generating Biohydrogen by Gasification
2.2.1. Generating Biohydrogen from Gasification of Terrestrial Biomass
2.2.2. Generating Biohydrogen via Gasification of Marine Biomass
2.2.3. Generating Biohydrogen from Gasification–Torrefaction
2.2.4. Generating Biohydrogen from Thermal Plasma Gasification
2.2.5. Catalysts for Generating Biohydrogen by Gasification
2.2.6. Use of Residual Water and Solid Residues to Generate Biohydrogen by Gasification
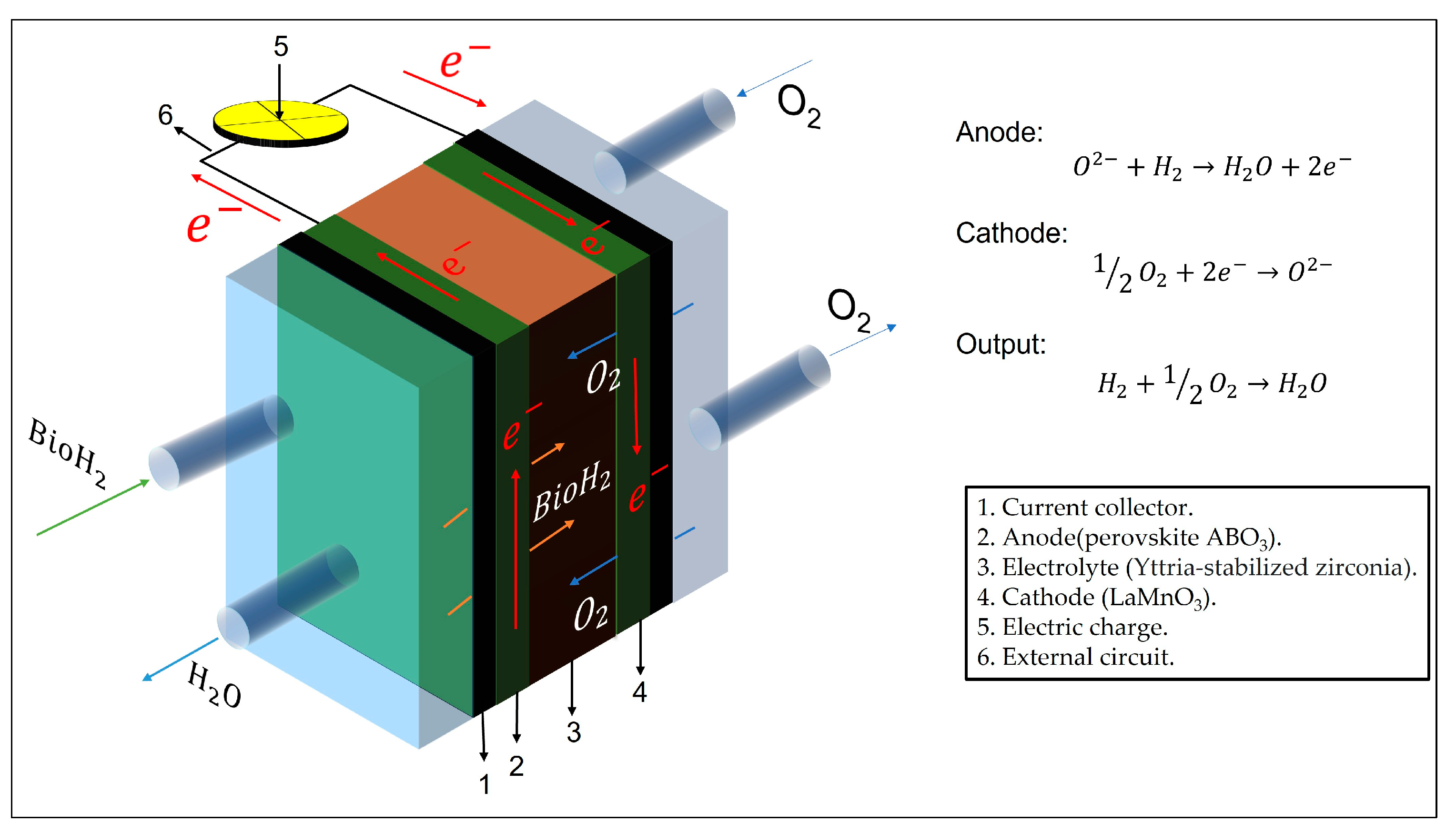
3. Challenges and Future Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, W.; de Souza, C.; Medeiros, A.; de Carvalho, J.; de Andrade, T.; Soccol, C.; Sydney, E. Hydrogen: Current advances and patented technologies of its renewable production. J. Clean. Prod. 2021, 286, 124970. [Google Scholar] [CrossRef]
- Silva, S.; Barros, R.; dos Santos, I.; de Cassia Crispim, A.; Tiago, G.; Lora, E. Technical and economic evaluation of using biomethane from sanitary landfills for supplying vehicles in the Southeastern region of Brazil. Renew. Energy 2022, 196, 1142–1157. [Google Scholar] [CrossRef]
- Badoga, S.; Dalai, A. Liquid Fuels from Oil Sands. In Sustainable Utilization of Natural Resources; CRC Press: Boca Raton, FL, USA, 2017; pp. 121–143. [Google Scholar] [CrossRef]
- Xia, S.; Li, K.; Xiao, H.; Cai, N.; Dong, Z.; Xu, C.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Pyrolysis of Chinese chestnut shells: Effects of temperature and Fe presence on product composition. Bioresour. Technol. 2019, 287, 121444. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Tangsathitkulchai, C.; Tangsathitkulchai, M. Characterization of products from slow pyrolysis of palm kernel cake and cassava pulp residue. Korean J. Chem. Eng. 2011, 28, 2262–2274. [Google Scholar] [CrossRef]
- Capunitan, J.; Capareda, S. Assessing the potential for biofuel production of corn stover pyrolysis using a pressurized batch reactor. Fuel 2012, 95, 563–572. [Google Scholar] [CrossRef]
- Donar, Y.; Sınağ, A. Catalytic effect of tin oxide nanoparticles on cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2016, 119, 69–74. [Google Scholar] [CrossRef]
- Prabhahar, R.; Nagaraj, P.; Jeyasubramanian, K. Promotion of bio oil, H2 gas from the pyrolysis of rice husk assisted with nano silver catalyst and utilization of bio oil blend in CI engine. Int. J. Hydrogen Energy 2020, 45, 16355–16371. [Google Scholar] [CrossRef]
- Waheed, Q.; Wu, C.; Williams, P. Hydrogen production from high temperature steam catalytic gasification of bio-char. J. Energy Inst. 2016, 89, 222–230. [Google Scholar] [CrossRef]
- Cheng, S.; Shu, J.; Xia, H.; Wang, S.; Zhang, L.; Peng, J.; Li, C.; Jiang, X.; Zhang, Q. Pyrolysis of Crofton weed for the production of aldehyde rich bio-oil and combustible matter rich bio-gas. Appl. Therm. Eng. 2019, 148, 1164–1170. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stahl, R.; Yanik, J. The slow and fast pyrolysis of cherry seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef]
- Yue, Y.; Lin, Q.; Irfan, M.; Chen, Q.; Zhao, X. Characteristics and potential values of bio-oil, syngas and biochar derived from Salsola collina Pall. in a fixed bed slow pyrolysis system. Bioresour. Technol. 2016, 220, 378–383. [Google Scholar] [CrossRef]
- Kongkasawan, J.; Nam, H.; Capareda, S. Jatropha waste meal as an alternative energy source via pressurized pyrolysis: A study on temperature effects. Energy 2016, 113, 631–642. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A.; Antonakou, E.; Papazisi, K.; Lappas, A.; Athanassiou, C. Investigating the potential for energy, fuel, materials and chemicals production from corn residues (cobs and stalks) by non-catalytic and catalytic pyrolysis in two reactor configurations. Renew. Sustain. Energy Rev. 2009, 13, 750–762. [Google Scholar] [CrossRef]
- Alagu, R.; Sundaram, E.; Natarajan, E. Thermal and catalytic slow pyrolysis of Calophyllum inophyllum fruit shell. Bioresour. Technol. 2015, 193, 463–468. [Google Scholar] [CrossRef]
- Reshad, A.; Tiwari, P.; Goud, V. Thermo-chemical conversion of waste rubber seed shell to produce fuel and value-added chemicals. J. Energy Inst. 2018, 91, 940–950. [Google Scholar] [CrossRef]
- Setter, C.; Silva, F.; Assis, M.; Ataíde, C.; Trugilho, P.; Oliveira, T. Slow pyrolysis of coffee husk briquettes: Characterization of the solid and liquid fractions. Fuel 2020, 261, 116420. [Google Scholar] [CrossRef]
- Kumari, P.; Mohanty, B. Hydrogen-rich gas production with CO2 capture from steam gasification of pine needle using calcium oxide: Experimental and modeling study. Int. J. Energy Res. 2020, 44, 6927–6938. [Google Scholar] [CrossRef]
- Niu, Y.; Han, F.; Chen, Y.; Lyu, Y.; Wang, L. Experimental study on steam gasification of pine particles for hydrogen-rich gas. J. Energy Inst. 2017, 90, 715–724. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C. A novel reforming method for hydrogen production from biomass steam gasification. Bioresour. Technol. 2009, 100, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Alobaid, F.; Epple, B. Process simulation of steam gasification of torrefied woodchips in a bubbling fluidized bed reactor using aspen plus. Appl. Sci. 2021, 11, 2877. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S. Experimental investigation of biomass devolatilization in steam gasification in a dual fluidised bed gasifier. Fuel 2017, 188, 628–635. [Google Scholar] [CrossRef]
- Rapagnà, S.; Gallucci, K.; Di Marcello, M.; Foscolo, P.; Nacken, M.; Heidenreich, S.; Matt, M. First Al2O3 based catalytic filter candles operating in the fluidized bed gasifier freeboard. Fuel 2012, 97, 718–724. [Google Scholar] [CrossRef]
- Pang, Y.; Yu, D.; Chen, Y.; Jin, G.; Shen, S. Hydrogen production from steam gasification of corn straw catalyzed by blast furnace gas ash. Int. J. Hydrogen Energy 2020, 45, 17191–17199. [Google Scholar] [CrossRef]
- Rapagnà, S.; Gallucci, K.; Foscolo, P. Olivine dolomite and ceramic filters in one vessel to produce clean gas from biomass. Waste Manag. 2018, 71, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Yusup, S.; Kamble, P.; Naqvi, M.; Watson, I. Assessment of energy flows and energy efficiencies in integrated catalytic adsorption steam gasification for hydrogen production. Appl. Energy 2018, 225, 346–355. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, Z.; Hantoko, D.; Zhong, L.; Rahim, D.; Fang, W.; Yan, M. Effect of alkali additives on desulfurization of syngas during supercritical water gasification of sewage sludge. Waste Manag. 2021, 131, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Chuayboon, S.; Abanades, S.; Rodat, S. Experimental analysis of continuous steam gasification of wood biomass for syngas production in a high-temperature particle-fed solar reactor. Chem. Eng. Process. Process. Intensif. 2018, 125, 253–265. [Google Scholar] [CrossRef]
- Norouzi, O.; Safari, F.; Jafarian, S.; Tavasoli, A.; Karimi, A. Hydrothermal gasification performance of Enteromorpha intestinalis as an algal biomass for hydrogen-rich gas production using Ru promoted Fe–Ni/γ-Al2O3 nanocatalysts. Energy Convers. Manag. 2017, 141, 63–71. [Google Scholar] [CrossRef]
- Korberg, A.; Thellufsen, J.; Skov, I.; Chang, M.; Paardekooper, S.; Lund, H.; Mathiesen, B. On the feasibility of direct hydrogen utilisation in a fossil-free Europe. Int. J. Hydrogen Energy 2023, 48, 2877–2891. [Google Scholar] [CrossRef]
- Bailey, C.; Bain, A.; Birk, J.; Hainsselin, M.; Kamal, M.; Linden, H.; Nahmias, D. The Green Hydrogen Report; National Renewable Energy Laboratory (NREL): Washington, DC, USA, 1995.
- Zhiznin, S.; Timokhov, V.; Gusev, A. Economic aspects of nuclear and hydrogen energy in the world and Russia. Int. J. Hydrogen Energy 2020, 45, 31353–31366. [Google Scholar] [CrossRef]
- Schönauer, A.; Glanz, S. Hydrogen in future energy systems: Social acceptance of the technology and its large-scale infrastructure. Int. J. Hydrogen Energy 2022, 47, 12251–12263. [Google Scholar] [CrossRef]
- Sun, H. Hydrogen energy is arousing great attention all over the world. Int. J. Hydrogen Energy 2021, 46, 2845–2846. [Google Scholar] [CrossRef]
- Sattarzadeh, M.; Ebrahimi, M.; Jazayeri, S. Maximum reduction in greenhouse gas emissions by complete replacement of natural gas with pure hydrogen as a sole low reactive fuel in a RCCI engine. Int. J. Engine Res. 2022, 24, 2677–2691. [Google Scholar] [CrossRef]
- Lindner, R. Green hydrogen partnerships with the G lobal South. Advancing an energy justice perspective on “tomorrow’s oil”. Sustain. Dev. 2023, 31, 1038–1053. [Google Scholar] [CrossRef]
- Oosthuizen, A.; Inglesi, R. The impact of policy priority flexibility on the speed of renewable energy adoption. Renew. Energy 2022, 194, 426–438. [Google Scholar] [CrossRef]
- Shamsi, M.; Obaid, A.; Farokhi, S.; Bayat, A. A novel process simulation model for hydrogen production via reforming of biomass gasification tar. Int. J. Hydrogen Energy 2022, 47, 772–781. [Google Scholar] [CrossRef]
- Li, B.; Mbeugang, C.; Liu, D.; Zhang, S.; Wang, S.; Wang, Q.; Xu, Z.X. Simulation of sorption enhanced staged gasification of biomass for hydrogen production in the presence of calcium oxide. Int. J. Hydrogen Energy 2020, 45, 26855–26864. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Chen, M.; Xin, W.; Yang, Z.; Kong, L. Hydrogen production via catalytic pyrolysis of biomass in a two-stage fixed bed reactor system. Int. J. Hydrogen Energy 2014, 39, 13128–13135. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.; Leung, M.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Ayala, N.; Sandoval, G. Bioenergía a partir de residuos forestales y de madera. Madera Bosques 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Foong, S.; Liew, R.; Lee, C.; Tan, W.; Peng, W.; Sonne, C.; Tsang, Y.; Lam, S. Strategic hazard mitigation of waste furniture boards via pyrolysis: Pyrolysis behavior, mechanisms, and value-added products. J. Hazard. Mater. 2022, 421, 126774. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Duman, G.; Uddin, M.; Yanik, J. Hydrogen production from algal biomass via steam gasification. Bioresour. Technol. 2014, 166, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Arin, G. Hydrogen from biomass via pyrolysis: Relationships between yield of hydrogen and temperature. Energy Sources 2004, 26, 1061–1069. [Google Scholar] [CrossRef]
- Demirbas, A. Production of combustible gas from triglycerides via pyrolysis. Energy Sources Part A 2009, 31, 870–875. [Google Scholar] [CrossRef]
- Gaudernack, B.; Lynum, S. Hydrogen from natural gas without release of CO2 to the atmosphere. Int. J. Hydrogen Energy 1998, 23, 1087–1093. [Google Scholar] [CrossRef]
- Fulcheri, L.; Probst, N.; Flamant, G.; Fabry, F.; Grivei, E.; Bourrat, X. Plasma processing: A step towards the production of new grades of carbon black. Carbon 2002, 40, 169–176. [Google Scholar] [CrossRef]
- Gao, N.; Salisu, J.; Quan, C.; Williams, P. Modified nickel-based catalysts for improved steam reforming of biomass tar: A critical review. Renew. Sustain. Energy Rev. 2021, 145, 111023. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Fernandez, E.; Cortazar, M.; Arregi, A.; Olazar, M.; Bilbao, J. Progress on catalyst development for the steam reforming of biomass and waste plastics pyrolysis volatiles: A review. Energy Fuels 2021, 35, 17051–17084. [Google Scholar] [CrossRef]
- Waheed, Q.; Williams, P. Hydrogen production from high temperature pyrolysis/steam reforming of waste biomass: Rice husk, sugar cane bagasse, and wheat straw. Energy Fuels 2013, 27, 6695–6704. [Google Scholar] [CrossRef]
- Duman, G.; Yanik, J. Two-step steam pyrolysis of biomass for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 17000–17008. [Google Scholar] [CrossRef]
- Saidi, M.; Faraji, M. Thermochemical conversion of neem seed biomass to sustainable hydrogen and biofuels: Experimental and theoretical evaluation. Renew. Energy 2024, 221, 119694. [Google Scholar] [CrossRef]
- Chen, G.; Andries, J.; Spliethoff, H. Catalytic pyrolysis of biomass for hydrogen rich fuel gas production. Energy Convers. Manag. 2003, 44, 2289–2296. [Google Scholar] [CrossRef]
- Garcıa, L.; Salvador, M.; Arauzo, J.; Bilbao, R. Catalytic pyrolysis of biomass: Influence of the catalyst pretreatment on gas yields. J. Anal. Appl. Pyrolysis 2001, 58, 491–501. [Google Scholar] [CrossRef]
- Chen, G.; Yao, J.; Liu, J.; Yan, B.; Shan, R. Biomass to hydrogen-rich syngas via catalytic steam reforming of bio-oil. Renew. Energy 2016, 91, 315–322. [Google Scholar] [CrossRef]
- Czernik, S.; Evans, R.; French, R. Hydrogen from biomass-production by steam reforming of biomass pyrolysis oil. Catal. Today 2007, 129, 265–268. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.; Feik, C.; Chornet, E. Hydrogen by catalytic steam reforming of liquid byproducts from biomass thermoconversion processes. Ind. Eng. Chem. Res. 2002, 41, 4209–4215. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Benito, P.; Bilbao, J.; Gayubo, A. Biomass to hydrogen-rich gas via steam reforming of raw bio-oil over Ni/La2O3-αAl2O3 catalyst: Effect of space-time and steam-to-carbon ratio. Fuel 2018, 216, 445–455. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Zhang, L.; Liu, S.; Lu, G. Steam reforming of acetic acid over Ni/ZrO2 catalysts: Effects of nickel loading and particle size on product distribution and coke formation. Appl. Catal. A Gen. 2012, 417, 281–289. [Google Scholar] [CrossRef]
- Takanabe, K.; Aika, K.; Seshan, K.; Lefferts, L. Sustainable hydrogen from bio-oil—Steam reforming of acetic acid as a model oxygenate. J. Catal. 2004, 227, 101–108. [Google Scholar] [CrossRef]
- Mohanty, P.; Patel, M.; Pant, K. Hydrogen production from steam reforming of acetic acid over Cu-Zn supported calcium aluminate. Bioresour. Technol. 2012, 123, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yan, C.; Zhao, X.; Huang, S.; Guo, C. Ni/La2O3-ZrO2 catalyst for hydrogen production from steam reforming of acetic acid as a model compound of bio-oil. Korean J. Chem. Eng. 2017, 34, 305–313. [Google Scholar] [CrossRef]
- Bimbela, F.; Oliva, M.; Ruiz, J.; García, L.; Arauzo, J. Hydrogen production by catalytic steam reforming of acetic acid, a model compound of biomass pyrolysis liquids. J. Anal. Appl. Pyrolysis 2007, 79, 112–120. [Google Scholar] [CrossRef]
- Borges, R.; Ferreira, R.; Rabelo, N.; Noronha, F.; Hori, C. Hydrogen production by steam reforming of acetic acid using hydrotalcite type precursors. Int. J. Hydrogen Energy 2018, 43, 7881–7892. [Google Scholar] [CrossRef]
- Fangzhu, J.; Hongman, S.; Chunfei, W.; Huajuan, L.; Yijiao, J.; Paul, T.; Jun, H. Effect of calcium addition on Mg-AlOx supported Ni catalysts for hydrogen production from pyrolysis-gasification of biomass. Catal. Today 2018, 309, 2–10. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Sun, L.; Xie, X.; Zhao, B.; Si, H.; Hua, D. Novel Ni-Al nanosheet catalyst with homogeneously embedded nickel nanoparticles for hydrogen-rich syngas production from biomass pyrolysis. Int. J. Hydrogen Energy 2021, 46, 1762–1776. [Google Scholar] [CrossRef]
- Llorca, J.; Homs, N.; Sales, J.; de la Piscina, P. Efficient production of hydrogen over supported cobalt catalysts from ethanol steam reforming. J. Catal. 2002, 209, 306–317. [Google Scholar] [CrossRef]
- Nabgan, W.; Abdullah, T.; Mat, R.; Nabgan, B.; Jalil; Firmansyah, L.; Triwahyono, S. Production of hydrogen via steam reforming of acetic acid over Ni and Co supported on La2O3 catalyst. Int. J. Hydrogen Energy 2017, 42, 8975–8985. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Investigation of steam reforming of acetic acid to hydrogen over Ni-Co metal catalyst. J. Mol. Catal. A Chem. 2007, 261, 43–48. [Google Scholar] [CrossRef]
- Choi, I.; Hwang, K.; Lee, K.; Lee, I. Catalytic steam reforming of biomass-derived acetic acid over modified Ni/γ-Al2O3 for sustainable hydrogen production. Int. J. Hydrogen Energy 2019, 44, 180–190. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, A.; Luo, S.; Yi, W.; Hu, S.; Zhang, Y. Catalytic steam reforming of biomass-derived acetic acid over two supported Ni catalysts for hydrogen-rich syngas production. ACS Omega 2019, 4, 13585–13593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Liang, T.; Yang, Z.; Yang, J.; Liu, S. Hydrogen generation from catalytic steam reforming of acetic acid by Ni/attapulgite catalysts. Catalysts 2016, 6, 172. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Liu, Y.; Zhai, Y.; Zhang, R.; Tang, X. Catalytic reforming of acetic acid as a model compound of bio-oil for hydrogen production over Ni-CeO2-MgO/olivine catalysts. Environ. Prog. Sustain. 2015, 34, 915–922. [Google Scholar] [CrossRef]
- Barati, M.; Babatabar, M.; Tavasoli, A.; Dalai, A.; Das, U. Hydrogen production via supercritical water gasification of bagasse using unpromoted and zinc promoted Ru/γ-Al2O3 nanocatalysts. Fuel Process. Technol. 2014, 123, 140–148. [Google Scholar] [CrossRef]
- Doshi, V.; Vuthaluru, H.; Bastow, T. Investigations into the control of odour and viscosity of biomass oil derived from pyrolysis of sewage sludge. Fuel Process. Technol. 2005, 86, 885–897. [Google Scholar] [CrossRef]
- Pokorna, E.; Postelmans, N.; Jenicek, P.; Schreurs, S.; Carleer, R.; Yperman, J. Study of bio-oils and solids from flash pyrolysis of sewage sludges. Fuel 2009, 88, 1344–1350. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Lebedynets, M.; Namieśnik, J.; Buszewski, B. Phenolics occurrence in surface water of the Dniester river basin (West Ukraine): Natural background and industrial pollution. Environ. Geol. 2007, 53, 67–75. [Google Scholar] [CrossRef]
- Uddin, M.; Daud, W.; Abbas, H. Potential hydrogen and non-condensable gases production from biomass pyrolysis: Insights into the process variables. Renew. Sustain. Energy Rev. 2013, 27, 204–224. [Google Scholar] [CrossRef]
- Bridgwater, A. Principles and practice of biomass fast pyrolysis processes for liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Lam, S.; Russell, A.; Lee, C.; Chase, H. Microwave-heated pyrolysis of waste automotive engine oil: Influence of operation parameters on the yield, composition, and fuel properties of pyrolysis oil. Fuel 2012, 92, 327–339. [Google Scholar] [CrossRef]
- Caprariis, B.; Filippis, P.; Scarsella, M.; Petrullo, A.; Palma, V. Biomass Gasification and Tar Reforming in a Two-stage Reactor. Energy Procedia 2014, 61, 1071–1074. [Google Scholar] [CrossRef][Green Version]
- Rincón, J.; Electo, S. Bioenergía: Fuentes, conversión y sustentabilidad. In La Red Iberoamericana de Aprovechamiento de Residuos Orgánicos en Producción de Energía, 1st ed.; Martínez, J.M.R., Lora, E.E.S., Eds.; CYTED: Bogotá, Colombia, 2015; p. 332. ISBN 978-9584 58880-0-5. [Google Scholar]
- Narayanan, D.; Vinithkrishna, N.; Rajkumar, S.; Thangaraja, J.; Sivagaminathan, M.; Devarajan, Y.; Varuvel, E. Techno-economic review assessment of hydrogen utilization in processing the natural gas and biofuels. Int. J. Hydrogen Energy 2022, 48, 21294–21312. [Google Scholar] [CrossRef]
- Elita, R.; Widjaya, G.; Les, B.; Catherine, H. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Qi, D.; Xiaoqi, Z.; Yuling, Z.; Xiaotong, J. Prediction and optimization of syngas production from a kinetic-based biomass gasification process model. Fuel Process. Technol. 2021, 212, 106604. [Google Scholar] [CrossRef]
- Zhenling, L.; Wanxi, P.; Mohsen, M.; Saeid, S.; Mehdi, B. Circulating fluidized bed gasification of biomass for flexible end-use of syngas: A micro and nano scale study for production of bio-methanol. J. Clean. Prod. 2016, 129, 249–255. [Google Scholar] [CrossRef]
- Hao, S.; Guang, Y.; Peixuan, X.; Yuchen, L.; Jun, Z.; Shurong, W.; Haiping, Y.; Hanping, C. Recent development of biomass gasification for H2 rich gas production. Appl. Energy Combust. Sci. 2022, 10, 100059. [Google Scholar] [CrossRef]
- Bishnu, A.; Animesh, D.; Prabir, B. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO. Int. J. Hydrogen Energy 2010, 35, 1582–1589. [Google Scholar] [CrossRef]
- Hakan, K.; Fehmi, A. Experimental results of gasification of walnut shell and pistachio shell in a bubbling fluidized bed gasifier under air and steam atmospheres. Fuel 2018, 214, 285–292. [Google Scholar] [CrossRef]
- Tuomi, S.; Kurkela, E.; Simell, P.; Reinikainen, M. Behaviour of tars on the filter in high temperature filtration of biomass-based gasification gas. Fuel 2015, 139, 220–231. [Google Scholar] [CrossRef]
- D’Orazio, A.; Rapagnà, S.; Foscolo, P.; Gallucci, K.; Nacken, M.; Heidenreich, S.; Dell’Era, A. Gas conditioning in H2 rich syngas production by biomass steam gasification: Experimental comparison between three innovative ceramic filter candles. Int. J. Hydrogen Energy 2015, 40, 7282–7290. [Google Scholar] [CrossRef]
- Jiang, H.; Zhongqing, Y.; Mingnv, G.; Linlin, G.; Li, Z.; Yunfei, Y.; Jingyu, R. Experimental study on the key factors affecting the gasification performance between different biomass: Compare citrus peel with pine sawdust. Int. J. Hydrogen Energy 2022, 47, 30428–30439. [Google Scholar] [CrossRef]
- Olive and Olive Oil Report. Ministry of Commerce. General Directorate of Tradesmen, Craftsmen and Cooperatives. 2018. Available online: https://ticaret.gov.tr/data/5d41e59913b87639ac9e02e8/3acedb62acea083bd15a9f1dfa551bcc.pdf (accessed on 9 October 2023).
- Özgün, T.; Nazlıcan, K.; Atakan, Ö.; Azize, A. Gasification performance of olive pomace in updraft and downdraft fixed bed reactors. Int. J. Hydrogen Energy 2023, 48, 22909–22920. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Ghesti, G.F.; Silveira, E.A.; Lamas, G.C.; Ferreira, R.; Costa, M. Waste-to-hydrogen via CO2/steam-enhanced gasification of spent coffee ground. Clean. Chem. Eng. 2022, 4, 100082. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Chen, Z.; Ding, D. An integrated process for hydrogen-rich gas production from cotton stalks: The simultaneous gasification of pyrolysis gases and char in an entrained flow bed reactor. Bioresour. Technol. 2015, 198, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Huijun, G.; Wanjun, G.; Laihong, S.; Tao, S.; Jun, X. Experimental investigation on biomass gasification using chemical looping in a batch reactor and a continuous dual reactor. Chem. Eng. J. 2016, 286, 689–700. [Google Scholar] [CrossRef]
- Yu, H.; Alex, C. The effect of biomass feeding location on rice husk gasification for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 40582–40589. [Google Scholar] [CrossRef]
- Janitha, C.; Rajan, J.; Henrik, K.; Britt, M.; Marianne, S. Air gasification of wood chips, wood pellets and grass pellets in a bubbling fluidized bed reactor. Energy 2021, 233, 121149. [Google Scholar] [CrossRef]
- Anil, K. Experimental investigation of a dual stage ignition biomass downdraft gasifier for deriving the engine quality gas. Ain Shams Eng. J. 2023, 14, 101912. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Sim, S.J.; Lee, J.H. Production of hydrogen from marine macro-algae biomass using anaerobic sewage sludge microflora. Biotechnol. Bioprocess Eng. 2009, 14, 307–315. [Google Scholar] [CrossRef]
- Olga, B.; Pavel, S. Production of hydrogen from renewable resources and its effectiveness. Int. J. Hydrogen Energy 2012, 37, 11563–11578. [Google Scholar] [CrossRef]
- Shobana, S.; Kumar, G.; Bakonyi, P.; Saratale, G.; Nemestóthy, N.; Bélafi, K.; Chang, J. A review on the biomass pretreatment and inhibitor removal methods as key-steps towards efficient macroalgae-based biohydrogen production. Bioresour. Technol. 2017, 244, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Roostaei, J.; Zhang, Y. Mixed culture of Chlorella sp. and wastewater wild algae for enhanced biomass and lipid accumulation in artificial wastewater medium. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Muhammad, A.; Takuya, O.; Takashi, M.; Takumi, K.; Norihiro, K.; Takao, K. Enhanced Energy Utilization System of Algae: Integrated Drying, Gasification and Combined Cycle. Energy Procedia 2015, 75, 906–911. [Google Scholar] [CrossRef]
- Lee, D. Production efficiency and economic benefit evaluation of biohydrogen produced using macroalgae as a biomass feedstock in Asian circular economies. Int. J. Hydrogen Energy 2022, 47, 40532–40551. [Google Scholar] [CrossRef]
- Muflih, A. Gasification of various biomasses including microalgae using CO2—A thermodynamic study. Renew. Energy 2018, 119, 598–607. [Google Scholar] [CrossRef]
- Abdul, R.; Xiaomin, C.; Fareed, H.; Asif, A.; Guozhao, J.; Binhai, C.; Weiguo, D.; Ming, Z. Hydrogen-rich energy recovery from microalgae (lipid-extracted) via steam catalytic gasification. Algal Res. 2020, 52, 102102. [Google Scholar] [CrossRef]
- Linlin, G.; Mingnv, G.; Jiang, H.; Zhongqing, Y. Experimental study on hydrogen production characteristics of kaolin supported Ni catalyzed by steam gasification of citrus peel. Fuel 2023, 340, 127431. [Google Scholar] [CrossRef]
- Gomes, J.; Mitoura, J.; Guirardello, R. Thermodynamic analysis for hydrogen production from the reaction of subcritical and supercritical gasification of the C. Vulgaris microalgae. Energy 2022, 260, 125030. [Google Scholar] [CrossRef]
- Carole, C.; Sylvain, S.; Jean, C. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Bustamante, V.; Carrillo, A.; Prieto, A.; Corral, J.; Hernández, C. Química de la biomasa vegetal y su efecto en el rendimiento durante la torrefacción: Revisión. Rev. Mex. Cienc. For. 2016, 7, 5–23. [Google Scholar] [CrossRef][Green Version]
- Jinsong, Z.; Qing, C.; Hui, Z.; Xiaowei, C.; Qinfeng, M.; Zhongyang, L.; Kefa, C. Biomass-oxygen gasification in a high-temperature entrained-flow gasifier. Biotechnol. Adv. 2009, 27, 606–611. [Google Scholar] [CrossRef]
- Lei, L.; Jinhao, Z.; Yan, Z.; Chongcong, L.; Changqi, Y. Impact of torrefaction on entrained-flow gasification of pine sawdust: An experimental investigation. Fuel 2021, 289, 119919. [Google Scholar] [CrossRef]
- Zainab, A.; Noemí, L.; Isabel, F.; Alberto, G.; Jesús, A.; José, S. Thermochemical valorization of argan nutshells: Torrefaction and air-steam gasification. Fuel 2023, 332, 125970. [Google Scholar] [CrossRef]
- Favas, J.; Monteiro, E.; Rouboa, A. Hydrogen production using plasma gasification with steam injection. Int. J. Hydrogen Energy 2017, 42, 10997–11005. [Google Scholar] [CrossRef]
- Tamayo, J.; Peña, L.; Vázquez, A.; Brito, Á. Hydrogen-Rich Syngas Production by Plasma Gasification of Existing Biomasses in Cuba. Rev. Cienc. Técnicas Agropecu. 2020, 29, 53–63. [Google Scholar]
- Ralph, M.; Baraka, S.; Diane, H. Using the G-H space to show heat and work efficiencies associated with nitrogen plasma gasification of wood. Chem. Eng. Sci. 2021, 243, 116793. [Google Scholar] [CrossRef]
- Qi, H.; Xu, H.; Zhang, J.; Xu, Z.; Zhong, L.; Cui, P.; Zhu, Z.; Wang, Y. Thermodynamic and techno-economic analyses of hydrogen production from different algae biomass by plasma gasification. Int. J. Hydrogen Energy 2023, 48, 35895–35906. [Google Scholar] [CrossRef]
- Houjun, Z.; Fang, C.; Jinli, Z.; You, H. Supercritical water gasification of fuel gas production from waste lignin: The effect mechanism of different oxidized iron-based catalysts. Int. J. Hydrogen Energy 2021, 46, 30288–30299. [Google Scholar] [CrossRef]
- Changqing, C.; Yupeng, X.; Liuhao, M.; Wenwen, W.; Jinwen, S.; Hui, J. Hydrogen production from supercritical water gasification of soda black liquor with various metal oxides. Renew. Energy 2020, 157, 24–32. [Google Scholar] [CrossRef]
- Peng, W.; Peng, X.; Laihong, S. Promotional effect of Ca on La1-xCaxFeO3 perovskites for ammonia production via steam catalytic gasification of microalgae. Chem. Eng. J. 2023, 455, 140483. [Google Scholar] [CrossRef]
- Lalsare, A.; Wang, Y.; Li, Q.; Sivri, A.; Vukmanovich, R.; Dumitrescu, C.; Hu, J. Hydrogen-rich syngas production through synergistic methane-activated catalytic biomass gasification. ACS Sustain. Chem. Eng. 2019, 7, 16060–16071. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, Z.; Jiao, W.; Li, L.; Zuo, Z.; Li, G.; Hao, Z.; Song, S.; Huang, J.; Fang, Y. Influencing factors and reaction mechanism for catalytic CO2 gasification of sawdust char using K-modified transition metal composite catalysts: Experimental and DFT studies. Energy Convers. Manag. 2020, 208, 112522. [Google Scholar] [CrossRef]
- Hu, J.; Jia, Z.; Zhao, S.; Wang, W.; Zhang, Q.; Liu, R.; Huang, Z. Activated char supported Fe-Ni catalyst for syngas production from catalytic gasification of pine wood. Bioresour. Technol. 2021, 340, 125600. [Google Scholar] [CrossRef]
- Gao, N.; Śliz, M.; Quan, C.; Bieniek, A.; Magdziarz, A. Biomass CO2 gasification with CaO looping for syngas production in a fixed-bed reactor. Renew. Energy 2021, 167, 652–661. [Google Scholar] [CrossRef]
- Sui, M.; Li, G.; Guan, Y.; Li, C.; Zhou, R.; Zarnegar, A. Hydrogen and syngas production from steam gasification of biomass using cement as catalyst. Biomass Convers. Biorefinery 2020, 10, 119–124. [Google Scholar] [CrossRef]
- Inayat, M.; Sulaiman, S.; Shahbaz, M.; Bhayo, B. Application of response surface methodology in catalytic co-gasification of palm wastes for bioenergy conversion using mineral catalysts. Biomass Bioenergy 2020, 132, 105418. [Google Scholar] [CrossRef]
- Cortazar, M.; Lopez, G.; Alvarez, J.; Amutio, M.; Bilbao, J.; Olazar, M. Behaviour of primary catalysts in the biomass steam gasification in a fountain confined spouted bed. Fuel 2019, 253, 1446–1456. [Google Scholar] [CrossRef]
- Mastuli, M.; Kamarulzaman, N.; Kasim, M.; Sivasangar, S.; Saiman, M.; Taufiq-Yap, Y. Catalytic gasification of oil palm frond biomass in supercritical water using MgO supported Ni, Cu and Zn oxides as catalysts for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 11215–11228. [Google Scholar] [CrossRef]
- Mastuli, M.; Kasim, M.; Mahat, A.; Asikin-Mijan, N.; Sivasangar, S.; Taufiq-Yap, Y. Structural and catalytic studies of Mg1−xNixO nanomaterials for gasification of biomass in supercritical water for H2-rich syngas production. Int. J. Hydrogen Energy 2020, 45, 33218–33234. [Google Scholar] [CrossRef]
- Mastuli, M.; Kamarulzaman, N.; Kasim, M.; Mahat, A.; Matsumura, Y.; Taufiq-Yap, Y. Catalytic supercritical water gasification of oil palm frond biomass using nanosized MgO doped Zn catalysts. J. Supercrit. Fluids 2019, 154, 104610. [Google Scholar] [CrossRef]
- Saha, B. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Gökkaya, D.; Sert, M.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal gasification of the isolated hemicellulose and sawdust of the white poplar (Populus alba L.). J. Supercrit. Fluids 2020, 162, 104846. [Google Scholar] [CrossRef]
- Okolie, J.; Mukherjee, A.; Nanda, S.; Dalai, A.; Kozinski, J. Catalytic supercritical water gasification of soybean straw: Effects of catalyst supports and promoters. Ind. Eng. Chem. Res. 2021, 60, 5770–5782. [Google Scholar] [CrossRef]
- Madenoğlu, T.; Yıldırır, E.; Sağlam, M.; Yüksel, M.; Ballice, L. Improvement in hydrogen production from hard-shell nut residues by catalytic hydrothermal gasification. J. Supercrit. Fluids 2014, 95, 339–347. [Google Scholar] [CrossRef]
- Kuo, J.; Lin, C.; Ho, C.; Hung, J. Influence of different fluidization and gasification parameters on syngas composition and heavy metal retention in a two-stage fluidized bed gasification process. Environ. Sci. Pollut. Res. 2021, 28, 22927–22935. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Alex, V.; Edward, L. The integration of low temperature supercritical water gasification with continuous in situ nano-catalyst synthesis for hydrogen generation from biomass wastewater. Chem. Eng. J. 2023, 455, 140845. [Google Scholar] [CrossRef]
- Bedoya, A.; Castrillón, J.; Ramirez, J.; Vásquez, J.; Arias, Z. Producción biológica de hidrógeno: Una aproximación al estado del arte. Dyna 2008, 75, 137–157. [Google Scholar]
- Levin, D.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Dutta, D.; De, D.; Chaudhuri, S. Hydrogen production by Cyanobacteria. Microb. Cell Factories 2005, 4, 36. [Google Scholar] [CrossRef]
- Noblecourt, A.; Christophe, G.; Larroche, C.; Fontanille, P. Hydrogen production by dark fermentation from pre-fermented depackaging food wastes. Bioresour. Technol. 2018, 247, 864–870. [Google Scholar] [CrossRef] [PubMed]
| Biomass | ≈Max. Yield H2 (%) | ≈Max. Temperature (°C) |
|---|---|---|
| Palm shell | 34 | 900 |
| Olive stalk | 16 | 850 |
| Walnut shell | 22 | 850 |
| Corn cob | 16 | 850 |
| Wheat straw | 54 | 750 |
| Sunflower | 17 | 850 |
| Cellulose | 15 | 700 |
| Bagasse | 38 | 600 |
| Corn stover | 17 | 700 |
| Palm kernel | 21 | 700 |
| Casava pulp | 28 | 800 |
| Coconut shell (CS) | 57 | 800 |
| Rice husk (RH) | 10 | 550 |
| Chestnut (Ch) | 18 | 800 |
| Sugar bagasse | 30 | 700 |
| CS + rhenium carbide | 57 | 800 |
| RH + silver | 14 | 550 |
| Ch + iron | 32 | 800 |
| Biomass | ≈Max. Yield Syngas (%) | ≈Max. Temperature (°C) |
|---|---|---|
| Lignin | 45 | 850 |
| Rice straw | 40 | 800 |
| Sawdust | 46 | 800 |
| Cherry | 22 | 600 |
| Calophyllum inophyllum | 40 | 500 |
| Sugarcane leaves | 55 | 650 |
| Rubber shell seed | 25 | 600 |
| Corn cob | 40 | 700 |
| Corn stalk | 30 | 700 |
| Palm shell | 65 | 900 |
| Cellulose | 22 | 700 |
| Coffee husk | 34 | 450 |
| Salsola collina | 45 | 500 |
| Pine wood | 27 | 700 |
| Palm kernel | 25 | 800 |
| Casava | 35 | 800 |
| Crofton weed | 46 | 800 |
| Jatropha waste meal | 22 | 500 |
| Biomass | ≈Max. Yield H2 | ≈Max. Temperature (°C) |
|---|---|---|
| Pine needle + CaO | 68%vol | 850 |
| Pine wood | 6%wt | 850 |
| Pine sawdust | 38%vol | 950 |
| Woodchips | 52%vol | 900 |
| Pine wood pellets | 2%wt | 820 |
| Almond shells+Olivine/Al2O3/NiO | 15%wt | 813 |
| Corn straw | 58%vol | 950 |
| Palm kernel shell | 49 mol/kg | 750 |
| Sewage sludge | 52%vol | 450 |
| Beech wood | 22 mol/kg | 1300 |
| Green macroalgae | 11 mol/kg | 600 |
| Sugarcane bagasse+Ni-Al2O3 | 39 mol/kg | 1050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Flores, J.J.; Alcaraz-Vera, J.V.; Ávalos-Rodríguez, M.L.; Guzmán-Mejía, E.; Rutiaga-Quiñones, J.G.; Pintor-Ibarra, L.F.; Guevara-Martínez, S.J. Thermochemical Production of Hydrogen from Biomass: Pyrolysis and Gasification. Energies 2024, 17, 537. https://doi.org/10.3390/en17020537
Alvarado-Flores JJ, Alcaraz-Vera JV, Ávalos-Rodríguez ML, Guzmán-Mejía E, Rutiaga-Quiñones JG, Pintor-Ibarra LF, Guevara-Martínez SJ. Thermochemical Production of Hydrogen from Biomass: Pyrolysis and Gasification. Energies. 2024; 17(2):537. https://doi.org/10.3390/en17020537
Chicago/Turabian StyleAlvarado-Flores, José Juan, Jorge Víctor Alcaraz-Vera, María Liliana Ávalos-Rodríguez, Erandini Guzmán-Mejía, José Guadalupe Rutiaga-Quiñones, Luís Fernando Pintor-Ibarra, and Santiago José Guevara-Martínez. 2024. "Thermochemical Production of Hydrogen from Biomass: Pyrolysis and Gasification" Energies 17, no. 2: 537. https://doi.org/10.3390/en17020537
APA StyleAlvarado-Flores, J. J., Alcaraz-Vera, J. V., Ávalos-Rodríguez, M. L., Guzmán-Mejía, E., Rutiaga-Quiñones, J. G., Pintor-Ibarra, L. F., & Guevara-Martínez, S. J. (2024). Thermochemical Production of Hydrogen from Biomass: Pyrolysis and Gasification. Energies, 17(2), 537. https://doi.org/10.3390/en17020537






